Abstract
Background
Spidroins are a unique family of large, structural proteins that make up the bulk of spider silk fibers. Due to the highly variable nature of their repetitive sequences, spidroin evolutionary relationships have principally been determined from their non-repetitive carboxy (C)-terminal domains, though they offer limited character data. The few known spidroin amino (N)-terminal domains have been difficult to obtain, but potentially contain critical phylogenetic information for reconstructing the diversification of spider silks. Here we used silk gland expression data (ESTs) from highly divergent species to evaluate the functional significance and phylogenetic utility of spidroin N-terminal domains.
Results
We report 11 additional spidroin N-termini found by sequencing ~1,900 silk gland cDNAs from nine spider species that shared a common ancestor > 240 million years ago. In contrast to their hyper-variable repetitive regions, spidroin N-terminal domains have retained striking similarities in sequence identity, predicted secondary structure, and hydrophobicity. Through separate and combined phylogenetic analyses of N-terminal domains and their corresponding C-termini, we find that combined analysis produces the most resolved trees and that N-termini contribute more support and less conflict than the C-termini. These analyses show that paralogs largely group by silk gland type, except for the major ampullate spidroins. Moreover, spidroin structural motifs associated with superior tensile strength arose early in the history of this gene family, whereas a motif conferring greater extensibility convergently evolved in two distantly related paralogs.
Conclusions
A non-repetitive N-terminal domain appears to be a universal attribute of spidroin proteins, likely retained from the origin of spider silk production. Since this time, spidroin N-termini have maintained several features, consistent with this domain playing a key role in silk assembly. Phylogenetic analyses of the conserved N- and C-terminal domains illustrate dramatic radiation of the spidroin gene family, involving extensive duplications, shifts in expression patterns and extreme diversification of repetitive structural sequences that endow spider silks with an unparalleled range of mechanical properties.
Background
There are numerous types of spider silks and each has its own suite of mechanical properties, including exceptional tensile strengths, extensibilities, and toughness [1,2]. This mechanical diversity is associated with the distinct functional demands of the different silk types and largely stems from variation in the molecular composition of the silk proteins [3,4]. An individual spider spins a multitude of silk types, with each type emerging from its own distinctive set of abdominal silk glands. This complex silk machinery enables spiders to utilize task-specific silks (e.g., for web assembly, egg-case construction, prey wrapping, etc.). Every fiber type is composed of one or more spidroin proteins (spidroin = spider fibroin; [5]). Spidroins synthesized by an individual spider are encoded by multiple gene paralogs, the result of gene duplication and divergence events [6-8]. The complement of spidroin paralogs found within a spider genome varies substantially across species from different families [6,7]. Determining the evolutionary relationships of spidroins is therefore an essential step to understanding spider silk diversification.
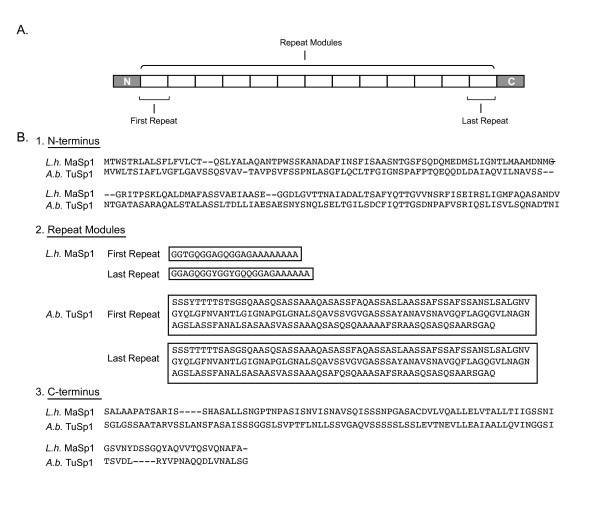
Spidroins are typically very large proteins (e.g., > 3000 amino acids, > 200 kDa) and exhibit a polymeric organization, where > 90% of the sequence is composed of highly homogenized tandem repeats [9-12]. Depending on the type of spidroin, these tandem repeats may contain combinations of amino acid sequence motifs that form structural modules such as crystalline beta-sheets, beta turns, or helices, that underlie the mechanical attributes of spider silks [4,13-15]. Flanking a spidroin's long core region of iterated repeats are short, non-repetitive amino (N) and carboxy (C) terminal domains (Figure 1). Sequence conservation of these terminal domains across spidroins, and their presence in silk fibers [16-18], imply they serve some critical role. For example, predicted signal peptides in the N-terminal domain are thought to regulate spidroin secretion from silk gland cells [19-21]; whereas experimental data suggest the N- and C- terminal domains contribute to fiber assembly [22-28].
Figure 1.
Spidroin molecular organization and comparison of domain sequences from two paralogs. A. Schematic of spidroin primary structure showing short, non-repetitive N- and C-terminal domains flanking a long region of tandem sequence repeat modules. B. A comparison of full-length, divergent spidroin paralogs encoding the dragline silk protein MaSp1 from Latrodectus hesperus (L.h.) [Genbank: EF595246] and the egg-case silk protein TuSp1 from Argiope bruennichi (A.b.) [Genbank: AB242144], showing their (1) N-terminal domains, (2) the first and last repeat in each sequence and (3) their C-terminal domains. Dashes are alignment gaps. Note the varying repeat sequence length and composition between L.h. MaSp1 and A.b. TuSp1, in comparison to the high similarity across repeat modules within a spidroin.
Reconstructing relationships among spidroins based on their repetitive regions is problematic because their extreme variability in length and sequence identity make them difficult to align [6,7,12]. The high variability observed between spidroin repetitive sequences results from mutations being spread across a gene by concerted evolution involving non-reciprocal recombination among intragenic repeats [10,29]. This scrambling and overwriting of repeated sequences violates assumptions of positional homology implied when they are aligned for phylogenetic construction [30,31]. Thus, despite the repetitive region composing the majority of a spidroin sequence, phylogenetic analyses of the spidroin gene family have relied mostly on the much shorter, more conserved C-terminal domain [6,8,21,32].
Spidroin C-terminal sequences are relatively straightforward to deduce via cDNA cloning and represent the overwhelming majority of existing sequence data available for gene family reconstruction. However, the short C-termini (encoded by ~300 bp) provide limited characters from which to infer evolutionary relationships among paralog lineages that could have arisen 300-400 million years ago [33]. Far fewer spidroin N-terminal sequences are known, due to the difficulties associated with direct N-terminal sequencing of silk proteins and cloning full-length spidroin cDNAs or genomic sequences that can be ~10-15 kb or longer [11,12,20]. The few published N-termini suggest promise as an additional source of phylogenetic characters, because they are approximately 50% longer than C-termini and appear to be more conserved [8,12,20,21,34].
The increasing efficiency of DNA sequencing has enabled us to assemble large-scale collections of expressed sequences (ESTs: Expressed Sequence Tags) from spider silk gland cDNA libraries. Through bioinformatic surveys of these data, we identified 11 more N-terminal spidroin sequences from nine species, nearly doubling the set available for phylogenetic inference. Notably, we report N-terminal spidroin sequences from a broad sampling of spider lineages, spanning > 240 million years of divergence, as well as from a greater diversity of functionally distinct silk proteins. We investigate the utility of these expanded N-terminal data, both separately and in combination with corresponding C-termini for resolving spidroin phylogeny and to trace the evolution of structural motifs that contribute to the extraordinary mechanical performance of spider silks. We also identify conserved sequence features of spidroin N-termini that are likely to be important for the production of native spider silk and the assembly of recombinant silk in vitro for biomimetic applications.
Methods
cDNA library construction and screening
Silk gland cDNA libraries were constructed from nine spider species representing eight families. Live spiders were anesthetized with CO2 and the following silk glands were dissected and then flash frozen in liquid nitrogen: (1) tubuliform glands from Argiope argentata (Araneidae); (2) minor ampullate glands from Latrodectus hesperus (Theridiidae); (3) flagelliform glands from Metepeira grandiosa (Araneidae); (4) large, ampullate shaped glands from Diguetia canities (Diguetidae); and combined silk gland tissue from (5) Agelenopsis aperta (Agelenidae), (6) Deinopis spinosa (Deinopidae), (7) Uloborus diversus (Uloboridae), (8) Kukulkania hibernalis (Filistatidae), and (9) Bothriocyrtum californicum (Ctenizidae). Total RNA was extracted from each tissue type by homogenization in TRIzol (Invitrogen, Carlsbad, CA) and further purified using the RNeasy Mini Kit (Qiagen, Valencia, CA). mRNA was isolated with oligo-(dT)-tagged magnetic beads (Invitrogen). cDNA was synthesized using Invitrogen's SuperScript Choice protocol, starting with the anchored oligonucleotide (dT)18V. cDNAs were fractionated by size using ChromaSpin 1000 columns (Clontech, Mountain View, CA), blunt-end ligated into pZErO-2 vector (Invitrogen), and electroporated into TOP10 Escherichia coli (Invitrogen). For each species, ~1800 colonies were arrayed and replicated onto nylon filters. Between 400-600 recombinant clones from each library were screened for size by visualization of plasmid DNA with gel electrophoresis using the method of Beuken et al. [35] and inserts ≥ 500 bp were sequenced using T7 or Sp6 primers.
The nylon filters of every cDNA library were screened with γ32P labeled oligonucleotide probes encoding poly-alanine (GCDGCDGCDGCDGCDGC) and alternating alanine and glycine (CCWGCWCCWGCWCCWGCWCC), motifs common to many spider silks [6]. We also used the following probes to screen specific libraries: (1) GMWGAWGCRAAWGCCATRTT, (2) CRAYMGMAGATGCRAATGCCAT (1-2 for Kukulkania hibernalis and Diguetia canities); (3) CGATGCGGCTGCTGCAGA, (4) GCCACGACCGAAGTCTCC, (5) CTGATGGGGTTGCTGTCC, (6) GCCTGGTGCTCTCGCCGT, (7) GCTATTTAGAGAGGGGTTGG, (8) CTGATTGCTGGTTTTGCC, (9) AACCGTTTGGAAATTTTG (3-9 for Diguetia canities); (10) CCWCCWGGWCCNNNWCCWCCWGGWCC, (11) CCWGGWCCTTGTTGWCCWGGWCC (10-11 for Metepeira grandiosa); and (12) CGATGTGGTGGTAGTTCT, (13) AGCGGATGAGAAGGCACT, (14) GGCACTGGAGAAAGCGCT, (15) ACTDGCTCCBACRCCRAC, (16) GAYTGGCTTGCGGCTTGRCT (12-16 for Agelenopsis aperta). Additional probes used in screening libraries from Argiope argentata were reported in Garb and Hayashi [29], from Uloborus diversus and Deinopis spinosa in Garb et al. [32], and Bothriocyrtum californicum in Garb et al. [7]. Probe-positive recombinant clones were sequenced with T7 and/or Sp6.
Characterization of spidroin N- and C-terminal domains
To identify putative spidroin N-terminal domain sequences, silk gland EST sequences were subjected to translated-Blast queries (BlastX; [36]) against the NCBI nr protein database. Collected sequences were also compiled in a private database against which we blasted published spidroin N-terminal sequences. Plasmids containing N-terminal coding regions were digested with restriction enzymes to estimate insert size. The longest clone of each N-terminal type, containing the maximal amount of upstream sequence, was selected for further characterization. These cDNAs were independently sequenced two times in their forward and reverse directions using T7 and Sp6. We also surveyed the literature and searched GenBank databases for published N-terminal sequences.
N-terminal sequences were identified as belonging to established spidroin classes by: 1) the presence of recurring amino acid motifs in adjacent repetitive sequence, diagnostic for particular spidroins (see Gatesy et al. [6]); 2) the silk gland from which they were isolated; and 3) their relationship to published N-termini based on preliminary phylogenetic analyses. Spidroin sequence nomenclature indicates the silk gland type from which it was initially isolated (Ma = major ampullate, Mi = minor ampullate, Tu = tubuliform, Ac = aciniform, or Flag = flagelliform), usually followed by "Sp" for spidroin, and often a number for distinct paralogs (e.g., MaSp1 and MaSp2 were the first two spidroins characterized from major ampullate silk glands). Sequences not readily assigned to these groups were designated by species name followed by "fibroin x", where x is a number identifying a distinct paralog (e.g., Bothriocyrtum californicum fibroin 1). However, it should be noted that various authors have occasionally assigned the same protein name to paralogous spidroins (e.g., paralogs from different species have been named "MaSp1").
For new and published spidroin N-terminal sequences, we associated each with its corresponding downstream C-terminal sequence. This is trivial in the four cases where full-length cDNA or genomic sequences have been reported [11,12]. However, the great majority of spidroin sequences represent partial transcripts that span either the N-terminal or C-terminal coding region adjacent to downstream or upstream repetitive sequence, respectively. Because spidroin repetitive sequence is extremely similar across its entire length (e.g., see Figure 1), previous work reporting N-termini have identified their probable downstream C-terminal sequence based on near identity of the adjacent repetitive regions in each [20,21,34]. In this paper, we similarly assigned a corresponding C-terminal sequence to an N-terminal sequence (from the same species) if their repetitive regions were nearly identical. GenBank accession numbers for newly reported and published sequences examined in this study are listed in Table 1.
Table 1.
Spider fibroin (spidroin) sequences analyzed in this study.
| N-terminus | Species | GenBank Accession | Reference | C-terminus Accession | Reference |
|---|---|---|---|---|---|
| B.c. fibroin1 | Bothriocyrtum californicum | HM752562 | This study | EU117162 | [7] |
| K.h. MaSp1 | Kukulkania hibernalis | HM752563 | This study | -- | -- |
| D.c. MaSp | Diguetia canities | HM752564 | This study | HM752565 | This study |
| D.c. MaSp-like | Diguetia canities | HM752566 | This study | HM752567 | This study |
| D.s. MaSp2 | Deinopis spinosa | HM752568 | This study | DQ399328, DQ399329a | [32] |
| A.ap. MaSp | Agelenopsis aperta | HM752573 | This study | AAT08436 | [55] |
| U.d. MiSp | Uloborus diversus | HM752574 | This study | ABD61597 | [32] |
| M.g. MiSp | Metepeira grandiosa | HM752575 | This study | HM752569 | This study |
| L.h. MiSp | Latrodectus hesperus | HM752570 | This study | HM752571 | This study |
| A.ap. TuSp1 | Agelenopsis aperta | HM752576 | This study | HM752572 | This study |
| A.a. TuSp1 | Argiope argentata | HM752577 | This study | AY953071 | [29] |
| A.b. TuSp1 | Argiope bruennichi | AB242144 | [11] | AB242144 | [11] |
| N.ct. TuSp1 | Nephila clavata | AB218974 | [64] | AB218973 | [64] |
| L.h. TuSp1 | Latrodectus hesperus | DQ379383 | [18] | AY953070 | [29] |
| A.t. MaSp2 | Argiope trifasciata | DQ059136S1 | [20] | DQ059136S2 | [20] |
| N.c. MaSp2 | Nephila clavipes | EU599240 | [34] | AY654297 | [17] |
| N.i. MaSp2 | Nephila inaurata madagascariensis | DQ059135 | [20] | AF350278 | [6] |
| N.c. MaSp1a | Nephila clavipes | EU599238 | [34] | AY654292 | [17] |
| N.c. MaSp1b | Nephila clavipes | EU599239 | [34] | AY654291 | [17] |
| L.h. MaSp1 | Latrodectus hesperus | EF595246 | [12] | EF595246 | [12] |
| L.h. MaSp2 | Latrodectus hesperus | EF595245 | [12] | EF595245 | [12] |
| L.g. MaSp1 | Latrodectus geometricus | DQ059133S1b | [20] | DQ059133S2 | [20] |
| E.a. MaSp | Euprosthenops australis | AM259067 | [21] | AJ973155 | [65] |
| N.c. Flag | Nephila clavipes | AF027972b | [19] | AF027973 | [19] |
| N.i. Flag | Nephila inaurata madagascariensis | AF218623S1 | [10] | AF218623S2 | [10] |
| A.v. Flag | Araneus ventricosus | AY945306 | -- | AY587193 | - |
N-terminal spidroin sequences were determined by conceptual translation using coding frames determined by BlastX searches. Rising et al. [21] identified the presence of a conserved translation initiation site (Met residue) in N-terminal sequences. Following this finding, we presumed that N-terminal transcripts lacked complete upstream coding information if their sequence did not overlap this region. We subjected the N-termini to SignalP http://www.cbs.dtu.dk/services/SignalP/ analyses, which predict the location of signal peptide cleavage sites. Superimposed Kyte-Doolittle [37] hydropathy plots of the N-termini were made with pepwindowall http://emboss.sourceforge.net/apps/cvs/emboss/apps/pepwindowall.html. N-terminal secondary structures were predicted using the Garnier et al. [38] method implemented in GOR IV http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_gor4.html.
Phylogenetic tree construction
N-terminal and C-terminal amino acid sequences were separately aligned with ClustalW, using default parameters as implemented in MacVector 7.0 (Oxford Molecular Group, Oxford, UK), then the output was refined manually. N-terminal sequences of Latrodectus geometricus MaSp1 and Nephila clavipes Flag were edited according to Rising et al. [21]. Protein sequence alignments were used to guide an alignment of encoding nucleotides using the program tranalign http://bioweb2.pasteur.fr/docs/EMBOSS/tranalign.html. Phylogenetic analyses were performed with N- and C-terminal alignments for protein and nucleotide sequences separately, and also in a combined analysis, concatenating the N- and C-terminal character matrices. Heuristic parsimony tree searches were conducted with PAUP* 4.0b [39], including 10,000 random taxon addition replicates and tree-bisection-reconnection branch swapping, treating gaps as missing data. Branch support was computed from 1000 bootstrap (BT) replicates, with three random taxon addition replicates per bootstrap replicate. Decay indices of tree nodes were determined using the program TreeRot v3 [40].
Bayesian phylogenetic analyses were also conducted separately for N-termini, C-termini, and the two in combination. Analyses were performed with MrBayes 3.1.2 [41], using the model recommended by ProtTest [42] for separate analyses of protein sequences, or by ModelTest [43] for separate and combined analyses of nucleotides. Combined nucleotide analyses were partitioned by N- and C-terminal domains, using the recommended model for each partition. Combined amino acid N- and C-terminal analyses implemented a "mixed" model, allowing switching between models plus a gamma distribution. Bayesian runs were executed for 5 × 106 generations, sampling trees every 1000 generations, and continued until split frequencies were below 0.01. Clade posterior probabilities (PP) were computed from a 50% majority-rule consensus of post burn-in trees (25% of each run, totaling 125,000 trees).
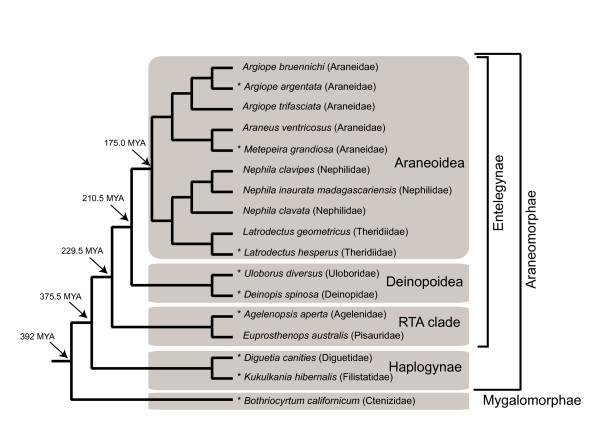
Root placement in spidroin gene family trees was estimated with reference to a phylogenetic hypothesis for spider species. This "species tree" included all species from which the analyzed spidroin genes were sampled and is a composite tree, summarized from published phylogenies (Figure 2). Relationships at the family level and above were determined from Coddington et al. [44], relationships among Nephila species were from Kuntner et al. [45], relationships within Araneidae are based on Scharff and Coddington [46] and relationships among Argiope species were from Elices et al. [47]. Each gene tree was reconciled with the fixed species tree in Notung 2.6 [48], to identify the rooted topology that minimized inferred gene duplications and losses (D/L). Default cost parameters in Notung (duplication = 1.5, loss = 1.0) were used to compute minimal D/L scores and re-root trees.
Figure 2.
Phylogeny of species examined in this study. Tree is a composite of published phylogenies [44-47]. Major lineages and approximate divergence dates estimated by Ayoub and Hayashi [33] are indicated. Asterisks mark species from which we report new N-terminal sequences. Branch lengths are not proportional to time.
Spidroin tree comparisons and character mapping
The resulting N- and C-terminal trees were visually compared to identify any well-supported (BT ≥ 70% or PP ≥ 0.95) but conflicting nodes [49]. Potential conflict between N- and C-termini was also evaluated with the partition homogeneity test (PHT), implemented in PAUP and excluding invariant characters. Null distributions were constructed from 1000 replicate character permutations, with most parsimonious trees for each replicate estimated from 10 random taxon addition replicates. Likelihood tree scores for different N- or C-terminal (and combined) phylogenetic hypotheses were evaluated relative to each dataset. Likelihood values for nucleotide trees were determined in PAUP*, using the recommended substitution model for the given dataset and allowing branch lengths to vary. Significant differences between best and alternative hypotheses were compared using the Shimodaira-Hasegawa (SH) test [50], with full optimization and 1000 bootstrap replicates. Likelihood values of trees derived from amino acid data were determined and compared to alternative trees with the SH test in TreePuzzle 5.2 [51]. For combined parsimony analyses, conflict and congruence for different nodes was also evaluated with Partitioned Branch support [52], computed with TreeRot v3, Hidden Branch Support and Partitioned Hidden Branch Support [53].
The combined amino acid tree was used to map N- and C-terminal domain synapomorphies. Unambiguously optimized apomorphic changes were determined using the "apolist" option in PAUP*, for both ACCTRAN and DELTRAN optimizations, and tracing each onto the combined tree to identify shared derived residues at each tree node. We scored exemplar repeat sequences from each sampled spidroin for the presence of amino acid motifs hypothesized to form specific secondary and tertiary structures [4]. These included poly-alanine, An (four or more contiguous alanines); two or more consecutive glycine-alanine couplets, (GA)n; two or more consecutive glycine-serine couplets, (GS)n; two or more GPGXn repeats, where P = proline and X = an amino acid from a small subset; and two or more tandem arrayed GGX. An, (GA)n, and (GS)n conform to beta-sheet structures that impart tensile strength, whereas repeating GPGXn motifs form beta-turns that confer extensibility, and the (GGX)n motif forms a 310 helix [4]. Gain or loss of these motifs at tree nodes was inferred by parsimony ancestral reconstruction using the combined N- and C-terminal domain trees in MacClade 4.0 [54].
Results
N-terminal sequence discovery
In total, we sequenced 1,921 silk gland cDNAs from nine spider species. BlastX searches of the resulting EST data identified 30 cDNA sequences containing putative spidroin N-termini. Blastclust analyses http://toolkit.tuebingen.mpg.de/blastclust#, which cluster highly similar sequences, grouped the 30 N-termini into 11 distinct sequence types. Each N-terminal cDNA represented a partial transcript that included some adjacent repetitive sequence. Except for the MaSp1 N-terminal sequence from the nursery web spider, Euprosthenops australis [21], previous reports of N-terminal spidroins were from eight species of the spider clade Araneoidea (ecribellate orbweavers; Figure 2). Our new sequences indicate the presence of a similar non-repetitive N-terminal domain in spidroins synthesized by eight additional species, six of which were non-araneoids from the lineages Deinopoidea (Uloborus and Deinopis), the RTA clade (Agelenopsis), Haplogynae (Kukulkania and Diguetia), and the suborder Mygalomorphae (Bothriocyrtum). These included sequences we hypothesize to be upstream of Agelenopsis aperta MaSp1 (GenBank accession AAT08436) and Kukulkania hibernalis MaSp1 (AAT08433) reported by Tian et al. [55]. In addition, we discovered N-terminal sequences from Latrodectus, Metepeira, and Uloborus for the minor ampullate spidroin MiSp, which constitutes temporary scaffold silk. Fourteen additional published N-terminal spidroin sequences, and one unpublished N-terminal sequence reported in GenBank as a "major ampullate dragline silk protein" (AY945306) but which we attribute to Flag because it flanks repetitive sequence characteristic of Flag spidroins, were included in subsequent analyses (Table 1). We were able to associate N-termini with putative downstream C-termini for all new and published sequences except for Kukulkania hibernalis MaSp1, the C-terminus of which is unknown. It was also not possible to determine whether the N-terminal sequence of MaSp2 from Deinopis spinosa was upstream of MaSp2a (DQ399329) or MaSp2b (DQ399328) C-terminal sequences, which are very similar to each other and possibly represent allelic variants [32]. For this reason, both MaSp2a and MaSp2b from D. spinosa were included in C-terminal analyses.
N-terminal sequence features
Alignment of the translated sequences showed that five of the 11 newly reported N-termini include the conserved methionine residue identified by Rising et al. [21] as the spidroin translation initiation site (Additional file 1). SignalP 3.0 predicted the presence and location of a signal peptide in nearly all N-terminal sequences, consistent with the targeting of these proteins for entry into the secretory pathway. Three sequences (A.a. TuSp1, A.v. Flag, and U.d. MiSp) were predicted to be non-secretory proteins, a possible artifact of their lack of some upstream sequence. The amino acid sequence motif "TTGXXN" identified by Rising et al. [21] as conserved across spidroin N-termini, does not appear in all of the new sequences we report here (Additional file 1). Of the 168 aligned residues in the N-terminal alignment, only three are present universally in all sequences (Additional file 1). These three residues are the start codon, an aspartic acid (position 70) and a glycine (position 140). However, 39% of all sites contained the same residue in at least half of the sequences. Average pairwise identity across N-terminal amino acid sequences was 37%, and corresponding C-termini shared an average of 35% identity (median pairwise identity for N-termini = 33%, C-termini = 30%). N-terminal regions had proportionately less length variation than C-terminal regions (complete N-terminal sequences ranged from 151-162 amino acids vs. 87-107 amino acids for C-terminal sequences). Superimposed Kyte-Doolittle plots indicated relative similarity in hydropathy profiles across N-termini (Additional file 2), with greatest hydrophobicity occurring in the first 10-20 residues, consistent with predictions that they include signal peptides, followed by alternating hydrophilic and hydrophobic regions. Secondary structural predictions for exemplars of the different N-termini consisted mostly of 4-6 alpha-helices (41-70%) that are connected by short intervening random coils and some extended strands (Additional file 3).
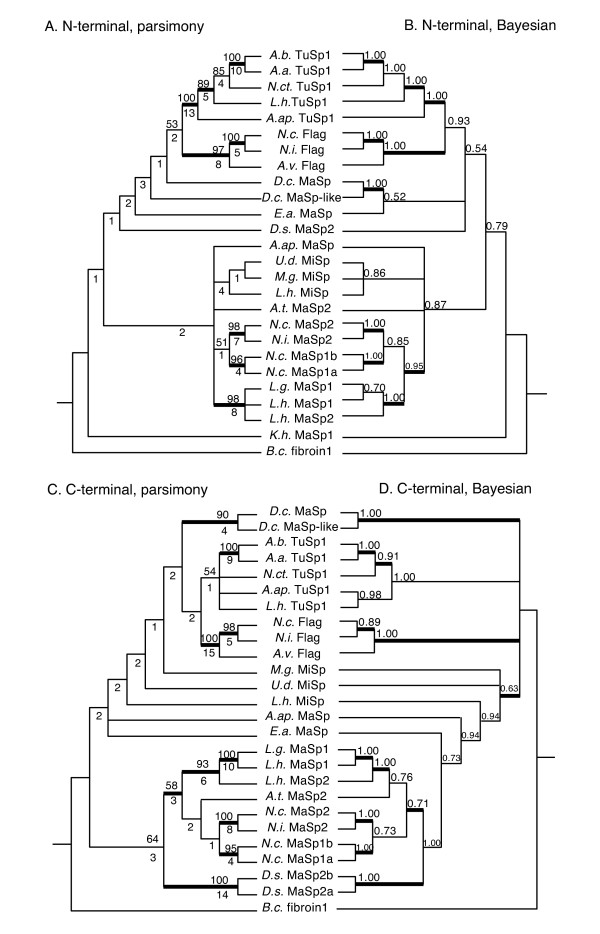
Phylogenetic analyses
At both the nucleotide (nu) and amino acid (aa) level, N-terminal sequences provided more variable (nu = 455, aa = 160) and parsimony informative (PI: nu = 415, aa = 144) characters than C-termini (variable: nu = 300, aa = 102; PI: nu = 283, aa = 96; Table 2). The consistency indices (CI) of most parsimonious trees, were similar for both N-termini and C-termini (e.g., Nterm aa CI = 0.634 vs. Cterm aa CI = 0.643; Table 2). Parsimony analyses of nucleotide data resulted in a single N-terminal tree and two C-terminal trees, whereas Bayesian consensus trees were less resolved (Additional file 4). Nearly all statistically supported nodes in the separate nucleotide trees were supported in the amino acid trees (Figure 3, Additional file 4), and the number of supported nodes did not markedly differ between N- and C-terminal trees (Table 2). Separate N- and C-terminal amino acid trees supported a TuSp1 clade (N-terminal: BT = 100, PP = 1.00; C-terminal: BT = 54, PP = 1.00) and Flag clade (N-terminal: BT = 97, PP = 1.00; C-terminal: BT = 100, PP = 1.00), but relationships among MaSp and MiSp sequences varied. Nevertheless, supported groups of more recently diverged sequences were mirrored in both N- and Cterminal trees (e.g., D.c. MaSp + D.c. MaSp-like; all Latrodectus MaSp sequences).
Table 2.
Summary statistics for spidroin N- and C-terminal domain character sets.
| Data | Chr. | Var. | PI | Len. | CI | RI | MPT | % distance ave (min-max) | branches BT ≥ 70% | branches PP ≥ 0.95 | D/L score Parsimony | D/L score Bayesian |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N nu | 504 | 455 | 415 | 2989 | 0.367 | 0.452 | 1 | 0.52(0.03-0.66) | 10 | 11 | 55.5(11/39) | 44.5(9/31) |
| C nu | 327 | 300 | 283 | 1877 | 0.392 | 0.497 | 2 | 0.55(0.01-0.72) | 11 | 13 | 43(10/28) | 50(10/35) |
| N aa | 168 | 160 | 144 | 1223 | 0.634 | 0.587 | 4 | 0.63(0.01-0.88) | 9 | 11 | 60(12/42) | 53.5(11/37) |
| C aa | 109 | 102 | 96 | 720 | 0.643 | 0.635 | 8 | 0.65(0-0.90) | 9 | 11 | 59(12/41) | 57(12/39) |
| N+C nu | 831 | 755 | 697 | 4877 | 0.376 | 0.459 | 1 | 0.53(0.02-0.65) | 12 | 13 | 54.5(11/38) | 50(10/35) |
| N+C aa | 277 | 262 | 239 | 1954 | 0.633 | 0.591 | 1 | 0.64(0.01-0.81 | 12 | 13 | 55.5(11/39) | 49(10/34) |
nu = nucleotide, aa = amino acid, chr = total # characters, Var. = variable characters, PI = parsimony informative characters, Len = tree length, CI = consistency index of most parsimonious tree(s) for given data, RI = retention index, MPT=# most parsimonious trees.
Figure 3.
Phylogenetic trees from separate analyses of spidroin N- and C-terminal domain sequences. A. N-terminal domain, consensus of 4 most parsimonious trees (MPTs), B. Bayesian consensus tree for N-terminus, C. C-terminal domain, consensus of 8 MPTs, D. Bayesian consensus for C-terminus; A, C: numbers above nodes are bootstrap (BT) values, below nodes decay index, thickened branch supported by ≥ 70 BT in parsimony nucleotide analyses (Additional file 4a, 4c); B, D: numbers above nodes are posterior probability (PP) values, thickened branch supported by ≥ 0.95 PP in Bayesian nucleotide analyses (Additional file 4b, 4d).
In parsimony and Bayesian nucleotide and amino acid analyses, the combination of N-and C-terminal data increased the number of strongly supported nodes (BT ≥ 70; PP ≥ 0.95) over those in separate N- and C-terminal trees (Figures 3, 4 and 5, Table 2, Additional files 4 and 5). The Bayesian consensus tree for the combined amino acid data was completely resolved and nearly identical to the single most parsimonious tree from that data (Figures 4 and 5) The main difference was in the placement of D.s. MaSp2, which in the parsimony tree grouped with E.a. MaSp, but was sister to the araneoid MaSp1 and MaSp2 sequences in the Bayesian consensus. Combined amino acid trees included a TuSp1 clade (BT = 100, PP = 1.00), a Flag clade (BT = 100, PP = 1.00), and a MiSp clade (BT = 55, PP = 1.00), but MaSp sequences were paraphyletic (Figures 4 and 5). Species tree reconciliation analyses with every spidroin phylogeny indicated that rooting on the branch leading to Bothriocyrtum californicum fibroin 1 minimized costs associated with gene duplications and losses.
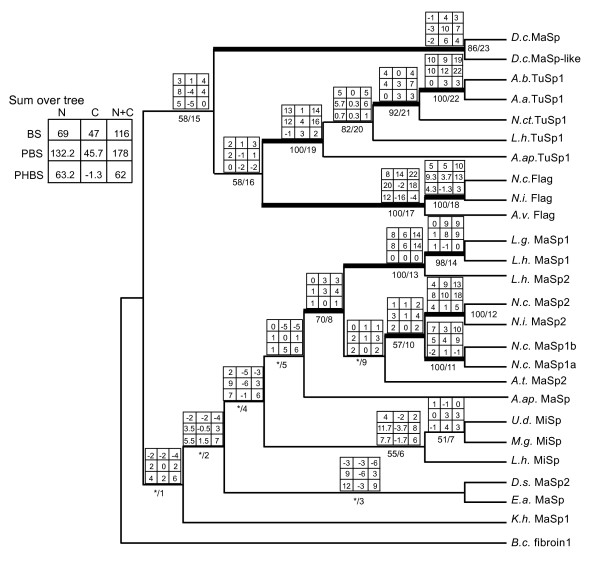
Figure 4.
Combined parsimony analysis of spidroin N- and C-termini showing consensus and conflict among domains. Matrix above branches shows character support by partition, and summed across tree in top left legend. Matrix columns from left to right: N-terminus, C-terminus, N+C termini; Rows from top to bottom: BS = branch support (decay index), PBS (partitioned branch support, and PHBS (partitioned hidden branch support). Below branches, left of the slash = bootstrap support (* = < 50%), right of the slash = node # referred to in text, thickened branches supported > 70% bootstrap replicates in parsimony nucleotide analysis (Additional file 5). Note that the K.h. MaSp1 C-terminus was coded as missing data, and the N-terminus of D.s. MaSp2 was concatenated with the D.s. MaSp2a C-terminus.
Figure 5.
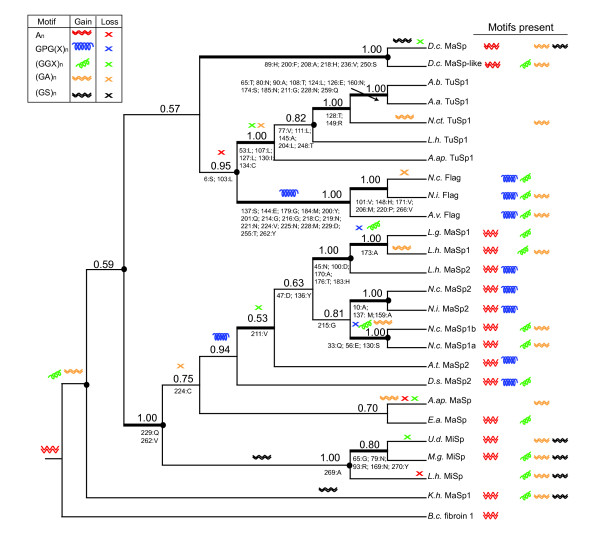
Bayesian consensus tree from spidroin N- and C-termini reconstructing structural motifs and gene duplications. 50% majority-rule consensus of post-burnin Bayesian trees from combined spidroin N+C terminal domains. Numbers above branches indicate PP values; thickened branches supported > 0.95 PP in Bayesian nucleotide analysis (Additional file 5b). Circles at nodes show inferred gene duplication events. Shown below branches are terminal domain amino acid synapomorphies by position (N-terminal positions from 1-168; C-terminal positions from 169-277). Gain of motif along a branch indicated as symbol in legend, and loss by an "X" of the same color. There are other equally parsimonious reconstructions for the evolution of (GA)n and (GGX)n motifs to those shown.
Evidence for any strongly supported, but conflicting nodes between N- and C-terminal trees was limited to the TuSp1 clade, which in the Bayesian C-terminal nucleotide analyses included all Flag sequences (PP = 0.98; Additional file 4). By contrast, the N-terminal Bayesian nucleotide tree showed a well-supported monophyletic TuSp1 (PP = 1.00), which was in agreement with the results of all other analyses. Also, L.h. TuSp1 grouped with A.ap.TuSp1 in the C-terminal amino acid Bayesian tree (PP = 0.98), which conflicts with it being sister to all other TuSp1 sequences in the N-terminal Bayesian amino acid tree (PP = 1.00; Figure 3). However, partition-homogeneity tests did not indicate significant incongruence between the N- and C-terminal characters (nu: P = 0.788; aa: P = 0.330). Comparisons of alternative topologies using the Shimodaira-Hasegawa (SH) test indicated there were significant differences in likelihood between topologies derived from separate N- or C-terminal data analyses (Table 3). But parsimony and Bayesian combined (N+C) data topologies were not significantly different from the best likelihood N- or C-terminal trees from separate data analyses. The sum of N- and C-terminal Partitioned Branch Support (PBS) values for all nodes in the combined parsimony tree (Figure 4) indicated that N-terminal data (PBS sum = 132.2) contributed much more support than the C-terminal data (PBS sum = 45.7). Nearly all N-terminal PBS scores were positive, whereas several C-terminal PBS scores were negative, the largest negative value being -6 (node 4, Figure 4). The Hidden Branch Support (HBS) values indicated that there was hidden support gained by combining the data (HBS sum = 62) and only 3 of 23 nodes indicated some hidden conflict (nodes 11, 16, and 17, Figure 4). Much of the hidden support emerged from the N-terminal data, while hidden conflict was mainly restricted to the C-terminal data (see PHBS values, Figure 4).
Table 3.
Summary of SH tests comparing alternative phylogenetic hypotheses.
| Data | model | Hypothesisa | -ln L Score | -ln L Difference | Prob. |
|---|---|---|---|---|---|
| N term nu | HKY+I+G | Nterm nu Highest PP | 10680.106 | BEST | 1.0000 |
| Nterm nu 1 of 1 MPT | 10687.329 | 7.222 | 0.557 | ||
| Cterm nu Highest PP | 10744.788 | 64.681 | 0.000* | ||
| Cterm nu 1 of 2 MPT | 10734.517 | 54.410 | 0.000* | ||
| Cterm nu 2 of 2 MPT | 10731.541 | 51.435 | 0.000* | ||
| N+C term nu Highest PP | 10689.373 | 9.266 | 0.431 | ||
| N+C term nu 1 of MPT | 10694.996 | 14.889 | 0.247 | ||
| C term nu | TrN+G | Nterm nu, Highest PP | 6812.899 | 28.163 | 0.036* |
| Nterm nu 1 of 1 MPT | 6815.562 | 30.826 | 0.016* | ||
| Cterm nu Highest PP | 6784.735 | BEST | 1.0000 | ||
| Cterm nu 1 of 2 MPT | 6791.424 | 6.688 | 0.544 | ||
| Cterm nu 2 of 2 MPT | 6791.653 | 6.917 | 0.574 | ||
| N+C term nu Highest PP | 6792.463 | 7.728 | 0.526 | ||
| N+C term nu 1 of MPT | 6795.710 | 10.974 | 0.333 | ||
| Nterm aa | WAG+G | Nterm aa Highest PP | 5630.28 | BEST | 1.0000 |
| Nterm aa 1 of 4 MPTs | 5644.44 | 14.15 | 0.4050 | ||
| Nterm aa 2 of 4 MPTs | 5646.50 | 16.22 | 0.3670 | ||
| Nterm aa 3 of 4 MPTs | 5635.67 | 5.39 | 0.7000 | ||
| Nterm aa 4 of 4 MPTs | 5633.38 | 3.09 | 0.8200 | ||
| Cterm aa Highest PP | 5665.89 | 35.60 | 0.0330* | ||
| Cterm aa 1 of 8 MPTs | 5666.24 | 35.95 | 0.0350* | ||
| Cterm aa 2 of 8 MPTs | 5668.54 | 38.25 | 0.0270* | ||
| Cterm aa 3 of 8 MPTs | 5662.15 | 31.86 | 0.0550 | ||
| Cterm aa 4 of 8 MPTs | 5666.87 | 36.59 | 0.0360* | ||
| Cterm aa 5 of 8 MPTs | 5674.10 | 43.82 | 0.0120* | ||
| Cterm aa 6 of 8 MPTs | 5669.18 | 38.89 | 0.0230* | ||
| Cterm aa 7 of 8 MPTs | 5676.45 | 46.16 | 0.0070* | ||
| Cterm aa 8 of 8 MPTs | 5664.36 | 34.04 | 0.0370* | ||
| N+C term aa Highest PP | 5640.57 | 10.29 | 0.5840 | ||
| N+C term aa 1 of 1 MPT | 5636.82 | 6.54 | 0.7770 | ||
| Cterm aa | JTT+G | Nterm aa Highest PP | 3404.20 | 38.83 | 0.0060* |
| Nterm aa 1 of 4 MPTs | 3415.87 | 50.50 | 0.0030 * | ||
| Nterm aa 2 of 4 MPTs | 3433.35 | 67.98 | 0.0000 * | ||
| Nterm aa 3 of 4 MPTs | 3438.46 | 73.09 | 0.0000 * | ||
| Nterm aa 4 of 4 MPTs | 3421.55 | 56.18 | 0.0040 * | ||
| Cterm aa Highest PP | 3365.37 | BEST | 1.0000 | ||
| Cterm aa 1 of 8 MPTs | 3378.26 | 12.89 | 0.3920 | ||
| Cterm aa 2 of 8 MPTs | 3378.20 | 12.82 | 0.3940 | ||
| Cterm aa 3 of 8 MPTs | 3381.57 | 16.20 | 0.2860 | ||
| Cterm aa 4 of 8 MPTs | 3381.57 | 16.20 | 0.2860 | ||
| Cterm aa 5 of 8 MPTs | 3382.67 | 17.30 | 0.2740 | ||
| Cterm aa 6 of 8 MPTs | 3381.54 | 16.16 | 0.2950 | ||
| Cterm aa 7 of 8 MPTs | 3382.62 | 17.25 | 0.2790 | ||
| Cterm aa 8 of 8 MPTs | 3381.54 | 16.16 | 0.2950 | ||
| N+C term aa Highest PP | 3376.32 | 10.95 | 0.4780 | ||
| N+C term aa 1 of 1 MPT | 3394.02 | 28.65 | 0.0310* |
a. Highest PP is Bayesian topology with highest posterior probability, * indicates topology statistically different from best topology for a given dataset and model. aa = amino acids, nu = nucleotides.
In the combined analyses, although the N-termini contributed more phylogenetic support, there were more synapomorphic residues in the C-terminal data (43) than in the N-termini (37; Figure 5). Considering the combined analysis results, ancestral reconstruction of structural motifs scored from exemplar repeats (Additional files 6 and 7) showed the unambiguous presence of An motifs at the root of the tree and its loss in three independent lineages (Figure 5). GPGXn repeats evolved at least twice to explain their presence in Flag sequences and in the clade containing MaSp2 sequences. (GS)n motifs independently arose in the MiSp lineage and in K.h. MaSp1. Alternative reconstructions were possible for (GA)n and (GGX)n evolution, as the presence or absence of these motifs at the root of the tree was equivocal (Figure 5).
Discussion
Conservation and diversity of spidroin N-termini
Despite years of intense research aimed at discovering the molecular basis for spider silk mechanics, determination of full-length spidroin sequences has rarely been achieved [11,12]. While many partial spidroin fragments containing C-termini have been characterized from divergent spider species, very few N-terminal sequences of these proteins are known and all but one were from araneoid species [11,12,18-21,34]. The limited number of the non-repetitive N-terminal domain sequences has restricted generalizations regarding its distribution across spidroins, as well its variability and potential functional significance. In this study we substantially expanded the number and diversity of spidroin N-terminal domain sequences by employing an EST approach, where > 1,900 silk gland cDNAs were sequenced at random. Using this method, we discovered that spidroins from the divergent spider lineages Mygalomorphae, Haplogynae, Agelenoidea, and Deinopoidea are also characterized by the presence of a non-repetitive N-terminal domain with high sequence similarity to those reported from distantly related araneoid spiders [11,12,18-20,34] and the pisaurid Euprosthenops australis [21].
The finding of an N-terminal domain in a mygalomorph spidroin is significant because it indicates that this molecular feature has been conserved for at least 240 million years of spider silk production, the minimal age that fossil evidence dates the divergence of mygalomorphs (tarantulas and their kin) and araneomorphs ("true spiders") from a common ancestor [56]. Molecular dating with multiple fossil calibration points estimates an even older divergence, as early as ~390 million years ago [33]. The conserved N-terminal domain may be a universal feature of spidroin protein architecture that has persisted since the spinning of the first spider silk, estimated to have occurred in the Devonian.
Our analyses show that spidroin N-termini are relatively conserved at multiple levels of molecular organization. In addition to sequence similarity, the different N-termini are similar in length (~151-162 amino acids) and share a common translation initiation site. After the first methionine, two other residues are identical across all spidroins (position 70 = D and position 140 = G), while their corresponding C-terminal domains do not have a single residue that is 100% conserved. The shared features of spidroin N-termini also include predicted signal peptides, hydropathy profiles characterized by similarly alternating hydrophobic and hydrophilic regions, and secondary structural predictions consisting of 4-6 alpha-helices. While previous studies noted some of these characteristics [20,21], our work substantially broadens their distribution across very distantly related species and spidroin paralogs that compose mechanically dissimilar silks.
Previous mass spectrometry (MS) work confirmed the presence of the TuSp1 N-terminal domain in egg-case silk fibers [18]. The Latrodectus hesperus MiSp N-terminus we report here contains the sequences (VWDSTATAEAFIGSFNS and MDDISSISDTIISAIER) that exactly match mass spectrometry peptide sequences collected from L. hesperus minor ampullate silk by LaMattina et al. [57] (peptide mass 2081.0 and 2434.1), excepting that all "I" residues were reported as "L" (I and L are difficult to distinguish with MS). These data indicate that the MiSp N-terminal sequence (beyond the signal cleavage site) is also present in minor ampullate silk fibers and given the conserved characteristics, further reinforces that this domain contributes to silk production beyond secretory signaling.
Though relatively similar at the structural level, the new N-terminal sequences we report reveal far more sequence diversity than previously known. Our work confirms that this domain is the most conserved region across spidroin paralogs, although it exhibits only slightly greater pairwise identity than do its corresponding C-termini. This additional N-terminal sequence diversity is intriguing because it may relate to differential mechanisms of fiber formation among functionally distinct silks. Fiber assembly processes are likely to vary across gland types because of the differences in the repetitive structural motifs of their constituent spidroins and the length of time they are stored in glands (e.g., major ampullate silk is used daily while tubuliform silk is used only a few times in a spider's lifetime). Examples of paralog-specific features are the two identically positioned cysteine residues that have evolved in araneoid and non-araneoid TuSp1 N-termini (positions 52 and 134, Additional file 1), suggesting their involvement in a biochemical mechanism particular to the assembly of spider egg-case silk fibers. These cysteines may participate in intra- or inter-molecular disulfide bridges, much like the conserved cysteines in lepidopteran heavy-chain fibroin [18,58].
Phylogenetic utility of spidroin N-termini
It is generally not controversial to combine sequence data from different regions of the same gene or protein for phylogenetic reconstruction. However, a number of features specific to spidroin genes suggest that their evolutionary dynamics may create phylogenetic conflict between the two termini. Recent work has demonstrated that there are multiple genomic copies of the MaSp1 dragline silk gene [8,21,34]. Detailed evolutionary analyses of Latrodectus MaSp1 and MaSp2 genes showed their encoded N-and C-termini do not form mutually exclusive clades, despite having markedly different repetitive region sequences (e.g., GPGXn motifs are abundant in MaSp2 but absent in MaSp1; [8]). Relationships among the N-termini of these sequences also conflicted with those from their C-termini, which was attributed to intergenic recombination between different MaSp1 copies and also between MaSp1 and MaSp2 [8]. Such recombination would introduce conflict in combined phylogenetic analyses. Ayoub and Hayashi [8] hypothesized that an alternative explanation for the unexpected groupings of MaSp1 and MaSp2 termini could be convergent evolution within a genome to facilitate co-expression and/or co-assembly in major ampullate glands. Convergent evolution of N- and/or C-termini would obscure their phylogenetic relationships in either separate or combined analyses.
Regardless of the potential for recombination and convergence, we primarily observe congruence among the well-supported nodes in trees separately constructed from N- and C-termini and a partition homogeneity test did not find strong evidence of character conflict between the two datasets. While there were significant differences in the likelihood scores of topologies produced by the N- and C-termini, neither N- nor C-terminal topologies were significantly different from the combined topologies. Our analyses did show repeated grouping of araneoid MaSp1 with MaSp2 sequences, but these relationships are mirrored in both N- and C-terminal trees where they were strongly supported. Some disagreement was found between N- and C-termini within the TuSp1 clade, suggesting the need for additional sequences to determine if this conflict persists or is a sampling artifact. Despite our best efforts to link N- and C-termini from the same protein, there is the possibility that in some cases the concatenated termini do not represent two ends of the same molecule, but instead are from different paralogs, introducing another potential source of phylogenetic conflict.
The combination of N- and C-termini produced improved phylogenies over separate analyses, based on the criteria of containing more strongly supported branches, increased branch support values, and being more robust to different methods of phylogenetic inference (i.e., the parsimony tree and Bayesian consensus were highly congruent). Combination of the data also revealed a much higher level of hidden branch support relative to hidden conflict [53], consistent with an overall increase in phylogenetic signal. However, the contribution of the two termini to combined analyses was imbalanced, as indicated by the partitioned branch support and hidden partitioned branch support values that showed N-termini provided greater support than C-termini. This result suggests that N-termini are more informative than C-termini for understanding spidroin relationships, perhaps because of their greater length and sequence conservation. Future work will focus on characterizing the presently unknown N-terminal domains of spidroins composing prey-wrapping silk (AcSp1; [59]) and cementing silk (PySp1 [60]) to further clarify spider silk diversification.
Evolution of spider silks
Although all spiders make silk, perhaps the greatest complexity of silk production is displayed by araneoid orb-weavers, which possess seven distinct gland types that manufacture different silks with diverse functional applications. Molecular characterizations of araneoid silks has established that six of these glands (major ampullate, minor ampullate, flagelliform, aciniform, tubuliform and pyriform glands) each express unique combinations of spidroin paralogs [9,19,29,59-61]. These paralogs encode proteins with varying proportions of structural motifs that underlie the signature mechanical properties of each fiber type. Outside of araneoids, there is tremendous variation in the number and types of silk glands, as well as the set of spidroin paralogs found across species. The evolution of this striking diversity can be investigated by jointly considering: 1) the phylogenetic relationships of spiders; 2) the distribution of silk glands among these lineages; and 3) the relationships of spidroins expressed by these glands and their sequence features.
In contrast to araneoids (part of the suborder Araneomorphae), spiders in the suborder Mygalomorphae (tarantulas and their kin) possess many primitive features of silk production, including homogeneous, acinous-shaped silk glands and uniform fiber types [62]. Consistent with their lesser glandular diversification, mygalomorphs also express fewer spidroin paralogs than araneomorphs and these paralogs are also relatively similar in sequence [7]. The glandular affiliation hypothesis of Hayashi and Lewis [19] proposed that spidroins evolved in association with the glands where they are primarily expressed, predicting a phylogenetic correlation between araneomorph gland type and spidroin paralogs. Our combined N- and C-terminal spidroin amino acid trees generally support this expectation: TuSp1, Flag and MiSp, expressed in tubuliform, flagelliform and minor ampullate silk glands, respectively, each form mutually exclusive clades. However, spidroins characterized from the major ampullate glands of the haplogyne species Kukulkania (K.h. MaSp1) and Diguetia (D.c. MaSp and D.c. MaSp-like) did not group with MaSp sequences from Entelegynae species. While all araneomorph spiders possess major ampullate glands, tubuliform glands are restricted to the Entelegynae clade, and flagelliform (and homologous pseudoflagelliform) glands subsequently evolved in the common ancestor of Araneoidea and Deinopoidea. Accordingly, tubuliform and flagelliform glands (and their expressed spidroins) may have originated as duplicates of major ampullate glands. There has been apparent duplication and loss of major ampullate glands among araneomorph spider lineages [63], such that the major ampullate glands of Haplogynae and Entelegynae spiders also may not be strictly homologous (identity of structures through inheritance from a common ancestor). Instead, some major ampullate glands may be serially homologous to each other (similarity of structures due to common developmental mechanisms). The non-monophyly of MaSp sequences may also reflect our greater sampling of major ampullate gland cDNAs from a wider range of species in distantly related spider families, as compared to our more limited sampling of other silk gland types in non-orbicularian species. Thus the grouping of TuSp1, Flag and MiSp into monophyletic clades could break down with further taxonomic sampling, suggesting that future work should substantially increase sampling of silk sequence types from a more diverse and numerous set of spider taxa.
The disjunct relationships of major ampullate spidroin termini are not entirely surprising given the diversity of their repetitive sequences. Major ampullate spidroins from orbicularian (araneoid + deinopoid) species, are largely characterized by iterations of An in combination with either tandem arrayed GGX in MaSp1 or GPGXn in MaSp2. The repetitive sequence of major ampullate spidroins from the haplogyne Kukulkania [55] and Diguetia (this study) are distinct from each other and from orbicularian MaSps (see Additional file 6). For instance, D.c. MaSps, characterized from the ampullate shaped glands of Diguetia contain An, but are unusual in also containing strings of glutamine (Qn). K.h. MaSp1, described from the major ampullate glands of Kukulkania [53], contains many iterations of (GA)n and (GS)n, and much less An than in orbicularian MaSps and no Qn like the D.c. MaSps. Swanson et al. [2] found that major ampullate silk fibers from divergent spider species exhibit substantial variability in their mechanical properties, which may correlate with the phylogenetic distribution of structural motifs we observe in MaSp repetitive sequences (Figure 5). Our results indicate that the structural module An, primarily associated with the high tensile strengths of major ampullate silk, was present in ancestral spidroins but was subsequently lost in some paralogs or expanded in others.
The relatively close relationship between the terminal domains of the egg-case silk protein TuSp1 and the orb-web capture spiral silk protein Flag is especially surprising given their dissimilar functions and repetitive sequence properties. A correlation between spidroin phylogeny and silk ecological use might predict a close relationship between Flag and other silk proteins used in orb-webs, such as the temporary scaffolding protein MiSp. Flag may alternatively be expected to share recent ancestry with orbicularian MaSp2 proteins, because both contain numerous iterations of the proline containing GPGXn structural module that forms elastic nano-springs [15]. Our phylogenetic hypothesis instead suggests more radical shifts in silk use subsequent to spidroin gene duplication, as well as convergent evolution of GPGXn modules that impart fibers with greater extensibility.
Conclusions
The presence of a similar, non-repetitive N-terminal domain in spidroin proteins across divergent spider lineages supports its participation in a general mechanism of spider silk production. Sequence conservation of these N-termini makes them an unequalled resource for reconstructing spidroin phylogeny. The improved understanding of spidroin relationships we provide using both N- and C-terminal domains shows that there is considerable evolutionary flexibility throughout the spider silk system, from the level of gene sequence motif to paralog number and silk gland expression pattern. This dynamic, labile nature of silk evolution is in stark contrast to the incredible homogeneity of repeats within some spidroins (e.g., 100% identity in consecutive 1026 bp repeats; [7]) and the consistently high-performing mechanical properties of silk fibers (e.g., dragline silks, [2]). Given the elevated rate of sequence rearrangement and turnover in the repetitive region, the spidroin N- and C-terminal domains are not only important for the biochemistry of silk fiber production, but also serve as signposts for retracing the history of the ancient and functionally diverse spider silks.
Authors' contributions
JEG and CYH conceived this study. JEG and NAA collected the data. JEG and CYH analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Supplementary Material
N-terminal alignment, top line shows residues in 50% or more sequences, boxed in region indicates most probable signal peptide region as predicted in SignalP. Sequence names abbreviated as in Table 1. Missing data indicated by X and alignment gaps by dashes.
Superimposed Kyte-Doolittle plots for N-terminal alignment indicating hydropathy. X-axis indicates residue position along alignment, Y-axis shows hydropathy score, where values above 0 indicate hydrophobicity and values below zero indicates hydrophilicity. Each line represents a different sequence. Breaks within lines correspond to gapped regions in sequence alignment.
Secondary structure predictions for representative spidroin N-terminal sequences. A-G. Distribution of three predicted structures 1: Alpha-helices (long, blue lines), 2. Extended strand (medium height, red lines) and 3. random coils (short, purple lines) predicted with GOR IV in varied spidroins, sequence names abbreviated as in Table 1; A: B.c. fibroin1, B: K.h. MaSp1, C: A.ap. TuSp1, D: D.c. MaSp, E: L.h. MiSp, F: D.s. MaSp2, G: N.i. Flag. Sequences from first residue following predicted signal peptide. H. Table showing percentage of three structures in each spidroin.
Spidroin terminal phylogenies based on nucleotides encoding protein in Additional file 1.; A. N-terminal parsimony tree, B. N-terminal Bayesian consensus tree; C: C-terminal parsimony strict consensus tree; D. C-terminal Bayesian tree; A, C Numbers above nodes are bootstrap values, numbers below nodes are decay indices; B, D numbers above nodes are clade posterior probability values.
Combined spidroin N+C terminal nucleotide analyses. A. 1 MPT; Above node, bootstrap support, below node, branch support (decay index). B. 50% majority-rule consensus of post-burnin Bayesian trees from combined N+C nucleotides, numbers indicate PP values.
Exemplar repeats used in motif coding analyses. Each exemplar represents a repeat taken from the complete sequence (e.g., N.c. MaSp1a), minor variants of these repeats are tandem iterated throughout the complete sequence.
Presence or absence of structural motifs in spidroin exemplar repeats. 0 = absent, 1 = present.
Contributor Information
Jessica E Garb, Email: Jessica_Garb@uml.edu.
Nadia A Ayoub, Email: ayoubn@wlu.edu.
Cheryl Y Hayashi, Email: cheryl.hayashi@ucr.edu.
Acknowledgements
We thank Oguchi Azubuike, Teresa DiMauro, Marshal Hedin, Chuck Kristensen, Nam Nguyen, Jim Starrett, Mark Stowe, Brook Swanson, and Victoria Vo for their help in providing spiders or collecting data. We thank Marie Heberstein for helpful comments and Alex Lancaster for computational assistance and resources. This work was funded by NSF grant DEB0236020 and U.S Army Research Office grants DAAD190210358 and W911NF0610455. Financial support was also provided by the University of Massachusetts Lowell.
References
- Blackledge TA, Hayashi CY. Silken toolkits: biomechanics of silk fibers spun by the orb web spider Argiope argentata (Fabricius 1775) J Exp Biol. 2006;209:2452–2461. doi: 10.1242/jeb.02275. [DOI] [PubMed] [Google Scholar]
- Swanson BO, Blackledge TA, Summers AP, Hayashi CY. Spider dragline silk: correlated and mosaic evolution in high-performance biological materials. Evolution. 2006;60:2539–2551. [PubMed] [Google Scholar]
- Gosline JM, Guerette PA, Ortlepp CS, Savage KN. The mechanical design of spider silks: from fibroin sequence to mechanical function. J Exp Biol. 1999;202:3295–3303. doi: 10.1242/jeb.202.23.3295. [DOI] [PubMed] [Google Scholar]
- Hayashi CY, Shipley NH. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. Int J Biol Macromol. 1999;24:271–275. doi: 10.1016/S0141-8130(98)00089-0. [DOI] [PubMed] [Google Scholar]
- Hinman MB, Lewis RV. Isolation of a clone encoding a second dragline silk fibroin. Nephila clavipes dragline silk is a two-protein fiber. J Biol Chem. 1992;267:19320–19324. [PubMed] [Google Scholar]
- Gatesy J, Hayashi C, Motriuk D, Woods J, Lewis R. Extreme diversity, conservation, and convergence of spider silk fibroin sequences. Science. 2001;291:2603–2605. doi: 10.1126/science.1057561. [DOI] [PubMed] [Google Scholar]
- Garb JE, DiMauro T, Lewis RV, Hayashi CY. Expansion and intragenic homogenization of spider silk genes since the Triassic: evidence from Mygalomorphae (tarantulas and their kin) spidroins. Mol Biol Evol. 2007;24:2454–2464. doi: 10.1093/molbev/msm179. [DOI] [PubMed] [Google Scholar]
- Ayoub NA, Hayashi CY. Multiple recombining loci encode MaSp1, the primary constituent of dragline silk, in widow spiders (Latrodectus: Theridiidae) Mol Biol Evol. 2008;25:277–286. doi: 10.1093/molbev/msm246. [DOI] [PubMed] [Google Scholar]
- Xu M, Lewis RV. Structure of a protein superfiber: spider dragline silk. Proc Natl Acad Sci. 1990;87:7120–7124. doi: 10.1073/pnas.87.18.7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi CY, Lewis RV. Molecular architecture and evolution of a modular spider silk protein gene. Science. 2000;287:1477–1479. doi: 10.1126/science.287.5457.1477. [DOI] [PubMed] [Google Scholar]
- Zhao A-C', Zhao T-F, Nakagaki K, Zhang Y-S, SiMa Y-H', Miao Y-G', Shiomi K, Kajiura Z, Nagata Y, Takadera M, Nakagaki M. Novel molecular and mechanical properties of egg case silk from wasp spider, Argiope bruennichi. Biochemistry. 2006;45:3348–3356. doi: 10.1021/bi052414g. [DOI] [PubMed] [Google Scholar]
- Ayoub NA, Garb JE, Tinghitella RM, Collin MA, Hayashi CY. Blueprint for a high-performance biomaterial: full-length spider dragline silk genes. PLoS ONE. 2007;2(6):e514. doi: 10.1371/journal.pone.0000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AH, Michal CA, Jelinski LW. Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science. 1996;271:84–87. doi: 10.1126/science.271.5245.84. [DOI] [PubMed] [Google Scholar]
- van Beek JD, Hess S, Vollrath F, Meier BH. The molecular structure of spider dragline silk: folding and orientation of the protein backbone. Proc Natl Acad Sci. 2002;99:10266–10271. doi: 10.1073/pnas.152162299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N, Oroudjev E, Mutz S, Cleveland JP, Hansma PK, Hayashi CY, Makarov DE, Hansma HG. Molecular nanosprings in spider capture-silk threads. Nat Mater. 2003;2:278–283. doi: 10.1038/nmat858. [DOI] [PubMed] [Google Scholar]
- Beckwitt R, Arcidiacono S. Sequence conservation in the C-terminal region of spider silk proteins (Spidroin) from Nephila clavipes (Tetragnathidae) and Araneus bicentenarius (Araneidae) J Biol Chem. 1994;269:6661–6663. [PubMed] [Google Scholar]
- Sponner A, Unger E, Grosse F, Weisshart K. Conserved C-termini of spidroins are secreted by the major ampullate glands and retained in the silk thread. Biomacromolecules. 2004;5:840–845. doi: 10.1021/bm034378b. [DOI] [PubMed] [Google Scholar]
- Hu X', Kohler K, Falick AM, Moore AMF, Jones PR, Vierra C. Spider egg case core fibers: trimeric complexes assembled from TuSp1, ECP-1, and ECP-2. Biochemistry. 2006;45:3506–3516. doi: 10.1021/bi052105q. [DOI] [PubMed] [Google Scholar]
- Hayashi CY, Lewis RV. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J Mol Biol. 1998;275:773–784. doi: 10.1006/jmbi.1997.1478. [DOI] [PubMed] [Google Scholar]
- Motriuk-Smith D, Smith A, Hayashi CY, Lewis RV. Analysis of the conserved Nterminal domains in major ampullate spider silk proteins. Biomacromolecules. 2005;6:3152–3159. doi: 10.1021/bm050472b. [DOI] [PubMed] [Google Scholar]
- Rising A, Hjälm G, Engström W, Johansson J. N-terminal nonrepetitive domain common to dragline, flagelliform, and cylindriform spider silk proteins. Biomacromolecules. 2006;7:3120–3124. doi: 10.1021/bm060693x. [DOI] [PubMed] [Google Scholar]
- Huemmerich D, Helsen CW, Quedzuweit S, Oschmann J, Rudolph R, Scheibel T. Primary structure elements of spider dragline silks and their contribution to protein solubility. Biochemistry. 2004;43:13604–13612. doi: 10.1021/bi048983q. [DOI] [PubMed] [Google Scholar]
- Sponner A, Vater W, Rommerskirch W, Vollrath F, Unger E, Grosse F, Weisshart K. The conserved C-termini contribute to the properties of spider silk fibroins. Biochem Biophys Res Commun. 2005;338:897–902. doi: 10.1016/j.bbrc.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Ittah S, Cohen S, Garty S, Cohn D, Gat U. An essential role for the C-terminal domain of a dragline spider silk protein in directing fiber formation. Biomacromolecules. 2006;7:1790–1795. doi: 10.1021/bm060120k. [DOI] [PubMed] [Google Scholar]
- Ittah S, Michaeli A, Goldblum A, Gat U. A model for the structure of the C-terminal domain of dragline spider silk and the role of its conserved cysteine. Biomacromolecules. 2007;8:2768–2773. doi: 10.1021/bm7004559. [DOI] [PubMed] [Google Scholar]
- Hedhammar M, Rising A, Grip S, Saenz Martinez A, Nordling K, Casals C, Stark M, Johansson J. Structural properties of recombinant nonrepetitive and repetitive parts of major ampullate spidroin 1 from Euprosthenops australis: implications for fiber formation. Biochemistry. 2008;47:3407–3417. doi: 10.1021/bi702432y. [DOI] [PubMed] [Google Scholar]
- Lin Z, Huang W, Zhang J, Fan J.-S, Yang D. Solution structure of eggcase silk protein and its implications for silk fiber formation. Proc Natl Acad Sci. 2009;106:8906–8911. doi: 10.1073/pnas.0813255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askarieh G, Hedhammar M, Nordling K, Saenz A, Casals C, Rising A, Johansson J, Knight SD. Self-assembly of spider silk proteins is controlled by a pH-sensitive relay. Nature. 2010;465:236–239. doi: 10.1038/nature08962. [DOI] [PubMed] [Google Scholar]
- Garb JE, Hayashi CY. Modular evolution of egg case silk genes across orbweaving spider superfamilies. Proc Natl Acad Sci. 2005;102:11379–11384. doi: 10.1073/pnas.0502473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi CY. In: Molecular Systematics and Evolution: Theory and Practice. DeSalle R, Giribet G, Wheeler W, editor. Birkhauser, Berlin; 2002. Evolution of spider silk proteins: insights from phylogenetic analyses; pp. 209–224. [Google Scholar]
- Phillips AJ. Homology assessment and molecular sequence alignment. J Biomed Inform. 2006;39:18–33. doi: 10.1016/j.jbi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Garb JE, DiMauro T, Vo V, Hayashi CY. Silk genes support the single origin of orb-webs. Science. 2006;312:1762. doi: 10.1126/science.1127946. [DOI] [PubMed] [Google Scholar]
- Ayoub NA, Hayashi CY. In: The Timetree of Life. Hedges SB, Kumar S, editor. Oxford University Press; 2009. Spiders (Araneae) pp. 255–259. [Google Scholar]
- Gaines WA IV, Marcotte WR Jr. Identification and characterization of multiple Spidroin 1 genes encoding major ampullate silk proteins in Nephila clavipes. Insect Mol Biol. 2008;17:465–474. doi: 10.1111/j.1365-2583.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuken E, Vink C, Bruggeman CA. One-step procedure for screening recombinant plasmids by size. Biotechniques. 1998;24:748–750. doi: 10.2144/98245bm10. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–32. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Garnier J, Osguthorpe DJ, Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978;120:97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Swofford D PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4 2006Sunderland, MA.: Sinauer Associates; 16924060 [Google Scholar]
- Sorenson MD, Franzosa EA. TreeRot, Version 3. Boston University, Boston, MA; 2007. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21:2104–2105. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Coddington JA, Giribet G, Harvey MS, Prendini L, Walter DE. In: Assembling the tree of life. Cracraft J, Donoghue M, editor. New York: Oxford University Press; 2004. Arachnida; pp. 296–318. [Google Scholar]
- Kuntner M, Coddington JA, Hormiga G. Phylogeny of extant nephilid orb-weaving spiders (Araneae, Nephilidae): testing morphological and ethological homologies. Cladistics. 2008;24:147–217. doi: 10.1111/j.1096-0031.2007.00176.x. [DOI] [Google Scholar]
- Scharff N, Coddington JA. A phylogenetic analysis of the orb-weaving spider family Araneidae (Arachnida, Araneae) Zool J Linn Soc. 1997;120:355–434. doi: 10.1111/j.1096-3642.1997.tb01281.x. [DOI] [Google Scholar]
- Elices M, Plaza GR, Arnedo MA, Prez-Rigueiro J, Torres FG, Guinea GV. Mechanical behavior of silk during the evolution of orb-web spinning spiders. Biomacromolecules. 2009;10:1904–1910. doi: 10.1021/bm900312c. [DOI] [PubMed] [Google Scholar]
- Vernot B, Stolzer M, Goldman A, Durand D. Reconciliation with non-binary species trees. J Comp Biol. 2008;15:981–1006. doi: 10.1089/cmb.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens JJ. Combining data sets with different phylogenetic histories. Syst Biol. 1998;47:568–581. doi: 10.1080/106351598260581. [DOI] [PubMed] [Google Scholar]
- Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol. 1999;16:1114–1116. [Google Scholar]
- Schmidt HA, Strimmer K, Vingron M, von Haeseler A. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics. 2002;18:502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- Baker RH, DeSalle R. Multiple sources of character information and the phylogeny of Hawaiian drosophilids. Syst Biol. 1997;46:654–673. doi: 10.1093/sysbio/46.4.654. [DOI] [PubMed] [Google Scholar]
- Gatesy JE, O'Grady P, Baker RH. Corroboration among data sets in simultaneous analysis: hidden support for phylogenetic relationships among higher level artiodactyl taxa. Cladistics. 1999;15:271–313. doi: 10.1111/j.1096-0031.1999.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Maddison DR, Maddison WP. MacClade 4: analysis of phylogeny and character evolution. Version 4.08. Sunderland, MA.: Sinauer Associates; 2005. [Google Scholar]
- Tian MZ, Liu CZ, Lewis RV. Analysis of major ampullate silk cDNAs from two non-orb-weaving spiders. Biomacromolecules. 2004;5:657–660. doi: 10.1021/bm034391w. [DOI] [PubMed] [Google Scholar]
- Selden PA, Gall JC. A Triassic mygalomorph spider from the northern Vosges, France. Palaeontology. 1992;35:211–235. [Google Scholar]
- La Mattina C, Reza R, Hu X, Falick AM, Vasanthavada K, McNary S, Yee R, Vierra CA. Spider minor ampullate silk proteins are constituents of prey wrapping silk in the cob weaver Latrodectus hesperus. Biochemistry. 2008;47:4692–4700. doi: 10.1021/bi800140q. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kajiyama N, Ishikura K, Waga S, Kukuchi A, Ohtomo K, Takagi T, Mizuno S. Determination of the site of disulfide linkage between heavy and light chains of silk fibroin produced by Bombyx mori. Biochim. Biophys. Acta. 1999;1432:92–103. doi: 10.1016/s0167-4838(99)00088-6. [DOI] [PubMed] [Google Scholar]
- Hayashi CY, Blackledge TA, Lewis RV. Molecular and mechanical characterization of aciniform silk: uniformity of iterated sequence modules in a novel member of the spider silk fibroin gene family. Mol Biol Evol. 2004;21:1950–1959. doi: 10.1093/molbev/msh204. [DOI] [PubMed] [Google Scholar]
- Blasingame E, Tuton-Blasingame T, Larkin L, Falick AM, Zhao L, Fong J, Vaidyanathan V, Visperas A, Geurts P, Hu X, La Mattina C, Vierra CA. Pyriform spidroin 1, a novel member of the silk gene family that anchors dragline silk fibers in attachment discs of the black widow spider, Latrodectus hesperus. J Biol Chem. 2009;284:29097–29108. doi: 10.1074/jbc.M109.021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin MA, Lewis RV. Spider minor ampullate silk proteins contain new repetitive sequences and highly conserved non-silk-like "spacer regions". Protein Sci. 1998;7:667–672. doi: 10.1002/pro.5560070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JW. The origin of the spinning apparatus in spiders. Biol Rev. 1987;62:89–113. [Google Scholar]
- Griswold CE, Coddington JA, Platnick NI, Forster RR. Towards a phylogeny of entelegyne spiders (Araneae, Araneomorphae, Entelegynae) J Arachnol. 1999;27:53–63. [Google Scholar]
- Zhao A, Zhao T, Sima Y, Zhang Y, Nakagaki K, Miao Y, Shiomi K, Kajiura Z, Nagata Y, Nakagaki M. Unique molecular architecture of egg case silk protein in a spider, Nephila clavata. J Biochem. 2005;138:593–604. doi: 10.1093/jb/mvi155. [DOI] [PubMed] [Google Scholar]
- Stark M, Grip S, Rising A, Hedhammar M, Engström W, Hjälm G, Johansson J. Macroscopic fibers self-assembled from recombinant miniature spider silk proteins. Biomacromolecules. 2007;8:1695–1701. doi: 10.1021/bm070049y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
N-terminal alignment, top line shows residues in 50% or more sequences, boxed in region indicates most probable signal peptide region as predicted in SignalP. Sequence names abbreviated as in Table 1. Missing data indicated by X and alignment gaps by dashes.
Superimposed Kyte-Doolittle plots for N-terminal alignment indicating hydropathy. X-axis indicates residue position along alignment, Y-axis shows hydropathy score, where values above 0 indicate hydrophobicity and values below zero indicates hydrophilicity. Each line represents a different sequence. Breaks within lines correspond to gapped regions in sequence alignment.
Secondary structure predictions for representative spidroin N-terminal sequences. A-G. Distribution of three predicted structures 1: Alpha-helices (long, blue lines), 2. Extended strand (medium height, red lines) and 3. random coils (short, purple lines) predicted with GOR IV in varied spidroins, sequence names abbreviated as in Table 1; A: B.c. fibroin1, B: K.h. MaSp1, C: A.ap. TuSp1, D: D.c. MaSp, E: L.h. MiSp, F: D.s. MaSp2, G: N.i. Flag. Sequences from first residue following predicted signal peptide. H. Table showing percentage of three structures in each spidroin.
Spidroin terminal phylogenies based on nucleotides encoding protein in Additional file 1.; A. N-terminal parsimony tree, B. N-terminal Bayesian consensus tree; C: C-terminal parsimony strict consensus tree; D. C-terminal Bayesian tree; A, C Numbers above nodes are bootstrap values, numbers below nodes are decay indices; B, D numbers above nodes are clade posterior probability values.
Combined spidroin N+C terminal nucleotide analyses. A. 1 MPT; Above node, bootstrap support, below node, branch support (decay index). B. 50% majority-rule consensus of post-burnin Bayesian trees from combined N+C nucleotides, numbers indicate PP values.
Exemplar repeats used in motif coding analyses. Each exemplar represents a repeat taken from the complete sequence (e.g., N.c. MaSp1a), minor variants of these repeats are tandem iterated throughout the complete sequence.
Presence or absence of structural motifs in spidroin exemplar repeats. 0 = absent, 1 = present.