Abstract
The core symptoms of autism spectrum disorder (ASD) include deficits in social interaction, impaired communication, and repetitive behaviors with restricted interests. Mouse models with behavioral phenotypes relevant to these core symptoms offer an experimental approach to advance the investigation of genes associated with ASD. Previous findings demonstrate that BTBR T+ tf/J (BTBR) is an inbred mouse strain that shows robust behavioral phenotypes with analogies to all three of the diagnostic symptoms of ASD. In the present study, we investigated the expression of social behaviors in a semi-natural visible burrow system (VBS), during colony formation and maintenance in groups comprising three adult male mice of the same strain, either C57BL/6J (B6) or BTBR. For comparative purposes, an extensively investigated three-chambered test was subsequently used to assess social approach in both strains. The effects of strain on these two situations were consistent and highly significant. In the VBS, BTBR mice showed reductions in all interactive behaviors: approach (front and back), flight, chase/follow, allo-grooming and huddling, along with increases in self-grooming and alone, as compared to B6. These results were corroborated in the three-chambered test: in contrast to B6, male BTBR mice failed to spend more time in the side of the test box containing the unfamiliar CD-1 mouse. Overall, the present data indicates that the strain profile for BTBR mice, including consistent social deficits and high levels of repetitive self-grooming, models multiple components of the ASD phenotype.
Keywords: autism, social behavior, visible burrow system, mouse models, BTBR
1. Introduction
Autism spectrum disorder (ASD) is a highly-prevalent and severe neurodevelopmental disorder defined in the DSM-IV by three fundamental symptoms [2,28]. Abnormal reciprocal social interactions include low levels of social approach, and other impairments such as deficits in non-verbal expression (e.g. deficits in eye-to-eye gaze and diminished expression of emotion as measured by lack of gesturing or facial expression) and markedly diminished peer relationships [11,15]. Deficits in social communication include delayed development of speech and poor expressive language [19,29]. Stereotyped, repetitive, and ritualistic behaviors, resistance to change a learned response, compulsions, obsessions, and other persistent behavioral patterns are components of the third diagnostic indicator of ASD [27,41]. While the etiology of ASD is not yet established, support for a strong genetic component is evident from the 90% concordance rate between monozygotic twins [4,5,18]. However, genetic studies to determine specific heritable factors underlying susceptibility for ASD have indicated that the great majority of cases involve the interaction between multiple genes and possible environmental factors [1,20,38].
The primary diagnostic indices of ASD are abnormal behaviors, rather than biochemical, neuroanatomical or other physiological symptoms [2,16,21]. Therefore, suitable animal models for ASD require a relationship to the types of social deficits that are considered the core symptoms of such disorder, including impairments in social interaction and deficiencies in other functional domains [12,31,33]. Advanced transgenic and recombinant technologies, and the sequencing of mouse genome, have made the mouse the species of choice for many researchers in the field of behavioral genetics [8,10,17]. Mice are a social species, displaying social investigation of an unfamiliar conspecific, communal nesting, sleeping in group huddles, aggression directed towards intruders, sexual approach and mating behavior patterns, parental care of the pups and juvenile play [26,39].
The use of inbred strains of mice provides valuable translational tools to assess the interplay of genetic and environmental factors in neurodevelopmental disorders [13,32,34,35,37]. Results obtained in recent studies reveal that the BTBR T+ tf/J (BTBR) inbred strain displays behavioral traits with face validity for all three diagnostic symptoms of ASD. Compared to the commonly used C57BL/6J (B6) strain, BTBR mice display low reciprocal social interactions, reduced social approach and impaired juvenile play [30], a behavioral phenotype with analogy to the first diagnostic symptom of ASD, social interaction deficits. Similarly, pairs of male BTBR mice spent the least time engaged in social interactions when compared to 129S1/SvImJ (129S1) and B6 inbred strains [8]. The sociability of BTBR pups was not rescued by cross-fostering such mice with B6 mothers, countering the hypothesis that sociability is regulated in this inbred strain by the maternal environment [46]. In regard to the second diagnostic symptom of ASD, communication deficits, a recent study showed that BTBR mice display an unusual pattern of vocalizations consisting of high levels of harmonics, two-syllable, and composite calls, but minimal numbers of chevron-shaped syllables, upward, downward, and short calls, when compared to the B6 strain [40]. With regard to the third diagnostic symptom of ASD, both adult and juvenile BTBR mice exhibit high levels of repetitive self-grooming [30,44,46]. This high level of self-grooming, like the BTBR deficit in sociality, was unaffected by cross-fostering BTBR with B6 mothers [46]. Repetitive behaviors in BTBR mice were also assessed with the use of the exploratory hole-board test [36], with data indicating reduced exploration and high preference for holes located in the corners of the chamber in the BTBR strain. In addition, BTBR was the only strain that did not demonstrate a shift in hole preference towards an appetitive olfactory stimulus, following home cage exposure to the food [36], suggesting that BTBR mice are resistant to change and display restricted interests.
The objective of the present study was to extend the previous findings indicating that the BTBR strain of mice is a useful model for ASD, with focus on the first diagnostic symptom, social interaction deficits. To reach this goal, we investigated BTBR and B6 social behaviors in two tests: the visible burrow system (VBS) and the three-chambered social approach test. The VBS is a semi-natural context which was designed to provide many of the features of the natural habitats of rodents, including multiple tunnels and burrows in addition to an open surface area [6,7,9]. A previous study from our research group investigated the social relationships of B6 mice colonies in the VBS [3], with results indicating that frontal approaches, along with huddling, may constitute particularly appropriate measures of mouse sociability in this context, i.e. pro-social or amicable behaviors that do not involve extrinsic approach motivations such as aggression or sexual interest. In our study, the expression of social behaviors in the VBS was evaluated during colony formation and maintenance in groups comprising three adult male mice of the same strain, either B6 or BTBR. For comparative purposes, an extensively investigated three-chambered test [32] was subsequently used to assess social approach in B6 and BTBR mouse strains. The comparison of strain effects on sociability measures between the VBS and the three-chambered test was used to facilitate an evaluation of the validity of the new ethological measures and enable an assessment of whether these measures offer useful additional information on sociability. Our study also provided additional information on the third diagnostic symptom of ASD, repetitive behaviors, once analysis of the VBS data included scoring the frequency of self-grooming in B6 and BTBR strains.
2. Materials and methods
2.1. Animals
Twelve B6 male mice aged 10-14 weeks at the first day of colony formation and twelve BTBR male mice, aged 11-14 weeks at this same date were used as subjects. B6 and BTBR mice for this study were offspring of breeding pairs obtained from the Jackson Laboratory (Bar Harbor, ME) and subjects were bred in the animal facilities of the University of Hawaii Laboratory Animal Service. Out-bred CD-1 stimulus mice were purchased from Charles River Labs (Company Location). Breeding pairs from both B6 and BTBR inbred strains were maintained by sibling mating. Subjects and stimulus mice were reared in standard polypropylene cages, 26.5 × 17 × 11.5 (H) cm, in a group of three to five male littermates after weaning at the 25 days of age, in a temperature-controlled room (22 ± 1°C). All subjects were maintained on a 12-h light/dark cycle (lights on at 06:00 a.m.), with free access to food and water in their home cages. All procedures were conducted in accordance with protocols approved by the University of Hawaii Institutional Animal Care and Use Committee.
2.2. Apparatus
2.2.1. VBS
Visible burrow systems were constructed as previously described [3], but with a reduction of the surface area, to improve the clarity of video-records of individual mice. Each colony was housed in a rectangular, galvanized metal bin, 86 × 61 × 26 (H) cm. Three chambers, each 12 × 7 × 6 (H) cm, were positioned behind a barrier wall extending across a short width (61 cm) of the bin, 30 cm from the end wall. This wall separated an open surface area (30 × 61 × 26 (H) cm) from the chambers in the other compartment. These chambers were connected to the wall via clear Plexiglas tubes 5 cm in diameter. Two of the three chambers, each connected to the surface area via a Z shaped tube, were connected to each other via a straight clear Plexiglas tube. The third chamber was connected only to the surface via a straight tube. The animals could pass freely between each chamber and the surface area, or between the two connected chambers, by these tubes. A water spout was located in the left corner of the surface area while food hoppers were located in the frontal wall of the bin. All dividing walls and chambers were constructed of black Plexiglas. A vertical extension of the surface area (30 × 61 × 30 (H) cm) made of clear Plexiglas was placed above the frontal part of the bin to prevent escape from the apparatus. The floor was covered by a layer of sawdust bedding (1 cm) in all chambers as well as the surface. A video camera was connected to a DVD recorder and mounted on the ceiling over the VBS allowing the recording of subject behavior from an upper view.
The experimental room was maintained on a 12-h light/dark cycle (lights on at 06:00 a.m.), being illuminated by fluorescent lamps during the light period and by infrared light during the dark phase. Temperature (22 ± 1°C) and humidity (70%) were also controlled in the experimental room.
2.2.2. Three-chambered social approach test
A 41 cm L × 70 cm W × 28 cm H three-chambered arena was used to assess sociability (Fig. 1). Since subject mice were black, white Plexiglas panels were installed on the back walls and the entire arena was placed on a white Plexiglas floor to provide a contrasting background. The two outside chambers contained an inverted empty black wire cup (Galaxy Pencil/Utility Cup Spectrum Diversified Designs, Inc., Streetsboro, OH) which housed stimulus mice. The experimental room was illuminated by standard fluorescent lamps (120 lx) and the tests were conducted from 10:00 am to 4:00 pm.
Fig. 1.
Three-chambered apparatus. Three interconnected chambers are separated by two manually operated sliding doors. Side compartments contain inverted wire cups to house a stimulus mouse. A steel weight and a clear Plexiglas cylinder are placed above the inverted cups to prevent lifting or climbing on top. The inset displays a front view demonstrating the clear Plexiglas window on the front of the apparatus.
2.3. Procedures
2.3.1. Observation of VBS colonies
Twenty-four hours prior to colony formation, subjects were marked for individual identification with a commercial crème-based hair dye (Jerome Russel, extra strength crème hair bleach). On day 1, each group of three male B6 or BTBR mice was moved from the rearing room to the testing room and placed in the VBS at the beginning of the dark period. Four VBS colonies were scored simultaneously in the experimental room. VBS colony grouping was maintained during 4 days. Four B6 colonies and four BTBR colonies were created and analyzed. All animals of a particular colony were previously unfamiliar to each other.
DVD recordings were made for 4 hours each, on days 1, 2 and 3 in the dark period and days 2, 3 and 4 in the light period. Behaviors were scored from these recordings by time sampling, with a 30-second sample being taken every 10 minutes for each mouse. Frequencies of ratings were the basic measures evaluated. Data were summed separately for light and dark periods, and also analyzed as a function of time in the VBS situation.
2.3.2. Definitions of behaviors
Social behaviors from the mouse ethogram (e.g. [25]) included huddle: lying in contact with another animal for more than 10 seconds of the 30-seconds time sample; being alone: remaining three body lengths away from the nearest neighbor, for more than 10 seconds of the 30-seconds time sample; allogrooming: lick or rub with paws, another animal; self-grooming: lick or rub self; approach to the front or back of another animal was defined in terms of a line bisecting the approached mouse, perpendicular to the long axis of its body; flight: rapid locomotion away from an approaching animal; chase: rapid locomotion toward another animal and follow: slow locomotion toward another animal that is moving.
Frequencies of behaviors were counted. Frequencies of huddling and being alone were then calculated as percentages of total time within a session by dividing the frequency of each behavior by 24, the highest possible number of occurrences.
2.3.3. Three-chambered social approach test
Immediately following removal from the VBS, mice were individually housed and assessed for sociability in a three-chambered apparatus [32] on the following day. Briefly, during a ten minutes habituation period, a subject mouse was placed in the middle chamber, the sliding doors were opened and the mouse given free access to the entire arena during which the duration of time in each of the two outside stimulus compartments was hand scored with stopwatches. Following the habituation phase, mice were placed back into the center, the doors were closed and a single unfamiliar male CD-1 mouse was placed in one of the two cups. The duration of time spent in each chamber was measured in a 10 minutes session and required all four paws to be in the compartment to be counted. The stimulus mouse placement was successively alternated between trials. The duration of time in which all four paws of the subject mouse was in each of the two compartments was compared
2.4. Statistical analysis
The behavioral data obtained in the VBS were analyzed by three-way analyses of variance (ANOVA) with days of testing (1-3) and lighting period (dark or light phase) as within subjects factors, and strain (B6 or BTBR) as the between subjects factor. Significant effects were followed by unpaired t-tests. Data from the three-chambered test were analyzed using within-strain repeated measures ANOVA for comparison of time spent in the empty cage side with time spent in the CD-1 mouse side. As time spent in each of the three chambers was not independent, the test condition factor compared time spent only in the right vs. left chambers. Center chamber times are shown in the graph for illustrative purposes. For all statistical analyses, a probability level of p<0.05 was considered significant but probabilities meeting more stringent criteria (e.g. p<0.01) are marked as such.
3. Results
3.1. VBS
Table 1 provides results of statistical tests for all measures of the VBS analysis, by strain, lighting period, and day, along with interaction effects. Although day vs. lighting effects were significant for all measures except chase/follow, strain is the major variable of interest and day vs. lighting effects are not described except in the context of significant strain vs. day vs. lighting interactions.
Table 1.
Summary of the statistic results obtained in the current VBS analysis
| ANOVA results |
|||||||
|---|---|---|---|---|---|---|---|
| Main effect |
|||||||
| Strain | Lighting period |
Days | Days x Strain |
Lighting x Strain |
Days x Lighting |
Days x Lighting x Strain |
|
| Approach front |
F(1,22)= 18.71; p<0.01** |
F(1,22)= 94.08; p<0.01** |
F(2,21)= 21.03; p<0.01** |
F(2,21)= 0.98; p=0.39 n.s. |
F(1,22)= 16.89; p<0.01** |
F(2,21)= 8.41; p<0.01** |
F(2,21)= 2.13; p=0.14 n.s. |
| Approach back |
F(1,22)= 5.09; p<0.05* |
F(1,22)= 77.62; p<0.01** |
F(2,21)= 9.84; p<0.01** |
F(2,21)= 0.39; p=0.67 n.s. |
F(1,22)= 3.63; p=0.07 n.s. |
F(2,21)= 10.27; p<0.01** |
F(2,21)= 0.82; p=0.45 n.s. |
| Flight | F(1,22)= 17.87; p<0.01** |
F(1,22)= 38.11; p<0.01** |
F(2,21)= 4.97; p<0.05* |
F(2,21)= 1.74; p=0.20 n.s. |
F(1,22)= 7.25; p<0.05* |
F(2,21)= 3.83; p<0.05* |
F(2,21)= 1.47; p=0.25 n.s. |
| Chase/ Follow |
F(1,22)= 6.65; p<0.05* |
F(1,22)= 23.05; p<0.01** |
F(2,21)= 0.93; p=0.40 n.s. |
F(2,21)= 0.87; p=0.43 n.s. |
F(1,22)= 5.76; p<0.05* |
F(2,21)= 1.23; p=0.31 n.s. |
F(2,21)= 1.26; p=0.30 n.s. |
| Self- grooming |
F(1,22)= 23.97; p<0.01** |
F(1,22)= 0.05; p=0.81 n.s. |
F(2,21)= 0.30; p=0.74 n.s. |
F(2,21)= 0.34; p=0.71 n.s. |
F(1,22)= 0.79; p=0.38 n.s. |
F(2,21)= 8.05; p<0.01** |
F(2,21)= 2.37; p=0.11 n.s. |
| Allo- grooming |
F(1,22)= 23.34; p<0.01** |
F(1,22)= 1.26; p=0.27 n.s. |
F(2,21)= 1.02; p=0.37 n.s. |
F(2,21)= 0.19; p=0.82 n.s. |
F(1,22)= 0; p=1.0 n.s. |
F(2,21)= 5.19; p<0.05* |
F(2,21)= 0.78; p=0.47 n.s. |
| Huddle | F(1,22)= 59.30; p<0.01** |
F(1,22)= 26.44; p<0.01** |
F(2,21)= 20.88; p<0.01** |
F(2,21)= 0.42; p=0.65 n.s. |
F(1,22)= 18.64; p<0.01** |
F(2,21)= 5.76; p<0.01** |
F(2,21)= 3.57; p<0.05* |
| Alone | F(1,22)= 59.30; p<0.01** |
F(1,22)= 26.44; p<0.01** |
F(2,21)= 20.88; p<0.01** |
F(2,21)= 0.43; p=0.65 n.s. |
F(1,22)= 18.64; p<0.01** |
F(2,21)= 5.76; p<0.01** |
F(2,21)= 3.57; p<0.05* |
p<0.05
p<0.01
3.1.1. Approach: Front and Back
Fig. 2 presents the mean frequency of frontal and back approaches for both strains over the three days. The main effects of strain were significant for both, reflecting that frontal and back approaches were significantly reduced in the BTBR strain. A significant lighting by strain interaction for frontal but not back approach enabled comparison of the former in terms of dark vs. light periods. The BTBR reduction in frontal approach was greater in the dark period. Interactions between strain and days, and strain by days by lighting period were not significant (see Table 1).
Fig. 2.

Individual frequencies (mean ± S.E.M.) of approaches to the front and approaches to the back of C57BL/6J and BTBR mice during the dark and light periods in the VBS. *Indicates significant differences between both strains; p<0.05; n=12 for each group.
3.1.2. Flight
Fig. 3 (upper panel) presents the mean frequencies of flight for both strains over the three days. Flight was significantly reduced in the BTBR strain. This difference was greater in the dark period. Interactions between strain and days, and strain by days by lighting period were not statistically significant (Table 1).
Fig. 3.

Individual frequencies (mean ± S.E.M.) of flight (upper panel) and chase/follow (lower panel) behaviors of C57BL/6J and BTBR mice during the dark and light periods in the VBS. *Indicates significant differences between both strains; p<0.05; n=12 for each group.
3.1.3. Chase/Follow
Fig. 3 (lower panel) presents mean frequencies of chase and follow behaviors for both strains over the three days. Chase/Follow was significantly reduced in the BTBR strain. This difference was greater in the dark period. The interactions between strain and days, days and lighting period, and the strain by days by lighting period were not significant (Table 1).
3.1.4. Self-grooming
Fig. 4 (upper panel) presents mean frequencies of self-grooming for both strains over the three days. Self-grooming was significantly increased in the BTBR strain. The interactions between strain and days, strain and lighting period, and strain by days by lighting period were not statistically significant (Table 1).
Fig. 4.

Individual frequencies (mean ± S.E.M.) of self-grooming (upper panel) and allo-grooming (lower panel) behaviors of C57BL/6J and BTBR mice during the dark and light periods in the VBS. *Indicates significant differences between both strains; p<0.05; n=12 for each group.
3.1.5. Allo-grooming
Fig. 4 (lower panel) presents the mean frequencies of allo-grooming for both strains over the three days. Allo-grooming was significantly reduced in the BTBR strain. Interactions between strain and days, strain and lighting period, and strain by days by lighting period were not significant (see Table 1).
3.1.6. Huddle
Fig. 5 (upper panel) presents the percentage of observations of huddling behavior for both strains over the three days. Huddling was significantly reduced in the BTBR strain in both dark and light periods. All subjects showed more huddling behavior during the light period. Interactions between strain and lighting period, and strain by days by lighting period (as well as days by lighting period) were all statistically significant (Table 1), with the BTBR strain showing less huddling during the light period, but not during the dark period, on day 1.
Fig. 5.
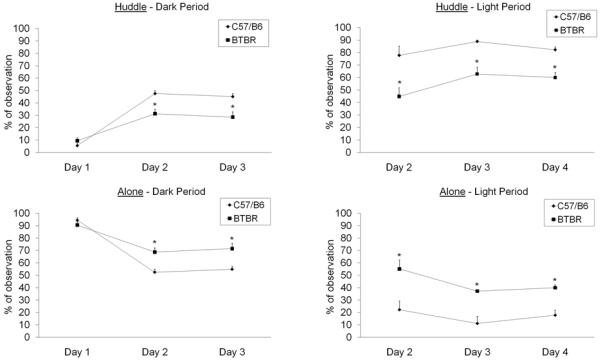
Percentage of observation (mean ± S.E.M.) verified for each huddle (upper panel) or being alone (lower panel) of C57BL/6J and BTBR mice during the dark and light periods in the VBS. *Indicates significant differences between both strains; p<0.05; n=12 for each group.
3.1.7. Alone
Fig. 5 (lower panel) presents the percentage of observations of alone in both strains over the three days. Alone was significantly increased in the BTBR strain in both dark and light periods. For all subjects, being alone was more prevalent during the dark period. Interactions between strain and lighting period, and the strain by days by lighting period (as well as days by lighting period) were all statistically significant (Table 1), with the BTBR strain showing more alone during the light period, but not during the dark period, on day 1.
Although data for huddle and alone appeared to be virtual mirror images of each other, it is notable that many other conditions were possible between the contact of huddling and the criterion of 3 body lengths apart for apart. The data are therefore independent.
3.2. Three-chambered social approach test
The analysis revealed that B6 mice showed a significant preference for spending time in the side of the test box containing the unfamiliar CD-1 mouse used as stimulus vs. the opposite side (p<0.05). However, BTBR mice did not show a significant preference for one side over the other (Fig. 6).
Fig. 6.
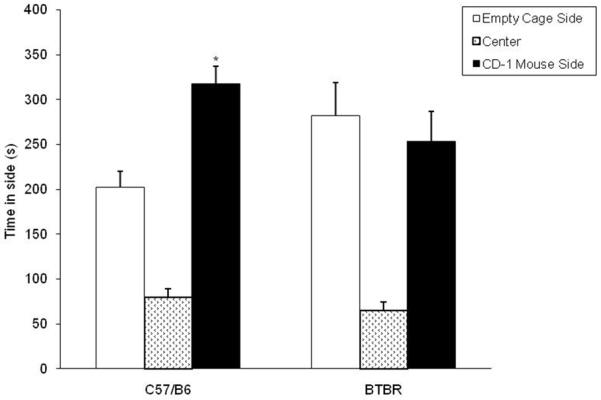
Time spent in each side (mean ± S.E.M.) during the three-chambered social approach test. *p<0.05, within-strain comparison; n=12 for each group.
4. Discussion
The lack of social approach and maintenance of reciprocal social relationships is a primary diagnostic indicator of ASD [2,42,43]. From an early age, autistic children spend less time in close proximity to other children and are less likely to focus attention on another child, in comparison to typically developing children [14,24]. Given the main importance of social impairment in ASD, this symptom is considered to be fundamental to a mouse model of such disorder [22,23]. In the current study, two tests (VBS and three-chambered social approach test) were used to assess the levels of sociability in B6 and BTBR strains of mice. While the former allows for a more naturalistic measurement of social behavior, the latter holds the behavior of the stimulus mouse constant, letting to the test mouse the freedom choice to interact with the other animal or not [21].
The effects of strain on these two situations were consistent and highly significant. In the VBS, BTBR mice showed reductions in all interactive behaviors: approach (front and back), flight, chase/follow, allo-grooming and huddling, along with increases in self-grooming and alone. These results were corroborated in the three-chambered social approach test: in contrast to B6, male BTBR mice failed to spend more time in the side of the test box containing the unfamiliar CD-1 mouse.
The effects on VBS measures obtained in the current study are in agreement with previous findings [3] with regard to the distribution of behaviors as a function of familiarity and light/dark period: approach front, approach back, flight, chase/follow and alone were all higher in dark periods while huddle was the only behavior that was significantly more common in the light period; self and allo-grooming were not influenced by light vs. dark periods. Taken together, the results obtained in these both VBS studies are in agreement with the proposal by Yang and coworkers (2008) of consistent dark vs. light period differences in the expression of social behaviors, especially when such behaviors are measured in the subject home environment. In parallel, this same research group points that social behaviors assessed in standard tests involving handling and novel situations (e.g. three-chambered social approach test) are not strongly influenced by circadian phase [44,45]. Significant day effects with reductions in approach front, approach back, flight, and alone, and increases in huddle, appeared to reflect a general reduction in defensiveness to the initially novel cohabitants of each colony over time, and triple interactions for huddle and alone reflected that strain interacted with these familiarity effects as well as activity period.
The results obtained in the present analysis are consistent with previous findings of consistent social deficits observed in the BTBR strain vs. high levels of sociability verified in B6 mice (for a review see [34]). More specifically, a significant decrease in the occurrence of reciprocal social interactions [8,30] as well a reduction in the number of front approaches measured in the juvenile play test [46] were verified in BTBR mice, as compared to B6. In addition, juvenile and adult BTBR mice displayed high levels of repetitive self-grooming [30,46]; low levels of social grooming were observed in juvenile BTBR mice, when compared to B6 [30]. Taken together, these findings are in agreement with the strain effects on approach front, huddle, alone, self-grooming and allo-grooming verified during the four days of colony grouping in the VBS. Previous studies [30,34] revealed that BTBR displayed normal scores on measures of exploratory locomotion, olfaction, learning, sensory reflexes, and low anxiety-like traits when compared to B6, indicating a specific deficit in sociability in the former strain.
In summary, the current study confirmed and extended previous findings indicating that BTBR mice display low levels of social behavior and high levels of repetitive self-grooming. Furthermore, the present results illustrate the importance of a detailed description of social behaviors in inbred strains of mice that can be used as a model to seek background genes specifically involved in ASD. Overall, these studies support the use of the BTBR strain as a mouse model for elucidating the genetics and neurobiology of sociability and other ASD-related phenotypes.
Acknowledgements
The present study was supported by the National Institute of Mental Health (NIMH) grant MH081845-01A2 to RJB. The authors also wish to thank Jacqueline N. Crawley for her help and advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].APA . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- [3].Arakawa H, Blanchard DC, Blanchard RJ. Colony formation of C57BL/6J mice in visible burrow system: identification of eusocial behaviors in a background strain for genetic animal models of autism. Behav Brain Res. 2007;176:27–39. doi: 10.1016/j.bbr.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- [5].Bespalova IN, Buxbaum JD. Disease susceptibility genes for autism. Ann Med. 2003;35(4):274–281. doi: 10.1080/07853890310005966. [DOI] [PubMed] [Google Scholar]
- [6].Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103(1):70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- [7].Blanchard RJ, Dulloog L, Markham C, Nishimura O, Compton JN, Jun A, et al. Sexual and aggressive interactions in a visible burrow system with provisioned burrows. Physiol Behav. 2001;72:245–254. doi: 10.1016/s0031-9384(00)00403-0. [DOI] [PubMed] [Google Scholar]
- [8].Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bouchard PR, Lynch CB. Burrowing behavior in wild house mice: variation within and between populations. Behav Genet. 1989;19:447–456. doi: 10.1007/BF01066170. [DOI] [PubMed] [Google Scholar]
- [10].Bucan M, Abel T. The mouse: genetics meets behaviour. Nat Rev Genet. 2002;3(2):114–123. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- [11].Clifford S, Young R, Williamson P. Assessing the early characteristics of autistic disorder using video analysis. J Autism Dev Disord. 2007;37:301–313. doi: 10.1007/s10803-006-0160-8. [DOI] [PubMed] [Google Scholar]
- [12].Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007a;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Crawley JN. Testing hypotheses about autism. Science. 2007b;318:56–57. doi: 10.1126/science.1149801. [DOI] [PubMed] [Google Scholar]
- [14].Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- [15].Dawson G, Toth K, Abbot R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychology. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- [16].DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Flint J, Mott R. Applying mouse complex-trait resources to behavioural genetics. Nature. 2008;456:724–727. doi: 10.1038/nature07630. [DOI] [PubMed] [Google Scholar]
- [18].Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- [19].Groen WB, Zwiers MP, van der Gaag RJ, Buitelaar JK. The phenotype and neural correlates of language in autism: an integrative review. Neurosci Biobehav Rev. 2008;32:1416–1425. doi: 10.1016/j.neubiorev.2008.05.008. [DOI] [PubMed] [Google Scholar]
- [20].Gupta AR, State MW. Recent advances in the genetics of autism. Biol Psychiatry. 2007;61:429–437. doi: 10.1016/j.biopsych.2006.06.020. [DOI] [PubMed] [Google Scholar]
- [21].Halladay AK, Amaral D, Aschner M, Bolivar VJ, Bowman A, DiCicco-Bloom E, et al. Animal models of autism spectrum disorders: information for neurotoxicologists. NeuroToxicology. 2009;30:811–821. doi: 10.1016/j.neuro.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hranilovic D, Bucan M. Social behavior as an endophenotype for psychiatric disorders: development of mouse models. Curr Genom. 2001;2:41–54. [Google Scholar]
- [23].Insel TR. Mouse models for autism: report from a meeting. Mamm Genome. 2001;12:755–757. doi: 10.1007/s00335-001-4006. [DOI] [PubMed] [Google Scholar]
- [24].Jahr E, Eikeseth S, Eldevik S, Aase H. Frequency and latency of social interaction in an inclusive kindergarten setting: a comparison between typical children and children with autism. Autism. 2007;11:349–363. doi: 10.1177/1362361307078134. [DOI] [PubMed] [Google Scholar]
- [25].Jones SE, Brain PF. An illustration of simple sequence analysis with reference to the agonistic behaviour of four strains of laboratory mouse. Behav Processes. 1985;11:365–388. doi: 10.1016/0376-6357(85)90003-8. [DOI] [PubMed] [Google Scholar]
- [26].Laviola J, Terranova ML. The developmental psychobiology of behavioural plasticity in mice: the role of social experiences in the family unit. Neurosci Biobehav Rev. 1998;23:197–213. doi: 10.1016/s0149-7634(98)00021-9. [DOI] [PubMed] [Google Scholar]
- [27].Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].LLaneza DC, DeLuke SV, Batista M, Crawley JN, Christodulu KV, Frye CA. Communications, interventions and scientific advances in autism: a commentary. Physiol Behav. 2010;100:268–276. doi: 10.1016/j.physbeh.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- [30].McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+ tf/J mice. Genes Brain Behav. 2008;7:152–263. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- [31].Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- [32].Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- [33].Moy SS, Nadler JJ, Magnuson TR, Crawley JN. Mouse models of autism spectrum disorder: the challenge for behavioral genetics. Am J Med Genet C Semin Med Genet. 2006;142:40–51. doi: 10.1002/ajmg.c.30081. [DOI] [PubMed] [Google Scholar]
- [34].Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008a;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, et al. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008b;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, et al. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29(7):349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- [39].Ricceri L, Moles A, Crawley JN. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res. 2007;176:40–52. doi: 10.1016/j.bbr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- [40].Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3(8):e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W, et al. Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. J Child Psychol Psychiatry. 2006;47:582–590. doi: 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- [42].Tager-Flusberg H, Joseph R, Folstein S. Current directions in research on autism. Ment Retard Dev Disabil Res Rev. 2001;7:21–29. doi: 10.1002/1098-2779(200102)7:1<21::AID-MRDD1004>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [43].Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45:135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- [44].Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, et al. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007b;1:1–9. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yang M, Weber MD, Crawley JN. Light phase testing of social behaviors: not a problem. Front Neurosci. 2008;2:186–191. doi: 10.3389/neuro.01.029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+ tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Devl Neuroscience. 2007a;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]



