Abstract
Background
Severe mental disorders are associated with elevated levels of inflammatory markers. In the present study, we investigated whether osteoprotegerin (OPG), a member of the tumour necrosis factor receptor family involved in calcification and inflammation, is elevated in patients with severe mental disorders.
Methods
We measured the plasma levels of OPG in patients with severe mental disorders (n = 312; 125 with bipolar disorder and 187 with schizophrenia) and healthy volunteers (n = 239).
Results
The mean plasma levels of OPG were significantly higher in patients than in controls (t531 = 2.6, p = 0.01), with the same pattern in bipolar disorder and schizophrenia. The increase was significant after adjustment for possible confounding variables, including age, sex, ethnic background, alcohol consumption, liver and kidney function, diabetes, cardiovascular disease, autoimmune diseases and levels of cholesterol, glucose and C-reactive protein.
Limitations
Owing to the cross-sectional design, it is difficult to determine causality.
Conclusion
Our results indicate that elevated OPG levels are associated with severe mental disorders and suggest that mechanisms related to calcification and inflammation may play a role in disease development.
Introduction
The underlying pathological mechanisms of severe mental disorders (i.e., schizophrenia and bipolar disorder) are still largely unknown. These disorders are highly heritable complex genetic disorders that involve interplay between genetic and environmental factors.1 Traditionally, schizophrenia and bipolar disorders have been considered distinct clinical entities. This assumption has, however, been challenged by new findings of similar genetic and biological factors that support a continuum hypothesis with overlapping genetic and pathophysiologic mechanisms.2
Several lines of evidence have implicated the immune system in the development of severe psychiatric disorders. In schizophrenia, multiple studies have implicated infection during fetal life and childhood as possible contributing factors in the development of the disorder,3 and several reports have suggested low-grade systemic inflammation in adult patients with schizophrenia.4 Recent studies have also reported increased levels of inflammatory markers in bipolar disorder.5–7 Inflammatory factors are involved in the response to mental stress,8,9 and the role of these mediators of inflammation in neurotransmission and plasticity in the central nervous system has recently been revealed, further suggesting a link between inflammatory cytokines and psychiatric disorders.10–12
Osteoprotegerin (OPG) is a soluble member of the tumour necrosis factor receptor superfamily. It inhibits osteoclastogenesis by binding the receptor activator of nuclear factor-κB ligand (RANKL), acting as a decoy receptor to competitively inhibit RANKL interaction with its receptor RANK. The OPG–RANKL–RANK axis has been shown to have pleiotropic effects on bone metabolism, endocrine function and the immune system. Osteoprotegerin is involved in vascular calcification in atherosclerosis.13–16 High serum levels of OPG are associated with cardiovascular disease and are strongly predictive of long-term mortality in patients with acute coronary syndromes, even after adjustment for conventional risk markers.17–20 Osteoprotegerin has also been found to predict mortality in apparently healthy individuals.21
The relation between OPG and cardiovascular disease is of particular interest for severe mental disorders, for which recent evidence indicates increased cardiovascular risk factors22,23 and mortality rates.24,25 It has been speculated that there may be common mechanisms involved in severe mental disorders and cardiovascular disease, and altered inflammation could be one such mechanism.26
Based on the link between OPG and both inflammation and cardiovascular disorders, our aim was to investigate differences in OPG levels in a large and well-characterized population of patients with schizophrenia and bipolar disorder and sex- and age-matched healthy controls. Our hypothesis was that OPG levels would be elevated in patients with schizophrenia and bipolar spectrum disorder, without differences between the 2 disorders.
Methods
Participants
We included patients from the ongoing Thematic Organized Psychosis research study at the University Hospitals in the Oslo area between 2004 and 2007. We included patients aged 18 to 65 years who were registered with the psychiatric service of any one of the participating hospitals, those who met the DSM-IV criteria for schizophrenia or bipolar spectrum disorders and who were willing and able to give written informed consent. We excluded patients with a history of moderate or severe head injury and those with a neurologic disorder or mental retardation (intelligence quotient < 70). In the current analyses, we included consecutively referred patients with valid measurements of immunological markers and who were not using immunosuppressive drugs, non-steroidal anti-inflammatory drugs or statins.
We randomly selected a representative control group of healthy volunteers from the statistical records from the same catchment area as the patients. The controls were contacted by letter inviting them to participate. In the current analyses, we included controls who had no history of medical problems, severe psychiatric disorders including alcohol or illicit substance abuse or dependency, or severe mental disorders in close relatives. In total, 239 consecutively recruited healthy controls had valid measurements of immunological parameters and were thus included.
All participants gave written informed consent to participation. The study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate. The biobank was approved by the Norwegian Directorate of Health.
Assessments
Trained clinical research personnel (psychiatrists and clinical psychologists) assessed all patients. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)27 was used for diagnostic purposes, and global symptomatology and functioning was measured by use of the Global Assessment of Functioning Scale (GAF; split version).28 Interrater reliability was good, with an overall kappa score of 0.77 (95% confidence interval [CI] 0.60–0.94) for diagnoses and an intraclass correlation coefficient (type 1.1) of 0.86 for both symptom and function GAF scores.
The control participants were assessed by use of a structured interview and screened with the Primary Care Evaluation of Mental Disorders (PRIME-MD).29 For all participants, the use of alcohol during the last 2 weeks before assessment was recorded. Blood samples were drawn between 8 am and 5 pm. The clinical chemistry parameters were analyzed at the Department of Clinical Chemistry, Oslo University Hospital, Ulleval, Oslo, Norway, on an Integra 800 (Roche Diagnostics) with standard methods. For immunological analysis, blood was drawn using ethylenediaminetetraacetic acid vials; the plasma was extracted the next working day and was stored at −80°C in multiple aliquots and thawed fewer than 3 times before analysis.
Immunological measures
We quantified the plasma levels of OPG in duplicate by enzyme immunoassay using commercially available matched antibodies (R&D Systems). The intra- and interassay coefficients of variation were less than 11%.30 Plasma concentrations of C-reactive protein (CRP) were measured by enzyme immunoassay as described elsewhere.31
Statistical analyses
We performed all statistical analyses using SPSS software for Windows (version 15.0). All tests were 2-sided with a preset level of significance of 0.05. The OPG values were normally distributed. To test if the variance differed between groups, we used the Levene test for equality of variances. We performed a multiple linear regression analysis of the level of OPG to explore whether the group differences between patients and controls was due to confounding variables, which included age, sex, ethnic background, kidney function (creatinine), liver function (alanine aminotransferase), alcohol intake, autoimmune diseases, diabetes and glucose, known cardiovascular disease, and cholesterol and high-sensitivity CRP (hsCRP) levels. These variables were entered as independent variables, and group membership (patient v. control) was entered into the analysis during the final step. Differences in the level of OPG across the 2 diagnostic groups together with the controls were analyzed by use of a 1-way analysis of variance with a Tamhane post-hoc correction.
Results
Participants
We included a total of 312 patients; 187 with a DSM-IV diagnosis of schizophrenia spectrum disorder (schizophrenia, n = 143; schizophreniform, n = 11; schizoaffective disorder, n = 33) and 125 with a diagnosis of bipolar spectrum disorder (bipolar I disorder, n = 72; bipolar II disorder, n = 45; bipolar not otherwise specified, n = 8). The patients with bipolar disorder had a mean GAF score of 59 for symptoms and 57 for function. The patients with schizophrenia had GAF scores of 41 for symptoms and 42 for function. We included 239 consecutively recruited healthy controls.
There were no differences in age and sex between the patient group and the control group, but the controls had a significantly higher rate of European ancestry (Table 1). The schizophrenia group had higher proportion of men than the bipolar and control groups. At the time that blood samples were collected, 13% of patients in the bipolar disorder group and 7% of the schizophrenia group were not taking medication. None of the participants in the control group were taking any medications.
Table 1.
Participant characteristics
| Group; mean (SD)* |
||||
|---|---|---|---|---|
| Characteristic | Controls, n = 239 | All patients, n = 312 | Schizophrenia, n = 187 | Bipolar, n = 125 |
| Age, yr | 35.8 (11) | 34.3 (10) | 33.4 (12) | 35.7 (12) |
| Sex, % female | 56 | 50 | 42** | 62 |
| European descent, % | 99 | 81** | 76** | 90** |
| Education, yr | 14.2 (2.2) | 12.8 (2.5)** | 12.2 (2.4) | 13.6 (2.3) |
| Creatinine, μmol/L† | 71 (11) | 72 (15) | 71 (13) | 72 (17) |
| Alanine aminotransferase, U/L | 25 (18) | 29 (20)** | 30 (19) | 27 (21) |
| High-sensitivity CRP, mg/L | 0.78 (1.23) | 0.97 (1.85) | 0.87 (1.29) | 1.12 (2.45) |
| Cholesterol, mmol/L† | 5.1 (1.0) | 5.3 (1.1) | 5.3 (1.1) | 5.3 (1.2) |
| Glucose, mmol/L† | 4.99 (0.84) | 5.21 (1.34)** | 5.21 (1.36) | 5.20 (1.27) |
| Diabetes or cardiovascular disease, % of participants | 0 | 5 | 5 | 5 |
| Autoimmune disease, % of participants | 0 | 8.3 | 9.5 | 6.5 |
| Alcohol units consumed in the previous 2 weeksठ| 9 (10) | 9 (20) | 7 (17) | 13 (23) |
| Substance abuse, no. (%) of participants¶ | 0 | 23 (7) | 13 (7) | 10 (8) |
| Smoking, no (%) of participants | 0 | 168 (55) | 102 (56) | 66 (53) |
| GAF, symptom/function | — | 48/48 | 41/42 | 59/57 |
CRP = C-reactive protein; GAF = Global Assessment of Functioning;27 SD = standard deviation.
Unless otherwise indicated.
Data missing for 5 controls, 7 schizophrenia and 6 bipolar disorder patients.
One unit of alcohol = 10 mL.
Data missing for 28 controls, 11 schizophrenia and 2 bipolar disorder patients.
Use of illegal drugs in the previous 2 weeks.
p < 0.05, significantly different compared with controls.
In total, 70% of the patients (n = 216) were taking antipsychotics: olanzapine (n = 105, 34%), quetiapine (n = 24, 8%), ziprasidone (n = 18, 6%), risperidone (n = 16, 5%), aripiprazole (n = 13, 4%), perfenazine (n = 15, 4%), clozapine (n = 11, 4%), amisulpride (n = 3, 1%), zuclopenthixol (n = 4, 1%), haloperidol (n = 3, 1%) and chlorprothixene (n = 2, 1%). A total of 31% were taking mood-stabilizing agents: lamotrigine (n = 44, 14.1%), valproate (n = 26, 8.3%), lithium (n = 23, 7.4%), carbamazepine (n = 5, 1.3%) and topiramate (n = 1, 0.3%). A total of 32% were taking antidepressive medications: selective serotonin reuptake inhibitors (n = 65, 21%), venlafaxine (n = 21, 6.7%) and mirtazapine (n = 14, 4.5%).
Of the patients, 2% were regularly taking medications for somatic disorders (insulin, cetirizin, omeprazol, metoprolol and diclofenac). None used anticoagulants. Fifty-five percent of the patients were current smokers (56% of the schizophrenia patients, 54% of the bipolar disorder patients). We did not have information about the smoking habits of the controls, but among patients, there was no correlation between smoking and OPG levels. Of the patients, 7% had used illegal substances in the previous 2 weeks, 1% had diabetes, 4% had cardiovascular disease and 4% had an autoimmune disorder (autoimmune thyroiditis n = 8, psoriasis n = 2, ankylosing spondylitis n = 1, celiac disease n = 1, regional enterocolitis n = 1).
Osteoprotegerin levels
The mean OPG level was 10% higher in the patient group (mean 2.78 ng/mL, standard deviation [SD] 1.47 ng/mL) than in the control group (mean 2.52 [SD 0.92] ng/mL; t531 = 2.6, p = 0.011; Table 2). The comparison of OPG levels across the 3 groups (schizophrenia, bipolar disorders and controls) indicated a significant between-group difference (F550 = 3.1, p = 0.046); however, none of these group differences was statistically significant after correction for multiple testing (bipolar disorder v. controls, p = 0.08; schizophrenia v. controls, p = 0.20; schizophrenia v. bipolar disorder, p = 0.94).
Table 2.
Osteoprotegerin levels in study participants
| Group | Osteoprotegerin level, ng/mL, mean (SD) [25%–75% percentile] |
|---|---|
| Controls, n = 239 | 2.52 (0.92) [1.92–2.97] |
| All patients, n = 312 | 2.78* (1.47) [2.00–3.16] |
| Schizophrenia, n = 187 | 2.75 (1.50) [1.98–3.07] |
| Bipolar disorder, n = 125 | 2.84 (1.41) [2.04–3.22] |
Significantly different from controls (t test, p < 0.05).
We performed further analyses of the mean OPG levels according to diagnosis subtype: schizophrenia (n = 143), mean 2.72 (SD 1.36) ng/mL; schizophreniform (n = 11), mean 2.25 (SD 0.67) ng/mL; schizoaffective disorder (n = 33), mean 3.04 (SD 2.13) ng/mL; bipolar 1 (n = 72), mean 2.76 (SD 0.05) ng/mL; bipolar not otherwise specified (n = 8), mean 2.22 (SD 0.34) ng/mL; and bipolar 2 (n = 45), mean 3.06 (SD 0.92) ng/mL. Although there were some differences between the diagnostic subgroups, they were not statistically significant.
The regression analyses indicated that OPG levels were significantly elevated in the patient group compared with the control group after adjustment for possible confounders (t485,12, 473 = −1.97, p = 0.049; Table 3). There were slightly more women in the control group (56%) than in the patient group (50%). Women had higher OPG levels (mean 2.84 [SD 1.35] ng/mL) than men (mean 2.48 [SD 1.14] ng/mL; p = 0.001), but the group-by-sex interaction term was not significant in the whole sample (p = 0.35).
Table 3.
Multiple linear regression analysis of the relation between severe mental disorders and osteoprotegerin levels after adjustment for potential confounding variables*
| Variable | Standardized regression coefficient, β† | t | Significance‡ |
|---|---|---|---|
| Age | 0.11 | 2.17 | 0.029 |
| Sex | 0.15 | 2.80 | 0.030 |
| European background | −0.06 | −1.20 | 0.005 |
| Alcohol use in the previous 2 weeks | −0.03 | −0.74 | 0.231 |
| Creatinine level | −0.01 | −0.25 | 0.460 |
| Glucose level | 0.04 | 0.76 | 0.799 |
| Cholesterol level | 0.07 | 1.48 | 0.450 |
| Alanine transferase level | 0.06 | 1.14 | 0.141 |
| Autoimmune disease | 0.04 | 0.85 | 0.257 |
| Diabetes or cardiovascular disease | −0.10 | −2.10 | 0.398 |
| High-sensitivity CRP level | 0.15 | 3.33 | 0.036 |
| Group (severe mental disorders v. control) | −0.09 | −1.97 | 0.049 |
CRP = C-reactive protein.
n = 486 (285 patients and 201 controls). Osteoprotegerin is the dependent variable, and the possible confounding variables are shown as independent factors in the table.
Adjusted r2 for the whole model = 0.076. Degrees of freedom = 485 (12,473). F for the whole model = 4.3.
p < 0.001 for the model.
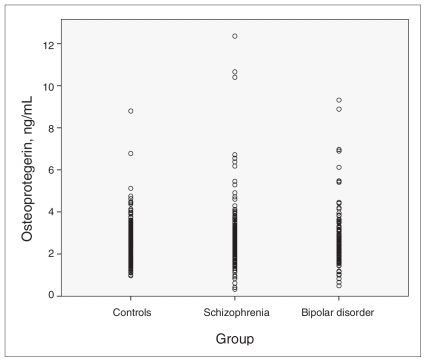
The known risk factors for cardiovascular disease, cholesterol and hsCRP, were weakly, but significantly, correlated with OPG level (cholesterol, r = 0.14; hsCRP, r = 0.17; p < 0.001). A diagnosis of cardiovascular disease or diabetes was not correlated with OPG level. Osteoprotegerin level was weakly negatively correlated with creatinine level (r = −0.09, p = 0.04). The variation in OPG levels was higher in patients than in controls (F = 9.5, p = 0.002; Fig. 1).
Fig. 1.
Distribution of the osteoprotegerin levels in individual patients, showing a higher variation among patients than among controls (p = 0.002).
Subgroup analyses
The use of antipsychotic drugs may be associated with inflammatory response.32 We therefore performed subgroup analyses by medication. Patients not taking antipsychotics (n = 80) had significantly higher OPG levels than did controls (mean 2.93 [SD 1.41] ng/mL v. mean 2.52 [SD 0.92] ng/mL; t102 = 2.4, p = 0.019). There was no difference in mean OPG level between patients using first- and second-generation antipsychotics (first generation, n = 25, mean 2.57 [SD 0.80] ng/mL; second generation, n = 192, mean 2.78 [SD 1.57] ng/mL; t259 = −0.65, p = 0.65). This suggests that medication use did not confound the results.
Patients who had used illegal drugs within the 2 weeks before testing did not have different OPG levels than the patients who had not used illegal drugs. Within the patient group as a whole, high OPG levels were positively correlated with age (r = 0.15, p < 0.001) and female sex (r = 0.15, p < 0.001).
The bipolar group included more women than men. Because both the women and bipolar patients tended to have higher OPG levels, we tested whether sex differences were confounding the differences in OPG levels between the schizophrenia and bipolar groups. Multiple linear regression analyses by OPG level in patients revealed that the group-by-sex interaction term was significant (t284 = 2.0, p = 0.049). However, there were no significant differences in OPG levels between the schizophrenia and bipolar groups after adjustment for group, sex and other potential confounders. Adding group-by-sex as a factor did not affect the result.
The levels of OPG were not significantly correlated with smoking status in patients. To further investigate whether the elevated OPG levels were confounded by smoking, we compared OPG levels in nonsmoking patients and controls. The OPG levels in nonsmoking patients (n = 136) were significantly higher than in the controls (mean 2.84 [SD 1.51] ng/mL v. mean 2.52 [SD 0.92] ng/mL; t194 = 2.2, p = 0.026). The non-smoking patients had similar OPG levels as patients who smoked (mean 2.74 [SD 1.47] ng/mL; t312 = 0.57, p = 0.58).
To test whether elevated body mass index (BMI) in the patient group was a confounding variable, we analyzed the subgroup of individuals for whom BMI was available (297 patients and 29 controls). There were no significant correlations between OPG levels and BMI (r = 0.03, p = 0.6). We compared OPG levels in nonobese participants (BMI < 30; n = 239 patients with mean BMI of 24.1 [SD 3.26]) with the control group. The patients with normal BMIs had significantly higher OPG levels than the controls (mean 2.79 [SD 1.5] v. mean 2.52 [SD 0.92]; t391 = 2.3, p = 0.021). We also controlled for BMI-related metabolic factors (i.e., glucose and cholesterol) in the analysis (Table 3).
Discussion
Our main finding was an increase in the plasma levels of OPG in patients with severe mental disorders, with a similar increase in schizophrenia and bipolar disorders. As we expected, OPG levels were correlated with age, sex, hsCRP and cholesterol but not with smoking or BMI. However, the association between OPG and severe mental disorders was independent of these potential confounding variables. Our findings add to those of an association between inflammation markers and severe mental disorders.4,6,33
To the best of our knowledge, this is the first study to address the levels of OPG in schizophrenia or bipolar disorder. The role of OPG has been investigated in other psychiatric disorders. One of these studies found that OPG levels were increased in young women with major depressive disorder,34 whereas another study reported reduced OPG levels in female patients with depression and a history of anorexia.35 However, in these studies, very few women were evaluated (i.e., 13 and 24, respectively). We included a large number of patients of both sexes, and our findings suggest that alterations in OPG levels could reflect or be involved in the pathophysiology of severe mental disorders. At present, however, how OPG is related to disease mechanisms is largely unknown.
Osteoprotegerin may be related to inflammatory endothelial processes resulting in calcification,36,37 which leads to cardiovascular disorders and may also affect the brain.13,15 In this way, OPG could affect the blood–brain barrier and induce neurotoxic effects, resulting in the smaller brain volumes that have been associated with OPG38 as well as with severe mental disorders.39,40
We found that OPG levels were correlated with cardiovascular risk factors such as cholesterol and hsCRP. The increase in OPG levels in the severe mental illness groups remained significant after adjustment for these factors. Patients without diabetes or cardiovascular disease did not have lower levels of OPG. Other studies involving patients with cardiovascular disorders from Scandinavia17,41,42 have reported OPG levels in similar ranges as ours. Thus, we previously reported that OPG levels comparable to those found in the patient groups in the present study (> 2.9 ng/mL) are associated with increased cardiovascular morbidity or mortality in patients with stroke or acute coronary syndrome.17,42 However, we have no prospective data, and further studies should examine whether OPG levels predict cardiovascular mortality in patients with severe mental disorders. Osteoprotegerin is a predictor of cardiovascular disease in apparently healthy individuals and in patients with acute coronary syndrome independent of other cardiovascular risk factors, and the levels of OPG are not correlated with smoking habits or BMI.17,21,43,44 It is tempting to hypothesize that increased OPG levels could also be related to the increased mortality from cardiovascular disease in severe mental disorders.22
The molecular mechanisms involved in the effect of OPG are largely unknown. However, OPG has been shown to promote matrix metalloproteinase (MMP)-9 activity, which was recently suggested to be involved in the pathogenesis of schizophrenia and bipolar disorder.45,46 Osteoprotegerin promotes MMP activity both directly and indirectly (i.e., by stabilizing RANKL activity).45,47,48
The role of OPG in calcium homeostasis could also be pathophysiologically relevant. Calcium signals regulate OPG expression,49,50 and a calcium antagonist traditionally used to prevent cardiovascular disease was recently found to have neuroprotective effects.51 Although speculative, any pathophysiological relevance of OPG could be is connected to cyclooxygenase (COX). Osteoprotegerin expression is related to COX-2 activity,52 and treatment with a COX-2 inhibitor has been shown to be an effective adjunctive treatment in schizophrenia.53 However, at present, the relation between calcium homeostasis, COX-1, OPG and mental disorders is unknown. Taken together, OPG level appears to be a stable and reliable marker not only of bone homeostasis and vascular calcification but also of inflammation, potentially mirroring 2 interacting pathogenic processes in severe mental disorders.
Limitations
The present study was cross-sectional, as are most studies in the field of immunology in psychiatry. This limits the discussion of causality. Although we controlled for many important confounding variables, we cannot rule out other confounders. Smoking could affect the findings, but it did not affect the OPG levels in patients. Antipsychotic medication has been found to be associated with both inflammatory and anti-inflammatory effects;54 thus, we cannot rule it out as a confounding variable. However, this is not likely because the levels of OPG were not correlated with medication use, and the unmedicated patients had higher levels of OPG than the medicated patients.
There was a larger standard deviation in OPG levels among patients than among controls. This could be of potential interest for understanding the role of OPG in severe mental disorders, and it should be further examined. It would also be of interest to study OPG in major depressive disorder, in which inflammation has been implicated.
Conclusion
The main result of the present study was an increase in the inflammatory marker OPG in severe mental disorders; this increase was present after adjustment for possible confounding factors. This further supports a role of immunological factors in the pathology of severe mental disorders; however, additional studies are needed to investigate the pathophysiological mechanisms.
Acknowledgements
The study was supported by a grant to the Thematic Organized Psychosis study group from the University of Oslo, the Research Council of Norway (no. 167153/V50, no. 163070/V50) and the South-East Norway Health Authority (no. 2004-123, no. 2007-050).
Footnotes
Competing interests: None declared.
Contributors: Drs. Hope, Melle and Andreassen conceived the study and its design and acquired and analyzed the data. Drs. Aukrust and Ueland contributed to the study conception and the analysis and interpretation of data. Drs. Agartz, Eiel Steen, Djurovic and Lorentzen contributed to data acquisition. Drs. Hope and Andreassen wrote the manuscript, which was reviewed by all other authors. All authors approved the final version submitted for publication.
References
- 1.Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9:527–40. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein P, Yip B, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AS. The risk for schizophrenia from childhood and adult infections. Am J Psychiatry. 2008;165:7–10. doi: 10.1176/appi.ajp.2007.07101637. [DOI] [PubMed] [Google Scholar]
- 4.Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–8. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Brietzke E, Kapczinski F. TNF-alpha as a molecular target in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1355–61. doi: 10.1016/j.pnpbp.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Kim YK, Jung HG, Myint AM, et al. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. 2007;104:91–5. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Padmos RC, Hillegers MH, Knijff EM, et al. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch Gen Psychiatry. 2008;65:395–407. doi: 10.1001/archpsyc.65.4.395. [DOI] [PubMed] [Google Scholar]
- 8.Webster Marketon JI, Glaser R. Stress hormones and immune function. Cell Immunol. 2008;252:16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Brydon L, Walker C, Wawrzyniak A, et al. Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain Behav Immun. 2009;23:217–24. doi: 10.1016/j.bbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–10. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- 11.McAfoose J. Evidence of a cytokine model for cognitive function. Neurosci Biobehav Rev. 2009;33:355–66. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Viviani B, Bartesaghi S, Corsini E, et al. Cytokines role in neurode-generative events. Toxicol Lett. 2004;149:85–9. doi: 10.1016/j.toxlet.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Corallini F, Rimondi E, Secchiero P. TRAIL and osteoprotegerin: A role in endothelial physiopathology? Front Biosci. 2008;13:135–47. doi: 10.2741/2665. [DOI] [PubMed] [Google Scholar]
- 14.Jorsal A, Tarnow L, Flyvbjerg A, et al. Plasma osteoprotegerin levels predict cardiovascular and all-cause mortality and deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetologia. 2008;51:2100–7. doi: 10.1007/s00125-008-1123-8. [DOI] [PubMed] [Google Scholar]
- 15.Frost ML, Grella R, Millasseau SC, et al. Relationship of calcification of atherosclerotic plaque and arterial stiffness to bone mineral density and osteoprotegerin in postmenopausal women referred for osteoporosis screening. Calcif Tissue Int. 2008;83:112–20. doi: 10.1007/s00223-008-9153-2. [DOI] [PubMed] [Google Scholar]
- 16.Baud’huin M, Lamoureux F, Duplomb L, et al. RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cell Mol Life Sci. 2007;64:2334–50. doi: 10.1007/s00018-007-7104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omland T, Ueland T, Jansson AM, et al. Circulating osteoprotegerin levels and long-term prognosis in patients with acute coronary syndromes. J Am Coll Cardiol. 2008;51:627–33. doi: 10.1016/j.jacc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 18.Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86:631–7. doi: 10.1210/jcem.86.2.7192. [DOI] [PubMed] [Google Scholar]
- 19.Anand DV, Lim E, Darko D, et al. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol. 2007;50:2218–25. doi: 10.1016/j.jacc.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Nybo M, Rasmussen LM. The capability of plasma osteoprotegerin as a predictor of cardiovascular disease: a systematic literature review. Eur J Endocrinol. 2008;159:603–8. doi: 10.1530/EJE-08-0554. [DOI] [PubMed] [Google Scholar]
- 21.Semb A, Ueland T, Aukrust P, et al. Osteoprotegerin and soluble receptor activator of nuclear factor-kappaB ligand and risk for coronary events: a nested case-control approach in the prospective EPIC-Norfolk population study 1993–2003. Arterioscler Thromb Vasc Biol. 2009;29:975–80. doi: 10.1161/ATVBAHA.109.184101. [DOI] [PubMed] [Google Scholar]
- 22.Birkenaes AB, Opjordsmoen S, Brunborg C, et al. The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study. J Clin Psychiatry. 2007;68:917–23. doi: 10.4088/jcp.v68n0614. [DOI] [PubMed] [Google Scholar]
- 23.Spelman LM, Walsh PI, Sharifi N, et al. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet Med. 2007;24:481–5. doi: 10.1111/j.1464-5491.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- 24.Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–50. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 25.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–31. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 26.Hanson DR, Gottesman II. Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet. 2005;6:7. doi: 10.1186/1471-2350-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Miriam G, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York (NY): Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 28.Pedersen G, Hagtvet KA, Karterud S. Generalizability studies of the Global Assessment of Functioning-Split version. Compr Psychiatry. 2007;48:88–94. doi: 10.1016/j.comppsych.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–56. [PubMed] [Google Scholar]
- 30.Ueland T, Bollerslev J, Godang K, et al. Increased serum osteoprotegerin in disorders characterized by persistent immune activation or glucocorticoid excess–possible role in bone homeostasis. Eur J Endocrinol. 2001;145:685–90. doi: 10.1530/eje.0.1450685. [DOI] [PubMed] [Google Scholar]
- 31.Wu TL, Tsao KC, Chang CP, et al. Development of ELISA on microplate for serum C-reactive protein and establishment of agedependent normal reference range. Clin Chim Acta. 2002;322:163–8. doi: 10.1016/s0009-8981(02)00172-9. [DOI] [PubMed] [Google Scholar]
- 32.Sugino H, Futamura T, Mitsumoto Y, et al. Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:303–7. doi: 10.1016/j.pnpbp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Drexhage R, Knijff E, Padmos R, et al. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10:59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- 34.Kahl KG, Greggersen W, Rudolf S, et al. Bone mineral density, bone turnover, and osteoprotegerin in depressed women with and without borderline personality disorder. Psychosom Med. 2006;68:669–74. doi: 10.1097/01.psy.0000237858.76880.3d. [DOI] [PubMed] [Google Scholar]
- 35.Kahl KG, Rudolf S, Dibbelt L, et al. Decreased osteoprotegerin and increased bone turnover in young female patients with major depressive disorder and a lifetime history of anorexia nervosa. Osteoporos Int. 2005;16:424–9. doi: 10.1007/s00198-004-1711-5. [DOI] [PubMed] [Google Scholar]
- 36.Guldiken B, Guldiken S, Turgut B, et al. Serum osteoprotegerin levels in patients with acute atherothrombotic stroke and lacunar infarct. Thromb Res. 2007;120:511–6. doi: 10.1016/j.thromres.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Strand M, Soderstrom I, Wiklund PG, et al. Polymorphisms at the osteoprotegerin and interleukin-6 genes in relation to first-ever stroke. Cerebrovasc Dis. 2007;24:418–25. doi: 10.1159/000108431. [DOI] [PubMed] [Google Scholar]
- 38.Jefferson AL, Massaro JM, Wolf PA, et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68:1032–8. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–66. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moorhead TW, McKirdy J, Sussmann JE, et al. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Knudsen ST, Foss CH, Poulsen PL, et al. Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. Eur J Endocrinol. 2003;149:39–42. doi: 10.1530/eje.0.1490039. [DOI] [PubMed] [Google Scholar]
- 42.Jensen JK, Ueland T, Atar D, et al. Osteoprotegerin concentrations and prognosis in acute ischaemic stroke. J Intern Med. 2009;267:410–7. doi: 10.1111/j.1365-2796.2009.02163.x. [DOI] [PubMed] [Google Scholar]
- 43.Hofsø D, Ueland T, Hager H, et al. Inflammatory mediators in morbidly obese subjects: associations with glucose abnormalities and changes after oral glucose. Eur J Endocrinol. 2009;161:451–8. doi: 10.1530/EJE-09-0421. [DOI] [PubMed] [Google Scholar]
- 44.Gannagé-Yared MH, Yaghi C, Habre B, et al. Osteoprotegerin in relation to bydy weight, lipid parameters insulin sensitivity, adipocytokines, and C-reactive protein in obese and non-obese young individuals: results from both cross-sectional and interventional study. Eur J Endocrinol. 2008;158:353–9. doi: 10.1530/EJE-07-0797. [DOI] [PubMed] [Google Scholar]
- 45.Rybakowski J, Skibinska M, Kapelski P, et al. Functional polymorphism of the matrix metalloproteinase-9 (MMP-9) gene in schizophrenia. Schizophr Res. 2009;109:90–3. doi: 10.1016/j.schres.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Rybakowski J, Skibinska M, Leszczynska-Rodziewicz A, et al. Matrix metalloproteinase gene and bipolar mood disorder. Neuromolecular Med. 2009;11:128–32. doi: 10.1007/s12017-009-8072-3. [DOI] [PubMed] [Google Scholar]
- 47.Theoleyre S, Wittrant Y, Couillaud S, et al. Cellular activity and signaling induced by osteoprotegerin in osteoclasts: involvement of receptor activator of nuclear factor kappaB ligand and MAPK. Biochim Biophys Acta. 2004;1644:1–7. doi: 10.1016/j.bbamcr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Ueland T, Yndestad A, Oie E, et al. Dysregulated osteoprotegerin/ RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461–8. doi: 10.1161/01.CIR.0000165119.62099.14. [DOI] [PubMed] [Google Scholar]
- 49.Takami M, Takahashi N, Udagawa N, et al. Intracellular calcium and protein kinase C mediate expression of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in osteoblasts. Endocrinology. 2000;141:4711–9. doi: 10.1210/endo.141.12.7852. [DOI] [PubMed] [Google Scholar]
- 50.Bergh JJ, Xu Y, Farach-Carson MC. Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel. Endocrinology. 2004;145:426–36. doi: 10.1210/en.2003-0319. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Hu X, Liu Y, et al. Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology. 2009;56:580–9. doi: 10.1016/j.neuropharm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Coon D, Gulati A, Cowan C, et al. The role of cyclooxygenase-2 (COX-2) in inflammatory bone resorption. J Endod. 2007;33:432–6. doi: 10.1016/j.joen.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Akhondzadeh S, Tabatabaee M, Amini H, et al. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–85. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 54.Drzyzga L, Obuchowicz E, Marcinowska A, et al. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun. 2006;20:532–45. doi: 10.1016/j.bbi.2006.02.002. [DOI] [PubMed] [Google Scholar]