Abstract
Objective
Dehydration challenges can increase the chemical composition of surface fluid overlying vocal fold epithelia (hypertonic surface fluid). The vocal fold epithelium is posited to act as a barrier, shielding the lamina propria from perturbations in the airway lumen. However, the effects of hypertonic surface fluid on the barrier functions of vocal fold epithelia have not been quantified. We, therefore, sought to investigate whether hypertonic surface fluid compromises epithelial barrier function. We examined the effects of hypertonic surface fluid on vocal fold epithelial resistance, paracellular pathway morphology, and tight junction protein integrity.
Study Design
Ex vivo, between group design.
Setting
Laboratory.
Methods
Porcine vocal folds (n = 24) were exposed to hypertonic or isotonic challenge and examined by electrophysiology, transmission electron microscopy, and Western blot analyses.
Results
Hypertonic, but not isotonic, challenge significantly reduced transepithelial resistance. This decrease in resistance was observed immediately after the challenge and was consistent with the appearance of dilated paracellular pathway morphology. However, hypertonic challenge did not alter protein levels of occludin, zona occludens-1, E-cadherin, or β-catenin.
Conclusion
Hypertonic surface fluid alters epithelial barrier function in the vocal folds. Specifically, exposure to hypertonic challenges increases epithelial permeability. Given the important role of the vocal fold epithelium in shielding the underlying mucosa from inhaled pathogens and pollutants, our data provide the impetus for future studies on pharmacological treatments aimed at restoring the hydration level and chemical composition of vocal fold surface fluid.
In the vocal folds, stratified squamous epithelial cells, with their intervening tight junctions, present a physical barrier to the entry of environmental toxins into the underlying lamina propria. The structural barrier provided by the vocal fold epithelium is also of functional significance. In healthy epithelium, electrolytes and water are selectively transported across tight junctions (paracellular pathway) and through the epithelial cells (transcellular pathway).1 This regulated transport of molecules is essential to maintain optimal tissue homeostasis. Disruption of the structural barrier increases paracellular permeability and can result in physiological consequences including uncontrolled access of airway lumen contents into vocal fold lamina propria and altered cell volume.
Injurious agents such as cigarette smoke have been shown to increase paracellular permeability and induce tight junction dysfunction in distal airway epithelia.2 In addition, patients with gastroesophageal reflux disease present with morphological markers of increased esophageal epithelial paracellular permeability.3 Laryngopharyngeal reflux has been shown to decrease E-cadherin, a tight junction protein in vocal fold epithelia.4 Another common challenge to the vocal fold epithelium arises from concentrated (hypertonic) surface fluid. The anatomical location of the larynx places the vocal folds at risk for surface dehydration during oral breathing, which can increase the tonicity of surface fluid. Healthy vocal fold epithelia reduce the gradient of hypertonic surface fluid by secreting water in the short term.5 However, the effects of persistent hypertonic surface fluid on vocal fold epithelial structure and function are unclear. In the stratified squamous epithelia of the esophagus, hypertonic surface fluid increases paracellular permeability and may exacerbate the effects of gastric reflux.6
We examined the effects of physiologically relevant hypertonic surface fluid on the permeability of vocal fold epithelia and the structural integrity of tight junctions. Markers of epithelial permeability include transepithelial resistance (RT) and paracellular pathway morphology.7 Specifically, findings of decreased RT and dilated paracellular pathway after hypertonic surface fluid exposure would be consistent with a hypothesis of increased epithelial permeability. A decrease in the concentrations of tight junction proteins after hypertonic challenge would suggest compromised structural integrity of vocal fold epithelium. We employed a combination of electrophysiological, transmission electron microscopic (TEM), and Western blot analyses to quantify the effects of hypertonic surface fluid on vocal fold epithelia. Specifically, RT was compared in native vocal fold epithelia exposed to hypertonic and isotonic surface fluid for two hours. To confirm that hypertonic challenge dilated the paracellular pathway, we compared the morphology (length and width) of the paracellular pathway in vocal fold epithelia exposed for two hours to hypertonic and isotonic surface fluids. Finally, to determine whether hypertonic surface fluid affects the integrity of the tight junctions we compared the expression of four tight junction proteins in vocal fold epithelia exposed to hypertonic and isotonic challenge. The four tight junction proteins investigated were E-cadherin, β-catenin, occludin, and zona occludens-1 (ZO-1). A finding of increased epithelial permeability would suggest that a persistent hypertonic fluid environment may increase the risk for vocal fold injury. Ultimately these data would provide an improved physiological rationale for pharmacological treatments that restore the composition of vocal fold surface fluid and thereby reduce susceptibility to vocal fold injury from noxious agents, such as cigarette smoke and gastric reflux.
Materials and Methods
Porcine Tissue Model
Adult porcine larynges were transported from the slaughterhouse to the laboratory in cold saline within 20 minutes of sacrifice. Each larynx was hemisected along the midsagittal plane. The vocal fold epithelium along with the superficial layer of lamina propria, hereafter referred to as vocal fold (VF), was dissected from the underlying ligament and exposed to hypertonic and isotonic challenges for two hours, as described in subsequent text. All experiments involved paired vocal folds; one VF from each larynx was exposed to hypertonic challenge and the contralateral VF was exposed to isotonic challenge (sham). The VFs were then prepared for electrophysiological testing (n = 14), TEM analyses (n = 4), or Western blot analyses (n = 6). The sample size was selected to yield statistical significance at a 0.05 alpha level on the basis of previous published research.6,8,9 All data collection procedures followed guidelines approved by Purdue University. This study did not include human subjects, so approval by an institutional review board was not required. All experiments on excised larynges were completed according to approved protocols at Purdue University.
Challenge Solutions
The isotonic solution was Hanks balanced salt solution (HBSS, mM: NaCl, 136.8; dextrose, 5.6; KCl, 5.6; NaHCO3, 4.2; CaCl2, 1.3; MgSO4, 0.8; KH2PO4, 0.4; Na2HPO4, 0.3). The hypertonic solution was prepared by adding 250 mM NaCl to HBSS.
Transepithelial Resistance (RT)
Freshly dissected VFs were mounted on the Lucite chamber of an Ussing System (World Precision Instruments, Sarasota, FL). Two voltage and two current electrodes (Ag+/AgCl electrodes with 3 mol/L KCl/agar salt bridges) were placed on either side of the tissue for measurement of open-circuit potential difference and short-circuit current by means of a current-voltage clamp.10 RT was measured, from Ohms law, as the ratio of potential difference/short-circuit current by measuring the current deflection to an imposed 2-mV pulse. RT was measured at baseline (between 45-60 minutes) after mounting the tissue. Next, the luminal surface of the Ussing system was drained and refilled with hypertonic solution (n = 7) or isotonic solution (n = 7), and RT was monitored at 10 minutes, one hour, and two hours postchallenge. The system was calibrated before each experiment. Raw RT data were converted to difference measures to represent the shift in resistance from baseline. Specifically, RT values at 10 minutes, one hour, and two hours were subtracted from baseline RT values. A negative RT value indicated decreased resistance from baseline and vice versa. To examine whether the challenges had differential effects on RT, we used a mixed-model analysis of variance with challenge as the between-factor and time as the within-factor. The alpha level was set to 0.05.
Paracellular Pathway Morphology
Freshly dissected VFs were mounted on Lucite chambers and exposed to either hypertonic challenge (n = 2) or isotonic challenge (n = 2) as described previously. Next, VF samples were diced and prepared for TEM by microwave processing (Pelco 3451 laboratory microwave system; Ted Pella, Redding, CA). For primary fixation, samples were immersed in 2.5 percent glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) (microwave time sequence: 60 seconds off, 40 seconds on, 180 seconds off). VF samples were then rinsed with 0.1 M sodium cacodylate buffer (40 seconds on ×2) and secondary fixation was completed in 1 percent osmium tetroxide in 0.1 M sodium cacodylate buffer (60 seconds off, 40 seconds on, 180 seconds off) followed by distilled water rinse (40 seconds on ×2). An ethyl alcohol (ETOH) series was used for VF sample dehydration. VF samples were immersed in 10, 30, and 50 percent ETOH (40 seconds on ×3) and held overnight at 70 percent ETOH. Final dehydration was completed the following day by using 90 percent and 100 percent (×2) ETOH and then propylene oxide for 10 minutes each. Stepwise infiltration was completed with Epon resin, and the samples were embedded in flat molds with partially polymerized resin in the bottom. Samples plus additional Epon resin were added to the molds and allowed to polymerize for 48 hours at 60°C. Ultrathin sections (90 nm) were cut and placed on 100 mesh Formvar and carbon-coated copper grids, stained with 2 percent uranyl acetate in 70 percent methyl alcohol for 10 minutes, and then stained with lead citrate for 3 minutes. The sections were viewed with an FEI/Phillips CM-10 transmission electron microscope (FEI Company, Hillsboro, OR) under low magnification (×4800) to confirm the nature of the tissue. High magnification (×37,000) images were saved to measure the dimensions of the paracellular pathway. No less than 10 representative images were selected from each VF sample and subjected to blinded, qualitative, and quantitative analyses (ImageJ software package; National Institutes of Health, Bethesda, MD). Hence, a total of 21 images were obtained and measured. The diffraction gradient was used as a referent. The dependent variable was length of the paracellular pathway. Length data were not normally distributed. Hence, a Wilcoxon signed-ranks test was used to compare paracellular pathway length in vocal folds exposed to hypertonic and isotonic challenge. The alpha level was set to 0.05.
Tight Junction Protein Concentrations
Freshly dissected vocal folds (n = 3) were mounted on modified Lucite chambers and subjected to hypertonic and isotonic challenges as described previously. Next, VFs were removed from the chamber and snap frozen in liquid nitrogen in preparation for analysis. The VFs were diced, sonicated, and lysed with M-PER (mammalian protein extraction solution; Pierce, Rockford, IL). Equivalent amounts of protein were separated by 7.5 percent sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were blocked overnight in 5 percent nonfat milk, rinsed, and then incubated at room temperature for one hour with primary antibodies against the following tight junctions: E-cadherin (1:1000; Tranduction Laboratories, San Jose, CA), β-catenin (1:1000; Transduction Laboratories), occludin (1: 1000; Zymed, Carlsbad, CA), and ZO-1 (1:1000; Zymed). β-Actin (1:1000; Zymed) served as the loading control. After being washed, the blots were incubated for one hour at room temperature with horseradish peroxidise–labeled secondary antibody (Bio-Rad Western Blot Kit; Bio-Rad Laboratories, Hercules, CA). This was then used according to the manufacturer's recommended protocol to detect specifically labeled bands.
Results
Hypertonic Challenge Decreased Transepithelial Resistance
RT decreased significantly after exposure to hypertonic but not isotonic challenge (F = 8.19, df = 1, 12, P = 0.01) (Fig 1). This reduction in RT was observed immediately after challenge and maintained for the duration of the challenge as evident from the nonsignificant main effect for time (F = 1.077, df = 2, 24, P = 0.32). In Figure 1, a positive RT value suggests an increase in resistance from baseline, whereas a negative value suggests a decrease from baseline. Specifically, RT values remained positive across all three time points in vocal folds exposed to isotonic challenge but decreased by 120 Ω · cm2 in vocal folds exposed to hypertonic challenge.
Figure 1.
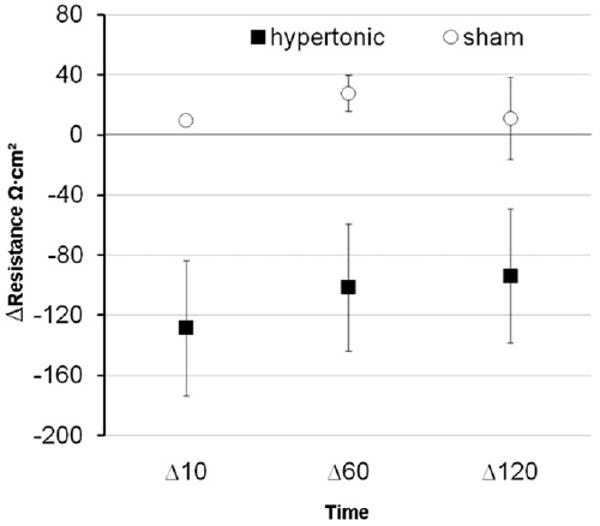
Transepithelial resistance (RT) in response to both sham challenge and hypertonic challenge over time. Negative ΔRT values suggest a decrease in resistance from baseline. Error bars represent standard error of the mean.
Hypertonic Challenge Altered Paracellular Pathway Morphology
The qualitative appearance of the paracellular pathway substantially differed between vocal folds subjected to isotonic and hypertonic challenge. As shown in Figure 2, the paracellular pathway was longer and more tortuous in vocal folds exposed to hypertonic challenge than in the isotonic challenge. Quantitative measurement on 21 different images revealed that the paracellular pathway was significantly longer after luminal exposure to hypertonic challenge (Z = −2.34, P = 0.02) (Fig 3). In addition, although not quantified, the width of the paracellular pathway appeared greater in vocal folds exposed to hypertonic challenge. The increased length and width of the paracellular pathway after hypertonic challenge will hereafter be referred to as dilated morphology.
Figure 2.
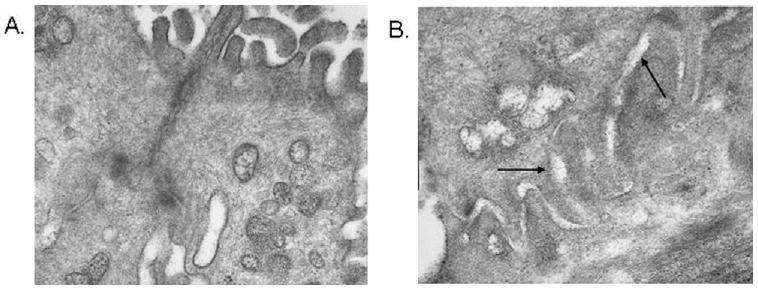
Representative transmission electron microscopic images (original magnification: ×37,000) of the paracellular pathway following sham (A) and hypertonic (B) challenge. Arrows represent the dilated paracellular pathway.
Figure 3.
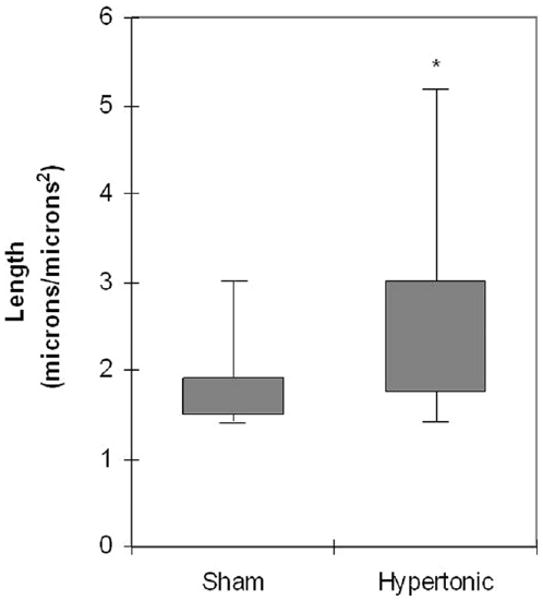
Length of the paracellular pathway in vocal fold epithelia following sham and hypertonic challenge (*P = 0.02). Shaded area represents the middle 50th quartile; error bars represent the range.
Hypertonic Challenge Did Not Alter E-cadherin, β-catenin, Occludin, or ZO-1 Protein Levels
Western blot analysis was then performed to characterize the molecular ramifications of the hypertonic challenge. Figure 4 shows a representative blot for each protein. As shown in the figure, the hypertonic challenge had no effect on any of the tight junction proteins of interest.
Figure 4.
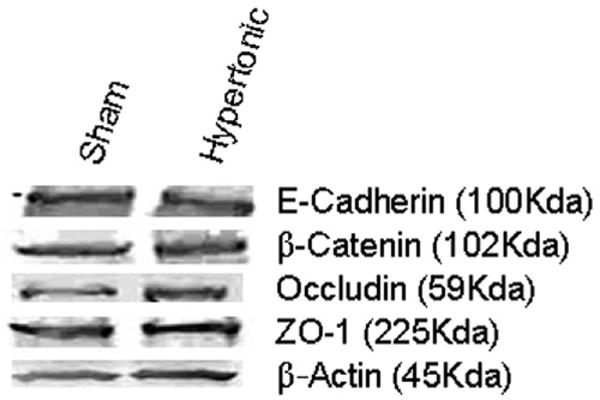
Representative blots of tight junction proteins in vocal fold epithelia following sham and hypertonic challenge.
Discussion
The vocal folds are subjected to a variety of environmental perturbations, most commonly dehydration, gastric reflux, and pollutant exposure, that alter the chemical composition of the surface fluid. To date, the bulk of investigation has focused on the sequelae of chemical injury to vocal fold lamina propria. The role of noxious agents in compromising epithelium structure and function has largely been ignored. The epithelium provides the tissue interface between the airway lumen and lamina propria and is particularly important in shielding the underlying subepithelial tissue from environmental perturbations, while potentially being vulnerable to the very same challenges. Therapeutic approaches to reduce susceptibility to laryngeal pathologies, resulting from chemical trauma to the vocal fold surface, necessitate understanding the effects of such stimuli on epithelial structure and function. Literature from other systems suggests that hypertonic challenges are likely to increase the risk for tissue injury and inflammation8,11 by compromising epithelial barrier function. However, the effects of these challenges on the vocal fold surface are unknown. Therefore, as a first step, we quantified the effects of chemical injury induced by hypertonic surface fluid on epithelial permeability.
Hypertonic challenges significantly decreased vocal fold RT and lengthened the paracellular pathway. Together, these findings suggest increased permeability of vocal fold epithelium following exposure to hypertonic surface fluid. Airway epithelial hyperpermeability and consequent loss of barrier function increase the likelihood that inhaled particulate may be transported into tissue,12 thereby compromising the defensive properties of the epithelial barrier. Our findings of increased epithelial permeability with hypertonic challenge are consistent with observations made in other stratified squamous epithelia. For example, hypertonic NaCl lowered RT and dilated paracellular pathways in rabbit esophageal epithelia.13 A causality relationship between increased permeability and compromised epithelial defense has not been demonstrated directly; however, research has demonstrated that noxious stimuli such as cigarette smoke increase airway epithelial permeability and neutrophil infiltration in smokers in vivo.14 Likewise, conditions such as inflammatory bowel disease may be characterized by increased paracellular permeability.15
The current data offer potential applications for pharmacological treatments. It appears that controlled and transient hypertonic saline challenges may induce leaky epithelial barrier function without damaging the structural integrity of the tissue. Controlled hypertonic saline could therefore be investigated as a means to enhance the access of inhaled pharmacological agents to the underlying mucosa. Specifically, the transport of hydrophilic molecules may be enhanced by leaky paracellular pathway.16 To our knowledge, there is no evidence that hypertonic saline is used therapeutically to enhance drug bioavailability. While the therapeutic implications of agents that selectively enhance paracellular permeability await further study, the negative effects of increased epithelial permeability in compromising tissue defense should not be overlooked. A hyperpermeable barrier induced by exposure to hypertonic surface fluid could increase the access of airborne particulates into tissue. Reversing the composition of the surface fluid toward isotonic levels may help restore barrier function and vocal fold homeostasis.
The structural integrity of the epithelial barrier appeared to remain intact as evidenced by similar expression of tight junction proteins after the hypertonic and isotonic challenges. The reason for the incongruence in findings of increased epithelial permeability in the presence of intact epithelial tight junction protein expression is unclear. It is possible that the two-hour challenge duration employed in the current study may have been too short to elicit structural alterations as captured by tight junction protein assays. It is also possible that agents such as hypertonic saline may modify epithelial resistance by primarily changing ion conductance through the transcellular and paracellular pathways, without perturbing the tight junctions themselves. Because epithelial resistance is influenced by the resistance of the transcellular and paracellular pathways, future investigations will investigate alternative markers of epithelial integrity, such as the paracellular permeability of tracers including dextran and [14C] sucrose, in addition to immunolocalization studies to examine whether hypertonic challenges alter tight junction protein location.
The finding of increased permeability of vocal fold epithelia in a hypertonic environment was substantiated by both electrophysiology and TEM methodology. However, some potential caveats in the methods warrant further discussion. First, it is possible that tissue processing may have altered the morphology of the paracellular pathway. To control for these effects, we incorporated in every experiment a control group in which vocal fold epithelia were exposed to sham challenge and processed identically to the experimental tissue. Second, the current investigation targeted four tight junction proteins; however, it is possible that claudin proteins that make up the tight junction complex may be more vulnerable to hypertonic challenge. Finally, the porcine animal model was selected on the basis of anatomical and histological similarities to human vocal folds.17,18 For example, the distribution of tight junction proteins is similar in porcine and human vocal fold epithelia.19 However, the transition from ciliated columnar epithelia to stratified squamous epithelia in the porcine upper respiratory tract follows a different distribution than that in the human airway.20 Furthermore, the immunological function of the porcine vocal folds may differ from that of human vocal folds,20 thereby affecting the nature of defensive response to surface challenges. The effect of hypertonic surface fluid on the barrier function of human vocal fold epithelia awaits further study. In conclusion, our observations suggest that a hypertonic challenge may not be directly detrimental to epithelial integrity. However, by increasing epithelial permeability, such challenges may make the vocal fold tissue more vulnerable to inhaled insults and mechanical stress.
Acknowledgments
We thank Debbie Sherman and Chia-Ping Huang at the Purdue Life Science Microscopy Facility and Mira Stankovich for assistance with electron microscopy. A debt of gratitude is also owed to Dr Jie Chen for her technical assistance.
Sponsorships: This work was funded in part by grant #008690 from the National Institutes of Health/National Institute on Deafness and other Communication Disorders, as well as Hackers for Hope, The Garban Fund, and the Langeloth Foundation.
Footnotes
Author Contributions: Mahalakshmi Sivasankar, study concept and design, data acquisition, analysis, and interpretation, article drafting and revision, approval of final version of article; Elizabeth Erickson, study design, data acquisition and interpretation, article drafting and revision, approval of final version of article; Mark Rosenblatt, study design, data analysis and interpretation, article revision, approval of final version of article; Ryan C. Branski, study concept and design, data acquisition, analysis, and interpretation, article drafting and revision, approval of final version of article.
Disclosures: Competing interests: None.
Contributor Information
Mahalakshmi Sivasankar, Department of Speech, Language, and Hearing Sciences, Purdue University, West Lafayette, IN.
Elizabeth Erickson, Department of Speech, Language, and Hearing Sciences, Purdue University, West Lafayette, IN.
Mark Rosenblatt, Department of Ophthalmology, Weill Cornell Medical College, New York, NY.
Ryan C. Branski, Head and Neck Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY.
References
- 1.Fisher K, Telser A, Phillips J, et al. Regulation of vocal fold transepithelial water fluxes. J Appl Physiol. 2001;91:1401–11. doi: 10.1152/jappl.2001.91.3.1401. [DOI] [PubMed] [Google Scholar]
- 2.Olivera D, Boggs S, Beenhouwer C, et al. Cellular mechanisms of mainstream cigarette smoke-induced lung epithelial tight junction permeability changes in vitro. Inhal Toxicol. 2007;19:13–22. doi: 10.1080/08958370600985768. [DOI] [PubMed] [Google Scholar]
- 3.Tobey N, Carson J, Alkiek R, et al. Dilated intercellular spaces: a morphological feature of acid reflux-damaged human esophageal epithelium. Gastroenterol. 1996;111:1200–5. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 4.Reichel O, Mayr D, Durst F, et al. E-cadherin but not beta-catenin expression is decreased in laryngeal biopsies from patients with laryngopharyngeal reflux. Eur Arch Otolaryngol. 2008;265:937–42. doi: 10.1007/s00405-007-0568-6. [DOI] [PubMed] [Google Scholar]
- 5.Sivasankar M, Fisher K. Vocal fold epithelial response to luminal osmotic perturbation. J Speech Lang Hear Res. 2007;50:886–98. doi: 10.1044/1092-4388(2007/063). [DOI] [PubMed] [Google Scholar]
- 6.Long J, Marten E, Tobey N, et al. Luminal hypertonicity and the susceptibility of rabbit esophagus to acid injury. Dis Esophagus. 1998;11:94–100. doi: 10.1093/dote/11.2.94. [DOI] [PubMed] [Google Scholar]
- 7.Tobey N, Hosseini S, Argote C, et al. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am J Gastroenterol. 2004;99:13–22. doi: 10.1046/j.1572-0241.2003.04018.x. [DOI] [PubMed] [Google Scholar]
- 8.Katsuyama I, Arakawa T. A convenient rabbit model of ocular epithelium damage induced by osmotic dehydration. J Occup Pharmacol Ther. 2003;19:281–9. doi: 10.1089/108076803321908400. [DOI] [PubMed] [Google Scholar]
- 9.Yankaskas JR, Gatzy JT, Boucher RC. Effects of raised osmolarity on canine tracheal epithelial ion transport function. J Appl Physiol. 1987;62:2241–5. doi: 10.1152/jappl.1987.62.6.2241. [DOI] [PubMed] [Google Scholar]
- 10.Sivasankar M, Nofziger C, Blazer-Yost B. cAMP regulation of ion transport in porcine vocal fold mucosa. Laryngoscope. 2008;118:1511–7. doi: 10.1097/MLG.0b013e3181772d63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlando R. Pathogenesis of gastroesophageal reflux disease. Am J Med Sci. 2003;326:274–8. doi: 10.1097/00000441-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Boucher R. Chemical modulation of airway epithelial permeability. Environ Health Perspect. 1980;35:3–12. doi: 10.1289/ehp.80353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long J, Marten E, Tobey N, et al. Effects of luminal hypertonicity on rabbit esophageal epithelium. Am J Physiol. 1997;273:G647–54. doi: 10.1152/ajpgi.1997.273.3.G647. [DOI] [PubMed] [Google Scholar]
- 14.Morrison D, Rahman I, Lannan S, et al. Epithelial permeability, inflammation, and oxidative stress in the air spaces of smokers. Am J Respir Crit Care Med. 1999;159:473–9. doi: 10.1164/ajrccm.159.2.9804080. [DOI] [PubMed] [Google Scholar]
- 15.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Mot. 2007;19:548–52. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 16.Ward P, Tippin T, Thakker D. Enhancing paracellular permeability by modulating epithelial tight junctions. Pharmaceut Sci Tech Today. 2000;3:346–58. doi: 10.1016/s1461-5347(00)00302-3. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi Y, Shin T, Sugihara H. Reconstruction of the laryngeal mucosa. A three-dimensional collagen gel matrix culture. Arch Otolaryngol Head Neck Surg. 1996;122:649–54. doi: 10.1001/archotol.1996.01890180057014. [DOI] [PubMed] [Google Scholar]
- 18.Hahn M, Kobler J, Zeitels S, et al. Quantitative and comparative studies of the vocal fold extracellular matrix II: collagen. Ann Otol Rhinol Laryngol. 2006;115:225–32. doi: 10.1177/000348940611500311. [DOI] [PubMed] [Google Scholar]
- 19.Gill GA, Buda A, Moorghen M, et al. Characterization of adherens and tight junctional molecules in normal animal larynx; determining a suitable model for studying molecular abnormalities in human laryngopharyngeal reflux. J Clin Pathol. 2005;58:1265–70. doi: 10.1136/jcp.2004.016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker E, Haverson K, Stokes C, et al. The larynx as an immunological organ: immunological architecture in the pig as a large animal model. Clin Exp Immunol. 2005;143:6–14. doi: 10.1111/j.1365-2249.2005.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


