Abstract
Background
Oxidative damage in the central nervous system is increasingly recognized as an important pathological process in many diseases. Previously, our laboratory found that oxidative damage to lipids and proteins was increased in postmortem brain tissue from patients with bipolar disorder and schizophrenia. In the current study, we analyzed oxidative damage to nucleic acids in the CA1, CA3 and dentate gyrus regions of postmortem hippocampus tissue from patients with bipolar disorder, schizophrenia and major depression.
Methods
We examined oxidative damage to nucleic acids by performing immunohistochemistry with a monoclonal antibody that recognizes both 8-hydroxyguanosine in RNA and 8-hydroxy-2’-deoxyguanosine in DNA.
Results
We found that the amount of oxidative damage to nucleic acids was elevated in the CA1, CA3 and dentate gyrus regions of the hippocampus among patients with bipolar disorder, schizophrenia and major depressive disorder. This damage was predominantly in the cytoplasm, suggesting that the damage was primarily to RNA. Compared with oxidative damage in control samples, the magnitude of damage was high in patients with schizophrenia, modest in patients with bipolar disorder and lower in patients with major depression.
Limitations
The interpretation of our results is limited by a number of factors, including the retrospective review of patient history, the relatively small sample size and the inclusion of patients who had substance abuse and were undergoing various drug treatments at the time of death.
Conclusion
Our results suggest that oxidative damage to RNA, rather than to DNA, occurs in vulnerable neurons of the brain in patients with major mental illness and may contribute to the pathology of these disorders. The magnitude of RNA oxidative damage may be associated with the severity of mental illness.
Introduction
Oxidative damage results from an overproduction of reactive oxygen species (ROS) that overwhelms the cellular antioxidant capacity. Brain cells are more vulnerable than other cells to oxidative damage because the brain consumes about 20% of the body’s total oxygen, although it constitutes less than 2% of total body weight. The significance of oxidative damage as a component of many disease processes in the central nervous system is being increasingly recognized. Oxidative damage has been found in neurologic disorders such as Parkinson disease and Alzheimer disease,1,2 and it was recently identified in mental illnesses such as bipolar disorder and schizophrenia. For example, increased lipid peroxidation and decreased activity of the antioxidant defence enzymes superoxide dismutase and catalase were found in the plasma of patients with bipolar disorder,3,4 the expression of the antioxidant enzyme glutathione s-transferase A4 and M3 subtypes was reduced in postmortem brain samples from patients with bipolar disorder,5 and increased nitric oxide radicals and decreased levels of glutathione and related antioxidant enzymes were found in the postmortem brain tissue of schizophrenia patients.6,7 These findings suggest that the process of oxidative damage may play an important role in the pathology of bipolar disorder and schizophrenia. Recently, many studies have shown that mood-stabilizing drugs and antipsychotic drugs inhibit oxidative damage and increase various antioxidant enzymes,8–14 suggesting that the process of oxidative damage may be targeted by these drugs. In a clinical study involving twins, the bipolar twin had increased lipid peroxidation compared with the healthy twin.15 This elevated lipid peroxidation was normalized after mood-stabilizing drug treatment,15 suggesting that antioxidative action may have a therapeutic indication in this disorder.
Previously, our laboratory found that oxidative damage to lipids and proteins was elevated in the postmortem brain tissue of patients with bipolar disorder and schizophrenia.16,17 Reactive oxygen species react not only with lipids and proteins but also with nucleic acids, thereby inducing oxidative damage to DNA and RNA. Guanine in DNA and RNA is more sensitive to ROS attacks than are the other bases. Reactive oxygen species oxidize guanine and generate 8-oxo-7,8-dihydroguanosine (8-OHG) in RNA and 8-oxo-7,8-dihydro-2-deoxyguanosine (8-OHdG) in DNA.
The hippocampus is a medial temporal structure involved in the Papez circuitry18 that is responsible for emotions and is susceptible to damage during chronic stress. Recent studies have indicated the presence of cellular damage and volumetric changes in this brain region of patients with mood disorders and schizophrenia.19,20 Because one factor that may be important in these changes is oxidative stress, we analyzed the amount of oxidative damage to nucleic acids in the CA1, CA3 and dentate gyrus regions of postmortem hippocampus tissue from patients with bipolar disorder, schizophrenia and major depressive disorder.
Methods
Postmortem brain tissue
We obtained human postmortem sections from the Stanley Medical Research Institute’s brain collection. We included sections from the anterior hippocampus of patients with bipolar disorder, schizophrenia and major depressive disorder as well as nonneurologic, nonpsychiatric controls. The tissue sections were 10-μm thick, formaldehyde-fixed, paraffin-embedded, slide-mounted coronal sections. The sections were matched for age, sex, postmortem interval, pH and mRNA quality. Diagnoses were retrospectively established by 2 senior psychiatrists using DSM-IV criteria. The participants’ demographic information is summarized in Table 1. Detailed clinical information, diagnostic procedures and other demographic information for these participants are available in previously published studies.21,22
Table 1.
Demographic characteristics of study participants
| Group; mean (SEM) [range]* |
||||
|---|---|---|---|---|
| Characteristic | Control participants | Major depressive disorder | Bipolar disorder | Schizophrenia |
| Age, yr | 48 (2.7) [29–68] | 47 (2.3) [30–65] | 42 (2.9) [25–61] | 45 (3.3) [25–62] |
| Sex, male:female | 9:6 | 9:6 | 9:6 | 9:6 |
| PMI, h | 23.7 (2.4) [8–42] | 27.5 (2.7) [7–47] | 32.6 (4.0) [13–62] | 33.7 (3.7) [12–61] |
| pH | 6.3 (0.1) [5.8–6.6] | 6.2 (0.1) [5.8–6.5] | 6.2 (0.1) [5.8–6.5] | 6.2 (0.1) [5.8–6.6] |
PMI = postmortem interval; SEM = standard error of the mean.
Unless otherwise indicated.
Immunohistochemistry
We hydrated the tissue sections through graded ethanol baths, and the paraffin was removed by use of xylene. The tissue sections were then rehydrated, blocked with 5% normal goat serum with 0.3% Triton X-100 for 1 hour at 22°C and incubated with a monoclonal antibody that recognizes both 8-OHG in RNA and 8-OHdG in DNA (1:100; QED Bioscience Inc.) and an antibody for β3-tubulin (1:200; Chemicon International) at 4°C overnight. After washing the sections with phosphate-buffered saline, we incubated them in a 1:1000 dilution of Alexa-568–conjugated antimouse IgG (Invitrogen Canada Inc.) and a 1:1000 dilution of Alexa-488–conjugated anti-chicken IgG (Invitrogen) for 2 hours at room temperature. After washing, we covered the sections with fluorescent antifade mounting media (Invitrogen) and a cover slip.
We examined the fluorescence-labelled sections at 40× magnification using an Eclipse E600 microscope with NIS-Elements Advanced Research version 3.0 software (Nikon Canada). We captured images from 5 fields of each region. In the CA1 and CA3 regions, we examined the intensity of each positively labelled cell. Regions of the field without specific immunoreaction were used as background. We captured all images with uniform threshold and intensity settings. We quantified the fluorescence intensity of each field using image analysis software. The fluorescence intensities of the CA1 and CA3 regions for each slide were calculated as follows:
Fluorescence intensity = (intensity of cell 1 − intensity of the background) + (intensity of cell 2 − intensity of background) + … + (intensity of cell n − intensity of the background).
Because the cells in the dentate gyrus region were so dense, we could not count the individual cells. Thus, we examined the total intensity of 5 fields. This intensity was calculated as follows:
Fluorescence intensity = intensity of 5 fields − intensity of the background.
We processed and evaluated 2 adjacent sections for each patient. Immunohistochemical and imaging analysis were performed in a blinded fashion on 4 groups in parallel.
Statistical analyses
We assessed the differences between groups by use of 1-way analyses of variance followed by post-hoc testing (Tukey honestly significant difference). Because we analyzed data for the CA1, CA3 and dentate gyrus hippocampal subregions, we considered p values less than 0.0167 (p = 0.05/3) to be significant because of the Bonferroni inequality adjustment for multiple comparisons. We used 2-tailed independent-samples t tests to assess the effects of sex. We examined the contributions of age, postmortem interval and pH by Pearson correlation analysis. All statistical analyses were performed with SPSS 16.0 for Windows.
Results
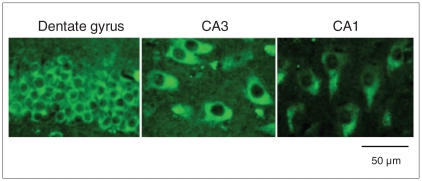
We found that oxidative damage to the nucleic acids was predominantly located in the cytoplasm, not the nucleus (Fig. 1). We found an overall significant effect of diagnosis on oxidative damage across the 4 groups in the neurons of the dentate gyrus (F3,56 = 12.138, p < 0.001; Fig. 2), CA3 (F3,56 = 7.783, p < 0.001; Fig. 3) and CA1 (F3,56 = 11.907, p < 0.001; Fig. 4) in patients with major depressive disorder, bipolar disorder and schizophrenia.
Fig. 1.
Immunohistochemical staining of 8-oxo-7,8-dihydroguanosine (8-OHG) and 8-oxo-7,8-dihydro-2-deoxyguanosine (8-OHdG) in the dentate gyrus, CA3 and CA1 regions of postmortem tissue from patients with bipolar disorder. The sections were stained with a monoclonal antibody that recognizes both 8-OHG in RNA and 8-OHdG in DNA.
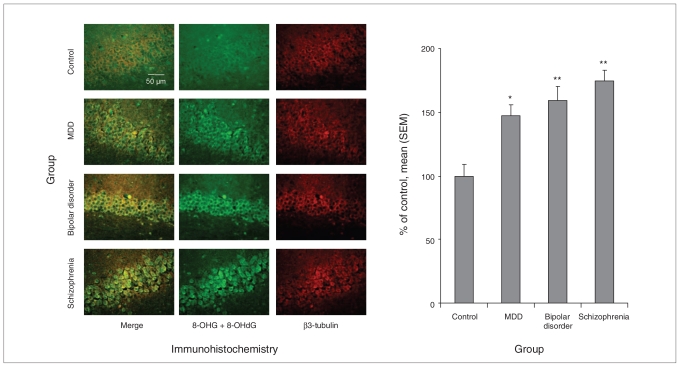
Fig. 2.
RNA oxidative damage in the dentate gyrus region of postmortem hippocampus tissue from controls and patients with major depressive disorder (MDD), bipolar disorder or schizophrenia. We assessed the damage to the nucleic acids via immunohistochemical staining with an antibody that recognizes 8-oxo-7,8-dihydroguanosine (8-OHG) in RNA and 8-oxo-7,8-dihydro-2-deoxyguanosine (8-OHdG) in DNA (n = 15 patients per group; original magnification × 40). *p < 0.0167, **p < 0.0033 compared with control. SEM = standard error of the mean.
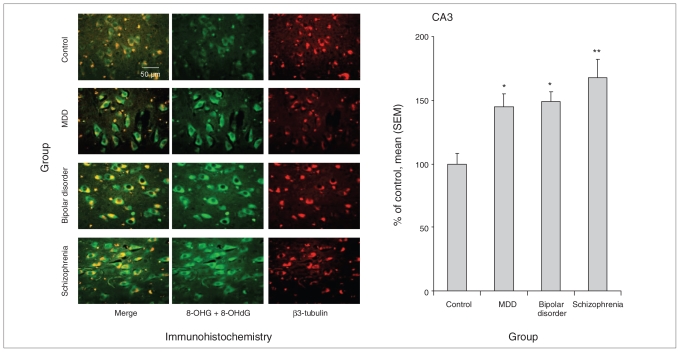
Fig. 3.
RNA oxidative damage in the CA3 region of postmortem hippocampus tissue from controls and patients with major depressive disorder (MDD), bipolar disorder or schizophrenia. Damage to nucleic acids was assessed by immunohistochemistry with an antibody for both 8-oxo-7,8-dihydroguanosine (8-OHG) in RNA and 8-oxo-7,8-dihydro-2-deoxyguanosine (8-OHdG) in DNA (n = 15 patients per group; original magnification × 40). An average of 50, 60, 60 and 65 positive cells were counted in the control, MDD, bipolar disorder and schizophrenia groups, respectively. *p < 0.0167, **p < 0.0033 compared with control. SEM = standard error of the mean.
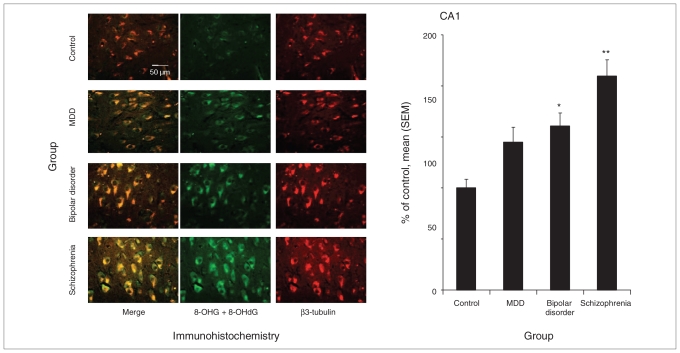
Fig. 4.
RNA oxidative damage in the CA1 region of postmortem hippocampus from controls and patients with major depressive disorder (MDD), bipolar disorder and schizophrenia. Damage to nucleic acids was assessed using immunohistochemistry with an antibody for both 8-oxo-7,8-dihydroguanosine (8-OHG) in RNA and 8-oxo-7,8-dihydro-2-deoxyguanosine (8-OHdG) in DNA (n = 15 patients per group; original magnification × 40). An average of 56, 64, 68 and 71 positive cells were counted in controls, MDD, bipolar disorder and schizophrenia groups, respectively. *p < 0.0167, **p < 0.0033 compared with control. SEM = standard error of the mean.
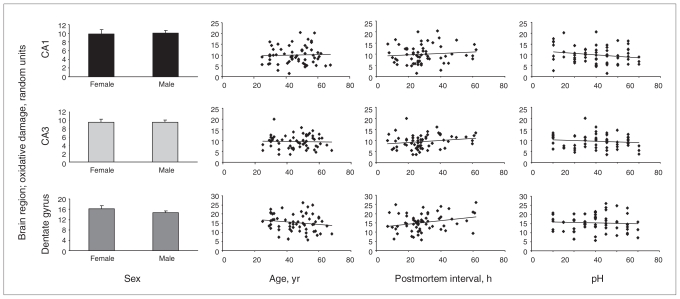
We assessed the effect of sex, age, postmortem interval and pH on nucleic acid oxidative damage in CA1, CA3 and dentate gyrus regions (Fig. 5). We found no difference between men and women and no correlation between nucleic acid oxidative damage and age or pH in these 3 regions. Although nucleic acid oxidative damage was not correlated with postmortem interval in the CA1 and CA3 regions, the damage was positively correlated with postmortem interval in the dentate gyrus (r = 0.264, p = 0.042). However, when postmortem interval was used as a covariate, the univariate analysis of variance still indicated that oxidative damage was significantly different among the diagnostic groups (F4,55 = 9.241, p < 0.001).
Fig. 5.
Difference in the amount of oxidative damage to RNA between men and women and the correlation between RNA oxidative damage and age, postmortem interval and pH in the postmortem hippocampus of control patients, patients with major depressive disorder, bipolar disorder or schizophrenia (n = 60; 36 men and 24 women). We used 2-tailed independent t tests to assess the effects of sex. The contributions of age, postmortem interval and pH were examined by Pearson correlation analysis.
Post-hoc comparisons for the dentate gyrus and CA3 regions revealed that the oxidative damage was significantly increased in all 3 mental illness groups. In the dentate gyrus (Fig. 2), nucleic acid oxidative damage was increased by 147% in patients with major depressive disorder (p = 0.004), 159% in patients with bipolar disorder (p < 0.001) and 175% (p < 0.001) in patients with schizophrenia. In the CA3 region (Fig. 3), nucleic acid oxidative damage was increased by 145% in major depressive disorder (p = 0.016), 149% in bipolar disorder (p = 0.007) and 168% (p < 0.001) in schizophrenia.
In the CA1 region (Fig. 4), nucleic acid oxidative damage was significantly increased in patients with bipolar disorder and schizophrenia compared with controls. Damage was increased by 161% in bipolar disorder (p = 0.010) and 210% in schizophrenia (p < 0.001). Although nucleic acid oxidative damage was increased by 144% in patients with major depressive disorder, it was not statistically significant (p = 0.09).
Discussion
In this study, we found that oxidative damage to nucleic acids in neurons was significantly increased in the CA1, CA3 and dentate gyrus regions of postmortem hippocampus tissue from patients with major depressive disorder, bipolar disorder and schizophrenia. This suggests that nucleic acid oxidative damage in vulnerable neurons has an important role in the pathological process of mental illness. Our results also indicate that this oxidative damage predominantly occurs in the cytoplasm, not the nucleus, of the cells in the CA1, CA3 and dentate gyrus regions. Because DNA is located in the nucleus and RNA is located in the cytoplasm, our results suggest that RNA but not DNA is damaged by oxidative stress in these diseases. RNA is more vulnerable to oxidative attack than DNA, possibly because the bases in single-strand RNA are not protected by hydrogen bonding as they are in double-strand DNA.23–25 The pattern of predominant oxidative damage to cytoplasmic RNA rather than nuclear DNA may be reversible after pharmacological intervention. We also found that the magnitude of RNA oxidative damage was highest in patients with schizophrenia, whereas it was more modestly increased in patients with bipolar disorder and less so in patients with major depression. These findings suggest that RNA oxidative damage may be associated with the severity of these mental illnesses.
RNA oxidation is generally associated with reduced protein expression because oxidized bases on RNA transcripts can slow the translation process and induce translation errors.26,27 Recent proteomic studies have identified many downregulated proteins in the postmortem brain tissue of patients with major depressive disorder, bipolar disorder and schizophrenia.28–31 It will be interesting to determine whether RNA oxidative damage contributes to the downregulation of these proteins.
Because most of the patients in our study had been taking various medications including antidepressants, mood stabilizers and antipsychotics, increased RNA oxidative damage may be related to drug treatment. Ideally, it would be best to compare RNA oxidative damage in unmedicated and medicated patients. However, in our patients, only 2 patients with major depression, 3 with bipolar disorder and 3 with schizophrenia were not taking any medication at the time of death. In addition, 5 of these 8 patients also had an alcohol or substance abuse problem. Therefore, it would not be practical to perform this comparison. Many recent studies, including ours,8–11 have shown that antidepressants, mood stabilizers and atypical antipsychotics inhibit oxidative damage in animal models and cells,8–14 which suggests that RNA oxidative damage in patients with mental illness is not likely a result of treatment with medication.
Increased RNA oxidative damage in patients with bipolar disorder, schizophrenia and major depressive disorder may result from dysfunctional mitochondria because mitochondria are the major source of ROS. A growing body of evidence suggests that bipolar disorder and schizophrenia are illnesses that are particularly associated with mitochondrial dysfunction.32–37 Studies involving brain imaging have shown that adenosine-5’-triphosphate (ATP) and phosphocreatine are decreased in the frontal lobes and basal ganglia in patients with mood disorders.32–35 It has also been reported that the density of mitochondria was decreased in the caudate nucleus and putamen of patients with schizophrenia.36 In addition, mitochondrial ATP production rates have been found to be decreased in the muscle of patients with major depressive disorder.37 These findings suggest that dysfunctional mitochondria impair energy production in these common mental illnesses.
Recent studies have shown that lactate levels in cerebrospinal fluid are significantly increased in patients with bipolar disorder and schizophrenia compared with controls,38 which suggests that mitochondrial dysfunction induces a shift in energy production from more efficient oxidative phosphorylation in the mitochondrial electron transport chain to less efficient anaerobic glycolysis. Mitochondrial ATP is produced via oxidative phosphorylation coupled to electron transport chain complexes I–V. Studies have shown deletions and mutations in genes coding for complex I subunits in patients with bipolar disorder.39 Recent DNA microarray analyses, including ours,40 in postmortem samples from the frontal cortex and hippocampus found that the expression of many mRNAs coding for subunits of complexes I–V was decreased in patients with bipolar disorder.40,41 A proteomic study has also shown that subunits of complex I and complex III are downregulated in postmortem samples of the prefrontal cortex of schizophrenia patients.30 Complexes I and III are the main sites where electrons are leaked to oxygen, resulting in the production of ROS that can cause oxidative damage.
Reactive oxygen species can be also produced by various oxidases.42 Monoamine oxidases (MAOs) degrade monoamine neurotransmitters and have an important role in the pathology of depression. Monoamine oxidase inhibitors are a pharmacological option for the treatment of depression. It has been reported that the density of MAO-A is increased in the brain of patients with depression.43 The results of these studies, together with ours,40 suggest that RNA oxidative damage in bipolar disorder, schizophrenia and major depressive disorder may result from overproduction of ROS caused by mitochondrial dysfunction or abnormal function of MAOs.
Limitations
The interpretation of our results is limited by a number of factors, including the retrospective review of patient history, the relatively small sample size and the inclusion of patients with substance abuse and those undergoing various drug treatments at the time of death. Therefore, confirmation in larger samples will be required if more definitive conclusions are to be made.
Conclusion
We found that RNA oxidative damage was significantly increased in the postmortem hippocampus of patients with major depressive disorder, bipolar disorder and schizophrenia. Together with our previous findings,16 this suggests that oxidative damage in the brain may contribute to the pathological process of mental illnesses and that antioxidative stress may be an alternative approach to pharmacological treatment of these psychiatric disorders.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (L.T.Y. and J.-F.W.), Stanley Medical Research Institute (L.T.Y. and J.-F.W.) and National Alliance for Research on Schizophrenia and Depression Young Investigator Awards (J.-F.W.).
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study. Dr. Che acquired the data, which he and Drs. Wang, and Young analyzed. Drs. Che, Wang and Young wrote the article, which they and Dr. Shao reviewed. All authors approved the final version submitted for publication.
References
- 1.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–9. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 2.Nunomura A, Moreira PI, Lee HG, et al. Neuronal death and survival under oxidative stress in Alzheimer and Parkinson diseases. CNS Neurol Disord Drug Targets. 2007;6:411–23. doi: 10.2174/187152707783399201. [DOI] [PubMed] [Google Scholar]
- 3.Kuloglu M, Ustundag B, Atmaca M, et al. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20:171–5. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- 4.Ranjekar PK, Hinge A, Hegde MV, et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–22. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 5.Benes FM, Matzilevich D, Burke RE, et al. The expression of proapoptosis genes is increased in bipolar disorder, but not in schizophrenia. Mol Psychiatry. 2006;11:241–51. doi: 10.1038/sj.mp.4001758. [DOI] [PubMed] [Google Scholar]
- 6.Yao JK, Leonard S, Reddy RD. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao JK, Leonard S, Reddy RD. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr Bull. 2004;30:923–34. doi: 10.1093/oxfordjournals.schbul.a007142. [DOI] [PubMed] [Google Scholar]
- 8.Shao L, Young LT, Wang JF. Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol Psychiatry. 2005;58:879–84. doi: 10.1016/j.biopsych.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 9.Cui J, Shao L, Young LT, et al. Role of glutathione in neuro-protective effects of mood stabilizing drugs lithium and valproate. Neuroscience. 2007;144:1447–53. doi: 10.1016/j.neuroscience.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Wang JF, Shao L, Sun X, et al. Glutathione S-transferase is a novel target for mood stabilizing drugs in primary cultured neurons. J Neurochem. 2004;88:1477–84. doi: 10.1046/j.1471-4159.2003.02276.x. [DOI] [PubMed] [Google Scholar]
- 11.Shao L, Cui J, Young LT, et al. The effect of mood stabilizer lithium on expression and activity of glutathione s-transferase isoenzymes. Neuroscience. 2008;151:518–24. doi: 10.1016/j.neuroscience.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 12.Wei Z, Bai O, Richardson JS, et al. Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. J Neurosci Res. 2003;73:364–8. doi: 10.1002/jnr.10668. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Xu H, Dyck LE, et al. Olanzapine and quetiapine protect PC12 cells from beta-amyloid peptide(25–35)-induced oxidative stress and the ensuing apoptosis. J Neurosci Res. 2005;81:572–80. doi: 10.1002/jnr.20570. [DOI] [PubMed] [Google Scholar]
- 14.Frey BN, Valvassori SS, Reus GZ, et al. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci. 2006;31:326–32. [PMC free article] [PubMed] [Google Scholar]
- 15.Frey BN, Andreazza AC, Kunz M, et al. Increased oxidative stress and DNA damage in bipolar disorder: a twin-case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:283–5. doi: 10.1016/j.pnpbp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Wang JF, Shao L, Sun X, et al. Increased oxidative stress in anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord. 2009;11:523–9. doi: 10.1111/j.1399-5618.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 17.Andreazza AC, Shao L, Wang JF, et al. Mitochondrial complex activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–8. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- 18.Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725–43. [Google Scholar]
- 19.Beyer JL, Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar Disord. 2002;4:89–104. doi: 10.1034/j.1399-5618.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 20.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–62. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 21.Dowlatshahi D, MacQueen G, Wang JF, et al. G protein-coupled cyclic AMP signaling in postmortem brain of subjects with mood disorders: effects of diagnosis, suicide, and treatment at the time of death. J Neurochem. 1999;73:1121–6. doi: 10.1046/j.1471-4159.1999.0731121.x. [DOI] [PubMed] [Google Scholar]
- 22.Knable MB, Barci BM, Webster MJ, et al. Stanley Neuropathology Consortium. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609–20. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- 23.Brégeon D, Sarasin A. Hypothetical role of RNA damage avoidance in preventing human disease. Mutat Res. 2005;577:293–302. doi: 10.1016/j.mrfmmm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Wu J, Deleo CJ. RNA damage and surveillance under oxidative stress. IUBMB Life. 2006;58:581–8. doi: 10.1080/15216540600946456. [DOI] [PubMed] [Google Scholar]
- 25.Nunomura A, Hofer T, Moreira PI, et al. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol. 2009;118:151–66. doi: 10.1007/s00401-009-0508-1. [DOI] [PubMed] [Google Scholar]
- 26.Shan X, Tashiro H, Lin CL. The identification and characterization of oxidized RNAs in Alzheimer’s disease. J Neurosci. 2003;23:4913–21. doi: 10.1523/JNEUROSCI.23-12-04913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong Q, Shan X, Chang Y, et al. RNA oxidation: a contributing factor or an epiphenomenon in the process of neurodegeneration. Free Radic Res. 2008;42:773–7. doi: 10.1080/10715760802311187. [DOI] [PubMed] [Google Scholar]
- 28.Beasley CL, Pennington K, Behan A, et al. Proteomic analysis of the anterior cingulate cortex in the major psychiatric disorders: evidence for disease-associated changes. Proteomics. 2006;6:3414–25. doi: 10.1002/pmic.200500069. [DOI] [PubMed] [Google Scholar]
- 29.Pennington K, Beasley CL, Dicker P, et al. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry. 2008;13:1102–17. doi: 10.1038/sj.mp.4002098. [DOI] [PubMed] [Google Scholar]
- 30.Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–97. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- 31.Martins-de-Souza D, Gattaz WF, Schmitt A, et al. Alterations in oligodendrocyte proteins, calcium homeostasis and new potential markers in schizophrenia anterior temporal lobe are revealed by shotgun proteome analysis. J Neural Transm. 2009;116:275–89. doi: 10.1007/s00702-008-0156-y. [DOI] [PubMed] [Google Scholar]
- 32.Kato T, Shioiri T, Murashita J, et al. Lateralized abnormality of high energy phosphate metabolism in the frontal lobes of patients with bipolar disorder detected by phase-encoded 31P-MRS. Psychol Med. 1995;25:557–66. doi: 10.1017/s003329170003347x. [DOI] [PubMed] [Google Scholar]
- 33.Deicken RF, Fein G, Weiner MW. Abnormal frontal lobe phosphorous metabolism in bipolar disorder. Am J Psychiatry. 1995;152:915–8. doi: 10.1176/ajp.152.6.915. [DOI] [PubMed] [Google Scholar]
- 34.Volz HP, Rzanny R, Riehemann S, et al. 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur Arch Psychiatry Clin Neurosci. 1998;248:289–95. doi: 10.1007/s004060050052. [DOI] [PubMed] [Google Scholar]
- 35.Moore CM, Christensen JD, Lafer B, et al. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry. 1997;154:116–8. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- 36.Kung L, Roberts RC. Mitochondrial pathology in human schizophrenic striatum: a post-mortem ultrastructural study. Synapse. 1999;31:67–75. doi: 10.1002/(SICI)1098-2396(199901)31:1<67::AID-SYN9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Gardner A, Johansson A, Wibom R, et al. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord. 2003;76:55–68. doi: 10.1016/s0165-0327(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 38.Regenold WT, Phatak P, Marano CM, et al. Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: implications for the mitochondrial dysfunction hypothesis. Biol Psychiatry. 2009;65:489–94. doi: 10.1016/j.biopsych.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munakata K, Tanaka M, Mori K, et al. Mitochondrial DNA 3644T to C mutation associated with bipolar disorder. Genomics. 2004;84:1041–50. doi: 10.1016/j.ygeno.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Sun X, Wang JF, Tseng M, et al. Down regulation in components of mitochondrial electron transport chain in post-mortem frontal cortex from subjects with bipolar disorder. J Psychiatry Neurosci. 2006;31:189–96. [PMC free article] [PubMed] [Google Scholar]
- 41.Konradi C, Eaton M, MacDonald ML, et al. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–8. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- 42.Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and nonelectron transport chain sources. Antioxid Redox Signal. 2005;7:1140–9. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- 43.Meyer JH, Ginovart N, Boovariwala A, et al. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. 2006;63:1209–16. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]