Abstract
Background/Aims
Diagnostic pars plana vitrectomy is a useful technique in the diagnosis of intraocular lymphoma (IOL); however, the role of transconjunctival sutureless vitrectomy (TSV) has not been fully explored for this indication. The purpose of this study was to review our experience with 25-gauge TSV for the diagnosis of IOL.
Methods
Patients who underwent 25-gauge TSV for the diagnosis of IOL (primary, secondary or recurrent) from two tertiary referral centres were reviewed. Demographic data and underlying medical conditions were reviewed. Preoperative and postoperative visual acuities (VA) and ophthalmic examination data were assessed. Cytopathology, flow cytometry, cytokine and gene rearrangement studies were assessed.
Results
Twelve patients underwent 25-gauge diagnostic TSV with a median follow-up time of 37 weeks. B-cell or T-cell IOL was diagnosed based on cytology in 3/12 patients (25%, 95% CI 8.9 to 53.2%) and in eight patients (67%, 95% CI 39.1 to 86.1%) using adjunctive diagnostic testing. VA stabilised or improved in 11 eyes (92%). Mean VA improved from 20/95 to 20/66 (p=0.055, paired t test).
Conclusions
25-Gauge TSV is safe and effective for obtaining vitreous specimens for the evaluation of IOL. The availability of expert ophthalmic pathological consultation, flow cytometry, cytokine evaluation and gene rearrangement studies were essential to the diagnosis.
Introduction
Diagnostic pars plana vitrectomy (PPV) is the primary method of obtaining intraocular fluid for the evaluation of lymphoma.1–5 However, other reported methods to obtain ocular fluid for the diagnosis of lymphoma have included anterior chamber paracentesis, fine needle aspiration biopsies of the vitreous, and chorioretinal biopsies.6 7 Recently, the advent of 25-gauge transconjunctival sutureless vitrectomy (TSV) has improved surgical efficiency, with improved patient comfort, decreased operative times and possibly decreased postoperative inflammation.8 9
Two in vitro studies investigating 25-gauge versus 20-gauge vitrectomy for obtaining vitreous specimens demonstrated adequate cellularity and flow cytometric staining using either gauge.10 11 However, 25-gauge TSV has not been specifically assessed in patients for the highly specialised testing that is required for the diagnosis of T-cell and B-cell intraocular lymphoma (IOL). We reviewed our experience with 25-gauge diagnostic TSV for the diagnosis of intraocular B-cell and T-cell IOL with the currently available diagnostic vitreous testing.
Methods
This study was a non-comparative, retrospective, consecutive case series of patients who underwent 25-gauge diagnostic vitrectomy for suspected IOL. The medical records of patients were reviewed for demographic data (age, sex), risk factors for lymphoma (eg, HIV status, human T cell lymphotrophic virus-1 infection). Ophthalmic data collected included preoperative and postoperative visual acuity, postoperative intraocular pressure and ophthalmic examination findings, including postoperative complications (ie, endophthalmitis, retinal detachment, glaucoma or hypotony with intraocular pressure <5 mmHg).
Clinical evaluation
Patients were evaluated preoperatively, on postoperative day 1, at postoperative week 1, and as indicated by the clinician thereafter. Vitreous specimens were sent for cytopathological evaluation, immunohistochemistry, flow cytometry, cytokine evaluation (ie, interleukin (IL)-10, IL-6, IL-2), and gene rearrangement studies (ie, IgH gene rearrangement for B-cell lymphoma, T-cell receptor gene rearrangement for T-cell lymphoma). Most patients also had MRI performed and a lumbar puncture to evaluate for brain and cerebrospinal fluid involvement, respectively.
Surgical technique
Consultative services (ie, flow cytometry laboratory, cytopathology) were contacted prior to surgery. During surgery, a 25-gauge infusion cannula was secured 4.0 mm (phakic) or 3.5 mm (pseudophakic) posterior to the limbus. After introduction of 25-gauge trocars superotemporally and superonasally using an oblique entry with conjunctival displacement, an undiluted vitreous specimen (specimen 1) was obtained with the infusion on air to maintain intraocular pressure. Approximately 1.5–2.0 ml of vitreous could be aspirated into a syringe using this technique. Next, the infusion was turned to fluid, residual air was removed, and additional vitrectomy was performed with the vitreous cutter for an additional 3.0–4.0 ml of diluted vitreous specimen (specimen 2) for flow cytometry evaluation. A complete core and peripheral vitrectomy were subsequently performed. The vitreous cassette (specimen 3) was then sent to cytology or microbiology as needed for appropriate testing. The cannulae were removed and the conjunctiva was reposited over each sclerotomy site; subconjuctival antibiotics and dexamethasone were then administered. In four patients, an air–fluid exchange was performed prior to removal of the 25-gauge cannulae at the surgeon's discretion.
Diagnostic testing
Table 1 outlines the diagnostic testing performed on each specimen. Following diagnostic TSV, the vitreous specimens were delivered within 15–30 min to the appropriate laboratory facilities. No special transport mediums (ie, RPMI) were used in the transport of vitreous fluid.
Table 1.
Specimen collection and vitreous testing
| Specimen | Transport media/ | Vitreous fluid | Infusion line | Volume typically collected (ml) | Diagnostic testing |
|---|---|---|---|---|---|
| 1 | 3 ml syringe (RPMI or direct transfer) | Undiluted | Air | 1.5–2.0 | Cytopathology, immunohistochemistry, gene rearrangement studies with microdissection/PCR, cytokine evaluation |
| 2 | 3 ml syringe (no special media for flow cytometry) | Minimally diluted | Fluid | 3.0–4.0 | Flow cytometry, microbiology or PCR testing if flow cytometry unavailable or unnecessary |
| 3 | Vitreous cassette | Diluted | Fluid | >50 | Microbiology |
Specimens should be delivered immediately to cytopathology and flow cytometry laboratories.
Specimens sent for PCR should be delivered either immediately or on ice to the microbiology/virology laboratory (depending on preference of processing laboratory).
Cytopathological evaluation was performed on the undiluted vitreous specimen (specimen 1) to evaluate features consistent for lymphoma or reactive lymphocytosis. The slides were prepared as previously described2 12 and reviewed by an experienced ophthalmic or cytopathologist.1 Immunohistochemical evaluation was also performed for cell surface markers indicative of monoclonal or polyclonal lymphoid populations and macrophages.12 13 Gene rearrangement studies were performed and tailored to the specific subtype of lymphoma according to previously established protocols.2 14 15 The supernatant fraction from the centrifuged undiluted vitreous specimen was transferred to the immunopathology laboratory for cytokine diagnostics in six patients.16–19
Flow cytometry was performed on the diluted vitreous specimen (specimen 2) with antibodies detecting cell surface markers for T- and B-cells, κ and λ light-chain markers, and macrophages. When a specific neoplastic condition was suspected (eg, HTLV-1-associated adult T-cell leukaemia/lymphoma, recurrent CNS B-cell lymphoma), the panel of surface markers was determined according to the standard protocols recommended by the flow cytometry laboratory of each institution.3 4 20 For patients with clinical features suspicious for B-cell lymphoma, cell surface markers included CD19, CD20, CD22, CD45, CD3, CD4, CD8, and κ and λ light chain. For patients with possible T-cell lymphoma, cell surface markers included CD3, CD8, CD4, CD7, CD2, CD25, and CD52.
Microbiology laboratory personnel performed testing for infectious aetiologies of uveitis using the vitreous cassette (specimen 3). Tests included PCR for Herpes simplex virus, Varicella zoster virus, cytomegalovirus, Epstein–Barr virus and toxoplasmosis. Gram stain, bacterial, fungal and mycobacterial cultures were performed when appropriate.
The final diagnosis for each patient was determined by a combination of the clinical history, ophthalmic evaluation, diagnostic testing results and response to treatment during the follow-up period. Consultation with oncology, neuro-oncology and haematology services was obtained when necessary for patient management.
Results
Twelve eyes of 12 patients with suspected IOL were evaluated with the 25-gauge diagnostic TSV. The mean age was 57 (range 24–79) years. The median follow-up time following diagnostic TSV was 37 (range 10–54) weeks. Individual patient data are summarised in table 2. Visual acuity remained stable (±1 line) or improved (≥ 2 lines) in 11 eyes (92%, 95% CI 64.6 to 98.5%), with six eyes (50%, 95% CI 25 to 50%) improving by two or more lines following diagnostic TSV. The mean preoperative logarithm of the minimum angle of resolution (logMAR) visual acuity (VA)±SD was 0.68±0.89 (Snellen VA 20/95). The mean postoperative logMAR VA was 0.52±0.85 (Snellen VA 20/66, p=0.055, paired t test).
Table 2.
Clinical and laboratory features of patients who underwent 25-gauge diagnostic TSV
| Patient no./age/sex | Medical co-morbidities | Preoperative findings | Preoperative VA | Final VA | Total F/U (weeks) | AFX | Post-operative findings/complications | Vitreous testing for T- or B-cell IOL | MRI brain | Lumbar puncture | Final Dx |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/64/M | Systemic B-cell lymphoma | Vitritis, RPE change | 20/20 | 20/40 | 54 | No | Cataract | CYTO, FC§, CYK§, IGH§ | Normal | No cytological evidence of lymphoma | B-cell IOL |
| 2/24/M | HTLV-1, CNS ATL | Vitritis, retinal vasculitis | 20/80 | 20/63 | 50 | No | Transient hypotony | CYTO, FC | Normal | – | HTLV-associated IU |
| 3/54/F | HTLV-1, CNS ATL | Vitritis, retinal vasculitis | 20/125 | 20/25 | 38 | No | None | CYTO§, FC§, TCR§, CYK§ | Normal | T-cell leukaemia/lymphoma | T-cell IOL |
| 4/65/F | Systemic T-cell lymphoma | Vitritis | 20/25 | 20/25 | 19 | No | Transient hypotony | CYTO, IHC, FC, TCR, CYK | Normal | No cytological evidence of lymphoma | Idiopathic IU |
| 5/72/F | Healthy | Vitritis, RPE changes | 20/40 | 20/20 | 36 | No | Cataract | CYTO, IHC, FC§, CYK, IGH | Small vessel ischaemic changes | No cytological evidence of lymphoma | Idiopathic IU* |
| 6/27/F | HIV, tuberculosis | Vitritis | 20/40 | 20/20 | 43 | No | Transient hypotony | CYTO, FC, CYK | Right frontal lobe lesion | No cytological evidence of lymphoma | Tuberculosis-associated IU |
| 7/79/F | CLL | Vitritis, Prior CRVO | HM | CF | 44 | No | Transient hypotony | CYTO, FC, IGH§ | Small vessel ischaemic changes | – | B-cell IOL |
| 8/70/F | HTLV-1, ATL | Vitritis, retinal vasculitis | LP | CF | 10 | Yes | CMV retinitis, NVG | CYTO, FC§, TCR§ | Normal | – | T-cell IOL |
| 9/52/M | Intra-abdominal mass near right renal pelvis | Vitritis, RPE changes | 20/25 | 20/25 | 25 | No | None | CYTO§, FC, CYK§, IGH§ | Normal | No cytological evidence of lymphoma | B-cell IOL |
| 10/65/M | Left frontal lobe mass, unclear aetiology | Vitritis, RPE changes | 20/20 | 20/20 | 39 | Yes | None | CYTO, FC, κ | Left frontal lobe mass | – | Probable B-cell IOL† |
| 11/69/M | Liver transplant | Vitritis | 20/70 | 20/40 | 26 | Yes | None | CYTO, FC, κ | Normal | – | Probable B-cell IOL |
| 12/42/F | CNS lymphoma | Vitritis | 20/50 | 20/40 | 11 | Yes | None | CYTO§,, FC, κ | Left parietal lobe mass | – | B-cell IOL |
AFX, air–fluid exchange performed at conclusion of vitrectomy procedure; ATL, adult T-cell leukaemia/lymphoma; CF, counting fingers; CLL, chronic lymphocytic leukaemia; CMV, cytomegalovirus; CNS, central nervous system; CRVO, central retinal vein occlusion; CYK, cytokine evaluation (ie, interleukin (IL)-10, IL-6, IL-2); CYTO, cytopathology; Dx, diagnosis; FC, flow cytometry; F/U, follow-up; HM, hand motions; HTLV, human T-cell lymphotrophic virus; IGH, IgH gene rearrangement studies; IHC, immunohistochemistry; IOL, intraocular lymphoma; IU, intermediate uveitis; NVG, neovascular glaucoma; RPE, retinal pigment epithelium; TCR, T-cell receptor gene rearrangement studies; TSV, transconjunctival sutureless vitrectomy; VA, visual acuity; κ, κ light chain gene rearrangement studies.
Patient 5 showed a monoclonal B-cell population by flow cytometry; however cytopathological evaluation, cytokine testing and IgH rearrangement studies were consistent with an inflammatory process.
A diagnostic brain biopsy was offered to this patient, but the patient deferred further intervention.
Indicates positive test.
The diagnosis of lymphoma (ie, primary, recurrent, or secondary ocular involvement) was established in eight patients (67%, 95% CI 39.1 to 86.1%) using this combination of vitreous assessment. Cytological evaluation showed morphological changes consistent with lymphoma in three of 12 patients assessed (25%, 95% CI 8.9 to 53.2%). In the other five patients, gene arrangements studies, cytokine studies or flow cytometry were needed to establish a diagnosis. Table 3 summarises the key findings from the vitreous specimens for each of the diagnostic testing modalities. Final diagnoses included intraocular B-cell lymphoma (6), intraocular T-cell lymphoma (2), HTLV-associated intermediate uveitis (1), tuberculosis-associated intermediate uveitis (1) and idiopathic intermediate uveitis (2).
Table 3.
Summary of vitreous testing on 25-gauge diagnostic TSV specimens
| Patient | Cytopathology | Immunohistochemistry | Flow cytometry | Gene rearrangement | Cytokine (pg/ml) | Microbiology, PCR |
|---|---|---|---|---|---|---|
| 1 | Scattered lymphocytes, monocytes | – | CD45bright, CD19dim, monoclonal κ light chain (identical to prior lymphoma) | IgH gene rearrangement detected | IL-6 <15.6, IL-10 75.5 | Gram stain, bacterial and fungal cultures negative |
| 2 | Rare, small lymphocytes | – | No evidence of T-cell lymphoma | – | – | HSV, CMV, VZV PCR negative, gram stain, bacterial, fungal cultures negative |
| 3 | Atypical lymphoid cells with irregular nuclei, lymphocytes, monocytes | – | T-cell leukaemia/lymphoma, Abnormal CD3dim population detected | TCR gamma-gene rearrangement detected | IL-6 843, IL-10 203, IL-2 18.4 | HSV, VZV PCR negative, gram stain, bacterial, fungal cultures negative |
| 4 | Lymphocytes, macrophages | Few CD68 cells (macrophages) | No evidence of T-cell lymphoma | No TCR gene rearrangement detected | IL-6 78.4, IL-10 76.0, IL-2 <15.6 | Gram stain, bacterial and fungal cultures negative |
| 5 | Isolated atypical lymphoid cells, lymphocytes, macrophages | CD20 negative, κ+ and λ+, CD68+, CD4+, CD8 cells | CD19+κ+, CD20-λ-, consistent with B-cell lymphoma | No IgH gene rearrangement detected | IL-10 <15.6, IL-6 79.0 | Gram stain, bacterial and fungal cultures negative, CMV, HSV, VZV PCR negative |
| 6 | Lymphocytosis, negative for malignant cells | – | No evidence of B-cell lymphoma | – | IL-6 <15.6, IL-10 <23.4 | EBV PCR positive, Toxo PCR negative, Gram stain, bacterial, fungal, mycobacterial culture negative |
| 7 | No evidence of lymphoma | – | Small population of B-cells | IgH gene rearrangement detected | – | PCR for CMV, VZV, HSV, EBV, Toxo, and bacterial and fungal cultures negative. |
| 8 | No evidence of lymphoma | – | 98% T-cells, CD3+, CD4+ and CD8+ T-cells, no evidence of lymphoma | TCR gene rearrangement detected | – | PCR for CMV, VZV, HSV, EBV, Toxo, and bacterial and fungal cultures negative. |
| 9 | Atypical lymphoid cells with irregular nuclei and condensed chromatin | – | No evidence of B-cell lymphoma | IgH gene rearrangement detected | IL-10 84.8 IL-6 17.7 | CMV, HSV, VZV PCR negative, Gram stain, bacterial and fungal culture negative. |
| 10 | Insufficient quantity for analysis | – | T-cell predominance, no evidence of lymphoma | κ light chain gene rearrangement detected | – | PCR for CMV, VZV, HSV, EBV, Toxo negative, bacterial and fungal cultures negative. |
| 11 | Insufficient quantity for analysis | – | T-cell predominance, no evidence of lymphoma | κ light chain gene rearrangement detected | – | PCR for CMV, VZV, HSV, EBV, Toxo negative, bacterial and fungal cultures negative. |
| 12 | Atypical lymphoid cells with irregular nuclei | – | T-cell predominance, no evidence of lymphoma | κ light chain gene rearrangement detected | – | PCR for CMV, VZV, HSV, EBV, Toxo negative, bacterial and fungal cultures negative. |
CMV, cytomegalovirus; EBV, Epstein–Barr virus; Micro, microbiology; HSV, Herpes simplex virus; IL, interleukin; Toxo, toxoplasmosis; VZV, Varicella zoster virus.
The diagnostic yields for the individual tests are summarised in table 4. Cytopathological evaluation and flow cytometry were performed in all 12 eyes and only two samples were deemed to be insufficient for cytopathological evaluation. Cytopathology with immunohistochemistry was positive in three eyes with lymphoma (25%). Flow cytometry was positive in four of 12 patients (33%), although one test was thought to be a false positive (see below). Gene arrangement studies were performed in 10 of 12 eyes and were positive in all eight patients diagnosed with IOL; interestingly, gene rearrangement studies had the highest diagnostic yield in this study. Cytokine concentrations (IL-10, IL-6 or IL-2) were evaluated in six eyes. IL-10:IL-6 ratios >1.0, which has been previously associated with IOL, was considered strongly suggestive for B-cell lymphoma18 and the presence of IL-2, which is not normally found in vitreous fluid, was considered positive. Overall, cytokine evaluation had a diagnostic yield of 37.5%. PCR testing and vitreous fluid culture had the lowest diagnostic yields of 2.7% and 0%, respectively, in this series.
Table 4.
Diagnostic yield of testing performed on vitreous specimens
| Diagnostic test | Number of tests performed | Number of positive tests (%) |
|---|---|---|
| Cytopathology/immunohistochemistry | 12 | 3 (25) |
| Flow cytometry | 12 | 4 (33.3) |
| Cytokine evaluation* | 8 | 3 (37.5) |
| Gene rearrangement studies | 10 | 8 (80) |
| PCR testing for HSV, CMV, VZV, EBV, toxoplasmosis, Mycobacterium tuberculosis | 37 | 1 (2.7) |
| Culture (bacterial, fungal, mycobacteria) | 25 | 0 (0) |
IL-10:IL-6 ratio considered one test.
CMV, cytomegalovirus; EBV, Epstein–Barr virus; HSV, Herpes simplex virus; VZV, Varicella zoster virus.
Of the eight patients diagnosed with IOL, six patients demonstrated B-cell lymphoma features while two patients were diagnosed with T-cell lymphoma. Of the six patients with B-cell lymphoma, one patient (patient 1) was diagnosed with recurrent primary IOL, two patients (patients 7 and 12) previously diagnosed with chronic lymphocytic leukaemia were diagnosed with new-onset secondary ocular involvement, and one patient was diagnosed with systemic non-Hodgkin's lymphoma (patient 9) by diagnostic vitrectomy. Patient 9 had been diagnosed with an abdominal mass abutting the renal pelvis and presented with a unilateral panuveitis (figure 1).
Figure 1.
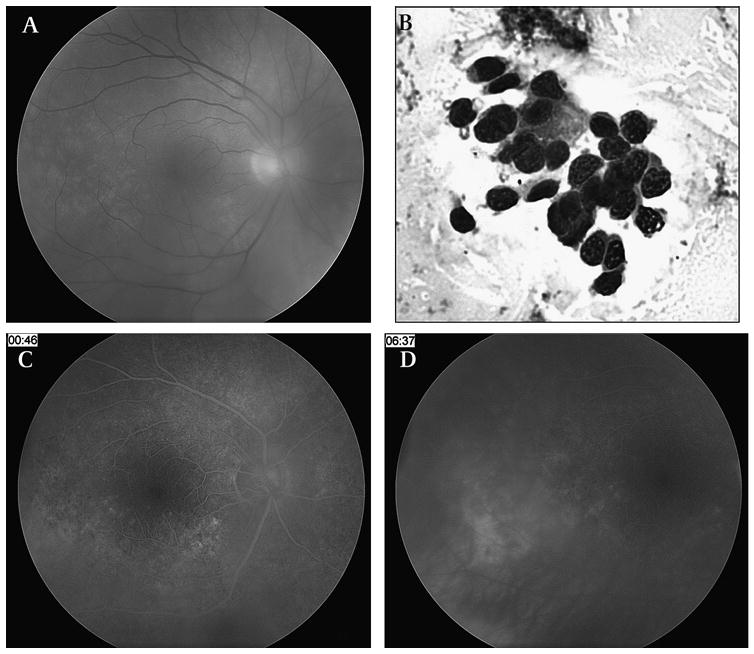
Patient 9 was a 52-year-old male patient who had a history of an intra-abdominal mass impinging on the right renal pelvis. Ophthalmic examination showed 1–2+ vitreous cell, trace vitreous haze and retinal pigment epithelium (RPE) mottling with a ‘leopard-skin’ pattern (A). Diagnostic vitrectomy revealed large atypical lymphocytes consistent with intraocular lymphoma (B). Fluorescein angiography showed mottled hyperfluorescence (C) and an area of late leakage with a serous detachment temporal to the fovea (D).
In two patients, the diagnosis of B-cell IOL was deemed probable based on monoclonal restriction of a κ light chain gene rearrangement. Patient 10 had a left frontal mass but refused further invasive testing including repeat vitrectomy or a brain biopsy. Patient 11 deferred chemotherapy or radiation and is being followed closely by his oncologists, as no CNS findings were observed and the vitritis has not recurred at 26 weeks follow-up following his diagnostic vitrectomy.
Both patients who were diagnosed with intraocular involvement by T-cell lymphoma (patients 3 and 8) had previously been diagnosed with human T-cell lymphotrophic virus-1 (HTLV-1)-associated systemic adult T-cell leukaemia/lymphoma. Vitritis and retinal vasculitis were seen in both patients (figure 2). Diagnostic testing was tailored for intraocular T-cell lymphoma in these patients, including flow cytometry for T-cell surface markers, T-cell receptor (TCR) gene rearrangement studies and cytokine assessment of IL-2.
Figure 2.
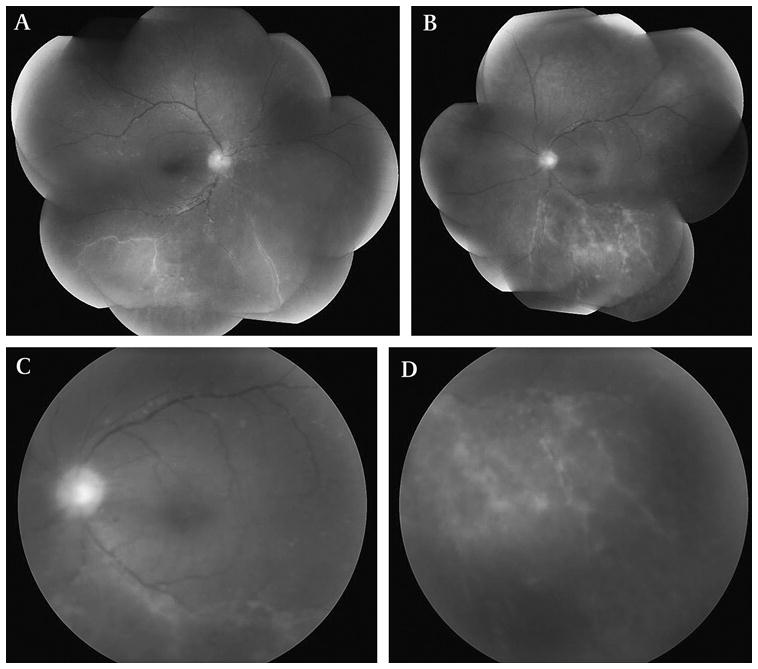
Patient 3 was a 54-year-old Caribbean woman with a history of systemic HTLV-1-associated T-cell lymphoma who presented with bilateral vitritis and retinal vasculitis (A, B). Fundus photograph of posterior pole revealed 1–2+ vitreous haze with 2+ vitreous cells (C) and prominent peripheral retinal vascular sheathing (D). Vitreous cytology, flow cytometry and T-cell receptor gene rearrangement studies from a diagnostic transconjunctival sutureless vitrectomy (TSV) specimen were consistent with intraocular T-cell lymphoma.
None of the patients in this series developed intraoperative retinal breaks requiring treatment, retinal detachment, or endophthalmitis following surgery. One patient developed neovascular glaucoma and cytomegalovirus retinitis secondary to immunosuppression from disseminated HTLV-1 associated T-cell leukaemia. Transient postoperative hypotony was observed in four individuals, but had resolved by the 1-week post-operative visit. None of these patients underwent an air–fluid exchange following diagnostic TSV. No difference was found in the proportion of patients who experienced hypotony when comparing those patients who underwent air–fluid exchange versus patients who did not undergo air–fluid exchange at the conclusion of the surgical procedure (p=0.14, Fisher's exact test). Cataract was observed in two patients in the postoperative period. Both patients had documented cataract prior to their diagnostic vitrectomy procedure, and these were thought to be age-related (ie, both patients were >60 years old). One patient (patient 5) underwent an uncomplicated cataract extraction, while the other patient (patient 1) has deferred cataract surgery.
Discussion
In this retrospective series of patients who underwent 25-gauge diagnostic TSV, the diagnosis of B- and T-cell lymphoma was made in 67% of eyes and was established by cytopathological evaluation in 25% of cases. Gene rearrangement studies had the highest diagnostic yield of 80% and were positive in all eight patients with IOL (primary or secondary). Visual acuity improved or remained stable in most eyes (92%) and no significant intra- or postoperative complications were seen.
Transient hypotony was seen in four eyes; however, no evidence of hypotony or consequent complications was observed at subsequent follow-up. Transient hypotony following 25-gauge surgery has been documented to occur in 4–20% of cases.21 Two of four instances of postoperative hypotony occurred in the setting of immune-mediated inflammation, and the rate of hypotony may be higher in these individuals. Due to the tertiary referral nature of the two practice settings, follow-up was limited in several patients and it is possible that delayed retinal complications could have developed outside the follow-up period. Despite this possibility, the low incidence of postoperative adverse events was encouraging.
The diagnosis of IOL is challenging for a number of reasons. Small quantities of vitreous fluid make surgical technique, instrumentation and timing critical to establishing the diagnosis. Besides the precise surgical technique of harvesting the vitreous specimen, expert ophthalmic cytopathological evaluation and flow cytometry services are also essential to establishing a diagnosis of lymphoma. Identification of lymphoma cells with cytopathology has long been considered the gold standard for the diagnosis of IOL; however, adjunctive measures to identify lymphoma were extremely helpful in this series of patients. For example, in one patient with recurrent IOL (patient 1), flow cytometry demonstrated monoclonal restriction of lymphocytes with CD19dimCD45bright, κ light chain positivity, although cytology was negative. This flow cytometry signature was identical to his previously seen lymphoma and indicative of recurrent disease. Another patient (patient 8) with a history of adult T-cell leukaemia/lymphoma was positive for a T-cell receptor gene rearrangement study despite a negative cytopathological evaluation. Cytokine evaluations were also helpful as adjunctive tests in the evaluation of IOL. Both patients diagnosed with B-cell lymphoma had IL-10:IL-6 ratios >1.0, which is consistent with IOL.18 Although an IL-10:IL-6 ratio <1.0 has also been documented in early IOL17 and elevated IL-10 level may be found in other uveitic conditions, the diagnosis of primary IOL should be considered in presence of an elevated IL-10:IL-6 ratio and suspicious clinical and laboratory findings.
The diagnostic yield of each of the tests performed in this series of patients compares favourably with prior reports of diagnostic vitrectomy for uveitis of unclear aetiology. Margolis et al recently reported the identification of a specific cause for uveitis with diagnostic vitrectomy in nine of 45 patients (20%) with yields of 14.3% for cytological analyses and 14.7% for flow cytometry.5 Davis et al reported the identification of an aetiology for infectious uveitis or intraocular neoplasm in 48 of 78 patients (61.5%) who underwent diagnostic vitrectomy. They described a 31% sensitivity of vitreous cytology in patients with lymphoma; adjunctive testing including flow cytometry and non-ocular tissue biopsies were important to the diagnosis in some cases.3
Interestingly, one patient (patient 5) demonstrated monoclonality by flow cytometry suggestive of B-cell lymphoma while cytopathology, immunohistochemistry, cytokine evaluation and gene rearrangement studies were consistent with idiopathic intermediate uveitis. Prominent monoclonal B-cell populations by flow cytometry have been described in histologically reactive lymphoid proliferations in non-ocular tissues,22 which may explain the findings in our patient.
Potential advantages conferred by small-gauge sutureless vitrectomy include shorter surgical times and potentially decreased postoperative inflammation.9 This may be relevant to the patients with uveitis undergoing diagnostic PPV, as intraocular surgery in uveitis patients may complicated by corneal oedema, postoperative fibrin formation, glaucoma,23 hypotony and poor wound healing.
While the mechanical action of vitrectomy does not appear to significantly alter the morphology or cellular characteristics of lymphoma cells lines for cytopathological analysis24 a clinical study directly comparing 20-, 23- and 25-gauge vitrectomy for diagnostic purposes has not been performed. One prior in vitro study evaluating 25- versus 20-gauge vitrectomy demonstrated adequate vitreous cellular material for cytopathology and flow cytometry in isolated vitreous mixed with cultured human lymphoma cells.10 Flow cytometry demonstrated similar mean cellular viability in both the 20- and 25-gauge vitrectomy groups. A second study using human lymphoma cells mixed in culture medium confirmed adequate samples for flow cytometry with cells harvested using either gauge vitreous cutter.11
A recent study evaluating the fluidics of 20-, 23 and 25- gauge vitrectomy showed that in both small- and 20-gauge systems, the fluidics were determined by lumen size, flow rate and cut rate; all of the available systems and instrumentation demonstrated laminar fluidics.25 Because the TSV systems do not contribute significant turbulent flow properties, it is unlikely that significant shearing forces of the TSV system would cause morphological alterations in the cells to be analysed.
This retrospective series suggests that 25-gauge TSV is a safe and effective technique for obtaining an adequate vitreous specimen and establishing a diagnosis of B- and T-cell lymphoma. Collaboration with an experienced cytopathologist, flow cytometry laboratory and personnel who are familiar with gene rearrangement studies are also important for appropriate analysis of vitrectomy specimens.
Acknowledgments
Funding This research is supported by Intramural Research Program of the National Eye Institute, National Institutes of Health. SY has received support from the Heed Ophthalmic Foundation and the Ronald G. Michels Foundation.
Footnotes
This research was presented in part at the American Society of Retinal Specialists annual meeting in Maui, Hawaii, USA, in October 2008.
Competing interests None.
Ethics approval This study was conducted with the approval of the National Eye Institute, National Institutes of Health; Bascom Palmer Eye Institute, University of Miami Miller School of Medicine.
Contributors The contributions of the authors SY, EDW, LJF, TAA, KKW, MS-S, PR, HNS, CCC and RBN are as follows: conception and design (SY, EDW, HNS, CCC, RBN), acquisition of data (SY, EDW, LJF, TAA, KKW, MSS, PR, HNS, CCC, RBN), drafting of the manuscript (SY, EDW, LJF, TAA, RBN), critical revision of the manuscript for important intellectual content (SY, EDW, LJF, TAA, KKW, MSS, PR, HNS, CCC, RBN), obtaining funding (TAA, EDW, CCC, RBN), administrative, technical, or material support (SY, EDW, LJF, TAA, KKW, MSS, PR, HNS, CCC, RBN) and supervision (EDW, TAA, MSS, PR, HNS, CCC, RBN).
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Whitcup SM, de Smet MD, Rubin BI, et al. Intraocular lymphoma. Clinical and histopathologic diagnosis. Ophthalmology. 1993;100:1399–406. doi: 10.1016/s0161-6420(93)31469-7. [DOI] [PubMed] [Google Scholar]
- 2.Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc. 2003;101:275–92. [PMC free article] [PubMed] [Google Scholar]
- 3.Davis JL, Miller DM, Ruiz P. Diagnostic testing of vitrectomy specimens. Am J Ophthalmol. 2005;140:822–9. doi: 10.1016/j.ajo.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 4.Davis JL, Viciana AL, Ruiz P. Diagnosis of intraocular lymphoma by flow cytometry. Am J Ophthalmol. 1997;124:362–72. doi: 10.1016/s0002-9394(14)70828-1. [DOI] [PubMed] [Google Scholar]
- 5.Margolis R, Brasil OF, Lowder CY, et al. Vitrectomy for the diagnosis and management of uveitis of unknown cause. Ophthalmology. 2007;114:1893–7. doi: 10.1016/j.ophtha.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Martin DF, Chan CC, de Smet MD, et al. The role of chorioretinal biopsy in the management of posterior uveitis. Ophthalmology. 1993;100:705–14. doi: 10.1016/s0161-6420(93)31585-x. [DOI] [PubMed] [Google Scholar]
- 7.Shields JA, Shields CL, Ehya H, et al. Fine-needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick Lecture. Ophthalmology. 1993;100:1677–84. doi: 10.1016/s0161-6420(93)31418-1. [DOI] [PubMed] [Google Scholar]
- 8.Kellner L, Wimpissinger B, Stolba U, et al. 25-gauge vs 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trial. Br J Ophthalmol. 2007;91:945–8. doi: 10.1136/bjo.2006.106799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakhanpal RR, Humayun MS, de Juan E, Jr, et al. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology. 2005;112:817–24. doi: 10.1016/j.ophtha.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 10.Fortun JA, Carvounis PE, Albini TA, et al. Comparison of cytologic and flow cytometry features of vitreous samples obtained using 20- or 25-gauge vitrectomy for the diagnosis of lymphoma. Invest Ophthalmol Vis Sci. 2009;48 E-Abstract 2212. [Google Scholar]
- 11.Trikha R, Yeung CC, Modjtahedi SP, et al. Evaluation of 25 gauge and 20 gauge vitrectomy on cell viability and diagnostic yield for B-cell lymphoma in culture using flow cytometry. Invest Ophthalmol Vis Sci. 2009;50 E-Abstract 5750. [Google Scholar]
- 12.Davis JL, Solomon D, Nussenblatt RB, et al. Immunocytochemical staining of vitreous cells. Indications, techniques, and results. Ophthalmology. 1992;99:250–6. doi: 10.1016/s0161-6420(92)31984-0. [DOI] [PubMed] [Google Scholar]
- 13.Lopez JS, Chan CC, Burnier M, et al. Immunohistochemistry findings in primary intraocular lymphoma. Am J Ophthalmol. 1991;112:472–4. doi: 10.1016/s0002-9394(14)76269-5. [DOI] [PubMed] [Google Scholar]
- 14.Shen DF, Zhuang Z, LeHoang P, et al. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105:1664–9. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy KP, Sloane JP, Kabarowski JH, et al. A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol. 1992;1:173–9. [PubMed] [Google Scholar]
- 16.Wolf LA, Reed GF, Buggage RR, et al. Vitreous cytokine levels. Ophthalmology. 2003;110:1671–2. doi: 10.1016/S0161-6420(03)00811-X. [DOI] [PubMed] [Google Scholar]
- 17.Buggage RR, Velez G, Myers-Powell B, et al. Primary intraocular lymphoma with a low interleukin 10 to interleukin 6 ratio and heterogeneous IgH gene rearrangement. Arch Ophthalmol. 1999;117:1239–42. doi: 10.1001/archopht.117.9.1239. [DOI] [PubMed] [Google Scholar]
- 18.Buggage RR, Whitcup SM, Nussenblatt RB, et al. Using interleukin 10 to interleukin 6 ratio to distinguish primary intraocular lymphoma and uveitis. Invest Ophthalmol Vis Sci. 1999;40:2462–3. [PubMed] [Google Scholar]
- 19.Chan CC, Whitcup SM, Solomon D, et al. Interleukin-10 in the vitreous of patients with primary intraocular lymphoma. Am J Ophthalmol. 1995;120:671–3. doi: 10.1016/s0002-9394(14)72217-2. [DOI] [PubMed] [Google Scholar]
- 20.Stetler-Stevenson M, Braylan RC. Flow cytometric analysis of lymphomas and lymphoproliferative disorders. Semin Hematol. 2001;38:111–23. [PubMed] [Google Scholar]
- 21.Chen E. 25-Gauge transconjunctival sutureless vitrectomy. Curr Opin Ophthalmol. 2007;18:188–93. doi: 10.1097/ICU.0b013e328133889a. [DOI] [PubMed] [Google Scholar]
- 22.Kussick SJ, Kalnoski M, Braziel RM, et al. Prominent clonal B-cell populations identified by flow cytometry in histologically reactive lymphoid proliferations. Am J Clin Pathol. 2004;121:464–72. doi: 10.1309/4EJ8-T3R2-ERKQ-61WH. [DOI] [PubMed] [Google Scholar]
- 23.Soheilian M, Mirdehghan SA, Peyman GA. Sutureless combined 25-gauge vitrectomy, phacoemulsification, and posterior chamber intraocular lens implantation for management of uveitic cataract associated with posterior segment disease. Retina. 2008;28:941–6. doi: 10.1097/IAE.0b013e31816ed5c7. [DOI] [PubMed] [Google Scholar]
- 24.Conlon MR, Craig I, Harris JF, et al. Effect of vitrectomy and cytopreparatory techniques on cell survival and preservation. Can J Ophthalmol. 1992;27:168–71. [PubMed] [Google Scholar]
- 25.Hubschman JP, Gupta A, Bourla DH, et al. 20-, 23-, and 25-gauge vitreous cutters: performance and characteristics evaluation. Retina. 2008;28:249–57. doi: 10.1097/IAE.0b013e31815ec2b3. [DOI] [PubMed] [Google Scholar]


