Abstract
Background
Compliance represents a major determinant for the effectiveness of pharmacotherapy. Compliance reports summarising electronically compiled compliance data qualify healthcare needs and can be utilised as part of a compliance enhancing intervention. Nevertheless, evidence-based information on a sufficient level of compliance is scarce complicating the interpretation of compliance reports. The purpose of our pilot study was to determine the compliance of ambulatory Alzheimer patients to antidementia drugs under routine therapeutic use using electronic monitoring. In addition, the forgiveness of donepezil (i.e. its ability to sustain adequate pharmacological response despite suboptimal compliance) was characterised and evidence-based guidance for the interpretation of compliance reports was intended to be developed.
Methods
We determined the compliance of four different antidementia drugs by electronic monitoring in 31 patients over six months. All patients were recruited from the gerontopsychiatric clinic of a university hospital as part of a pilot study. The so called medication event monitoring system (MEMS) was employed, consisting of a vial with a microprocessor in the lid which records the time (date, hour, minute) of every opening. Daily compliance served as primary outcome measure, defined as percentage of days with correctly administered doses of medication. In addition, pharmacokinetics and pharmacodynamics of donepezil were simulated to systematically assess therapeutic undersupply also incorporating study compliance patterns. Statistical analyses were performed with SPSS and Microsoft Excel.
Results
Median daily compliance was 94% (range 48%-99%). Ten patients (32%) were non-compliant at least for one month. One-sixth of patients taking donepezil displayed periods of therapeutic undersupply. For 10 mg and 5 mg donepezil once-daily dosing, the estimated forgiveness of donepezil was 80% and 90% daily compliance or two and one dosage omissions at steady state, respectively. Based on the simulation findings we developed rules for the evidence-based interpretation of donepezil compliance reports.
Conclusions
Compliance in ambulatory Alzheimer patients was for the first time assessed under routine conditions using electronic monitoring: On average compliance was relatively high but variable between patients. The approach of pharmacokinetic/pharmacodynamic in silico simulations was suitable to characterise the forgiveness of donepezil suggesting evidence-based recommendations for the interpretation of compliance reports.
Background
Alzheimer's disease presents the most common type of dementia, accounting for 50%-60% of all cases [1]. In 2001, more than 24 million people worldwide were suffering from dementia, a number that is expected to double every 20 years up to 81 million in 2040 due to increase in life expectancy [1]. Cholinesterase inhibitors such as donepezil, galantamine and rivastigmine and the NMDA-receptor modulator memantine present the first-line pharmacotherapy for Alzheimer's disease [2,3]. Prescription data from a large statutory health insurance in Germany show that donepezil and memantine are the market leaders, each representing approximately one third of all defined daily doses of all four agents [4]. These agents can improve symptoms, primarily in the domains of cognition (e.g. ADAS-cog score) and global function [5]. Effectiveness should be evaluated in two to four months intervals [6].
Within outcomes research compliance presents a treatment modifier, which highly impacts the effectiveness of pharmacotherapy [7]. Studies investigating compliance show a variety of measures of medication usage and varying terminologies (e.g. compliance, adherence, persistence) complicating the interpretation and comparison of those studies [8,9]. The Medication Compliance and Persistence Work Group of the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) defined medication compliance as "the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen"[8]. Persistence may be referred to as "the duration of time from initiation to discontinuation". Today, no overarching term combines these two concepts [8].
Since the end of the 1970s electronic monitoring has been used to compile dose administration histories of ambulatory patients [10]. The so called medication event monitoring system (MEMS) consists of a vial with a microprocessor in the lid which records the time (date, hour, minute) of every opening [11]. In contrast to traditional compliance assessment methods such as pill count, patient diaries or patient self-report, the method of electronic monitoring demonstrated to be a more reliable tool allowing a detailed analysis of patient medication taking behaviour over time [12]. However, as indirect method actual ingestion of the medicine cannot be measured [10] and compliance may be underestimated (e.g. in the case that a weekly pill-box is used instead) [13]. In addition, MEMS in itself presents a compliance enhancing intervention for patients who are informed about the purpose of the device. This may lead to an overestimation of their "usual" medication taking behaviour. Nevertheless, electronic monitoring has been recognised closest to a 'gold standard' for compliance measurement [11]. To our knowledge, compliance studies among Alzheimer patients using electronic monitoring have not been conducted until now. Previous studies have employed pharmacy refill data to investigate medication taking behaviour [14-17]. Reports summarising MEMS data characterise compliance-time patterns in detail and can be utilised as part of a compliance enhancing intervention where a healthcare professional provides feedback to the patient on his/her medication taking behaviour [18]. This was termed measurement-guided medication management (MGMM).
The advent of electronic monitoring has also advanced research on the question "how much compliance is enough?" being closely related to the concept of forgiveness. Urquhart defined forgiveness as the "drug's post-dose duration of action minus the prescribed interval between doses" [10]. Researchers in the HIV area have adopted a more general definition of forgiveness as ability of a regimen to achieve and sustain adequate pharmacological response (in this case viral suppression) despite suboptimal compliance [19]. In the present work forgiveness is used in the latter sense, specifying the former as forgiveness according to Urquhart. The crucial 'experiment' for measuring how much compliance is sufficient, presents the controlled, blinded substitution of placebos for active drug [20]. This is frequently not ethically possible and has only been pursued in the field of e.g. oral contraception, hypertension and depression [20,21]. Furthermore, a correlation between compliance and clinical outcome was established in observational studies [22,23].
The capacity for forgiveness of drugs may differ substantially, depending on their pharmacokinetic (PK, i.e. the drug exposition) and pharmacodynamic (PD, i.e. the drug effect) properties, e.g. clearance and steepness of concentration-effect relationship. Thus, given a known pharmacokinetic/pharmacodynamic (PK/PD) relationship, in silico studies were also suggested for the characterisation of forgiveness [24,25]. In the case of donepezil, the degree of inhibition of peripheral cholinesterase has been identified as a PD biomarker [26]. In this work we defined "time with therapeutic undersupply" (TTU) as a parameter. To avoid TTU, the daily dosages of cholinesterase inhibitors to achieve a consistent red blood cell (RBC) cholinesterase inhibition of at least 40% corresponded to those causing improvements in ADAS-cog and functional activity scores [27].
The aim of our pilot study was to determine the compliance of ambulatory Alzheimer patients to antidementia drugs under routine therapeutic use by means of electronic monitoring over a six months period. In addition, we performed pharmacokinetic/pharmacodynamic in silico simulations using the pilot study compliance pattern data and published PK/PD models to characterise the forgiveness of donepezil, the most frequently prescribed antidementia drug. Ultimately, we aimed at developing a recommendation for the evidence-based interpretation of donepezil compliance reports which may form the basis of a MGMM intervention.
Methods
Study design
The current research was part of a sequential phase II trial (pilot study) within the Medical Research Council framework [28] (guidance for the development and evaluation of complex interventions) investigating the impact of a pharmaceutical care intervention on ambulatory Alzheimer patients' compliance and other outcomes such as knowledge in pharmacotherapy and caregivers' health-related quality of life. Our pharmaceutical care program in the second part of the trial should include a complex intervention including MGMM. The study was approved by the respective ethics committee (Ethics Board 4) of the Charité - Universitaetsmedizin Berlin, Germany. Patients had to fulfil the following inclusion criteria: diagnosis of Alzheimer's disease; prescription of an antidementia drug (ATC-code N06D); living in ambulatory setting; sufficient command of the German language (patient and caregiver); ability to consent (patient). Patients who lived in residential homes or who intended to move further away during the study period were not eligible according to study protocol. Patients were consecutively recruited from the above mentioned clinic. The study design comprised a one month run-in phase followed by a main phase lasting six months. All study individuals screened between March 2006 and July 2007 were Alzheimer patients of the gerontopsychiatric clinic of the Charité - Universitaetsmedizin Berlin, Germany. The recruited patients were a convenience sample and comprised the standard care group of the phase II trial.
Standard care denoted community pharmacists provided their usual dispensing service that included appropriate drug information and advice for patients according to the Ordinance on the Operation of Pharmacies [29]. No defined compliance enhancing intervention to the patient or caregiver was offered. Having obtained written informed consent from patients and caregivers, the antidementia drug was repacked into a MEMS container. Patients and caregivers were informed about the monitoring device. Each patient/caregiver was given the instruction to open the MEMS container when they wanted to take a medicine, to remove and take the prescribed dose, then promptly close the device. Additionally, patients' regular community pharmacy was informed and trained how to perform and document the repackaging procedure.
Compliance
Compliance was determined using MEMS 6 TrackCap (Aardex Ltd., Zug, Switzerland). Refills of MEMS vials were performed and documented in patients' regular community pharmacies. Subsequently, refill events and self-reported non-usage periods (e.g. due to hospital stays) were removed from the dataset. Daily compliance in the main phase served as primary outcome measure, defined as percentage of days with the correct number of MEMS openings (e.g. two openings for a twice daily drug) acting on the usual assumption that opening corresponded to drug intake. Daily compliance was calculated for each individual month as well as the total main phase comprising six months. Additionally patients were dichotomised into 'compliers' (daily compliance ≥ 80% according to the commonly employed cut-off criterion) and 'non-compliers' (daily compliance < 80%) [30]. For descriptive data analysis of MEMS recordings of our study, means or medians were calculated as measure of central tendency. According to the attributes of the respective variables, we calculated range, quartiles and 95% confidence interval. In contrast to the large amount of MEMS data the sample size of patients was small. In this part, no statistical test or extrapolations were performed based on individual data. Statistical analyses were performed with SPSS 15 (SPSS Inc., Chicago, Ill, USA).
Forgiveness
To characterise the forgiveness of donepezil, PK/PD in silico simulations were performed using Microsoft Excel 2003 (Microsoft Corp., Redmond, WA, USA). Three approaches (A, B and C) were applied via trace-driven simulations [31] using different compliance patterns as input (function): Approach A used the compliance data from the pilot study to evaluate therapeutic undersupply for all individual patients taking donepezil. Approaches B and C served as sensitivity analyses to characterise the forgiveness of donepezil. For approach B, discrete daily compliance values (0%-100%) were simulated using a step size of 10%. These selected compliance patterns were created by the pseudo-random number generator in Microsoft Excel, for a period of 200 days. Eventually for approach C, scenarios of 1-7 dosage omissions at steady-state after a 14 days run-in phase and with a 7 days follow-up phase were simulated. The utilised PK and PD models are summarised in Table 1.
Table 1.
Pharmacokinetic and pharmacodynamic models for donepezil.
| Model type (name) & equation | Variables and parameters | Reference | |
|---|---|---|---|
|
Pharmacokinetic model (two compartment model) |
|||
| for D = 5 mg: A = 3.502 ng/mL B = 1.209 ng/mL α = 0.445 h-1 β = 0.014 h ka = 1.319 h-1 lag time = 0.96 h |
for D = 10 mg: A = 4.536 ng/mL B = 1.234 ng/mL α = 0.542 h-1 β = 0.015 h-1 ka = 1.696 h-1 lag time = 0.68 h |
[32] | |
|
Pharmacodynamic model (Emax model) |
Emax = 100.8% EC50 = 15.6 ng/mL |
[34] | |
Parameter values and reference to literature, utilised for the in silico simulation.
C = total plasma drug concentration [ng/mL]
t = time [h]
D = dose
A, B = intercepts of the two exponential terms
α, β = macro (hybrid) rate constants
ka = absorption rate constant
E = effect, i.e. inhibition of peripheral cholinesterase, %
Emax = maximum effect
EC50 = drug concentration at half of maximum effect
For donepezil, linear PK was assumed [27].The plasma concentration-time course of donepezil for long-term treatment with (irregular) multiple dosing (up to 200 days with Δt = 1 min) was generated using a previously published PK model [32], applying the principle of superposition, as explained in [33]. In case of approach C where dosage omissions at steady state conditions were analysed, the dosing interval between the last and next dose administered was accordingly extended, e.g. doubled (48 h instead of 24 h) for one missing dose.
The resulting plasma concentration-time course of donepezil (for multiple dosing) was directly linked to the PD model (see Table 1) describing the inhibition of peripheral cholinesterase that served as PD biomarker [34]. The main outcome variable for all three simulations was the "time with therapeutic undersupply" (TTU). TTU was defined as time (percentage or hour) with the PD biomarker below the minimum therapeutic inhibition, that is < 40% inhibition of peripheral cholinesterase. Additionally, for approach C forgiveness according to Urquhart was determined as the "drug's post-dose duration of action minus the prescribed interval between doses" [10].
Results
Compliance
From 39 patients who fulfilled the inclusion criteria 31 (79%) were enrolled into the study. The main reason (6 out of 8 refusals) for nonparticipation was MEMS-related inconveniences (e.g. incompatibility of MEMS and use of weekly pill-boxes).
Fifty percent of all patients were between 71 and 82 years old and both sexes were almost equally represented. Duration of antidementia pharmacotherapy at inclusion ranged from drug-naïve to 6.5 years with 2-12 concomitantly administered drugs. The majority of patients were on a once daily antidementia regimen with donepezil being the most prevailing drug. Moreover, in more than two-thirds of all patients the caregiver only was responsible for pharmacotherapy (Table 2). In total, 6507 MEMS openings were recorded over 5399 days.
Table 2.
Characteristics of the patients at baseline (n = 31) and study characteristics.
| Characteristics | |
|---|---|
| n (%) | |
| Sex: women | 17 (55) |
| Responsibility for pharmacotherapy | |
| Patient only | 2 (6) |
| Patient supported by caregiver | 6 (19) |
| Caregiver only | 22 (71) |
| Professional care only | 1 (3) |
| Antidementia drugs in MEMSa | |
| Donepezil | 12 (39) |
| Galantamine | 12 (39) |
| Memantine | 7 (23) |
| Rivastigmine | 1 (3) |
| Regimen of antidementia drug in MEMSa | |
| Once daily | 24 (75) |
| Twice daily | 8 (25) |
| median (range) | |
| Age in years | 76 (47-96) |
| Duration of MEMS monitoring in daysb | 180 (140-180) |
| Duration of antidementia pharmacotherapy at inclusion in months | 18 (0-78) |
| Number of regularly administered drugs | 6 (2-12) |
MEMS, medication event monitoring system; a one patient was started on donepezil (MEMS monitoring during 49 days), but later in the course of the study he was switched to memantine (MEMS monitoring during 111 days); b eight out of 31 patients (26%) had non-monitored periods (e.g. due to hospital stays) or incomplete follow-up of less than 180 days.
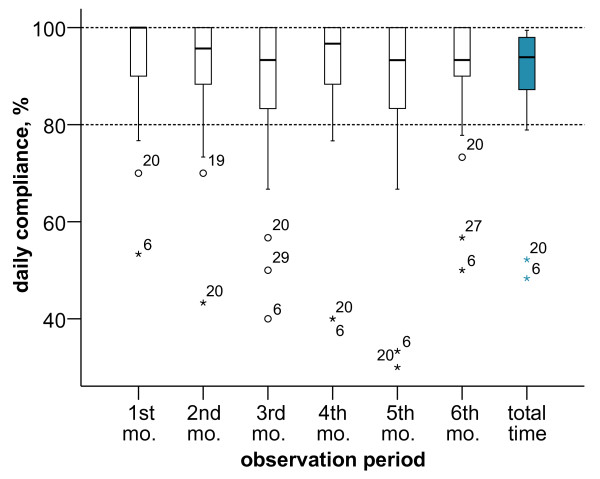
Median compliance of all patients in the main phase was 94% with a large range of 48% to 99% (lower quartile: 87%, upper quartile: 98%). In comparison to the first month, median compliance decreased by 7% points in the sixth month (Fig. 1).
Figure 1.
Daily compliance during study observation period for each month and the entire observation period. The ends of the whiskers represent the lowest data value within 1.5 times the box height from the lower box edge and the highest data value within 1.5 times the box height from the upper box edge, respectively. Index numbers represent individual patients ID: if associated with circle, this ID was regarded as outlier (1.5-3 times box height from the box edge), if with a star as extreme value (> 3 times box height from the box edge); mo. = month.
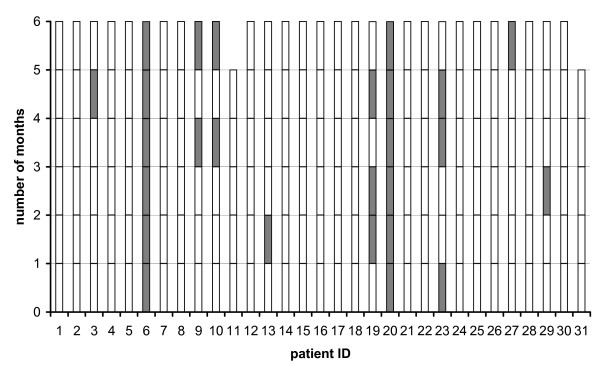
After dichotomisation of compliance (daily compliance 80% and more or < 80%), intraindividual compliance patterns by month revealed that 10 patients (32%, 95% confidence interval: 17%-51%) were at least one month non-compliant (Fig. 2). Among these, two patients were non-compliant throughout the main phase.
Figure 2.
Intraindividual daily compliance during the observation period. Compliant months (daily compliance ≥ 80%, white bars) or non-compliant months (daily compliance < 80%, grey bars); patient #11 and patient #31 participated five months only.
Forgiveness of donepezil
To determine their possible therapeutic undersupply individual compliance patterns of the twelve patients of the study comprising 1873 MEMS recordings with either 5 mg (n = 3) or 10 mg (n = 9) once daily donepezil were simulated (approach A). In the 5 mg donepezil dosing group, two patients (#13 and #23) out of three were undersupplied during certain time periods. Moreover, for both patients it was found that one occasionally omitted dose (dosing interval ≈48 h) did not cause therapeutic undersupply. However, series of one dose omission triggered the appearance of undersupplied periods. Very interestingly, daily compliance in the main phase for patient #13 and #23 was 87.2% and 80.0%, respectively, i.e. they were regarded as compliant according to the commonly employed 80% cut-off criterion. None of the patients taking 10 mg donepezil displayed any undersupplied periods.
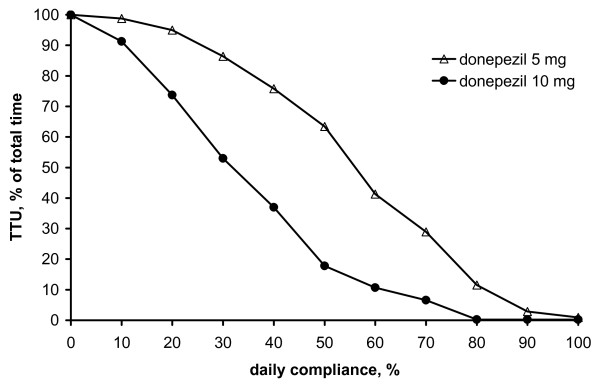
Simulations of discrete compliance values are displayed in Fig. 3 (approach B). For 10 mg (5 mg) donepezil negligible therapeutic undersupply was observed with daily compliance exceeding 80% (90%) leading to 0.3% (2.9%) TTU of total time. Fig. 3 revealed a sigmoidal relationship between TTU and discrete compliance values. For efficacious pharmacotherapy, however, TTU of total time should be negligible, i.e. close to zero.
Figure 3.
Forgiveness characterisation: Simulation approach B. Time with therapeutic undersupply (TTU) in percentage of total time versus discrete daily compliance values.
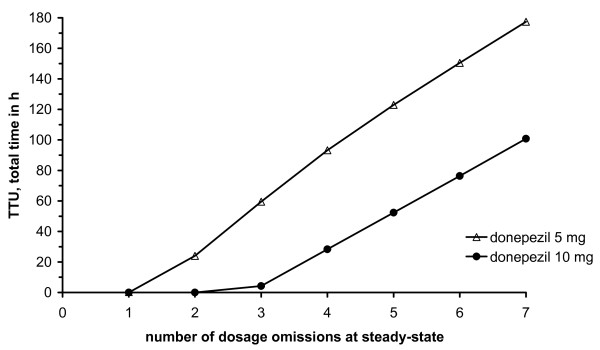
Approach C characterised the influence of 1-7 dosage omissions on the inhibition of cholinesterase (Fig. 4): Initially, both curves ran parallel to the abscissa. Subsequently, TTU linearly increased with a higher number of dosage omissions with comparable slope. In the case of 5 mg donepezil two or more dosage omissions caused therapeutic undersupply. For 10 mg three or more dosage omissions at steady state led to therapeutic undersupply. Forgiveness according to Urquhart was calculated as 68.4 h for 10 mg and 28.3 h for 5 mg donepezil, respectively, i.e. the PD effect lasted for almost 3 d and more than 1 d beyond the dosing interval, respectively.
Figure 4.
Forgiveness characterisation: Simulation approach C. Total time with therapeutic undersupply (TTU) in hours versus number of dosage omissions at steady-state.
Evidence-based interpretation of compliance reports for donepezil
Based on our results, for 5 mg donepezil, a monthly daily compliance less than 90% or two consecutive dosage omissions were regarded as therapeutically relevant non-compliance. For 10 mg donepezil we regarded a monthly daily compliance of less than 80% or three consecutive dosage omissions as a trigger of therapeutically relevant non-compliance.
Discussion
In this study, we provide evidence from non-invasively acquired data that compliance of ambulatory Alzheimer patients in their daily life seems to be relatively high with on average one non-compliant day out of ten. Nevertheless, about one third of all patients were at least one out of six months non-compliant employing the frequently used 80% cut-off criterion. PK/PD in silico simulations revealed that for 10 mg (5 mg) donepezil forgiveness of donepezil was estimated as 80% (90%) daily compliance or two (one) dosage omissions at steady-state, respectively. Based on these results, evidence-based recommendations for the detection of relevant non-compliance were developed to aid the interpretation of compliance reports as part of e.g. a MGMM.
Electronic monitoring of medication events - based on electronic detection of opening a container - presents an indirect method of estimating when and how much drug has been taken. Direct measures such as directly observed therapy and therapeutic drug monitoring may be feasible in certain areas (e.g. intensive care units) but not in others, as in our setting and patient population due to the high effort required. Errors in compliance estimation will occur if a patient opens the container without taking the drug or takes another number of tablets than the one prescribed. In addition, apparently irrational openings could also be detected in the investigated population (in 61% of all patients, but only in approximately 1% of all openings). Arnet and Haefeli recognised so called "curiosity events" in nearly 20% of patients' electronic monitoring systems which were due to showing the device to relatives or friends or uncertainty whether they had closed the electronic monitoring device [35].
Denhaerynck et al. provided a form to their patients to document deviations from "normal" usage of the monitoring device (e.g. during hospital stays) as well as conducted a structured interview with each patient at the end of the study [36]: Uncensored data showed a 3.4% higher non-compliance than the censored data (incorporating information of forms and interviews). Hence, non-censoring could be considered as an overestimation of non-compliance [36]. Nevertheless, another study revealed that uncorrected electronic monitoring data could successfully project measured plasma concentrations of drugs [37]. In our study, pharmacy refills and self-reported non-usage periods (e.g. due to hospital stays) were excluded from further analysis which may relevantly improve the quality of the data.
Denhaerynck et al. investigated the compliance enhancing effect in transplant patients by omitting the first month of electronic monitoring from their analysis: This only resulted in a 0.4% decrease in daily compliance. They concluded that the compliance enhancing effect of electronic monitoring only had minor clinical significance [36]. Nevertheless, the run-in phase of one month as part of our study design may reduce this effect, thus giving a more realistic picture of medication taking behaviour.
Electronic monitoring presents the most reliable method for the determination of compliance despite certain disadvantages [11]. Three studies determined persistence for donepezil and rivastigmine in treatment naïve patients using pharmacy-refill data [14-17]. Nearly one third of all patients stopped donepezil or rivastigmine therapy within 60 days of starting therapy [15]. There were no marked differences in persistence between both drugs [15,17]. Especially during the dosage up-titration period, adverse drug reactions (mainly gastrointestinal) occur that usually cease later in the course [38]. With the exception of one patient there were no treatment naïve participants in our study being particularly prone to adverse drug reactions. This patient was down-titrated to 5 mg donepezil from 10 mg due to adverse drug effects but did not stop therapy. In the study conducted by Roe and co-workers 14% of all patients who continued therapy for at least 6 months showed gaps in supply for at least six weeks [16]. In our study, around one third of all patients were considered non-compliant (80% cut-off criterion) in at least one month demonstrating how much more sensitive electronic monitoring has to be regarded compared to pharmacy refill data. During the course of the study, median compliance slightly decreased by 7% points. In future, a longer study should be performed to assess whether the decrease will be more substantial over a longer period.
Inhibition of peripheral cholinesterase served as a biomarker of pharmacological response in Alzheimer's disease. Jann et al. concluded that daily doses of cholinesterase inhibitors to achieve a consistent peripheral cholinesterase inhibition of at least 40% corresponded to those leading to an improvement in cognition and functional activity scores [27]. Elsewhere, positive associations were reported between the AChE inhibition and change in ADAS-cog and CIBIC plus [34]. Unfortunately, the strength of association was not reported by Rogers et al. and thus the predictive value of RBC AChE inhibition has been discussed [34,39] it nevertheless provides evidence of potential efficacy. Hence, 40% inhibition served as a reasonable cut-off below which only minor efficacy has to be expected.
Donepezil was chosen for characterising the forgiveness in the in silico analysis due to the following reasons: (i) most widely used acetylcholinesterase inhibitor, (ii) high number of MEMS data available (2720 monitored days), and (iii) an available PK as well as PD model and an established dose-response relationship.
The PK model included the long half-life of donepezil of 50 h. Since population pharmacokinetic models that provide variability parameters are not available, PK variability could not be employed in the in silico study. Moreover, for approaches B and C, investigating the influence of daily compliance and dosage omission on therapeutic coverage/undersupply, a high magnitude of PK variability could provide a more sophisticated picture of the outcome. Future in silico simulations for donepezil should account for population PK models that quantify variability, when available. In addition, subgroup analysis for confounders should be performed with a larger data base considering the metabolising isoenzymes, cytochrome P450 3A4 and the 2D6 which can be induced or inhibited by comedication. Besides, the current simulation had to be limited to donepezil because for other antidementia drugs literature data was mostly insufficient but highly warranted. For galantamine, a PK model was available but no PD model [40]. An exception presented rivastigmine as oral capsules where a PK/PD model was described in the literature [41]. Due to the low number of rivastigmine data of our study (one patient only), its low market share (13%) [4] and increasing acceptance of a transdermal formulation with differing PK/PD characteristics lacking published PK models we did not find it adequate to perform a simulation.
From three patients being prescribed donepezil who were at least one month non-compliant (daily compliance < 80%) two displayed periods of therapeutic undersupply. Especially the combination of a drug holiday (dosing interval exceeding 96 h) and several single dose omissions triggered therapeutic undersupply. The long half-life of donepezil (~50 h) might at first glance exclude this drug from being interesting for forgiveness analysis. But as shown in our investigation, forgiveness is in fact an issue for donepezil in certain non-compliance patterns (e.g. several dose omissions). These patterns in electronic monitoring reports present an indicator of potential insufficient therapeutic coverage and have to be discussed with the patient/caregiver [18]. In the absence of electronic monitoring patients or caregivers should be questioned in an empathic, non-patriarchic style about their medication taking behaviour. An old, still widely-held idea going back to research in the cardiovascular field in the 1960 s, was that taking 80% of the prescribed doses generally qualifies as satisfactory compliance [42]. This view, however, has to be regarded as pharmacodynamically naïve since forgiveness of each drug product is determined by its individual dosage form, PK and PD [43]. Our results reveal that 80% compliance already results in more than 10% therapeutic undersupply for 5 mg donepezil daily whereas 10 mg daily maintains almost full therapeutic coverage, i.e. for 5 mg donepezil we now suggest 90% compliance to serve as a cut-off.
Both donepezil dosages forgive a common compliance error: one occasionally omitted tablet. Compared to other therapeutic areas donepezil exhibited a high degree of forgiveness. If a single gestagen-only pill is taken more than three hours late, there will be a need of back-up contraception [44]. The forgiveness of once daily antihypertensives atenolol and betaxolol can be estimated as about 6 hours and more than 48 hours [45]. These results were generated by controlled (verum only group), blinded trials partially substituting verum against placebo, whereas we have implemented in silico simulations, in this way providing a lower level of evidence. Studies early in the era of antiretroviral therapy demonstrated the need for > 95% compliance in order to achieve and sustain viral suppression [19]. High rates of viral suppression could also be attained at more moderate compliance with newer antiretroviral regimens (e.g. lopinavir/ritonavir) [23]. Beyond this, sparse or no evidence is available on forgiveness of major therapeutic classes.
Conclusions
For the first time, this pilot study assessed compliance under routine therapeutic use of ambulatory Alzheimer patients using electronic monitoring. Compliance in ambulatory Alzheimer patients was relatively high but variable. The approach of pharmacokinetic/pharmacodynamic in silico simulations was suitable to characterise the forgiveness of donepezil giving evidence-based recommendations for the design of the intervention part of our study (MGMM-guided complex intervention of the pharmaceutical care program) and for the interpretation of compliance reports. Information on patients' compliance (percentage and pattern) should be incorporated in decisions whether to continue therapy in the case of therapeutic failure.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
OS (having full access to all the data in the study) and CK designed the study, acquired, analyzed and interpreted the data, responsibility for the integrity of the data and the accuracy of the data analysis and drafted the manuscript. AS and ASP were involved in the study concept and design, analysis and interpretation of the data and the critical revision of the manuscript for important intellectual content. CS was involved in the design, analysis, and interpretation of the simulation study. IF was involved in the analysis and interpretation of the compliance data. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Oliver Schwalbe, Email: oschwalb@zedat.fu-berlin.de.
Christian Scheerans, Email: chscheer@zedat.fu-berlin.de.
Ines Freiberg, Email: freiberg.ines@gmx.de.
Andrea Schmidt-Pokrzywniak, Email: andrea.schmidt-pokrzywniak@medizin.uni-halle.de.
Andreas Stang, Email: andreas.stang@medizin.uni-halle.de.
Charlotte Kloft, Email: charlotte.kloft@pharmazie.uni-halle.de.
Acknowledgements
We gratefully acknowledge the financial support for the study of the "Foerderinitiative Pharmazeutische Betreuung e.V.". We are indebted to all patients and caregivers participating in the study. "Foerderinitiative Pharmazeutische Betreuung e.V." had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- Blennow K, Leon MJ de, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- AKdÄ. Arzneimittelkommission der deutschen Ärzteschaft - Therapieempfehlungen Demenz. 2008.
- Burns A, Iliffe S. Alzheimer's disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- GEK Arzneimittelverordnungsreport. 2009.
- Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, Booker L, Oremus M. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- National service framework for older people. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4071283.pdf
- Kozma CM, Reeder CE, Schulz RM. Economic, clinical, and humanistic outcomes: a planning model for pharmacoeconomic research. Clin Ther. 1993;15:1121–1132. [PubMed] [Google Scholar]
- Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- WHO. Adherence to Long-Term Therapies - Evidence for action. Geneva. 2003.
- Urquhart J. The electronic medication event monitor. Lessons for pharmacotherapy. Clin Pharmacokinet. 1997;32:345–356. doi: 10.2165/00003088-199732050-00001. [DOI] [PubMed] [Google Scholar]
- Cramer JA. Microelectronic systems for monitoring and enhancing patient compliance with medication regimens. Drugs. 1995;49:321–327. doi: 10.2165/00003495-199549030-00001. [DOI] [PubMed] [Google Scholar]
- Spilker B. In: Patient compliance in medical practice and clinical trials. Cramer J, Spilker B, editor. New York: Raven Press; 1991. Methods of assessing and improving compliance in clinical trials; pp. 37–56. [Google Scholar]
- Bova CA, Fennie KP, Knafl GJ, Dieckhaus KD, Watrous E, Williams AB. Use of electronic monitoring devices to measure antiretroviral adherence: practical considerations. AIDS Behav. 2005;9:103–110. doi: 10.1007/s10461-005-1685-0. [DOI] [PubMed] [Google Scholar]
- Kogut SJ, El-Maouche D, Abughosh SM. Decreased persistence to cholinesterase inhibitor therapy with concomitant use of drugs that can impair cognition. Pharmacotherapy. 2005;25:1729–1735. doi: 10.1592/phco.2005.25.12.1729. [DOI] [PubMed] [Google Scholar]
- Mauskopf JA, Paramore C, Lee WC, Snyder EH. Drug persistency patterns for patients treated with rivastigmine or donepezil in usual care settings. J Manag Care Pharm. 2005;11:231–251. doi: 10.18553/jmcp.2005.11.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Anderson MJ, Spivack B. How many patients complete an adequate trial of donepezil? Alzheimer Dis Assoc Disord. 2002;16:49–51. doi: 10.1097/00002093-200201000-00008. [DOI] [PubMed] [Google Scholar]
- Suh DC, Thomas SK, Valiyeva E, Arcona S, Vo L. Drug persistency of two cholinesterase inhibitors: rivastigmine versus donepezil in elderly patients with Alzheimer's disease. Drugs Aging. 2005;22:695–707. doi: 10.2165/00002512-200522080-00006. [DOI] [PubMed] [Google Scholar]
- Rosen MI, Ryan CE, Rigsby MO. Motivational Enhancement and MEMS Review to Improve Medication Adherence. Behav Med. 2002;19:183–190. [Google Scholar]
- Shuter J. Forgiveness of non-adherence to HIV-1 antiretroviral therapy. J Antimicrob Chemother. 2008;61:769–773. doi: 10.1093/jac/dkn020. [DOI] [PubMed] [Google Scholar]
- Urquhart J. Pharmionics: research on what patients do with prescription drugs. Pharmacoepidemiol Drug Saf. 2004;13:587–590. doi: 10.1002/pds.1004. [DOI] [PubMed] [Google Scholar]
- Urquhart J. Defining the margins for errors in patient compliance with prescribed drug regimens. Pharmacoepidemiol Drug Saf. 2000;9:565–568. doi: 10.1002/pds.540. [DOI] [PubMed] [Google Scholar]
- Choo PW, Rand CS, Inui TS, Lee ML, Ma CC, Platt R. A pharmacodynamic assessment of the impact of antihypertensive non-adherence on blood pressure control. Pharmacoepidemiol Drug Saf. 2000;9:557–563. doi: 10.1002/pds.539. [DOI] [PubMed] [Google Scholar]
- Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95% J Acquir Immune Defic Syndr. 2007;45:4–8. doi: 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- Nony P, Boissel JP. Use of sensitivity functions to characterise and compare the forgiveness of drugs. Clin Pharmacokinet. 2002;41:371–380. doi: 10.2165/00003088-200241050-00004. [DOI] [PubMed] [Google Scholar]
- Boissel JP, Nony P. Using pharmacokinetic-pharmacodynamic relationships to predict the effect of poor compliance. Clin Pharmacokinet. 2002;41:1–6. doi: 10.2165/00003088-200241010-00001. [DOI] [PubMed] [Google Scholar]
- Williams PJ, Ette EI. In: Pharmacometrics - The Science of Quantitative Pharmacology. Ette EI, Williams PJ, editor. Hoboken: Wiley; 2007. Pharmacometrics: Impacting Drug Development and Pharmacotherapy; pp. 1–19. [Google Scholar]
- Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet. 2002;41:719–739. doi: 10.2165/00003088-200241100-00003. [DOI] [PubMed] [Google Scholar]
- Medical Research Council. MRC Health Services and Public Research Board. Framework for development and evaluation of RCTs for complex interventions to improve health. 2000. http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC003372
- Apothekenbetriebsordnung (Ordinance on the Operation of Pharmacies) in the version of the publication of 26th September, 1995 (Federal Law Gazette I, page 1195) last amended pursuant to the Amendment Act for the Federal Pharmacists Regulation and other legislation dated 20st July 2007 (Federal Law Gazette I, page 1574)
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Law AM. Simulation, Modeling and Analysis. 4th. New York: McGraw-Hill; 2007. [Google Scholar]
- Mihara M, Ohnishi A, Tomono Y, Hasegawa J, Shimamura Y, Yamazaki K, Morishita N. Pharmacokinetics of E2020, a new compound for Alzheimer's disease, in healthy male volunteers. Int J Clin Pharmacol Ther Toxicol. 1993;31:223–229. [PubMed] [Google Scholar]
- Thron CD. Linearity and superposition in pharmacokinetics. Pharmacol Rev. 1974;26:3–31. [PubMed] [Google Scholar]
- Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- Arnet I, Haefeli WE. Overconsumption detected by electronic drug monitoring requires subtle interpretation. Clin Pharmacol Ther. 2000;67:44–47. doi: 10.1067/mcp.2000.103821. [DOI] [PubMed] [Google Scholar]
- Denhaerynck K, Schafer-Keller P, Young J, Steiger J, Bock A, de Geest S. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol. 2008;8:5. doi: 10.1186/1471-2288-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijens B, Tousset E, Rode R, Bertz R, Mayer S, Urquhart J. Successful projection of the time course of drug concentration in plasma during a 1-year period from electronically compiled dosing-time data used as input to individually parameterized pharmacokinetic models. J Clin Pharmacol. 2005;45:461–467. doi: 10.1177/0091270004274433. [DOI] [PubMed] [Google Scholar]
- Eisai. Aricept© summary of product characteristics. Eisai GmbH Frankfurt/Main Deutschland. 2007.
- Sramek JJ, Cutler NR. RBC cholinesterase inhibition: a useful surrogate marker for cholinesterase inhibitor activity in Alzheimer disease therapy? Alzheimer Dis Assoc Disord. 2000;14:216–227. doi: 10.1097/00002093-200010000-00006. [DOI] [PubMed] [Google Scholar]
- Piotrovsky V, van Peer A, van Osselaer N, Armstrong M, Aerssens J. Galantamine population pharmacokinetics in patients with Alzheimer's disease: modeling and simulations. J Clin Pharmacol. 2003;43:514–523. [PubMed] [Google Scholar]
- Gobburu JV, Tammara V, Lesko L, Jhee SS, Sramek JJ, Cutler NR, Yuan R. Pharmacokinetic-pharmacodynamic modeling of rivastigmine, a cholinesterase inhibitor, in patients with Alzheimer's disease. J Clin Pharmacol. 2001;41:1082–1090. doi: 10.1177/00912700122012689. [DOI] [PubMed] [Google Scholar]
- Urquhart J. How much compliance is enough? Pharm Res. 1996;13:10–11. doi: 10.1023/A:1016004611847. [DOI] [PubMed] [Google Scholar]
- Urquhart J. Pharmacodynamics of variable patient compliance: implications for pharmaceutical value. Adv Drug Deliv Rev. 1998;33:207–219. doi: 10.1016/S0169-409X(98)00029-5. [DOI] [PubMed] [Google Scholar]
- McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive review. Contraception. 1994;50:S1–195. doi: 10.1016/0010-7824(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Johnson BF, Whelton A. A Study Design for Comparing the Effects of Missing Daily Doses of Antihypertensive Drugs. Am J Ther. 1994;1:260–267. doi: 10.1097/00045391-199412000-00003. [DOI] [PubMed] [Google Scholar]