Abstract
Hemangioblastomas frequently develop in patients with von Hippel-Lindau (VHL) disease, an autosomal dominant genetic disorder. The tumors are characterized by a dense network of blood capillaries, often in association with cysts. Although activation of receptor tyrosine kinase (RTK) signaling, including epidermal growth factor receptor (EGFR) has been implicated in the development of malignant brain tumors such as high-grade gliomas, little is known about the role of RTK signaling in hemangioblastomas. To address this issue, we examined hemangioblastoma tumor specimens using receptor tyrosine kinase (RTK) activation profiling and immunohistochemistry. Six human hemangioblastomas were analyzed with a phospho-RTK antibody array, revealing EGFR phosphorylation in all tumors. EGFR expression was confirmed by immunohistochemistry in all tumors analyzed and downstream effector pathway activation was demonstrated by positive staining for phospho-AKT. Our findings suggest that, in primary hemangioblastomas, RTK upregulation and signaling predominantly involves EGFR, providing an attractive molecular target for therapeutic intervention.
Keywords: Hemangioblastoma, EGFR, Receptor tyrosine kinases, Overexpression
Background
Hemangioblastomas are rare central nervous system (CNS) tumors that typically develop within the cerebellum and the spinal cord [1]. They are highly vascularized tumors characterized by abundant endothelial cell proliferation and cyst formation [2]. The tumors are mainly composed of hemangioblasts, i.e. endothelial cells [1]. Other cell types that are found within the tumors include mast cells, pericytes and stromal cells, which are believed to be the primary neoplastic cells [3, 4].
Patients with von Hippel-Lindau (VHL) syndrome, an autosomal-dominant disorder, are predisposed to developing hemangioblastomas. The underlying genetic defect is caused by mutations in the VHL gene on the short arm of chromosome 3, which acts as a tumor suppressor [5], predisposing to the development of a wide variety of benign and malignant tumors involving the kidneys, adrenal glands, CNS and the retina [6]. The VHL protein interacts with ElonginC, ElonginB and CUL2 in a complex referred to as VCB-CUL2. VCB-CUL2 targets other large proteins for degradation including the hypoxia-inducible factors (HIFs), resulting in overexpression of genes involved in the metabolic adaptation to oxygen deprivation [7]. Recent data indicates that loss of VHL prolongs receptor tyrosine kinase (RTK) turnover by delaying endocytosis-mediated receptor deactivation and thereby enhancing downstream signaling [8].
EGFR is the first of a family of four closely related membrane RTKs (ErbB1, ErbB2 or HER2/neu, ErbB3, and ErbB4) that employ tyrosine kinase activity as the signal transduction initiator. Overexpression of EGFR has been documented across all stages of tumorigenesis, including pre-cancerous lesions, early cancers, as well as advanced cancers [9] and activation of the EGFR signaling pathway has been linked to tumor cell proliferation, survival, angiogenesis, and metastasis [10]. Increased expression of EGFR and transforming growth factor alpha (TGF-α) has been documented in a variety of human cancers such as early stage non-small-cell lung cancers [11], epithelial cancers [12], as well as low-grade and high-grade gliomas [13].
EGFR is a 170 kDa transmembrane glycoprotein composed of an amino-terminal extracellular ligand-binding domain, a hydrophobic transmembrane helix, and a cytoplasmic domain, which contains the tyrosine kinase domain and a carboxy-terminal region containing critical tyrosine residues and receptor regulatory motifs [14]. Binding of ligands to the extracellular domain results in receptor oligomerization, activation of the receptor’s tyrosine kinase activity and receptor autophosphorylation in several C-terminal tyrosine residues. These phosphorylated tyrosines serve as binding sites for a number of cytoplasmic signal-transducing molecules. Activation of these pathways downstream of EGFR leads to cell proliferation, differentiation, migration/motility, adhesion, protection from apoptosis, enhanced survival, and gene transcription [9].
To investigate the role of RTK signaling in hemangioblastomas, we screened a series of six hemangioblastoma specimens from our Brain Tumor Bank for RTK activation by phospho-RTK profiling. In all six specimens examined, epidermal growth factor receptor (EGFR) was the most strongly and consistently phosphorylated RTK. EGFR protein expression was confirmed by immunohistochemistry and downstream signal activation was demonstrated by positive staining for phospho-AKT. We conclude that EGFR should be explored as a therapeutic target for the treatment of hemangioblastomas.
Materials and methods
Tumor samples
This study was conducted under a protocol approved by the institutional review board of New York University School of Medicine. Four cerebellar and two spinal cord hemangioblastoma specimens were studied. The age range for the three female and three male hemangioblastoma patients was 19–38 years. After surgical removal, the specimens were formalin-fixed and paraffin-embedded or frozen directly and stored at −80°C. In all cases, scant portions of adjacent cerebellar or spinal cord tissues were also present in the specimens.
Proteome profiler arrays
To investigate the activation/phosphorylation of RTKs, we used the Proteome Profiler arrays (R&D Systems, Wiesbaden, Germany), also called human phospho-RTK antibody array. The human phospho-RTK antibody array is a nitrocellulose membrane where forty-two different anti-RTK antibodies have been spotted in duplicate, including four positive controls and five negative controls.
To conduct a proteome profiler array experiment, the tumor specimens were thawed on ice and, using a disposable pestle, homogenized in NP-40 lysis buffer (150 mM NaCl, 1% Nonidet P-40, 1% deoxycholate, 0.1% SDS, 10 mM Tris–HCl, pH 8.0, 1 mM EDTA, pH 8.0) supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride; PMSF, 0.2 mM sodium orthovanadate and 1 µg/ml aprotinin). Cell lysates were gently rocked for 30 min at 4°C and then centrifuged at 14,000×g for 5 min (4°C), and the supernatants removed and stored at −80°C until use. A total of 500 µg of protein, as determined by the BCA assay, was used for each array and developed using SuperSignal West Pico Chemiluminescent kit (Pierce, Inc.) according to the manufacturer’s recommendations.
Immunohistochemistry
Immunohistochemistry was performed using mouse anti-human EGFR clone 31G7 (Invitrogen, Carlsbad, CA) and rabbit anti-human phosphorylated-AKT (p-AKT) clone 736E11 (Cell Signaling Technologies, Danvers, MA). Four-micron tissue sections were collected and deparaffinized in xylene (3 changes), rehydrated through graded alcohols and rinsed in distilled water. Heat-induced epitope retrieval was performed in 10 mM citrate buffer pH 6.0 for 20 min in a 1,200-Watt microwave oven at 90% power for p-AKT. Sections were allowed to cool for 30 min and then rinsed in distilled water. Antigen retrieval for EGFR was performed by incubating the slides with protease 3 (Ventana Medical Systems Tucson, AZ) for 6 min. Antibody incubations and detection were carried out at 37°C on a NEXes instrument (Ventana Medical Systems Tucson, AZ) using Ventana’s reagent buffer and peroxidase detection kit. In brief, endogenous peroxidase activity was blocked with hydrogen peroxide. pAKT was diluted 1:20 and EGFR was diluted 1:50, both were incubated overnight at room temperature. Primary antibody was detected using a cocktail of biotinylated goat anti-mouse and goat antirabbit antibodies, followed by application of streptavidinperoxidase conjugate. The immune complex was visualized with 3,3 diaminobenzidene and enhanced with copper sulfate. Slides where washed in distilled water, counterstained with hematoxylin, dehydrated and mounted with permanent media. Appropriate positive and negative controls were included with the study sections.
Results
We analyzed six surgically removed human hemangioblastoma specimens from six patients. All tumors had a proliferation index of <1%, as assessed by MIB-1 staining (data not shown) and were analyzed by phospho-RTK profiling.
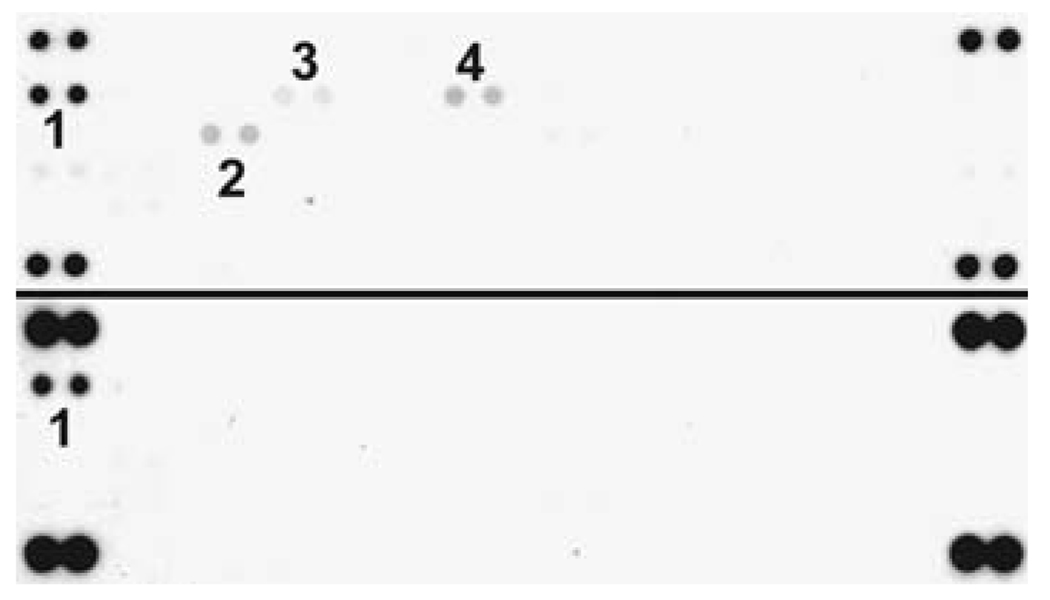
The phosphorylation/activation status of 42 RTKs was simultaneously tested using a membrane array of primary antibodies specific for each receptor. Homogenized tissue lysates were incubated overnight with the membrane and detected the following day with a pan-antiphosphotyrosine antibody. The results of RTK phosphorylation in the six hemangioblastoma specimens are summarized in Table 1. Most strikingly,we observed consistent phosphorylation of EGFR in all samples analyzed (Fig. 1, upper panel). In contrast, phosphorylation of additional receptors including FGFR2 and RON, TIE2, AXL, NGFR, and ROR was detected faintly and inconsistently. There was no detectable phosphorylation of the remaining RTKs tested by the phospho-array including ErbB2, ErbB3, FGFR1, FGFR3, FGFR4, insulin receptor, IGF-1 receptor, Dtk, Mer, MSP receptor, PDGFRα, PDGFRβ, SCF receptor, Flt-3, M-CSF receptor, c-Ret, ROR2, Tie-1, TrkA, TrkB, VEGFR1, VEGFR2, VEGFR3, MuSK, EphA1, EphA2, EphA3, EphA4, EphA6, EphA7, EphB1, EphB2, EphB4, and EphB6.
Table 1.
Patient characteristics and results summary
| Patient | Age | Sex | Tumor location |
VHL status |
IHC EGFR |
IHC p-AKT | p-EGFR | p-ErbB4 | p-FGFR2 | p-RON | p-AXL | p-TIE2 | p-NGFR | p-ROR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 | F | Spinal cord | VHL | ++ | ++ | ++ | + | + | + | − | − | − | − |
| 2 | 25 | F | Cerebellum | N/A | ++ | − | ++ | − | − | − | − | − | − | − |
| 3 | 32 | M | Spinal cord | Sporadic | ++ | − | + | − | − | − | − | − | − | + |
| 4 | 35 | M | Cerebellum | Sporadic | ++ | N/A | ++ | − | − | − | − | − | − | − |
| 5 | 36 | F | Cerebellum | Sporadic | ++ | + | ++ | − | − | − | + | + | + | − |
| 6 | 38 | M | Cerebellum | Sporadic | + | + | ++ | − | − | − | − | − | − | − |
Patient characteristics, tumor location, immunohistochemical staining (IHC) for EGFR, and phospho-RTK status (italics) of the six hemangioblastoma cases are listed. IHC is scored as + (weak) or ++ (strong). The level of RTK phosphorylation is indicated by the intensity of the duplicate dots scored from − (negative), + (weak), and ++ (strong). N/A: not available
Fig. 1.
RTK phosphorylation in hemangioblastoma specimens. Tumor tissue lysate was incubated on RTK arrays, and phosphorylation status was determined by subsequent incubation with antiphosphotyrosine horseradish peroxidase. Each RTK is spotted in duplicate and the pairs of dots in each corner of the membrane are positive controls for the assay. Two representative arrays are shown (Patient #1 upper panel, patient #2 lower panel). Positive phospho-RTK signals are (1) EGFR, (2) RON, (3) ErbB4, (4) FGFR2
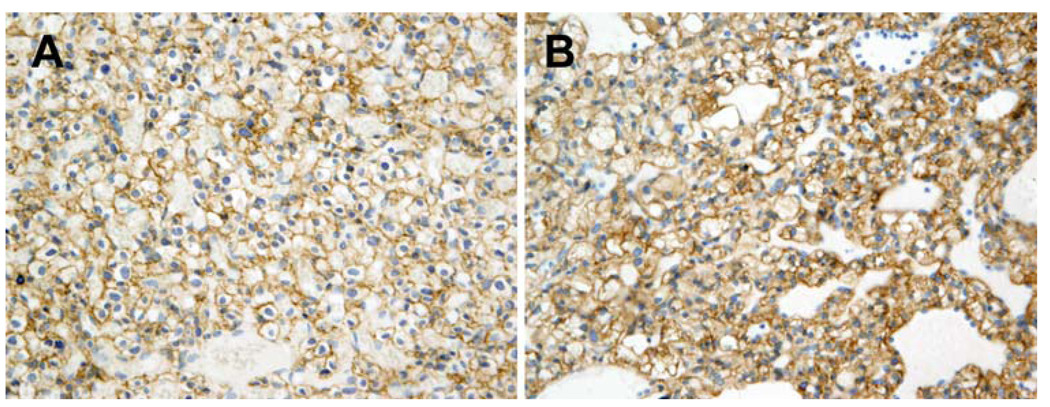
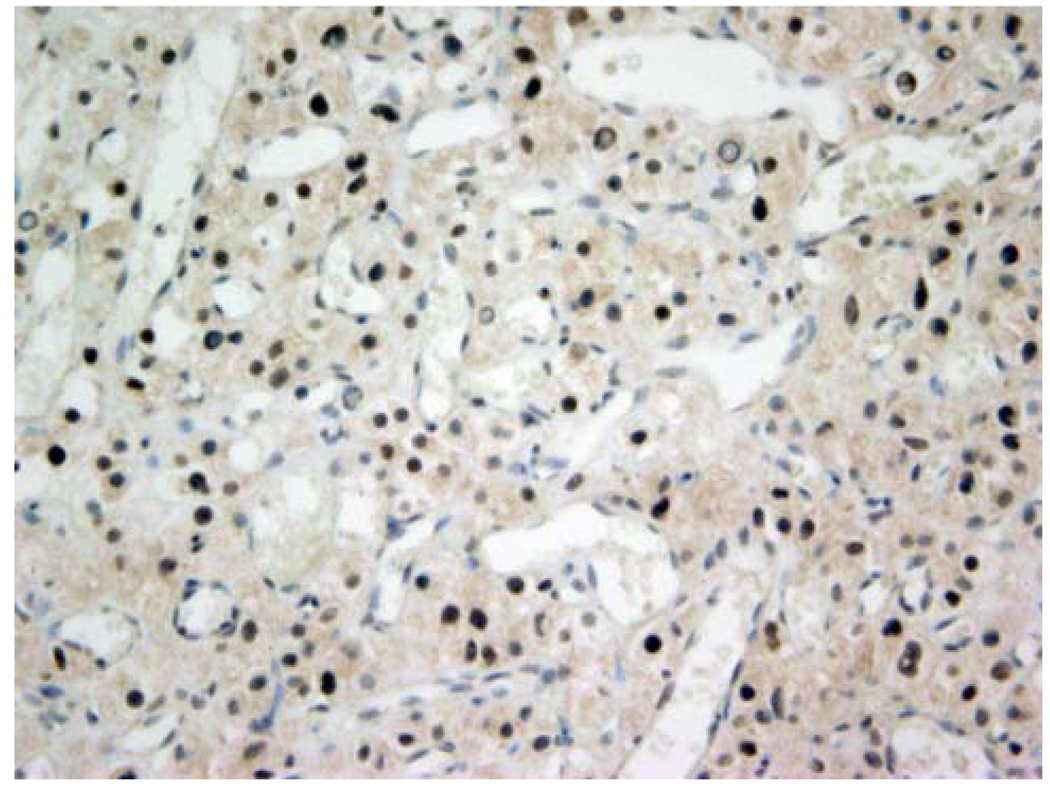
Based on the RTK array findings, we performed immunohistochemistry to confirm EGFR protein expression. All six hemangioblastoma specimens were highly vascular and showed the typical dense network of blood-filled capillaries in association with stromal cells and cystic areas. In all cases, scant portions of adjacent cerebellar or spinal cord tissues were also present in the specimens. A distinct boundary between highly vascularized tumor and normal brain tissue could be observed. Immunoreactivity for EGFR was strongest in the microvasculature of the hemangioblastomas including tumor cells and, to a lesser extent, in the interstitium (Fig. 2a, b). To investigate signal activation downstream of EGFR, we determined the phosphorylation status of AKT by immunohistochemistry. Three of five hemangioblastoma specimens available for testing stained positive for phospho-Akt (Fig. 3) indicating activation of the effector pathway.
Fig. 2.
Immunolocalization of EGFR in hemangioblastoma tissues. Immunohistochemical staining for EGFR was performed on hemangioblastoma tissue samples from six patients with similar results. Two representative specimens are shown, corresponding to patients #1 (a) and #4 (b) in Table 1. EGFR is highly expressed in human hemangioblastomas as shown by brown membranous staining in stromal cells characterized by centrally placed nuclei. Original magnification at 200×
Fig. 3.
EGFR downstream signal activation. Immunohistochemistry for phospho-AKT shows strong brown nuclear and cytoplasmic staining confirming AKT pathway activation. Representative sample shown corresponds to patient #1 (Table 1 and Fig. 2a). Original magnification at 200×
Discussion
Our findings are consistent with two prior immunohistochemical studies that found EGFR overexpression in hemangioblastomas [15, 16], although EGFR phosphorylation status and downstream signal activation were not assessed in these reports. EGFR overexpression in hemangioblastomas does not appear to be caused by EGFR gene amplifications [16], which can be observed in other malignancies. However, overexpression of EGFR via HIF has been described in vitro by translational upregulation [17], and more recently via deceleration of endocytosis by downregulation of Rab-5 mediated endosome fusion [8]. Our results indicate that these or similar mechanisms resulting in EGFR overexpression and phosphorylation are active in hemangioblastomas.
Receptor tyrosine kinases (RTKs) are a subclass of cell-surface growth-factor receptors with an instrinsic, ligand-controlled tyrosine-kinase activity. They regulate diverse functions in normal cells such as cell proliferation, migration, metabolism, differentiation and survival, as well as those that regulate intercellular communication during development [18]. RTK activity in resting, normal cells is tightly controlled. However, when they are mutated or structurally altered, RTKs become potent onco-proteins. Abnormal activation of RTKs in transformed cells has been shown to be causally involved in the development and progression of many human cancers.
Up-regulation of RTKs has been shown to be a recurring theme in the development of human cancers. In particular, recent studies have focused on the overexpression of EGFR. Ligand-induced activation of EGFR, a receptor tyrosine kinase, can instigate a wide range of cellular responses such as growth, differentiation, migration, and survival through various signaling pathways [19]. The dependence of certain cancer cells on EGFR for growth and survival has directed much attention to this receptor as a central target for cancer therapy [5, 20, 21]. EGFR overexpressing human cancers include renal [22], breast [12], ovarian [12], non-small-cell lung [11], prostate [23], and pancreatic [24].
In the normal brain, EGFR is expressed at very low levels [25]. However, in brain tumors, EGFR has been shown to be overexpressed. A study of astrocytomas, anaplastic astrocytomas, and glioblastomas has shown positive EGFR immunoreactivity being detected in 85% of tumors. Additionally, the expression of EGFR increased with the grade of malignancy in low-grade astrocytomas (67%), anaplastic astrocytomas (87%), and glioblastomas (100%) [26]. Overexpression of EGFR and increased stimulation of EGFR through autocrine growth factor loops, in particular through TGF-α, have been identified as a common mechanism of RTK deregulation in brain tumors including glioblastomas [13], medulloblastomas [27], and astrocytomas [28]. TGF-α mRNAs and EGFR mRNAs have been shown to be consistently co-expressed in capillary hemangioblastomas, suggesting the overexpression and activation of EGFR [16]. Selected growth factor receptors, including EGFR, have also been shown to be expressed uniformly high in capillary hemangioblastomas by immunocytochemistry [15].
In the present study, we assessed RTK phosphorylation and downstream signaling by phospho-RTK profiling and immunohistochemistry. We assayed snap-frozen, untreated hemangioblastomas from six diagnosed patients for evidence of RTK phosphorylation and found all of the specimens had EGFR phosphorylation. In contrast with normal brain specimens that had no detectable RTK activation [25], each of the six hemangioblastoma samples examined by antibody array profiling showed EGFR phosphorylation and some samples with multiple phosphorylated RTKs. In addition to the most prominently phosphorylated EGFR, other receptors include ErbB4, FGFR2, RON, TIE2, AXL, NGFR, and ROR (Fig. 1). We confirmed EGFR expression and downstream signal activation by immunohistochemistry for EGFR and phospho-AKT (Figs. 2, 3 and Table 1).
ErbB4, one of four members within the EGFR family of receptor tyrosine kinases, has been shown to be expressed at a higher level in low-grade gliomas than in high-grade gliomas. This suggests that ErbB4 may act as a suppressor of malignant transformation in these tumor types [29]. Additionally, ErbB4 expression has been shown to have an antagonistic effect on the clinical influence of other EGFR family members. A possible explanation for the apparently contradictory observations may be that ErbB4 expression seems to be differentially up- or down-regulated in different types of cancer due to co-expression of other EGFR family members [30].
Besides EGFR and ErbB4, the phosphorylation/activation of FGFR2, RON, and the TIE2 receptor have all been reported in the progression of various solid tumor types. For example, in the neovascularization of glioblastomas, FGFR2 has been shown to be up-regulated in response to resistance of endothelial cells to apoptosis [31]; RON (c-met) up-regulated in response to the hypoxic environment of the tumors [32]; and TIE2 receptor up-regulated in response to initiation of blood vessel sprouting [33].
All RTK arrays from our patient series showed strong EGFR phosphorylation with or without co-phosphorylation of other RTKs. These results suggest that hemangioblastoma tumor progression and development may require co-activation of more than one receptor tyrosine kinase. Previously, other RTKs such as FGFR2, VEGFR3, TIE2 and PDGFR have been shown to be activated in the development of other cancers and tumors [18]. Concomitant activation of multiple RTKs may provide a mechanism to reduce dependence on any single RTK for the maintenance of critical downstream signaling in a complex tumor environment, thereby rendering such tumors resistant to inhibition of a single RTK [35]. Consequently, targeting more than one signaling pathway may represent a promising therapeutic strategy. Based on VEGF and VEGF receptor expression [2], clinical trials using the VEGFR targeting agents sunitinib and PTK877 are already underway for patients with hemangioblastomas. Intriguingly, close functional interactions between EGFR and VEGFR signaling in tumors have been described in preclinical studies, and combined EGFR/VEGFR targeting strategies are being investigated in clinical trials for solid tumors [34]. Therefore, we propose that EGFR-targeting agents should be evaluated in preclinical and/or clinical trials for hemangioblastomas while considering combinations with VEGFR inhibitors or other anti-angiogenic agents.
Conclusions
In the present study involving six human hemangioblastomas, we demonstrate EGFR to be the most consistently overexpressed and activated RTK. In addition, we identified multiple activated RTKs which have not been previously associated with hemangioblastomas, such as RON, AXL and ROR. In conclusion, our results suggest that overexpression and activation of EGFR, and possibly other RTKs, may play a role in the growth of hemangioblastomas by providing autocrine or juxtacrine growth stimuli for the stromal cells. We propose that molecular targeted therapy directed against EGFR and other RTKs expressed in hemangioblastomas may be of therapeutic value and should be explored in further preclinical and clinical studies.
Acknowledgements
We thank Dr. Luis Chiriboga for his excellent technical assistance. This work was supported by the National Institutes of Health grants NRSA (GC) and R01 CA100426 (DZ), and the Pediatric Cancer Foundation (MAK).
Contributor Information
Gregory J. Chen, Department of Pathology, New York University School of Medicine, New York, NY, USA Microvascular and Molecular Neuro-Oncology Laboratory, New York University School of Medicine, New York, NY, USA.
Matthias A. Karajannis, Department of Pediatrics, New York University School of Medicine, New York, NY, USA New York University Cancer Institute, New York University School of Medicine, New York, NY, USA.
Elizabeth W. Newcomb, Department of Pathology, New York University School of Medicine, New York, NY, USA New York University Cancer Institute, New York University School of Medicine, New York, NY, USA.
David Zagzag, Email: david.zagzag@nyumc.org, Department of Pathology, New York University School of Medicine, New York, NY, USA; New York University Cancer Institute, New York University School of Medicine, New York, NY, USA; Microvascular and Molecular Neuro-Oncology Laboratory, New York University School of Medicine, New York, NY, USA; Division of Neuropathology, New York University School of Medicine, New York, NY, USA.
References
- 1.Richard S, Campello C, Taillandier L, Parker F, Resche F. Haemangioblastoma of the central nervous system in von Hippel-Lindau disease. French VHL Study Group. J Intern Med. 1998;243:547–553. doi: 10.1046/j.1365-2796.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 2.Wizigmann-Voos S, Breier G, Risau W, Plate KH. Up-regulation of vascular endothelial growth factor and its receptors in von Hippel-Lindau disease-associated and sporadic hemangioblastomas. Cancer Res. 1995;55:1358–1364. [PubMed] [Google Scholar]
- 3.Hussein MR. Central nervous system capillary haemangioblastoma: the pathologist’s viewpoint. Int J Exp Pathol. 2007;88:311–324. doi: 10.1111/j.1365-2613.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JY, Dong SM, Park WS, Yoo NJ, Kim CS, Jang JJ, Chi JG, Zbar B, Lubensky IA, Linehan WM, Vortmeyer AO, Zhuang Z. Loss of heterozygosity and somatic mutations of the VHL J Neurooncol tumor suppressor gene in sporadic cerebellar hemangioblastomas. Cancer Res. 1998;58:504–508. [PubMed] [Google Scholar]
- 5.Neumann HP, Lips CJ, Hsia YE, Zbar B. Von Hippel-Lindau syndrome. Brain Pathol. 1995;5:181–193. doi: 10.1111/j.1750-3639.1995.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaelin WG. Von Hippel-Lindau disease. Annu Rev Pathol. 2007;2:145–173. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 7.Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–2618. [PubMed] [Google Scholar]
- 8.Wang Y, Roche O, Yan MS, Finak G, Evans AJ, Metcalf JL, Hast BE, Hanna SC, Wondergem B, Furge KA, Irwin MS, Kim WY, Teh BT, Grinstein S, Park M, Marsden PA, Ohh M. Regulation of endocytosis via the oxygen-sensing pathway. Nat Med. 2009;15:319–324. doi: 10.1038/nm.1922. [DOI] [PubMed] [Google Scholar]
- 9.Kim ES, Khuri FR, Herbst RS. Epidermal growth factor receptor biology (IMC-C225) Curr Opin Oncol. 2001;13:506–513. doi: 10.1097/00001622-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Shien T, Tashiro T, Omatsu M, Masuda T, Furuta K, Sato N, Akashi-Tanaka S, Uehara M, Iwamoto E, Kinoshita T, Fukutomi T, Tsuda H, Hasegawa T. Frequent overexpression of epidermal growth factor receptor (EGFR) in mammary high grade ductal carcinomas with myoepithelial differentiation. J Clin Pathol. 2005;58:1299–1304. doi: 10.1136/jcp.2005.026096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veale D, Kerr N, Gibson GJ, Kelly PJ, Harris AL. The relationship of quantitative epidermal growth factor receptor expression in non-small cell lung cancer to long term survival. Br J Cancer. 1993;68:162–165. doi: 10.1038/bjc.1993.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckstein N, Servan K, Girard L, Cai D, von Jonquieres G, Jaehde U, Kassack MU, Gazdar AF, Minna JD, Royer HD. Epidermal growth factor receptor pathway analysis identifies amphiregulin as a key factor for cisplatin resistance of human breast cancer cells. J Biol Chem. 2008;283:739–750. doi: 10.1074/jbc.M706287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bredel M, Pollack IF, Hamilton RL, James CD. Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res. 1999;5:1786–1792. [PubMed] [Google Scholar]
- 14.Boonstra J, Rijken P, Humbel B, Cremers F, Verkleij A, en Henegouwen P. The epidermal growth factor. Cell Biol Int. 1995;19:413–430. doi: 10.1006/cbir.1995.1086. [DOI] [PubMed] [Google Scholar]
- 15.Bohling T, Hatva E, Kujala M, Claesson-Welsh L, Alitalo K, Haltia M. Expression of growth factors and growth factor receptors in capillary hemangioblastoma. J Neuropathol Exp Neurol. 1996;55:522–527. doi: 10.1097/00005072-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Reifenberger G, Reifenberger J, Bilzer T, Wechsler W, Collins VP. Coexpression of transforming growth factor-alpha and epidermal growth factor receptor in capillary hemangioblastomas of the central nervous system. Am J Pathol. 1995;147:245–250. [PMC free article] [PubMed] [Google Scholar]
- 17.Franovic A, Gunaratnam L, Smith K, Robert I, Patten D, Lee S. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci USA. 2007;104:13092–13097. doi: 10.1073/pnas.0702387104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 19.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 20.Ritter CA, Arteaga CL. The epidermal growth factor receptor-tyrosine kinase: a promising therapeutic target in solid tumors. Semin Oncol. 2003;30:3–11. doi: 10.1053/sonc.2003.50027. [DOI] [PubMed] [Google Scholar]
- 21.Hirata A, Uehara H, Izumi K, Naito S, Kuwano M, Ono M. Direct inhibition of EGF receptor activation in vascular endothelial cells by gefitinib (‘Iressa’, ZD1839) Cancer Sci. 2004;95:614–618. doi: 10.1111/j.1349-7006.2004.tb02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrides PE, Bock S, Bovens J, Hofmann R, Jakse G. Modulation of pro-epidermal growth factor, pro-transforming growth factor alpha and epidermal growth factor receptor gene expression in human renal carcinomas. Cancer Res. 1990;50:3934–3939. [PubMed] [Google Scholar]
- 23.Olapade-Olaopa EO, Moscatello DK, MacKay EH, Horsburgh T, Sandhu DP, Terry TR, Wong AJ, Habib FK. Evidence for the differential expression of a variant EGF receptor protein in human prostate cancer. Br J Cancer. 2000;82:186–194. doi: 10.1054/bjoc.1999.0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, Evans DB, Abbruzzese JL, Hicklin DJ, Radinsky R. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000;6:1936–1948. [PubMed] [Google Scholar]
- 25.Libermann TA, Razon N, Bartal AD, Yarden Y, Schlessinger J, Soreq H. Expression of epidermal growth factor receptors in human brain tumors. Cancer Res. 1984;44:753–760. [PubMed] [Google Scholar]
- 26.Hwang SL, Chai CY, Lin HJ, Howng SL. Expression of epidermal growth factor receptors and c-erbB-2 proteins in human astrocytic tumors. Kaohsiung J Med Sci. 1997;13:417–424. [PubMed] [Google Scholar]
- 27.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 28.Khatua S, Peterson KM, Brown KM, Lawlor C, Santi MR, LaFleur B, Dressman D, Stephan DA, MacDonald TJ. Overexpression of the EGFR/FKBP12/HIF-2alpha pathway identified in childhood astrocytomas by angiogenesis gene profiling. Cancer Res. 2003;63:1865–1870. [PubMed] [Google Scholar]
- 29.Andersson U, Guo D, Malmer B, Bergenheim AT, Brannstrom T, Hedman H, Henriksson R. Epidermal growth factor receptor family (EGFR, ErbB2-4) in gliomas and meningiomas. Acta Neuropathol. 2004;108:135–142. doi: 10.1007/s00401-004-0875-6. [DOI] [PubMed] [Google Scholar]
- 30.Junttila TT, Sundvall M, Maatta JA, Elenius K. Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants. Trends Cardiovasc Med. 2000;10:304–310. doi: 10.1016/s1050-1738(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 31.Auguste P, Gursel DB, Lemiere S, Reimers D, Cuevas P, Carceller F, Di Santo JP, Bikfalvi A. Inhibition of fibroblast growth factor/fibroblast growth factor receptor activity in glioma cells impedes tumor growth by both angiogenesis-dependent and-independent mechanisms. Cancer Res. 2001;61:1717–1726. [PubMed] [Google Scholar]
- 32.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 33.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 34.Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol. 2008;5:521–530. doi: 10.1038/ncponc1161. [DOI] [PubMed] [Google Scholar]
- 35.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C, Chin L, DePinho RA. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]