Abstract
Nitric oxide (NO) produced by macrophages is toxic to host tissues and invading pathogens and its regulation is therefore essential to suppress host cytotoxicity. Macrophage arginase 1 (Arg1) inhibits the production of NO by competing with NO synthases for arginine, the common substrate of NO synthases and arginases. Two signal transduction pathways control the production of Arg1 in macrophages. First, a pathway dependent on the Toll-like receptor (TLR) adaptor protein myeloid differentiation marker 88 (MyD88) induces the expression of Arg1 in intracellular infections, whereas a second pathway, which is dependent on signal transducer and activator of transcription 6 (STAT6) is required for the expression of Arg1 in alternatively-activated macrophages. We found that mycobacteria-infected macrophages produced soluble factors, including interleukin-6 (IL-6), IL-10, and granulocyte colony–stimulating factor (G-CSF), that induced the expression of Arg1 in an autocrine-paracrine manner. We further established that Arg1 expression was controlled by the MyD88-dependent production of IL-6, IL-10, and G-CSF rather than by cell-intrinsic MyD88 signaling to Arg1. Our data reveal that the MyD88-dependent pathway that induces expression of Arg1 after infection by mycobacteria requires the activation of STAT3 and may result in the development of an immunosuppressive niche in granulomas because of the induced production of Arg1 in surrounding uninfected macrophages.
Introduction
A major conundrum in understanding immunity to Mycobacterium tuberculosis (Mtb) is how populations of bacteria persist during the assault from cell-mediated immune responses that restrain bacterial growth, usually for the lifetime of an infected individual. A related question concerns the underlying mechanisms that suppress bacterial growth but fail to completely eliminate the bacteria, which leads to the generation of a reservoir of latently infected people, estimated at one third of humanity. Understanding the host-pathogen interplay in tuberculosis is essential for combining efficacious anti-tuberculosis drugs with new therapeutic approaches that might enhance immune-mediated clearance of the bacteria.
After its uptake in aerosols, Mtb is phagocytosed by macrophages in the lung. The early phagocyte response induces the recruitment of monocytes into the lung, providing the bacteria with additional host cells in which to replicate (1, 2). In macrophages, Mtb resides mainly within phagosomes in which the bacteria use multiple adaptive measures to avoid and compensate for antibacterial agents that are deployed by the host. Among the antimycobacterial strategies of the host, the free radical nitric oxide (NO), which is directly toxic to diverse intracellular pathogens including Mtb, is one of the most important (3, 4). Loss of inducible NO synthase (iNOS), the host enzyme that generates NO, by genetic ablation or pharmacologic inhibition leads to increased susceptibility to mycobacteria and early death in infected mice (5–8). Mtb blocks the recruitment of iNOS to the phagosomal membrane, possibly as a means of limiting exposure to NO (9). Another method of immune evasion focuses on the possibility that Mtb alters macrophage-mediated metabolism of L-arginine to reduce the amount of NO produced. The underlying mechanism of depletion of L-arginine involves increases in the expression of the gene encoding type 1 arginase (Arg1) in macrophages activated by mycobacteria through the Toll-like receptor (TLR) pathway. Arg1 suppresses the amount of NO that can be generated by competing with iNOS for L-arginine (10–13). We have shown that mice lacking Arg1 in macrophages have decreased Mtb loads compared to those of control mice; an effect that correlates with an enhanced NO response by both purified macrophages and macrophages within granulomatous lesions in experimental mycobacterial infections (14). Arg1-deficient macrophages also have an increased ability to kill Mtb relative to that of wild-type macrophages in vitro when macrophages are stimulated to produce increased amounts of iNOS by interferon-γ (IFN-γ), and of Arg1 by interleukin-4 (IL-4) and IL-10 (15). Thus, macrophage Arg1 limits the production of NO in vitro and in vivo, thereby inhibiting efficient clearance of the mycobacteria.
Mycobacteria induce the expression of Arg1 by a mechanism that requires the TLR adaptor protein myeloid differentiation marker 88 (MyD88) and the transcription factor CAAT/enhancer binding protein β (C/EBPβ) (14). The MyD88-C/EBPβ pathway is distinct from the well-understood signal transducer and activator of transcription 6 (STAT6)-dependent “alternative activation” pathway, which induces the expression of Arg1 in the context of T helper 2 (TH2)-type immunity, because STAT6 is not required for the expression of Arg1 during mycobacterial infection (14). Here, we reveal a cell-extrinsic pathway induced by mycobacterial infection that controls the expression of Arg1. We found that infection of macrophages by mycobacteria drove the production of IL-6, IL-10, and granulocyte colony–stimulating factor (G-CSF), which subsequently induced the expression of Arg1 in uninfected cells by the STAT3 signaling pathway. An implication of this finding is that mycobacterial-infected macrophages condition neighboring uninfected cells to partially suppress the production of NO, possibly as a niche-modification mechanism.
Results
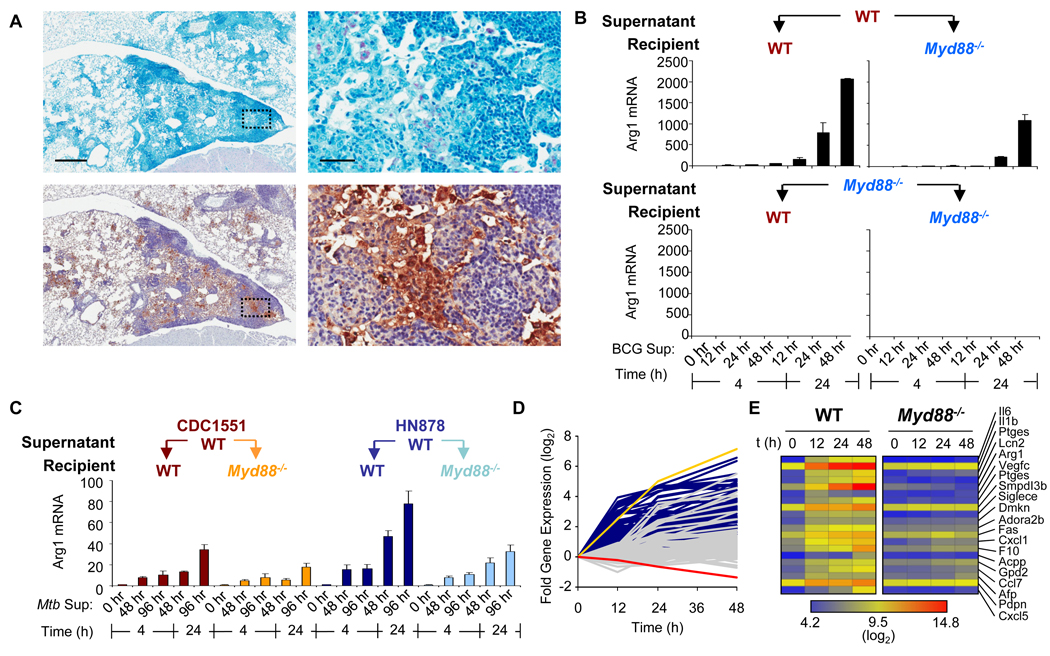
Expression of Arg1 is induced by a secreted factor(s) after infection by Mycobacterium spp
The infection of bone marrow–derived macrophages (BMDMs) by Mycobacterium bovis bacillus Calmette-Guérin (BCG) induces the expression of Arg1 through a pathway that is dependent on the transcription factor C/EBPβ and the TLR adaptor protein MyD88. (fig. S1) (14). In mice, Arg1 protein colocalized with macrophages in BCG-infected lungs in regions of inflammation (Fig. 1A). Previous studies in animal models of mycobacteria infection have demonstrated that infected cells produce type I interferons that function in an autocrine-paracrine way to induce expression of the gene encoding iNOS (16). Reasoning that a similar mechanism might be used by bacteria to induce the expression of Arg1, we designed a supernatant transfer experiment to determine whether BCG infected-macrophages produced a factor(s) that could induce the expression of Arg1 in uninfected cells. BMDMs from wild-type and Myd88−/− mice were left untreated or were infected with BCG. Culture supernatants collected at 12, 24, and 48 hours post-infection, were filter-sterilized and used to stimulate uninfected wild-type and Myd88−/− BMDMs for 4 and 24 hours. We found that wild-type and Myd88−/− BMDMs stimulated with supernatant from BCG-infected wild-type cells expressed Arg1, indicating that a soluble factor(s) induced the expression of Arg1 in an MyD88-independent manner (Fig. 1B). Supernatants from BCG infected Myd88−/− BMDMs were unable to induce the expression of Arg1 expression in BMDMs from either strain of mouse, which indicated that the factor(s) responsible for the induction of Arg1 expression was generated through an MyD88-dependent mechanism. Additionally, the expression of Arg1 in recipient cells cannot be attributed solely to mycobacterial material, which would have been present in supernatants from infected wild-type and Myd88−/− BMDMs. Thus, a key role of MyD88 in modulating the expression of Arg1 is to regulate the production of soluble factors that can induce Arg1 expression by an autocrine-paracrine mechanism. To determine if this phenomenon was common to other mycobacterial infections, wild-type BMDMs were infected with two clinical isolates of Mtb, CDC1551 and HN878, that activate the production of macrophage cytokines to different degrees (17). The culture supernatants were used to stimulate wild-type and Myd88−/− BMDMs. Consistent with the BCG experiments, supernatants from Mtb-infected BMDMs were able to induce the expression of Arg1 in uninfected wild-type and Myd88−/− cells (Fig. 1C).
Fig. 1.
Mycobacteria-infected cells secrete factor(s) that induce the expression of Arg1. (A) Wild-type mice (n = 4) were infected intranasally with BCG. Four weeks after infection, mice were sacrificed and lungs were prepared for histology by staining for acid-fast bacilli (pink, top) and for Arg1 (brown, bottom). Scale bars represent 500 µm (left panels) and 50 µm (right panels). (B) BMDMs from wild-type and Myd88−/− mice (n = 2 mice) were infected with BCG (at MOIs of 100 and 10) for 12, 24, and 48 hours. Supernatants (a 1:2 dilution of the combined supernatants from both sets of infections) were used to stimulate BMDMs from wild-type or Myd88−/− mice for either 4 or 24 hours. RNA was analyzed by qRT-PCR. Data shown are the mean fold-increase in Arg1 mRNA ± the standard deviation (SD), and are representative of two experiments (A and B). (C) BMDMs from wild-type mice were infected with Mtb strains CDC1551 and HN878 (n = 3). Supernatants from these infections were used to stimulate wild-type and Myd88−/− BMDMs (n = 3). RNA was analyzed by qRT-PCR. Data shown are the mean fold-increase in Arg1 mRNA ± the standard deviation (SD), and are from one experiment. (D and E) BMDMs from wild-type and Myd88−/− mice were infected with BCG over the indicated times. RNA from these infected cells was subjected to Affymetrix expression analysis. (D) Profiles of the fold-increase in gene expression of wild-type (dark blue traces, with Arg1 as the yellow trace) and Myd88−/− (gray traces, with Arg1 as the red trace) over time in log2 scale after infection with BCG. (E) Gene expression heat map clustered by secreted or extracellular GO terms.
To identify the secreted factor(s), we infected wild-type and Myd88−/− BMDMs with BCG for 12, 24, or 48 hours, or left cells uninfected and subjected purified RNA from these cells to Affymetrix transcript analysis (fig. S2). We focused on annotated messenger RNAs (mRNAs) with the gene ontology (GO) terms “extracellular space” and “extracellular region” that increased in abundance at least two-fold in wild-type BMDMs at 12 hours, but did not increase two-fold in abundance in Myd88−/− macrophages at the same time point. Eighty candidate probe sets were identified (Fig. 1D). A portion of the MyD88-dependent genes were not induced at 12 hours, but displayed a delayed induction between 12 and 48 hours that is consistent with previous microarray analysis of the mycobacterial infection of macrophages (18). We expected that the factor(s) that we were interested in would likely not be induced to appreciable amounts in Myd88−/− BMDMs, and therefore we modified our transcript analysis to include candidates that were induced ≥ two-fold at 12 hours in wild-type cells, but less than two-fold at all time points in Myd88−/− cells. As anticipated, Arg1 ranked in the highest cluster, and secreted factors, including IL-6 and IL-1β, were ranked at the top of the list (Fig. 1E).
C/EBPβ transcription requires MyD88 and is partially responsible for the expression of Arg1 after infection by BCG (fig. S1) (14). We next determined the role of C/EBPβ in the production of, and response to, secreted factors after infection by BCG. Fetal liver–derived macrophages (FLDMs) from wild-type or Cebpb−/− embryos were infected with BCG for 24 hours, and the supernatants were used to stimulate naïve FLDMs from mice of the same genotypes. Cebpb−/− cells showed inhibited production of Arg1 mRNA compared to wild-type cells receiving supernatant from BCG-infected wild-type cells (fig. S3A). Additionally, supernatants from BCG-infected Cebpb−/− cells were unable to induce the expression of Arg1 in wild-type cells to the same extent that was observed when wild-type cells were incubated with supernatant from BCG-infected wild-type cells. Together, our results suggest that C/EBPβ was partially required for both the production of, and the response to, the secreted factors that were responsible for the induction of Arg1, revealing the likely mechanism of the partial dependence of Arg1 expression on C/EBPβ in mycobacterial-infected macrophages (14).
The STAT3-activating cytokines IL-6, IL-10, and G-CSF are secreted from BCG-infected BMDMs and are critical for Arg1 expression
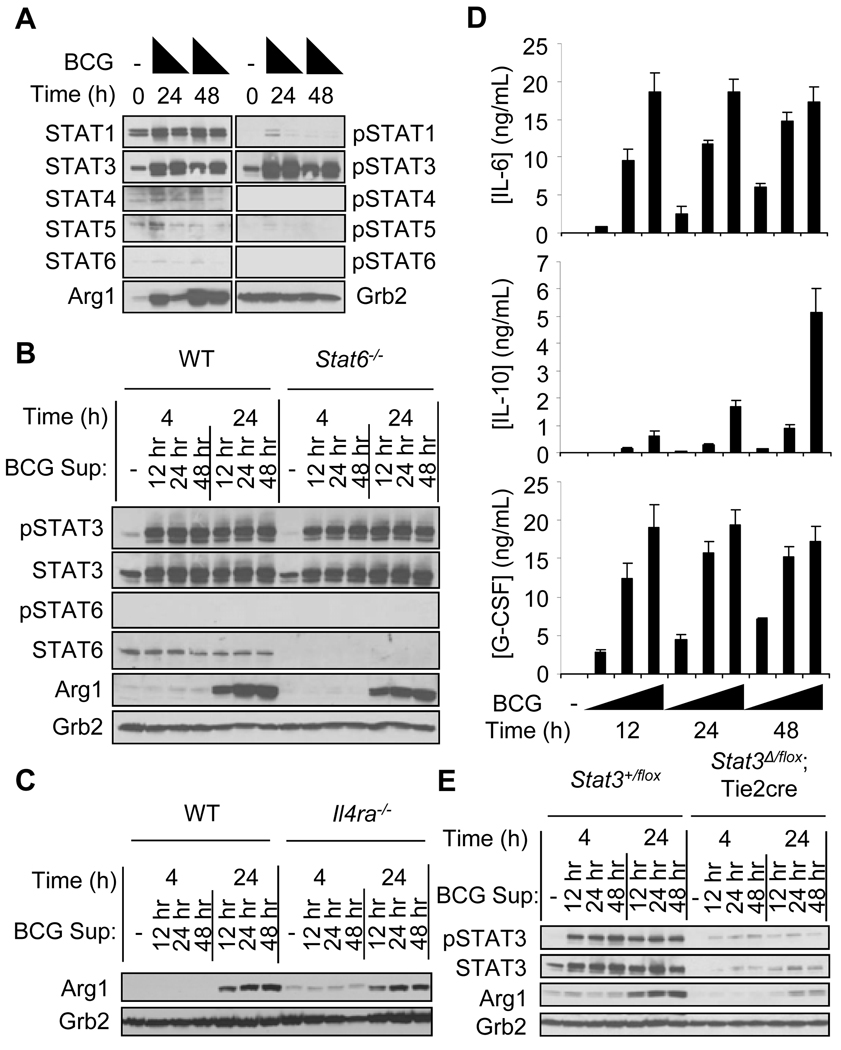
To isolate the secreted factor(s), we first determined the signaling mechanism activated by the autocrine-paracrine pathway that induces the expression of Arg1. Plausible candidates for soluble factors were the STAT6-activating cytokines IL-4 and IL-13, although our previous data suggested that STAT6 signaling was unlikely to play a role in the MyD88-dependent pathway of Arg1 expression (14). However, a recent study showed that the infection of macrophages by Francisella tularensis induces the production of IL-4 and IL-13, which signal in an autocrine-paracrine manner through STAT6 and cause macrophages to express genes characteristic of alternatively-activated macrophages, including Arg1 (19). To test the role of IL-4, IL-13, and STAT6, we first measured the patterns of STAT phosphorylation that accompanied mycobacterial infection. BMDMs from wild-type mice were infected with BCG and cell lysates were collected and analyzed for the activation of STATs (Fig. 2A). We could not detect tyrosine phosphorylation of STAT6, which is necessary for the expression of Arg1 in response to IL-4, IL-13, or both (13, 20–22). When supernatants were transferred from infected wild-type cells to uninfected wild-type or Stat6−/− BMDMs, there was no difference in the generation of Arg1 protein (Fig. 2B). Additionally, transfer of supernatant to BMDMs that lack the receptor subunit used by both IL-4 and IL-13 (Il4ra−/−) induced the production of Arg1 to the same extent as that produced by control cells (Fig. 2C). Thus, STAT6 signaling through the IL-4Rα chain was not required for the Arg1-inducing effects of the soluble factor(s), definitively excluding a role for IL-4 or IL-13 in Arg1 expression in our model. Next, we addressed whether the Arg1-expressing macrophages were alternatively activated. Of 25 genes commonly associated with alternative activation, only five were increased in expression in BCG-infected macrophages (fig. S4A). Additionally, the canonical alternative activation markers Chi3l3 and Retnla were not found after stimulation with supernatant from BCG-infected cells (fig. S4B). Therefore, although BCG infection and supernatant transfer induced the expression of Arg1 in macrophages, these cells were not alternatively activated.
Fig. 2.
The activation of STAT3, but not STAT6, is critical for the induction of Arg1 expression after infection with BCG. (A) BMDMs from wild-type mice were left untreated, or were infected with BCG (at MOIs of 100 or 10). Whole-cell lysates were analyzed by Western blotting for the indicated proteins. Data shown are from one experiment with pooled BMDMs from six mice. Culture supernatants from wild-type BMDMs infected with BCG for 12, 24, and 48 hours were used to stimulate BMDMs from wild-type and Stat6−/− mice (B), and BMDMs from wild-type and Il4ra−/− mice (C) for 4 and 24 hours. Whole-cell lysates were analyzed by Western blotting for the indicated proteins. Data shown are from one experiment with three mice (B) and from one experiment with one mouse (C). (D) IL-6, IL-10, and G-CSF were detected by Luminex in supernatants from BMDMs from wild-type mice infected with BCG at MOIs of 100, 10, and 1. (E) BMDMs from Stat3+/flox mice or Stat3Δ/flox;Tie2cre mice were stimulated with supernatants from BMDMs from wild-type mice infected with BCG at an MOI of 100. Whole-cell lysates were analyzed by Western blotting (n=1 based on deletion efficiency of STAT3). Data shown in D and E are from two experiments, except for the G-CSF data in (D) which are from one experiment.
Whereas STAT6 was not necessary for the induced expression of Arg1, STAT3 was tyrosine phosphorylated after infection with BCG (Fig. 2A). The STAT3 signaling pathway is activated by a large number of cytokines, including IL-6, IL-10, and G-CSF, which are made by activated macrophages. Indeed, IL-6, IL-10, and G-CSF accumulated in supernatants after the infection of wild-type BMDMs with BCG (Fig. 2D), but were not produced by BCG-infected Myd88−/− cells (fig. S5), and the abundance of IL-6 and G-CSF mRNAs was reduced in Cebpb−/− cells compared to those in wild-type cells after infection with BCG (fig. S3B). To determine whether STAT3 signaling was responsible for the induction of Arg1 expression, we used supernatants from BCG-infected wild-type BMDMs to stimulate BMDMs deficient in STAT3 signaling (Stat3Δ/flox;Tie2cre) or control BMDMs from Stat3+/flox mice (23). The induction of Arg1 expression was substantially reduced in Stat3Δ/flox ;Tie2cre BMDMs compared to that in control cells, confirming that the factor(s) secreted after BCG infection stimulated macrophages to express Arg1 in a manner dependent on STAT3 signaling (Fig. 2E). IL-6, IL-10, and G-CSF were produced after infection with BCG and were sufficient to induce Arg1 production in macrophages (Fig. 2D and fig. S6). Based on these data, we next re-applied the supernatant transfer technique to determine which STAT3-activating cytokine was necessary for induction of Arg1 expression.
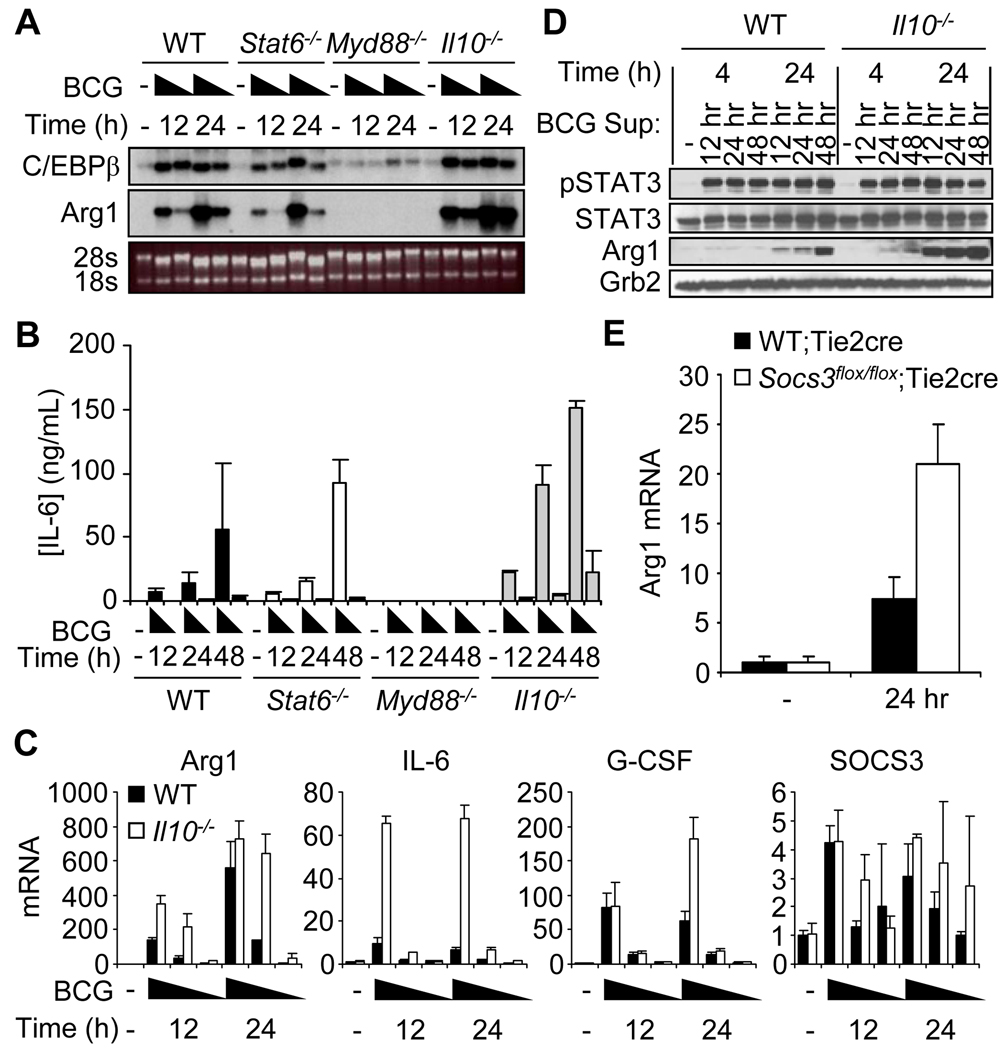
We first tested the requirement of IL-10 for Arg1 expression, because of a report that described the increased alternative activation of macrophages after mycobacterial infection, in which macrophages exhibited enhanced production of IL-10 relative to that of control macrophages (15). BMDMs from Il10−/− mice produced increased amounts of Arg1 mRNA relative to those of wild-type cells after infection with BCG (Fig. 3, A and C). We detected a substantial increase in the amount of IL-6 produced by Il10−/− cells compared to that in supernatants from infected wild-type BMDMs (Fig. 3, B and C). Although these data indicated that IL-10 alone was not responsible for STAT3-mediated induction of Arg1 expression, the infection of Il10−/− BMDMs provided an opportunity to stimulate uninfected BMDMs with supernatants that contained excessive amounts of IL-6. Correlating with the augmented amounts of IL-6, the abundance of Arg1 protein was increased when cells were stimulated with supernatant from BCG-infected Il10−/− BMDMs relative to that which occurred after stimulation with supernatant from BCG-infected wild-type cells (Fig. 3D). This phenomenon may also be attributed to increased G-CSF signaling, because G-CSF mRNA was modestly increased in infected Il10−/− BMDMs compared to that in wild-type cells, and the combination of IL-6 and G-CSF had a synergistic effect on the production of Arg1 protein (Fig. 3C and fig. S6C).
Fig. 3.
The expression of Arg1 is increased in BCG-infected macrophages from Il10−/− mice compared to that in cells from wild-type mice. (A to C) BMDMs from wild-type, Stat6−/−, Myd88−/−, and Il10−/− mice were left untreated or were infected with BCG (at MOIs of 100, 10, and 1). RNA was analyzed by gel electrophoresis followed by Northern blotting (A, MOIs of 100 and 10 are shown) or by qRT-PCR (C). (B) Culture supernatants from the above BCG-infected cells were collected and analyzed by ELISA for the presence of IL-6 (data from cells infected at MOIs of 100 and 10 are shown). Data are presented as the mean concentration of IL-6 (ng/ml) ± SD. (D) Culture supernatants from wild-type and Il10−/− BMDMs infected with BCG (combined supernatants from infections at MOIs of 100 and 10) were used to stimulate BMDMs from wild-type mice for 4 and 24 hours. Whole-cell lysates were analyzed by Western blotting for the indicated proteins. (E) Supernatants were collected from wild-type BMDMs infected with BCG (at an MOI of 10) for 24 hours. Supernatants were diluted 1:2 and used to stimulate BMDMs from wild-type;Tie2cre mice (n = 6 mice) or Socs3flox/flox;Tie2cre mice (n = 10 mice). Twenty-four hours after stimulation, RNA was analyzed by qRT-PCR. Data are from one experiment with at least three mice per group (A to D) or from two experiments combined and presented as the mean expression ± SD (E).
SOCS3 is an essential factor to block IL-6- and G-CSF-mediated activation of STAT3 signaling (24–27). Consequently, we determined whether SOCS3 also inhibited STAT3-mediated induction of Arg1 expression after infection with BCG. Whereas the abundance of SOCS3 mRNA was similar in BCG-infected wild-type and Il10−/− BMDMs (Fig. 3C), we observed that BMDMs from Socs3flox/flox;Tie2cre mice produced more Arg1 than did control BMDMs 24 hours after stimulation with supernatant from wild-type BMDMs infected with BCG at a multiplicity of infection (MOI) of 10 for 24 hours (Fig. 3E). These data are consistent with the current model for SOCS3-mediated inhibition of IL-6 and G-CSF signaling and reinforce the notion that excessive STAT3 signaling drives the expression of Arg1.
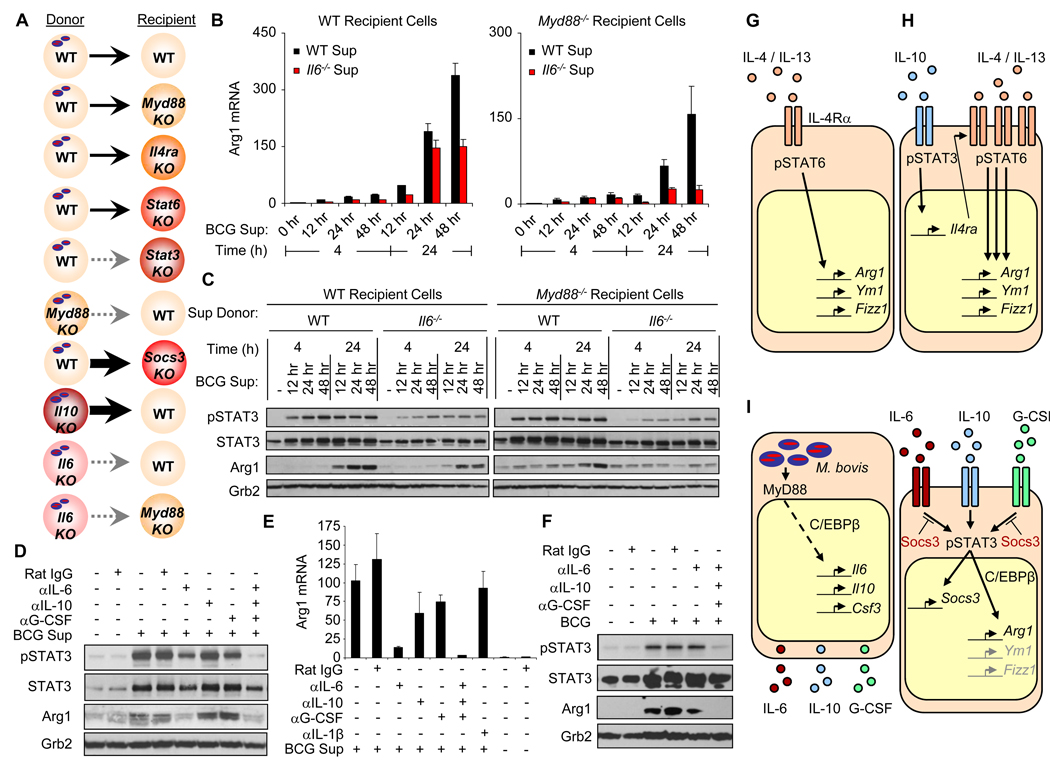
In contrast to G-CSF, IL-6 was likely the cytokine required for the majority of Arg1 production, because the amount of IL-6 mRNA produced after infection with BCG was greater than that of G-CSF (fig. S5B), and recombinant IL-6 was the better inducer of Arg1 production (fig. S6C). To verify the role of IL-6 in the production of Arg1, we again used the BCG-supernatant transfer system (Fig. 4A). Although there was only a modest decrease in the amount of Arg1 protein produced in infected Il6−/− BMDMs relative to that of infected wild-type BMDMs (fig. S7), wild-type or Myd88−/− BMDMs stimulated with supernatants from this infection showed a marked reduction in the amount of Arg1 produced compared to that in cells stimulated with supernatant from infected wild-type cells (Fig. 4, B and C). These data suggest that whereas IL-6 alone was not necessary for the production of Arg1 in BCG-infected cells, it was essential for the autocrine-paracrine response that induced the expression of Arg1 mediated by STAT3 after infection with BCG.
Fig. 4.
IL-6, IL-10, and G-CSF are required for STAT3-dependent production of Arg1 after infection with BCG. (A) Supernatant transfer model: solid arrows indicate the induced production of Arg1 and dashed gray arrows indicate the loss of Arg1 expression in supernatant "recipients" from BCG-infected "donors". (B and C) BMDMs from wild-type and Myd88−/− mice were stimulated with supernatants from BCG-infected (MOI = 100) wild-type and Il6−/− BMDMs. (D and E) Myd88−/− BMDMs were stimulated with supernatants from BCG-infected wild-type BMDMs (at an MOI of 100) for 48 hours in the presence of cytokine-neutralizing antibodies. (F) BMDMs from wild-type mice were infected with BCG (at an MOI of 100) for 24 hours in the presence of cytokine-neutralizing antibodies. (B and E) RNA was analyzed by qRT-PCR. (C, D, and F) Whole-cell lysates were analyzed by Western blotting. Data are presented as mean values ± the standard error of the mean (SEM) for B and E and are representative of two experiments (B to E) or one experiment (F). (G) Arg1 and other “alternatively activated” genes are expressed in macrophages after activation of STAT6 by stimulation of cells with IL-4 or IL-13. (H) IL-10 enhances this established model of alternative activation of macrophages by increasing the cell-surface abundance of IL-4Rα, the common receptor subunit for both IL-4 and IL-13. (I) BCG-infected macrophages produce IL-6, IL-10, and G-CSF after the activation of MyD88, thus inducing the STAT3-dependent expression of Arg1 without triggering alternative activation of macrophages.
We next addressed the potential that IL-6, IL-10, and G-CSF each might be involved in stimulating the expression of Arg1 when one of the other cytokines is incapable of signaling. Neutralizing antibodies against IL-6, IL-10, or G-CSF were added to supernatants from BCG-infected wild-type BMDMs (MOI = 100, 48 hours), which were then used to stimulate Myd88−/− BMDMs. When neutralizing antibody against IL-6 was added to the supernatant, the induction of Arg1 expression was substantially reduced (Fig. 4, D and E). Furthermore, the expression of Arg1 was completely inhibited when neutralizing antibodies against IL-6, IL-10, and G-CSF were present in the supernatant (Fig. 4, D and E). To determine whether the production of Arg1 after infection by BCG absolutely required the autocrine-paracrine STAT3 signal transduction pathway, wild-type BMDMs were infected with BCG (MOI = 100) for 24 hours with neutralizing antibody against IL-6 alone, or with neutralizing antibodies against IL-6, IL-10, and G-CSF. Although the neutralizing antibody against IL-6 had minimal effects, the production of Arg1 and the phosphorylation of STAT3 were completely inhibited in BCG-infected macrophages treated with neutralizing antibodies against IL-6, IL-10, and G-CSF (Fig. 4F). We expect that the neutralizing antibody against IL-6 was ineffective on its own because BCG-infected macrophages continuously produce IL-6, which likely has ongoing autocrine-paracrine effects before it can be inhibited by neutralizing antibody, whereas we were blocking a fixed (and diluted) amount of cytokine in the supernatant transfer experiments (Fig 4, D and E). Nevertheless, these data confirm that the expression of Arg1 in BCG-infected macrophages requires the autocrine-paracrine STAT3 signal transduction pathway by means of the production of, and the response to, combinations of IL-6, IL-10, and G-CSF.
Discussion
Mycobacterium spp. use diverse strategies to persist in the infected host (3). One mechanism involves the hijacking of the expression of Arg1 in macrophages. Mice unable to produce Arg1 in macrophages are able to control Mtb more efficiently than are wild-type mice, and these Arg1-deficient macrophages also have an increased ability to kill Mtb in vitro (14, 15). In contrast to the expression of Arg1 in alternatively activated macrophages (Fig. 4 G and H), Arg1 is induced independently of IL-4 and IL-13 signaling and STAT6 activation after infection by BCG. Here, we uncovered an autocrine-paracrine pathway involving MyD88-dependent production of IL-6, IL-10, and G-CSF after infection by BCG that acted on the infected cell and on surrounding uninfected cells through STAT3 to induce the production of Arg1 (Fig. 4I). Arg1 is constitutively produced in hepatocytes, yet its role in the liver varies considerably compared to that in macrophages (28). Whereas Arg1 in the liver is necessary for the removal of excess nitrogen by the urea cycle, Arg1 is induced in macrophages to regulate the production of NO during inflammation (14, 22). In addition, downstream metabolites that are produced by Arg1-mediated metabolism of L-arginine have roles in cell division, collagen synthesis, and wound healing (29). In contrast to Arg1 in macrophages, the expression of Arg1 in hepatocytes is constitutive, as would be expected for a protein that is essential in a fundamental metabolic process such as the elimination of nitrogen; however, macrophages can be activated to produce Arg1 in great amounts through the activation of STAT6 in response to stimulation by IL-4 or IL-13 in TH2-type responses (13, 20–22). In contrast to the STAT6 pathway, Arg1 is induced by MyD88, in part through TLR signaling, and does not require STAT6 (Fig. 2) (14). Indeed, the only STAT that was activated after infection by BCG was STAT3, as determined by measuring the abundances of tyrosine-phosphorylated STAT proteins.
We were not surprised to observe the robust activation of STAT3 after infection because we and others have detected the production of activators of STAT3, such as IL-6 and IL-10, by macrophages infected with BCG and other Mycobacterium spp. (30–32). Yet, the connection between the activation of STAT3 and the induction of Arg1 expression was unexpected. When supernatant from BCG-infected cells that lack IL-6 was transferred to uninfected macrophages, the production of Arg1 mRNA or protein was greatly reduced compared to that in macrophages stimulated with supernatant that contained IL-6 (Fig. 4, B and C). Other activators of STAT3, such as IL-10 and G-CSF, were also induced after infection with BCG and were responsible for the phosphorylation of STAT3 and the production of Arg1 that was retained in the absence of IL-6 (Fig. 4, D and E). IL-6, IL-10, and G-CSF have diverse biological roles, yet they may have redundant functions in inducing the production of Arg1 after infection. Although IL-6 was the most potent inducer of Arg1 expression (fig. S6), only the blockade of IL-6, IL-10, and G-CSF by neutralizing antibodies resulted in the complete inhibition of Arg1 production in BCG-infected macrophages (Fig. 4F). Thus, our data point to autocrine-paracrine functions of IL-6, IL-10, and G-CSF in regulating macrophage activity during mycobacterial infection.
Early reports described IL-6 as protective in Mtb immunity, because Il6−/− mice succumb to infection much faster than do wild-type mice (33). Although not described at the time, the increased lethality in Il6−/− mice may have been due to a defect in the development of TH17 cells, which is partially dependent on IL-6 signaling (34, 35). In addition, Saunders et al. showed that IL-6 is a cofactor for the early production of IFN-γ by T cells after infection with Mtb (36); however, the function of IL-6 in mycobacterial infection is more complex, because other groups have described its immunosuppressive role. Whereas IL-6 may be necessary for the production of IFN-γ, Nagabhushanam et al. demonstrated that Mtb-infected macrophages secrete IL-6, which acts in an autocrine-paracrine fashion to inhibit the IFN-γ response by reducing cell-surface major histocompatibility complex II (MHC II) on uninfected macrophages (37). VanHeyningen et al. also connected the production of IL-6 to suppressed T cell activity after infection with BCG (38). They identified IL-6 as the soluble factor that is responsible for this phenomenon, because suppression of T cells was reversed when neutralizing antibody against IL-6 was added to the BCG-conditioned media.
Importantly, macrophages infected with mycobacteria cannot be labeled as either classically- or alternatively-activated, which further illustrates the plasticity of macrophage function (fig. S4 and Fig. 4I). Similar to the autocrine-paracrine activity of type I interferons in the stimulation of iNOS activity and NO production after mycobacterial infection (16), we propose a model for the induction of Arg1 expression after BCG infection that involves the MyD88-dependent production of IL-6, IL-10, and G-CSF (Fig. 4I). This mechanism conceivably functions to condition infected and uninfected macrophages to promote bacterial survival. Future therapeutic approaches for tuberculosis and other intracellular infections would benefit from focusing on the surrounding uninfected cells and tissue sites in addition to the infected cells.
Materials and Methods
In vivo BCG infection
BCG Pasteur strain was inoculated intranasally in a 50-µl dose [containing ~5 × 106 colony-forming units (CFUs)]. Tissues were analyzed four weeks later.
In vitro mycobacteria cocultures
BCG cultures were washed in phosphate-buffered saline (PBS) and sonicated in a 10-ml volume to decrease clumping. Bacteria were diluted to generate a range of concentrations so that the approximate infection ratio of bacteria to macrophage noted was between 500:1 and 1:1. Mtb strains CDC1551 and HN878 were sonicated and used to infect BMDMs at a multiplicity of infection (MOI) of between 5 and 1. Filter-sterilized supernatants from infections were used at a 1:4 dilution for the stimulation of macrophages, unless otherwise noted. Rat immunogloubulin (IgG), obtained from eBioscience, and neutralizing antibodies against IL-6 and G-CSF, both from R & D Systems, and IL-10, obtained from eBioscience, were used at a final concentration of 1 µg/ml.
RNA analysis
RNA was isolated with Trizol (Invitrogen). Complementary DNA (cDNA) was synthesized with SuperScript II reverse transcriptase (Invitrogen) and analyzed by quantitative real-time polymerase chain reaction assay to determine the abundance of Arg1 mRNA normalized to that of glyceraldehye 3`-phosphate dehydrogenase (GAPDH). Primers and probes were obtained from Applied Biosystems. Data are presented as the mean fold-change in mRNA abundance over time. 3 µg of RNA was used for Northern blotting analysis. For Affymetrix microarray transcript analysis, BMDMs from 3 female C57BL/6 and 3 female Myd88−/− mice were plated at 5 × 106 cells per plate in 10-cm plates and infected with BCG (at an MOI of 10) for 12, 24, and 48 hours. RNA was analyzed on the Affymetrix 430v2 murine genome array chip.
Western blotting and histological analysis
For Western blotting analysis, protein lysates were separated by Tris-HCl-buffered 4 to 15% gradient SDS polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer to Protran membranes. Membranes were blocked in 3% milk in Tris-buffered saline containing 0.05% Tween and incubated with the appropriate antibodies against the indicated proteins. Grb2 was used as a loading control. Histology sections were stained by the St. Jude Veterinary Pathology Core with hematoxylin and eosin, standard staining for acid fast bacilli, and with antibody against Arg1.
Supernatant analysis
G-CSF, IL-6, and IL-10 were detected in culture supernatants by multiplex bead analysis provided by LINCOPlex (Millipore) and analyzed with Luminex technology.
Supplementary Material
Footnotes
This manuscript has been accepted for publication in Science Signaling. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencesignaling.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skold M, Behar SM. Tuberculosis triggers a tissue-dependent program of differentiation and acquisition of effector functions by circulating monocytes. J. Immunol. 2008;181:6349–6360. doi: 10.4049/jimmunol.181.9.6349. [DOI] [PubMed] [Google Scholar]
- 3.Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. 2008;3:399–407. doi: 10.1016/j.chom.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 5.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 7.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn JL, Scanga CA, Tanaka KE, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J. Immunol. 1998;160:1796–1803. [PubMed] [Google Scholar]
- 9.Davis AS, Vergne I, Master SS, Kyei GB, Chua J, Deretic V. Mechanism of inducible nitric oxide synthase exclusion from mycobacterial phagosomes. PLoS Pathog. 2007;3:e186. doi: 10.1371/journal.ppat.0030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modolell M, Corraliza IM, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur. J. Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- 11.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 1998;160:5347–5354. [PubMed] [Google Scholar]
- 12.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J. Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 13.Rutschman R, Lang R, Hesse M, Ihle JN, Wynn TA, Murray PJ. Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J. Immunol. 2001;166:2173–2177. doi: 10.4049/jimmunol.166.4.2173. [DOI] [PubMed] [Google Scholar]
- 14.El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, Basaraba RJ, Konig T, Schleicher U, Koo MS, Kaplan G, Fitzgerald KA, Tuomanen EI, Orme IM, Kanneganti TD, Bogdan C, Wynn TA, Murray PJ. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol. 2008;9:1399–1406. doi: 10.1038/ni.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber T, Ehlers S, Heitmann L, Rausch A, Mages J, Murray PJ, Lang R, Holscher C. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J. Immunol. 2009;183:1301–1312. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi S, Blumenthal A, Hickey CM, Gandotra S, Levy D, Ehrt S. Expression of many immunologically important genes in Mycobacterium tuberculosis-infected macrophages is independent of both TLR2 and TLR4 but dependent on IFN-alphabeta receptor and STAT1. J. Immunol. 2005;175:3318–3328. doi: 10.4049/jimmunol.175.5.3318. [DOI] [PubMed] [Google Scholar]
- 17.Manca C, Reed MB, Freeman S, Mathema B, Kreiswirth B, Barry CE, 3rd, Kaplan G. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect Immun. 2004;72:5511–5514. doi: 10.1128/IAI.72.9.5511-5514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi S, Nathan C, Schnappinger D, Drenkow J, Fuortes M, Block E, Ding A, Gingeras TR, Schoolnik G, Akira S, Takeda K, Ehrt S. MyD88 primes macrophages for full-scale activation by interferon-gamma yet mediates few responses to Mycobacterium tuberculosis. J Exp Med. 2003;198:987–997. doi: 10.1084/jem.20030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirey KA, Cole LE, Keegan AD, Vogel SN. Francisella tularensis live vaccine strain induces macrophage alternative activation as a survival mechanism. J. Immunol. 2008;181:4159–4167. doi: 10.4049/jimmunol.181.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 22.Pauleau AL, Rutschman R, Lang R, Pernis A, Watowich SS, Murray PJ. Enhancer-mediated control of macrophage-specific arginase I expression. J. Immunol. 2004;172:7565–7573. doi: 10.4049/jimmunol.172.12.7565. [DOI] [PubMed] [Google Scholar]
- 23.Panopoulos AD, Zhang L, Snow JW, Jones DM, Smith AM, El Kasmi KC, Liu F, Goldsmith MA, Link DC, Murray PJ, Watowich SS. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood. 2006;108:3682–3690. doi: 10.1182/blood-2006-02-003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, Rutschman R, Murray PJ. SOCS3 regulates the plasticity of gp130 signaling. Nat. Immunol. 2003;4:546–550. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- 25.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 26.Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, Cluse LA, Sutherland KD, Hartley L, Williams E, Zhang JG, Hilton DJ, Nicola NA, Alexander WS, Roberts AW. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 27.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 28.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J. Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 29.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djoba Siawaya JF, Beyers N, van Helden P, Walzl G. Differential cytokine secretion and early treatment response in patients with pulmonary tuberculosis. Clin. Exp. Immunol. 2009;156:69–77. doi: 10.1111/j.1365-2249.2009.03875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang S, Uematsu S, Akira S, Salgame P. IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J. Immunol. 2004;173:3392–3397. doi: 10.4049/jimmunol.173.5.3392. [DOI] [PubMed] [Google Scholar]
- 32.Newton SM, Smith RJ, Wilkinson KA, Nicol MP, Garton NJ, Staples KJ, Stewart GR, Wain JR, Martineau AR, Fandrich S, Smallie T, Foxwell B, Al-Obaidi A, Shafi J, Rajakumar K, Kampmann B, Andrew PW, Ziegler-Heitbrock L, Barer MR, Wilkinson RJ. A deletion defining a common Asian lineage of Mycobacterium tuberculosis associates with immune subversion. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15594–15598. doi: 10.1073/pnas.0604283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann SH. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect. Immun. 1997;65:4843–4849. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol. Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect. Immun. 2000;68:3322–3326. doi: 10.1128/iai.68.6.3322-3326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagabhushanam V, Solache A, Ting LM, Escaron CJ, Zhang JY, Ernst JD. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-gamma. J. Immunol. 2003;171:4750–4757. doi: 10.4049/jimmunol.171.9.4750. [DOI] [PubMed] [Google Scholar]
- 38.VanHeyningen TK, Collins HL, Russell DG. IL-6 produced by macrophages infected with Mycobacterium species suppresses T cell responses. J. Immunol. 1997;158:330–337. [PubMed] [Google Scholar]
- 39.Acknowledgements: We thank D. Bush and the Veterinary Pathology Core at St. Jude Children’s Research Hospital for the immunohistochemistry staining, and D. Hilton and W. Alexander for the gift of mice bearing conditional alleles of Socs3. Funding: This work was supported by NIH grants AI062921 to P.J.M. and 1F32CA138064 to J.E.Q.; Cancer Center Core Grant P30 CA21765; and the American Lebanese Syrian Associated Charities. Author contributions: J.E.Q., A.M.S., and A.A.D. did the majority of the experiments; G.N. performed the microarray analysis; M.K. and G.K. did the Mtb experiments; H.Z. and S.S.W. provided bones from Stat3+/flox and Stat3Δ/flox;Tie2cre mice; J.E.Q. and P.J.M. conceived and designed the project and wrote the manuscript. Competing interests: The authors have no conflicts of interest to disclose. Accession numbers: Microarray data have been deposited with the GEO database, accession number GSE22935.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.