Abstract
BACKGROUND
One of the most studied actions of juvenile hormone (JH) is its ability to modulate ecdysteroid signaling during insect development and metamorphosis. Previous studies in mosquitoes showed that 20-hydroxecdysone (20E) regulates vitellogenin synthesis. However, the action of JH and its mimics, e.g. methoprene, on female reproduction of mosquitoes remains unknown.
RESULTS
Here, we utilized a major malaria vector, Anopheles gambiae Giles, as a model insect to study the action of methoprene on female reproduction. Ecdysteroid titers and expression profiles of ecdysone-regulated genes were determined before and after a blood meal. An ecdysteroid peak was detected at 12 h post blood meal (PBM). The maximum expression of ecdysone-regulated genes, such as ecdysone receptor (EcR), hormone receptor 3 (HR3) and vitellogenin (Vg) gene, coincided with the ecdysteroid peak. Interestingly, topical application of methoprene, at 6 h PBM delayed ovarian development and egg maturation by suppressing the expression of ecdysone-regulated genes in female mosquitoes.
CONCLUSION
Our data suggest that ecdysteroids titers are correlated with Vg synthesis and methoprene affects vitellogenesis by modulating ecdysteroid action in A. gambiae.
Keywords: Ecdysone, juvenile hormone, gene expression, vitellogenesis, Anophelese gambiae
1 INTRODUCTION
Juvenile hormone (JH) and ecdysteroid (20-hydroxyecdysone, 20E, is an active ecdysteroid) are two major hormones that coordinately regulate insect growth, development, reproduction and other physiological processes. In anautogenous mosquitoes such as Aedes aegypti L. that require a blood meal to initiate oogenesis, ecdysteroids play a major role in regulating female reproduction, especially yolk protein synthesis and deposition during vitellogenesis stage (see 1 for a review). In Ae. aegypti, blood meals activate ecdysteroids biosynthesis in ovarian follicle cells. Then, the ecdysteroids are released into the hemolymph, taken up by the fat body, and converted into 20E. which regulates the transcription of vitellogenin (Vg), a yolk protein precursor, in the fat body. The ecdysteroid titers in female Ae. aegypti are correlated with vitellogenin synthesis and the expression patterns of genes involved in 20E action in the fat body.1, 2 The isoform-specific expression patterns of several ecdysone-regulated genes of Ae. aegypti [e.g. ecdysone receptor (AaEcR) and ultraspiracle (AaUSP)] suggest that there are distinct physiological functions for each isoform of receptors during vitellogenesis.3–5 The expression of Ae. aegypti hormone receptor 3 (AaHR3) correlates well with the ecdysteroid titers and Vg production with a peak at 24 h post blood meal (PBM).6 Furthermore, 20E induces the expression of AaHR3 gene in the previtellogenic fat body in vitro. Several lines of evidence suggest that AaEcR and ecdysone-induced protein 75 (AaE75) can bind directly to the promoter of the Vg gene and activate its transcription after a blood meal 7, 8.
In contrast, JH is the main player in regulating vitellogenin gene expression in the fat body and its uptake into the ovaries in several other insects such as locusts and cockroaches 9, 10. JH stimulates Vg synthesis in the fat body of the German cockroach, Blattella germanica (L.).11 In mosquitoes, it appears that JH acts mainly at the previtellogenic stage, an arresting stage before a blood meal. Corpora allata (CA), the source of JH, were found to be required during the previtellogenic phase, but not after a blood meal, for proper egg development.12, 13 Methoprene, a JH analog, has been commercially used as a mosquito larvacide for decades. Most of the methoprene-treated mosquitoes die during the pupal stage.14 In Ae. aegypti, methoprene can block midgut remodeling during the larval-pupal transition by interfering with the expression of genes involved in 20E action.14 The expression of many key genes such as EcRB, USPA and HR3 that are involved in 20E action were down-regulated by methoprene treatment. However, it is not known whether methoprene can also modulate 20E action in adult insects.
The African malaria mosquito, Anopheles gambiae Giles is the most important vector of malaria, which is responsible for more than 1 million deaths per year.15 Since most of the endocrinology studies on hormonal regulation have been concentrated on Ae. aegypti, little is known about hormonal regulation of female reproduction and vitellogenesis in A. gambiae. In this study, ecdysteroid titers in the female mosquito were measured and an ecdysteroid peak was detected at 12 h PBM. The expression patterns of ecdysone-regulated genes, such as EcR, HR3 and Vg, were correlated with ecdysteroid titers. Furthermore, topical application of methoprene delayed egg maturation and down-regulated the expression of genes involved in 20E action during the first gonotrophic cycle. Taken together, these data showed that methoprene down-regulates the expression of genes involved in 20E action and causes delay in vitellogenesis and egg maturation in A. gambiae.
2 MATERIALS AND METHODS
2.1 Mosquito strain and rearing
Anopheles gambiae G3 strain (catalog number: MRA-112; deposited by Dr. Mark Q. Benedict) was obtained from the MR4 (Malaria Research and Reference Reagent Resource Center) and reared at 27 °C with a 16 : 8 h light : dark cycle. Larvae were fed on a diet of Cichlid Power Flakes (M. Reed Enterprises, Sutter Creek, CA). Newly emerged adults (200–300) were placed in an approximately 2 L plastic cylindrical cage and fed on 10% sucrose. Blood feeding on rat was used for initiating vitellogenesis.
2.2 Determination of ecdysteroid titers
Ecdysteroid titers in the whole samples were determined as previously described.16–18 Briefly, staged individual female mosquitoes were collected and homogenized in 250 µL of ice-cold 75% aqueous methanol and centrifuged at 13,000 × g at 4 °C for 15 min. Supernatant was transferred to 6 × 50 mm borosilicate glass tubes. Pellets were resuspended in an additional 100 µL of methanol and kept on ice for 30 min. After centrifugation as above, the supernatant was combined with the previous sample accordingly. Samples were dried using a Speed-Vac and stored at −20 °C until measurement of ecdysteroid titers. An enzyme immunoassay (EIA) was used to estimate ecdysteroid titers. The EIA was performed in a 96-well microtiter plate and was based on the competition between ecdysteroid (in standards or samples) and a known amount of peroxidase-labeled conjugated ecdysone for the ecdysteroid antiserum that had been bound to the IgG-coated wells. The linear range of the assay was 0.5–40 pg. The ecdysteroid antiserum used in the EIA had a high affinity for α-ecdysone (E), 20E, makisterone A, 20, 26-dihydroxyecdysone, 26-hydroxyecdysone and 3-dehydroecdysone, but did not detect polar conjugates.
2.3 RNA isolation and cDNA synthesis
Total RNA was extracted from the abdominal fat body isolated from five female mosquitoes per stage using TRI reagent (Molecular Research Center Inc., Cincinnati, OH). Total RNA was then treated with DNase I (Ambion, Austin, TX) in a 50 µL total reaction volume following the manufacturer’s protocol. RNA concentration and purity were assessed using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA). cDNA synthesis by reverse transcription was performed using 2 µg of DNase I treated RNA and iScript cDNA synthesis kit (Biorad Laboratories, Hercules, CA) in a 20 µL reaction volume.
2.4 Quantitative real-time reverse-transcriptase polymerase chain reaction (qRT-PCR)
qRT-PCR was performed using MyiQ single color real-time PCR detection system (Biorad Laboratories, Hercules, CA). qRT-PCR was performed in a 20 µL total reaction volume containing 1 µL of cDNA, 1 µL each of 10 mM forward and reverse gene specific primers (Table. 1), 7 µL of water and 10 µL of supermix (Biorad Laboratories, Hercules, CA). PCR conditions were: 95 °C for 2 min followed by 40 cycles of 94 °C for 10 s, 60 °C for 20 s and 72 °C for 30 s. A standard curve was obtained using a ten-fold serial dilution of pooled cDNAs. Ribosomal protein S7 gene (AgS7RP, GenBank accession no. XM_001237575) was used as an internal control. The mRNA abundance of each gene was obtained relative to AgS7RP control by a standard-curve based method.19,20 Both the PCR efficiency and the correlation coefficient were taken into account prior to estimating the relative gene expression. Mean and standard errors for each time point were obtained from the averages of three independent biological replicates.
Table 1.
Primers used in qRT-PCR
| Symbol | Forward primer | Reverse primer |
|---|---|---|
| AgEcR | cgaacagcagcagctacaag | cctcctcgttgggtgagtta |
| AgUSP | agaaggagaaaccgatgctg | aaatgtccggcttcaggtc |
| AgHR3 | aatggcgtacgaggaaacac | gaaaacgtactgcgggtgat |
| AgVg | tacttcggcaacgtcatcag | cggtgtattgctgcttctca |
| AgS7RP | gtgttcggttccaaggtga | accggcacgtagatgatga |
2.5 Measurement of follicle length
Ovaries from different developmental stages were dissected in 1 × PBS. The individual primary follicle was separated and photographed using an Olymous CKX41 inverted microscope with DP12 digital microscope camera (Melville, NY). Follicle images were processed and follicle lengths were measured using ImageJ software (National Institute of Health, NIH). Follicle lengths of 6–8 female mosquitoes were measured at each stage.
2.6 Methoprene treatment
Methoprene (isopropyl (E,E)-(RS)-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate) was a gift from Wellmark International (Dallas, TX). Stock methoprene was dissolved in cyclohexane at a concentration of 1 µg µL−1 and stored at −20 °C until use. Blood-fed female mosquitoes collected at 6 h post blood meal (PBM) were briefly chilled on ice before methoprene treatment. Approximately 0.2 µL of various concentrations of methoprene was applied topically to the lateral side of the abdomen. All untreated controls received 0.2 µL cyclohexane. Ovaries were dissected and the length of primary follicles were measured as 9n Section 2.5 at 48 h PBM (42 h after methoprene treatment). Total RNA was extracted from pools of fat body collected from five insects at 18 h PBM (12 h after methoprene treatment). cDNAs prepared from the RNA were used in qRT-PCR.
2.7 Statistical analysis
Student’s t-Test and analysis of variance was performed using JMP 8.0 (SAS Institute Inc., Cary, NC) to examine significance of the differences among treatments (α = 0.05). Pair-wise comparisons were made using Tukey-Kramer HSD method.
3 RESULTS
3.1 Ecdysteroid titers before and after a blood meal
In anautogenous mosquitoes, 20E plays an important role in the synthesis of Vg in the fat body during vitellogenesis.1 A blood meal is considered as the signal that activates 20E cascade and Vg gene transcription. We measured whole body ecdysteroid titers using an EIA assay 16–18 during the first gonotrophic cycle. As shown in Fig. 1A, whole body ecdysteroid levels in the female mosquitoes were low (less than 10 pg insect−1) before a blood meal. Immediately after a blood meal on the 4th day after adult eclosion, whole body ecdysteroid levels increased dramatically to 82.8 pg insect−1 by 3 h PBM and 108 pg insect−1 by 6 h PBM. The maximum level of whole body ecdysteroids was detected at 12 h PBM (about 328.7 pg insect−1). The ecdysteroid levels then decreased gradually and reached 16.5 pg insect−1 by 48 h PBM when eggs were fully mature and females began oviposition. These data suggest that a blood meal triggers the biosynthesis and secretion of ecdysteroids in female A. gambiae.
Figure 1. Whole body ecdysteroid titers and the expression of vitellogenin gene (AgVg) of Anophelese gambiae before and after a blood meal.
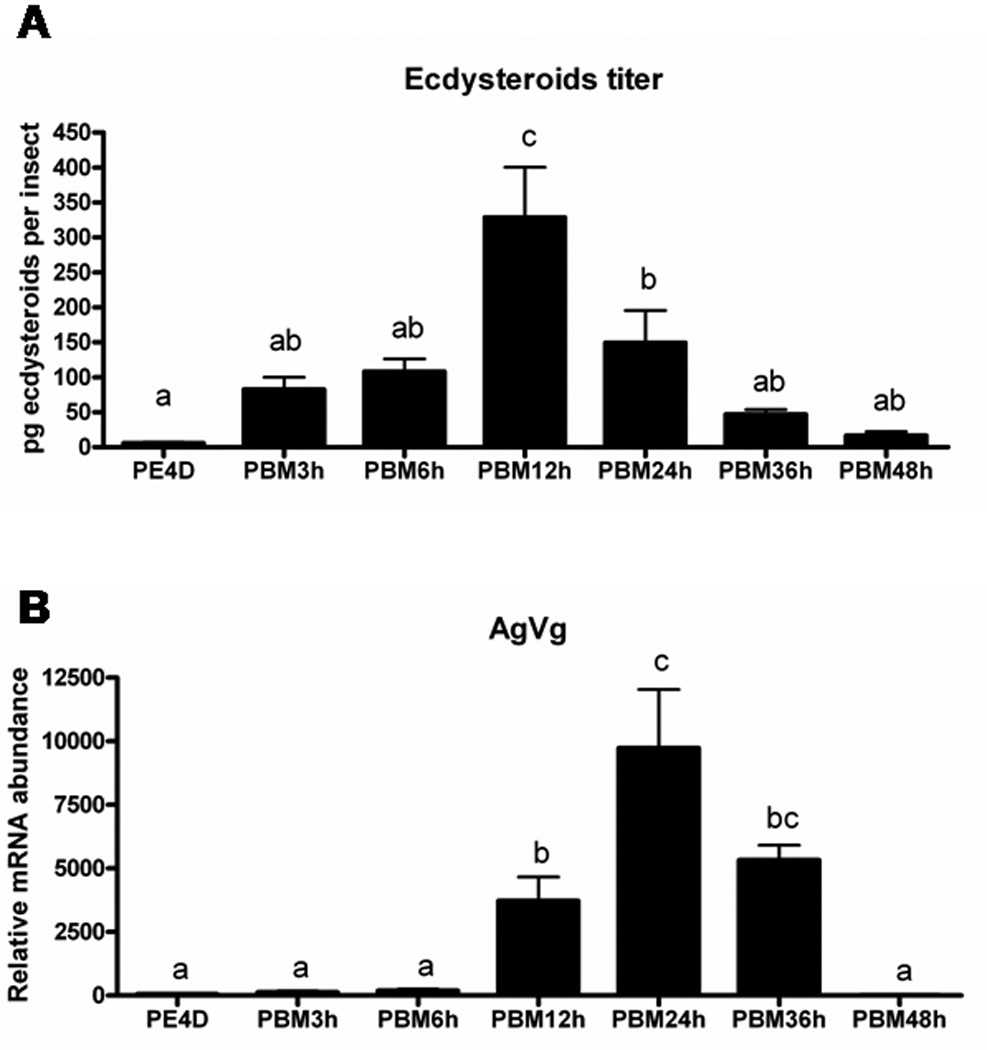
(A). Whole body ecdysteroid titers of A. gambiae. Ecdysteroid levels were estimated using enzyme immunoassay as previously described 16–18. Each time point represents mean ± SE of 5–10 individual insects. Means with the same letter are not significantly different (α ≤ 0.05; ANOVA). (B). The expression of AgVg of A. gambiae. mRNA abundance of AgVg in the fat body determined by quantitative real-time reverse-transcriptase PCR (qRT-PCR). Total RNA was extracted from pools of five fat bodies for each time point. The Y-axis denotes expression levels normalized using AgS7RP levels as an internal control. Mean ± SE of three replications are shown. Means with the same letter are not significantly different (α ≤ 0.05; ANOVA). PE4D: four days after eclosion. PBM: hours after blood meal.
3.2 Expression profiles of ecdysone-regulated genes and Vg gene
To study the role of 20E signaling during vitellogenesis of A. gambiae, we identified key genes involved in 20E action using bioinformatics approaches and quantified their mRNA levels using qRT-PCR. Anopheles gambiae genome sequences deposited in NCBI were searched using sequences of homolog genes from Ae. aegypti. We identified three ecdysone-regulated genes, ecdysone receptor (AgEcR, GenBank accession no. XM_320323), ultraspiracle (AgUSP, GenBank accession no. XP_320944), hormone receptor 3 (AgHR3, GenBank accession no. XM_319750), and one vitellogenin gene (AgVg, GenBank accession no. AAF82131) (Table 2). Since there are two isoforms of EcR and USP genes in Ae. aegypti, we compared amino acid identity between two species. We found that AgEcR is closer to AaEcRB (59.3%) and AgUSP is similar to AaUSPA (82.6%). Especially in the 5’-end, AgUSP and AaUSPA share a high similarity. Total RNA was extracted from fat bodies attaching to the abdominal integument (hereafter referred to as the fat body) at various stages and mRNA levels were quantified using qRT-PCR. AgVg mRNA levels increased significantly by 12 h PBM and reached the maximum levels by 24 h PBM (Fig. 1B). The Vg mRNA levels started to decrease by 36 h PBM and reached the minimum levels by 48 h PBM (Fig. 1B). Lower levels of AgEcR mRNA were detected prior to a blood meal in female adults at four-day post adult eclosion (PE4D) (Fig. 2A). The AgEcR mRNA levels increased significantly by 12 h PBM, then the mRNA levels decreased by 36 h PBM. In contrast, no significant differences were detected in the mRNA levels of AgUSP, a heterodimer partner for EcR, in the fat body dissected from insects prior to a blood meal or 12 h PBM and 24 h PBM (Fig. 2B). Interestingly, the mRNA levels of AgHR3, an early-late gene in 20E action, were lower prior to blood meal and started to increase after blood meal and reached the maximum levels by 24 h PBM (Fig. 2C). These data show that the expression patterns of Vg gene as well as genes involved in 20E action cascade are correlated with ecdysteroid titers, suggesting that 20E action cascade and Vg synthesis are activated after a blood meal in female A. gambiae.
Table 2.
Genes identified in Anopheles gambiae
| Anopheles Symbol | GenBank accession no. | Length (aa) | Identitya |
|---|---|---|---|
| AgEcR | XM_320323 | 477 | 52.0%/59.3%b |
| AgUSP | XP_320944 | 474 | 82.6%/77.8%b |
| AgHR3 | XM_319750 | 639 | 66.9% |
| AgVg | AAF82131 | 2051 | 53.7% |
Amino acid identity between Anopheles gambiae and Aedes aegypti
The first number is the identity between homologous in A. gambiae and isoform-A in A. aegypti, while the second one is comparing isoform-B in A. aegypti
Figure 2. The expression of ecdysone-regulated genes of Anopheles gambiae before and after a blood meal.
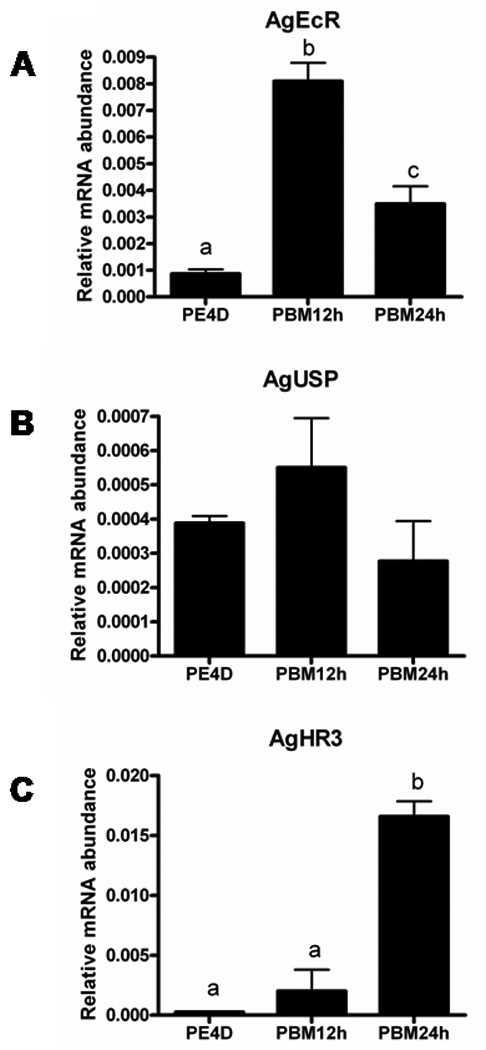
mRNA abundances of AgEcR (A), AgUSP (B) and AgHR3 (C) in the fat body collected at different stages. Total RNA was extracted from pools of five fat bodies for each time point. The Y-axis denotes expression levels normalized using AgS7RP levels as an internal control. Mean ± SE of three replications are shown. Means with the same letter are not significantly different (α ≤ 0.05; ANOVA). PBM: hours after blood meal.
3.3 Methoprene delays egg maturation
JH is one of the key hormones that regulate previtellogenic ovarian development in mosquitoes.9, 10 In Ae. aegypti, JH levels increase during the first two days after adult eclosion, and then the levels decline rapidly after a blood meal and remain low during vitellogenesis.21 One of JH analogues, methoprene, has been widely used as a larvacide for controlling mosquitoes. Various doses of methoprene (1 ng, 10 ng, 50 ng female−1) were topically applied to the abdomen of female adults at 6 h PBM. The primary follicle length increased rapidly after a blood meal and reached the maximum size at 48 h PBM in control insects that received no methoprene treatment (Fig. 3). As shown in Fig. 4E, the primary follicle length in methoprene-treated mosquitoes at 48 h PBM was significantly shorter than the length of the follicles in the control mosquitoes. Ovaries from control mosquitoes dissected at 48 h PBM contained fully mature oval shaped eggs (Fig. 4A). In contrast, the primary follicles of methoprene-treated mosquitoes were still round in shape and the size of these follicles was similar to that of the follicles of the ovaries dissected from control mosquitoes at 24 h PBM (Fig. 3). Interestingly, the digestion of blood meals in the midgut was not affected by methoprene treatment (see midgut pictures in Fig. 4A–D). Although methoprene application did not completely block yolk deposition, it did delay egg maturation and the growth of primary follicles.
Figure 3. Primary follicle length at different developmental stages of Anopheles gambiae.
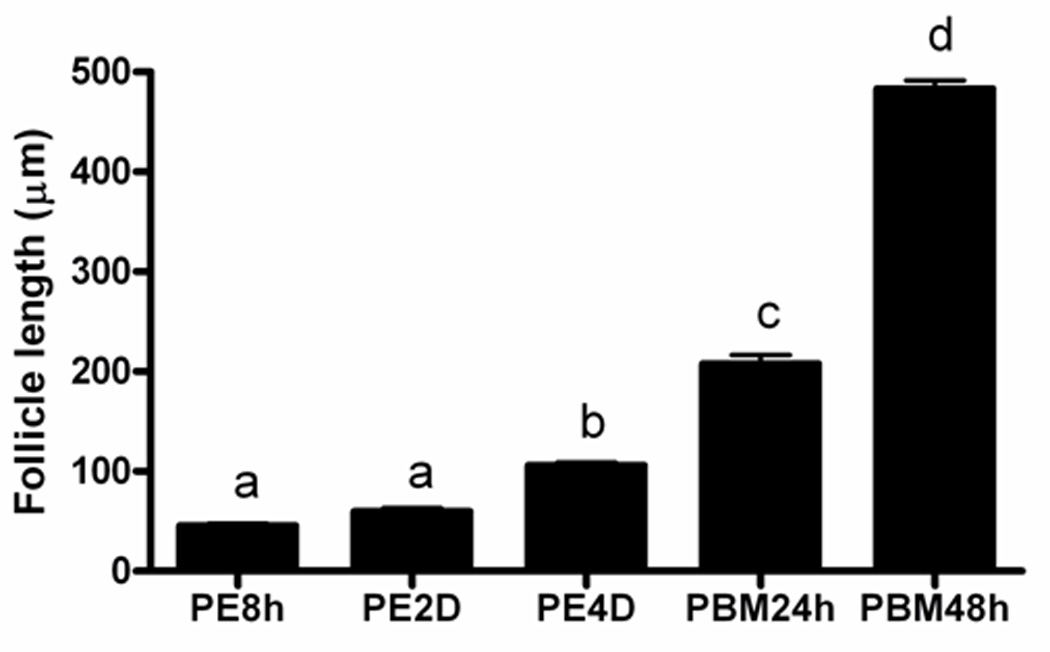
Ovaries collected from 6–8 females were used for follicle length measurement. Means with the same letter are not significantly different (α ≤ 0.05; ANOVA). PBM: hours after blood meal.
Figure 4. Effect of methoprene treatment on female reproduction of Anopheles gambiae.
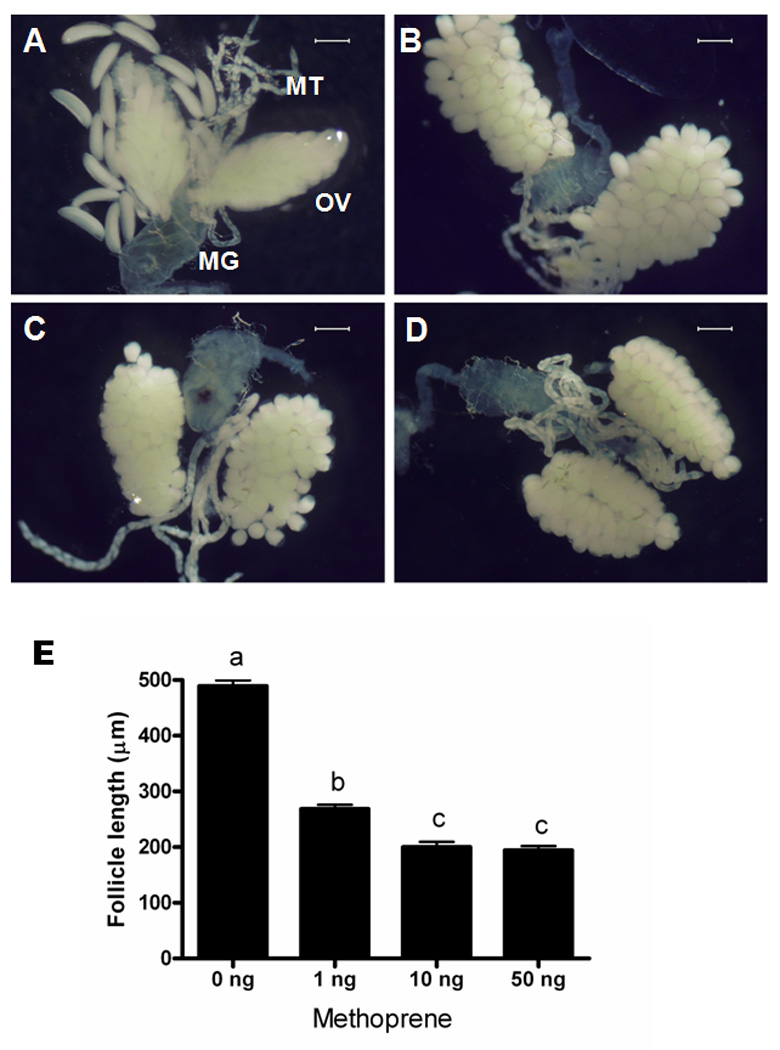
Ovaries dissected at 48 h PBM from cyclohexane treated (A), 1 ng methoprene treated (B), 10 ng methoprene treated (C), 50 ng methoprene treated females (D). (OV: ovary; MG: midgut; MT: malpighian tubules). Scale bar: 300 µm. (E) Primary follicle length from methoprene-treated females at 48 h PBM. Mean ± SE of follicles from 6–8 females are shown. Means with the same letter are not significantly different (α ≤ 0.05; ANOVA).
3.4 Methoprene modulates expression of ecdysone-regulated genes
To understand whether the delay in oocyte maturation caused by methoprene treatment is due to the block of yolk protein production and deposition, we measured and compared expression of ecdysone-regulated genes and Vg gene in the fat body dissected from both control and methoprene-treated (10 ng female−1) insects at 18 h PBM. As shown in Fig. 5, methoprene treatment suppressed the expression of AgEcR, AgUSP, AgHR3 and AgVg genes. The mRNA levels of AgHR3 were 7.6-fold lower in methoprene-treated mosquitoes when compared to that in cyclohexane-treated control insects. Although AgVg mRNA was only down-regulated 1.4-fold by methoprene, the reduction in Vg mRNA levels may have affected yolk protein synthesis and deposition, resulting in a delay in oocyte maturation.
Figure 5. Effects of methoprene treatment on the expression of ecdysone-regulated genes of Anopheles gambiae at 18 h PBM.
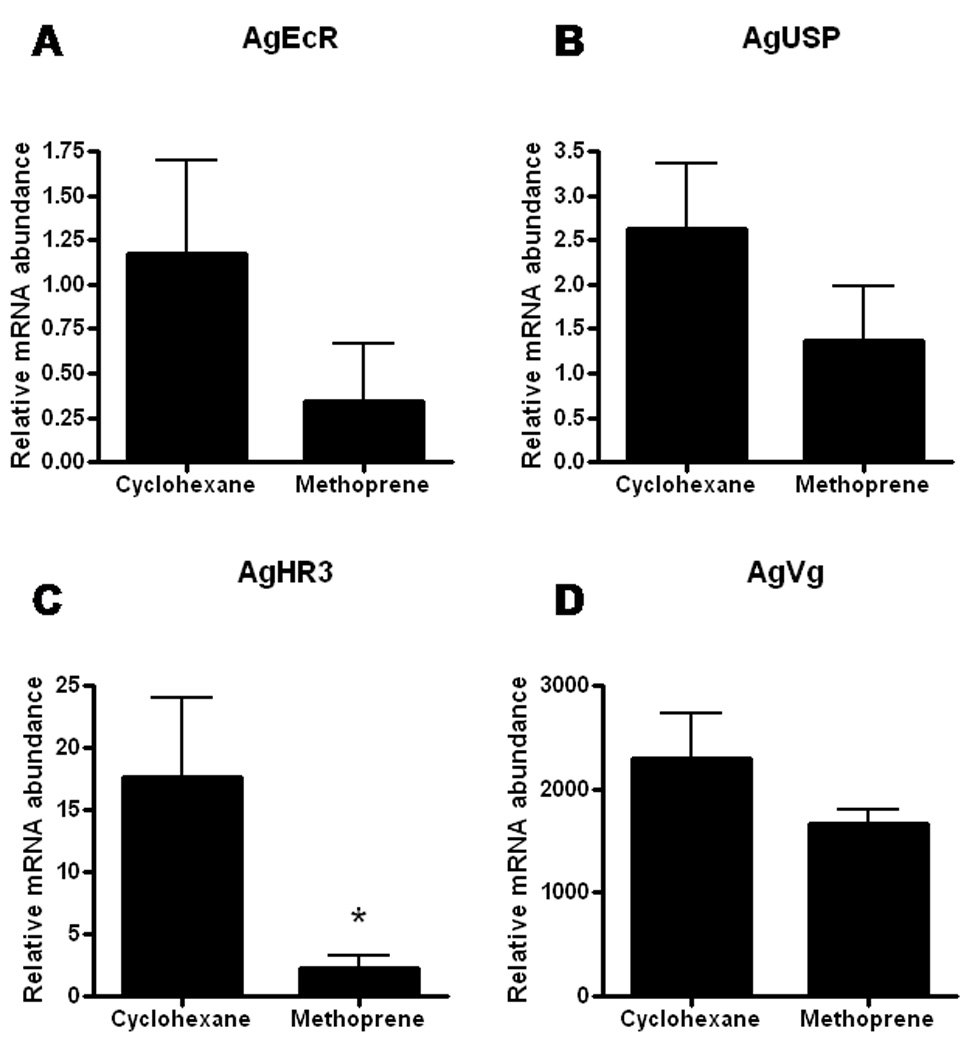
mRNA abundances of AgEcR (A), AgUSP (B), AgHR3 (C) and AgVg (D) in the fat body collected from cyclohexane and 10 ng methoprene treated female mosquitoes. Total RNA was extracted from pools of five fat bodies for each time point. The Y-axis denotes expression levels normalized using AgS7RP levels as an internal control. Mean ± SE of three replications are shown. Asterisk indicates significant differences between two treatments (α ≤ 0.05; Student’s t-Test).
4 DISCUSSION
Hormonal regulation of female reproduction and vitellogenesis has been well studied in many holometabolous insects, including the yellow fever mosquito, Ae. aegypti. 20E and JH are two major hormones that regulate oocyte development in the ovary and Vg synthesis in the fat body.1, 11 However, this information is lacking for the major malaria vector, A. gambiae. The data we report here suggest that blood meals activate 20E cascade and vitellogenesis, and the JH mimic methoprene delays egg maturation and vitellogenesis by modulating the expression of ecdysone-regulated genes in female A. gambiae. This common theme across taxa suggests conserved cross-talk between 20E and JH in controlling female reproduction in insects.
In Ae. aegypti, blood meals trigger the ecdysteroids biosynthesis in ovaries. The hemolymph ecdysteroid titers reach their maximum level at 18–20 h PBM (270 pg female−1).22 In our studies, the peak of ecdysteroid titers appears at 12 h PBM (328.7 pg female−1), which is similar to the data reported for Ae. aegypti. A recent study showed that ovarian ecdysteroids secretion in A. gambiae reached the maximum at 18 h PBM with a secretion of 142 pg ecdysone equivalents/ovary pair/5 h. 23 These studies also showed that ovaries from 24 h PBM insects secreted both E and 20E. Hagedorn et al22 were the first to discover that the ovaries of female Ae. aegypti are the source of ecdysteroids and 20E is the active form that stimulates Vg synthesis in the fat body. Only 20E but not E was found in the extracts of whole mosquitoes. This suggests that E is converted quickly into 20E following its release from the ovary. In contrast, the ovaries of A. gambiae secrete a 1:3 mixture of E and 20E, and no free E or 20E is stored in the ovaries.23 It is also interesting that the AgVg gene expression pattern is correlated with the ecdysteroid titers. The peak of AgVg mRNA was at 24 h PBM, 12 h following the appearance of ecdysteroids peak, suggesting a tight relationship between ecdysteroids and Vg synthesis.
In Ae. aegypti, 20E stimulates Vg synthesis in the fat body by acting through its receptor complex (EcR/USP) and a number of transcription factors involved in the 20E action cascade. In this study, we observed a correlation between ecdysteroid titers and the expression pattern of AgEcR gene, suggesting that, soon after synthesis and secretion, ecdysteroids induce the expression of their own receptors. 20E induction of its receptor gene has been reported in several other insect species.24, 25 The expression of AgHR3 was high at 24 h PBM, 12 h after the peaks of ecdysteroid titers and AgEcR mRNA levels. HR3 is considered as an early-late gene, which means that it is expressed after early genes such as E74, E75 and broad. HR3 can interact with EcR and βFTZF1, and function as a switch that defines the larval-prepupal transition in Drosophila melanogaster Meig.26 In Ae. aegypti and many other insects, HR3 gene expression is activated by 20E.6, 27, 28 The correlation between ecdysteroid titers and expression of key genes involved in the 20E action cascade suggests that in A. gambiae as in Ae. aegypti, ecdysteroids are one of the primary regulators of Vg synthesis.
JH is another key hormone that plays critical roles in regulation of insect metamorphosis and reproduction. In many cases, JH has antagonistic effects on 20E action. Exogenous application of methoprene blocks pupal cuticular protein synthesis and prevents metamorphoses in lepidopteran insects.29, 30 Methoprene application down-regulates the expression of ecdysone-regulated genes in midgut tissues and blocks programmed cell death (PCD) of larval midgut cells in Ae. aegypti 14 and Heliothis virescens F.31 JH also controls a wide variety of biological functions in adult insects, including vitellogenin synthesis in the female fat body, patency of the ovarian follicular epithelium,9, 10 and protein synthesis in the male accessory glands.32 In mosquito, JH titers are high during the previtellogenic stage at two days after adult eclosion, suggesting an important regulatory role of JH during that stage.21 A priming role for JH on the previtellogenic fat body has been proposed, since the mosquito fat body without prior exposure to JH loses its responsiveness to 20E.1 A blood meal causes an immediate decrease in JH levels when ecdysteroids and Vg synthesis begin.1 In the present study, as low as 1 ng methoprene topically applied to female mosquitoes caused at least 24 h delay in egg maturation. At 48 h PBM the primary follicles in untreated mosquitoes were nearly 50% larger than the follicles in methoprene-treated mosquitoes. The primary follicles in methoprene-treated mosquitoes were still round in shape, resembling those in untreated mosquitoes at 24 h PBM. Similar to its action in Ae. aegypti and H. virescens,14,31 methoprene has an antagonistic role on ecdysone action and down-regulates the expression of genes involved in ecdysone action in the fat body of female A. gambiae. Recent studies in Tribolium castaneum Hbst. showed that another JH analog, hydroprene, acted in a similar way by down-regulating the expression of key genes involved in 20E action cascade during midgut remodeling.33
In this study, the transcription of genes involved in regulation of Vg synthesis, such as AgEcR and AgHR3, is down-regulated in methoprene-treated insects, which may be due to the inhibition of 20E action by methoprene treatment. On the other hand, methoprene may inhibit the ecdysteroid biosynthesis. The inhibition of the ecdysteroid secretion from the prothoracic gland by JH or its analogues during the final instar larval stage has been reported in some lepidopterous insects.34, 35 In addition, the expression of AgVg was also suppressed nearly 30% by methoprene treatment. The reduction of vitellogenin synthesis in the fat body may result in decrease in Vg (or yolk protein) deposition, consequently delaying egg maturation. Based on our data, we propose a model for methoprene action in reproduction of A. gambiae (Fig. 6). We propose that methoprene affects A. gambiae reproduction by down regulating the expression of genes involved in ecdysteroid action and/or synthesis and secretion of ecdysterioids. The data included in this paper provide strong evidence for methoprene down regulation of expression of genes involved in ecdysteroid action.
Figure 6. Proposed model for methoprene action on female reproduction of Anopheles gambiae.
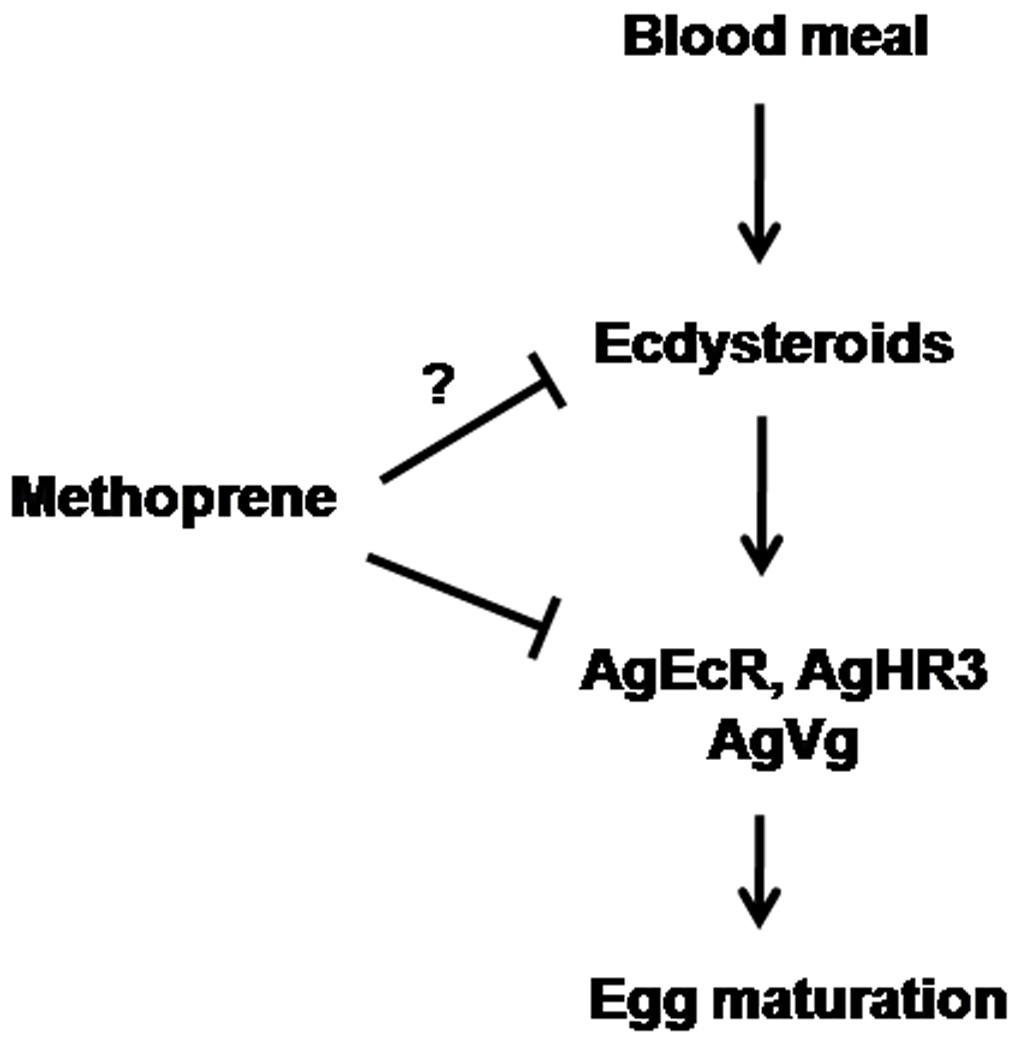
Although we propose that delaying egg maturation is probably related to reduction in Vg synthesis in the fat body, we cannot rule out that methoprene might target tissues other than fat body, e.g. ovary. Exogenous JH application can irreversibly block follicular maturation in Ae. aegypti, when application is done before 24 h PBM.36 20E also controls the formation of the vitelline envelope37, 38 and the separation of the secondary follicle from the germarium in Ae. aegypti.38 Ecdysone-regulated genes, such as EcR, are found to be highly expressed in the ovaries after a blood meal in Ae. aegypti.3 Therefore, methoprene could affect 20E action in ovaries and block follicle maturation. The direct action of methoprene on oogenesis needs further investigation. In summary, ecdysteroid titers of whole mosquito extracts and expression profiles of ecdysone-regulated genes of A. gambiae are similar to those of Ae. aegypti. In addition, we found that methoprene can delay egg maturation and down-regulate the expression of ecdysone-regulated genes in A. gambiae. Our results suggest that methoprene could be used for the control of A. gambiae, the major malaria vector, by interfering with egg maturation. Furthermore, the knowledge on hormonal regulation of female reproduction in A. gambiae would lead to the identification of new target sites for mosquito control.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health (GM070559-05). We thank Dr. Stephen Dobson for help with mosquito rearing and MR4 for providing Anopheles gambiae G3 strain. This is contribution number 10-08-045 from the Kentucky Agricultural Experimental Station.
REFERENCES
- 1.Raikhel AS, Kokoza VA, Zhu J, Martin D, Wang SF, Li C, Sun G, Ahmed A, Dittmer N, Attardo G. Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity. Insect Biochem Mol Biol. 2002;32:1275–1286. doi: 10.1016/s0965-1748(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 2.Dhadialla TS, Raikhel AS. Perspectives in Comparative Endocrinology. Canada: National Research Council of Canada; 1994. Endocrinology of mosquito vitellogenesis; pp. 275–281. [Google Scholar]
- 3.Cho WL, Kapitskaya MZ, Raikhel AS. Mosquito ecdysteroid receptor: analysis of the cDNA and expression during vitellogenesis. Insect Biochem Mol Biol. 1995;25:19–27. doi: 10.1016/0965-1748(94)00045-j. [DOI] [PubMed] [Google Scholar]
- 4.Kapitskaya M, Wang S, Cress DE, Dhadialla TS, Raikhel AS. The mosquito ultraspiracle homologue, a partner of ecdysteroid receptor heterodimer: cloning and characterization of isoforms expressed during vitellogenesis. Mol Cell Endocrinol. 1996;121:119–132. doi: 10.1016/0303-7207(96)03847-6. [DOI] [PubMed] [Google Scholar]
- 5.Wang SF, Li C, Zhu J, Miura K, Miksicek RJ, Raikhel AS. Differential expression and regulation by 20-hydroxyecdysone of mosquito ultraspiracle isoforms. Dev Biol. 2000;218:99–113. doi: 10.1006/dbio.1999.9575. [DOI] [PubMed] [Google Scholar]
- 6.Kapitskaya MZ, Li C, Miura K, Segraves W, Raikhel AS. Expression of the early-late gene encoding the nuclear receptor HR3 suggests its involvement in regulating the vitellogenic response to ecdysone in the adult mosquito. Mol Cell Endocrinol. 2000;160:25–37. doi: 10.1016/s0303-7207(99)00253-1. [DOI] [PubMed] [Google Scholar]
- 7.Kokoza VA, Martin D, Mienaltowski MJ, Ahmed A, Morton CM, Raikhel AS. Transcriptional regulation of the mosquito vitellogenin gene via a blood meal-triggered cascade. Gene. 2001;274:47–65. doi: 10.1016/s0378-1119(01)00602-3. [DOI] [PubMed] [Google Scholar]
- 8.Martin D, Wang SF, Raikhel AS. The vitellogenin gene of the mosquito Aedes aegypti is a direct target of ecdysteroid receptor. Mol Cell Endocrinol. 2001;173:75–86. doi: 10.1016/s0303-7207(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt GR, Davey KG. Cellular and molecular actions of juvenile hormone .2. Roles of juvenile hormone in adult insects. Adv Insect Physiol. 1996;26:1–155. [Google Scholar]
- 10.Engelmann F, editor. Juvenile Hormone action in Insect Reproduction. San Diego: Acdemic Press; 2003. [Google Scholar]
- 11.Maestro JL, Cobo J, Belles X. Target of rapamycin (TOR) mediates the transduction of nutritional signals into juvenile hormone production. J Biol Chem. 2009;284:5506–5513. doi: 10.1074/jbc.M807042200. [DOI] [PubMed] [Google Scholar]
- 12.Larsen JR, Bodenstein D. The humoral control of egg maturation in the mosquito. J Exp Zool. 1959;140:343–381. doi: 10.1002/jez.1401400208. [DOI] [PubMed] [Google Scholar]
- 13.Lea AO. Egg maturation in mosquitoes not regulated by the corpora allata. J Ins Physiol. 1969;15:537–541. [Google Scholar]
- 14.Wu Y, Parthasarathy R, Bai H, Palli SR. Mechanisms of midgut remodeling: juvenile hormone analog methoprene blocks midgut metamorphosis by modulating ecdysone action. Mech Dev. 2006;123:530–547. doi: 10.1016/j.mod.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Roll Back Malaria WHO, UNICEF. Geneva: WHO; World Malaria Report 2005. 2005
- 16.Margam VM, Gelman DB, Palli SR. Ecdysteroid titers and developmental expression of ecdysteroid-regulated genes during metamorphosis of the yellow fever mosquito, Aedes aegypti (Diptera: Culicidae) J Ins Phys. 2006;52:558–568. doi: 10.1016/j.jinsphys.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Parthasarathy R, Tan A, Bai H, Palli SR. Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech Dev. 2008;125:299–313. doi: 10.1016/j.mod.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kingan TG. A competitive enzyme-linked immunosorbent assay: applications in the assay of peptides, steroids, and cyclic nucleotides. Anal Biochem. 1989;183:283–289. doi: 10.1016/0003-2697(89)90481-8. [DOI] [PubMed] [Google Scholar]
- 19.Rutledge RG, Cote C. Mathematics of quantitative kinetic PCR and the application of standard curves. Nucleic Acids Res. 2003;31:e93. doi: 10.1093/nar/gng093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6:62. doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro AB, Wheelock GD, Hagedorn HH, Baker FC, Tsai LW, Schooley DA. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. J Ins Physiol. 1986;32:867–877. [Google Scholar]
- 22.Hagedorn HH, O'Connor JD, Fuchs MS, Sage B, Schlaeger DA, Bohm MK. The ovary as a source of alpha-ecdysone in an adult mosquito. Proc Natl Acad Sci U S A. 1975;72:3255–3259. doi: 10.1073/pnas.72.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pondeville E, Maria A, Jacques JC, Bourgouin C, Dauphin-Villemant C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc Natl Acad Sci U S A. 2008;105:19631–19636. doi: 10.1073/pnas.0809264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jindra M, Malone F, Hiruma K, Riddiford LM. Developmental profiles and ecdysteroid regulation of the mRNAs for two ecdysone receptor isoforms in the epidermis and wings of the tobacco hornworm, Manduca sexta. Dev Biol. 1996;180:258–272. doi: 10.1006/dbio.1996.0299. [DOI] [PubMed] [Google Scholar]
- 25.Hiruma K, Bocking D, Lafont R, Riddiford LM. Action of different ecdysteroids on the regulation of mRNAs for the ecdysone receptor, MHR3, dopa decarboxylase, and a larval cuticle protein in the larval epidermis of the tobacco hornworm, Manduca sexta. Gen Comp Endocrinol. 1997;107:84–97. doi: 10.1006/gcen.1997.6901. [DOI] [PubMed] [Google Scholar]
- 26.White KP, Hurban P, Watanabe T, Hogness DS. Coordination of Drosophila metamorphosis by two ecdysone-induced nuclear receptors. Science. 1997;276:114–117. doi: 10.1126/science.276.5309.114. [DOI] [PubMed] [Google Scholar]
- 27.Palli SR, Hiruma K, Riddiford LM. An ecdysteroid-inducible Manduca gene similar to the Drosophila DHR3 gene, a member of the steroid hormone receptor superfamily. Dev Biol. 1992;150:306–318. doi: 10.1016/0012-1606(92)90244-b. [DOI] [PubMed] [Google Scholar]
- 28.Palli SR, Ladd TR, Sohi SS, Cook BJ, Retnakaran A. Cloning and developmental expression of Choristoneura hormone receptor 3, an ecdysone-inducible gene and a member of the steroid hormone receptor superfamily. Ins Biochem Mol Biol. 1996;26:485–499. doi: 10.1016/0965-1748(96)00004-5. [DOI] [PubMed] [Google Scholar]
- 29.Riddiford LM, Palli SR, Hiruma K. Hormonal control of sequential gene expression in Manduca epidermis. Prog Clin Biol Res. 1990;342:226–231. [PubMed] [Google Scholar]
- 30.Wolfgang WJ, Riddiford LM. Larval cuticular morphogenesis in the tobacco hornworm, Manduca sexta, and its hormonal regulation. Dev Biol. 1986;113:305–316. doi: 10.1016/0012-1606(86)90166-1. [DOI] [PubMed] [Google Scholar]
- 31.Parthasarathy R, Palli SR. Developmental and hormonal regulation of midgut remodeling in a lepidopteran insect. Heliothis virescens. Mech Dev. 2007;124:23–34. doi: 10.1016/j.mod.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Parthasarathy R, Tan A, Sun Z, Chen Z, Rankin M, Palli SR. Juvenile hormone regulation of male accessory gland activity in the red flour beetle, Tribolium castaneum. Mech Dev. 2009;126:563–579. doi: 10.1016/j.mod.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parthasarathy R, Palli SR. Molecular analysis of juvenile hormone analog action in controlling the metamorphosis of the red flour beetle, Tribolium castaneum. Arch Ins Biochem Physiol. 2009;70:57–70. doi: 10.1002/arch.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rountree DB, Bollenbacher WE. The release of the prothoracicotropic hormone in the tobacco hornworm, Manduca sexta, is controlled intrinsically by juvenile hormone. J Exp Biol. 1986;120:41–58. doi: 10.1242/jeb.120.1.41. [DOI] [PubMed] [Google Scholar]
- 35.Sho S, Hiroshi I. Developmental arrest induced by juvenile hormone in larvae of Bombyx mori. Arc Ins Biochem Physiol. 1988;8:219–228. [Google Scholar]
- 36.Judson CL, de Lumen HZ. Some effects of juvenile hormone and analogues on ovarian follicles of the mosquito Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1976;13:197–201. doi: 10.1093/jmedent/13.2.197. [DOI] [PubMed] [Google Scholar]
- 37.Raikhel AS, Lea AO. Juvenile hormone controls previtellogenic proliferation of ribosomal RNA in the mosquito fat body. Gen Comp Endocrinol. 1990;77:423–434. doi: 10.1016/0016-6480(90)90233-c. [DOI] [PubMed] [Google Scholar]
- 38.Beckemeyer EF, Lea AO. Induction of follicle separation in the mosquito by physiological amounts of ecdysterone. Science. 1980;209:819–821. doi: 10.1126/science.209.4458.819. [DOI] [PubMed] [Google Scholar]


