Abstract
Background
Major depressive disorder is associated with both structural and functional alterations in the emotion regulation network of the central nervous system. The relation between structural and functional changes is largely unknown. Therefore, we sought to determine the relation between structural differences and functional alterations during the recognition of emotional facial expressions.
Methods
We examined 13 medication-free patients with major depression and 15 healthy controls by use of structural T1-weighted high-resolution magnetic resonance imaging (MRI) and functional MRI during 1 session. We set the statistical threshold for the analysis of imaging data to p < 0.001 (uncorrected).
Results
As shown by voxel-based morphometry, depressed patients had reductions in orbitofrontal cortex volume and increases in cerebellar volume. Additionally, depressed patients showed increased activity during emotion recognition in the middle frontal cortex, caudate nucleus, precuneus and lingual gyrus. Within this cerebral network, the orbitofrontal volumes were negatively correlated in depressed patients but not in healthy controls with changes in blood oxygen level–dependent signal in the middle frontal gyrus, caudate nucleus, precuneus and supplementary motor area.
Limitations
Our results are limited by the relatively small sample size.
Conclusions
This combined functional and structural MRI study provides evidence that the orbitofrontal cortex is a key area in major depression and that structural changes result in functional alterations within the emotional circuit. Whether these alterations in the orbitofrontal cortex are also related to persistent emotional dysfunction in remitted mental states and, therefore, are related to the risk of depression needs further exploration.
Introduction
Major depression is marked by negative mood and specific cognitive and behavioural alterations. Neuropsychologic studies have shown that depressed patients have a significant bias toward sad emotions.1 It has been reported that depressed patients interpret happy faces as neutral and neutral faces as sad.2 The processing of facial emotional expressions is an important factor for intact social functioning and communication, and major depression is associated with dysfunctional processing of faces. These findings from neuropsychologic studies are not linked to those from structural and functional magnetic resonance imaging (fMRI) studies.
Although previous region-of-interest studies have provided evidence for volume reductions in patients with major depression relative to healthy controls3 in the dorsolateral prefrontal cortex,4 orbitofrontal cortex,5–7 subgenual prefrontal cortex,8 anterior cingulate cortex,9,10 hippocampus,11,12 amygdala9,13 and basal ganglia,14,15 the number of voxel-based morphometry studies is still limited. Using voxel-based morphometry, Shah and colleagues16 showed reduced grey matter density in the left temporal cortex in patients with chronic depression and changes in the left hippocampus in patients with treatment-resistant depression.17 Right-sided hippocampal reductions18 and significant bilateral reductions of volume in the orbitofrontal cortex6 have been observed.
The findings of a recent voxel-based morphometry study support a relation between grey matter volume reduction, depressive symptoms and cognitive dysfunction.19 In this study,19 decreased grey matter volume of the hippocampus and cingulate cortex in patients relative to healthy controls was associated with executive dysfunction as measured by the Wisconsin Card Sorting Test. A progressive decline of grey matter density in depressed patients was shown in the hippocampus, anterior cingulum, left amygdala and right dorsomedial prefrontal cortex among patients with major depression and among those with a relatively bad illness course, indicating that depressive episodes are associated with neuroplastic brain changes,20 as formulated in the neuroplasticity hypothesis.21 However, the functional consequences of these structural alterations have not been thoroughly addressed. Some studies have found that hippocampal volume reduction is associated with increased executive dysfunction22 and poorer performance in verbal memory tasks.16,23
With regard to emotion recognition and processing, functional imaging studies have shown increased activation of the amygdala when either masked happy or fearful faces are presented.24 Surguladze and colleagues25 detected higher blood oxygen level–dependent (BOLD) responses to sad facial expressions within the fusiform gyrus, putamen and parahippocampal gyrus, whereas a similar fMRI investigation found decreased activity in patients relative to healthy controls in the left amygdala, ventral striatum and frontoparietal cortex.26 Chen and colleagues27 observed increased activity in the pre- and postcentral gyri, middle temporal gyrus, parahippocampal gyrus, striatum and brainstem of bipolar patients with depression after the presentation of fearful faces. In our previous study that investigated affective facial processing of sad and angry faces in medicated depressed patients by fMRI, depressed patients showed increased activity in the left middle cingulum and right prefrontal cortex relative to healthy controls.28 Recently, we showed that the connectivity between the orbitofrontal cortex and the cingulate, precuneus and cerebellar region is decreased and that connectivity between the orbitofrontal cortex and the dorsolateral prefrontal cortex and motor regions is increased in drug-free patients with major depression, indicating a functional dysbalance.29
To the best of our knowledge, there has been only one study that combined fMRI and voxel-based morphometry in patients with major depression.30 This study showed that hyperactivation of the anterior cingulate cortex of unmedicated patients during a Stroop task was inversely correlated with grey matter reduction in the orbitofrontal cortex.30 In the present study, which was based on a similar methodological approach, we used an emotional recognition task to analyze the correlation between structural and functional alterations in medication-free patients with major depression. Our aim was to investigate whether structural changes in the orbitofrontal cortex are associated with functional changes in the brain regions of the emotion regulation network.
Methods
Participants
We recruited 13 inpatients with major depression from the Department of Psychiatry of the Ludwig-Maximilians-University, Munich. Psychiatric diagnoses based on DSM-IV criteria and on the clinical version of the Structured Clinical Interview for DSM-IV (SCID-CV)31 were determined by consensus of at least 2 psychiatrists. All patients had been medication-free for at least 1 year before they attended our psychiatric service for treatment because of an acute episode.
We documented clinical variables using the Hamilton Rating Scale for Depression (HAM-D)32 on the day of the scan. We excluded patients and controls with a previous head injury with loss of consciousness, previous use of a cortisol medication, previous alcohol or substance abuse, neurologic diseases, aged less than 18 or more than 65 years, and those who were pregnant. We also excluded those with other mental illnesses and personality disorders.
We matched the patients with 15 healthy controls with respect to age, sex and handedness. We assessed demographic variables, medical history, trauma and other exclusion criteria in a structured interview. Neither the healthy controls nor their first-degree relatives had a history of neurologic or mental illness. Handedness for all participants was determined by use of the Edinburgh Handedness Inventory.33
After an extensive description of the study to the patients and controls, written informed consent was obtained. The study was approved by the research ethics committee of the Ludwig-Maximilians-University and was prepared in accordance to the ethical standards of the Declaration of Helsinki.
Emotional paradigm
We conducted 2 runs (one implicit and one explicit; the order was random). Stimuli consisted of faces from Gur and colleagues.34 The face-matching task was as described by Hariri and colleagues,35 with adjustments. We made changes with respect to the type of emotion, and we used explicit as well as implicit conditions. Because showing differences in implicit processing was a main aim of our previous study and because we found more subcortical areas activated in the implicit task,36 we also used this version in the present study. Instead of using sad and anxious faces, we included sad and angry faces because we wanted to focus on clear major depression without comorbidity of anxiety.
In total, 48 triplets of emotional faces (sad or angry) were arranged in a block design, resulting in 8 blocks of 6 triplets each; these blocks were presented interspersed with 9 control blocks. The control blocks consisted of 6 triplets each and presented simple geometrical, white shapes (squares, triangles, circles, ellipses). In the explicit trial, each triplet contained either 3 female or 3 male faces. Participants were instructed to choose which faces agreed in their emotional expression (Fig. 1). For the implicit trial, each triplet contained 1 male or female face as the target and 2 other faces of mixed sex (Fig. 1). Participants were asked to determine the sex that matched the target face. The target faces alternately showed angry and sad emotions.
Fig. 1.
Examples of the explicit and implicit triplets and the control stimuli used in this emotional paradigm.
Participants gave their responses with an fMRI-compatible response pad (Lumina LP-400 Response Pads for fMRI; Cedrus Corporation) using 2 keys to choose the right or left face in the lower line of the triplet. Each triplet was presented for 5.3 seconds, resulting in a total scan length of about 9 minutes for each trial (8 blocks with emotional faces, 9 control blocks with geometrical shapes). The order of the trials (explicit, implicit) and of the presented target stimuli was random.
Image acquisition
We acquired functional images on a 3-T MRT scanner (Signa HDx; GE Healthcare) using a T2*-weighted gradient echoplanar imaging sequence (repetition time [TR] 2100 ms, echo time [TE] 35 ms, flip angle 90°, matrix 64 × 64, field of view [FOV] 256 × 256 mm). Two functional runs of 265 contiguous volumes were acquired. The volumes comprised 37 axial slices of 4 mm thickness, covering the whole brain. Slices were positioned to the connecting line between anterior and posterior commissure.
We acquired structural T1-weighted MRI scans within the same session using a 3-dimensional fast spoiled gradient echo sequence (TR 6.9 ms, TE 3.2 ms, flip angle 15°, matrix 256 × 256, FOV 220 mm, slice thickness 1.4 mm, 248 slices).
Statistical analyses
Behavioural data
We calculated behavioural performance differences between healthy controls and depressed patients by use of SPSS. We analyzed the data separately for the implicit and explicit trials using 2-sample t tests for reaction time and errors.
Structural data
We used grey matter volume to measure between-group anatomic differences. The VBM5 toolbox (http://dbm.neuro.uni-jena.de/vbm/) uses the approach of Good and colleagues,37 which facilitates an optimal segmentation and registration of MRI scans to the standardized anatomic space of the Montreal Neurological Institute. Optimized voxel-based morphometry consists of a 2-step procedure starting with the construction of a study-specific, whole-brain template and grey matter, white matter and cerebrospinal fluid before accounting for the magnetic field properties of the scanner and the anatomic properties of the study cohort.
In the second step, the customized template and tissue priors are used for data segmentation, registration to and resegmentation in standard space. Thus, intersubject global brain size differences were removed, and homologous brain regions were brought into alignment.
Optimized voxel-based morphometry ensures the removal of a maximum number of nonbrain voxels from the data. The VBM5 toolbox extends the existing algorithms because it increases the quality of segmentation by applying a hidden Markov random field model to the segmented tissue classes at both steps of the protocol.38 We used only the grey matter volume maps for statistical analysis after smoothing with an 8-mm full-width at half-maximum Gaussian kernel.
Functional data
We used Statistical Parametric Mapping (SPM5) in MATLAB (MathWorks) with the following preprocessing steps: realigning all volumes to the sixth scan to correct for participant motion (exclusion criteria: more than 3 mm), coregistration of the functional and structural data sets, spatial normalization into a standard stereotactic space, using a Montreal Neurological Institute template and smoothing the data with an 8-mm isotropic Gaussian kernel. We calculated statistical parametric maps based on a voxel-by-voxel method using a general linear model.39
In the first-level analysis, the statistical design matrix included 2 sessions (implicit and explicit) with 2 regressors. Each regressor consisted of a boxcar function convolved with an estimated hemodynamic response function. Thus, the expected hemodynamic response to the experimental stimulus was modelled using the relative contributions of a delayed boxcar reference wave function as well as confounding variables (whole-brain activity and low-frequency variations). After parameter estimation, contrast images were constructed for explicit triplets contrasted with control stimuli and implicit triplets contrasted with control stimuli. Each participant’s contrast images were entered into the second-level analysis, which used 2-sample t tests for intergroup differences (differences between patients and controls) and 1-sample t tests for intragroup analysis (activation only in patients or only in controls) to show mean activations from the contrasts.
Structural and functional data
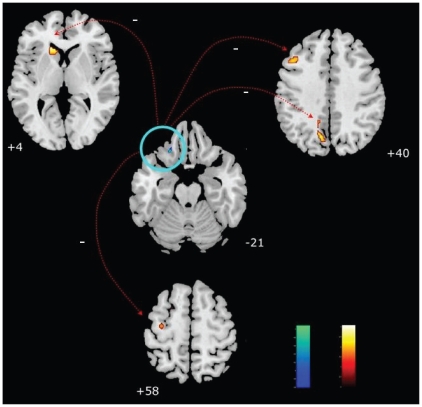
To test the effects of structural alterations in depressed patients on BOLD signals, we extracted the first eigenvariate from the most significant differences in grey matter volume. We performed a correlation analysis between the functional signal changes and these eigenvariates in SPM5. Next, the eigenvariates from the significant results of the correlation analysis were extracted to calculate the correlation coefficients by the use of SPSS of functional and structural data (Fig. 2).
Fig. 2.
Negative correlation of the orbitofrontal cortex volume reduction (blue) and cerebral activity of dorsolateral prefrontal cortex, caudatus, precuneus and supplementary motor area during emotion recognition (orange) in patients with major depression (p < 0.001, uncorrected).
We used the WFU PickAtlas40 to create a mask that included all areas that showed an intergroup difference in cerebral activity. We used this as an exclusive mask in the regression analysis. The orbitofrontal cortex volumes were used as dependent variables, and the BOLD activations in the whole brain were used as explanatory variables. First, we performed separate regression analyses in depressed patients and healthy controls to investigate the relation between measures of structural volume and cerebral activity. Next, we compared the regression slopes for depressed patients and healthy controls by means of a 2-sample t test in SPM5. Additionally, we performed a correlation analysis for the depressed patients’ scores on the HAMD and their cerebral activity.
For all analyses, age and sex were covariates of no interest. All results were based on a statistical threshold of p < 0.001 (uncorrected). The anatomic localization of significant voxels was identified using the SPM toolbox’s automated anatomic labelling, which was described by Tzourio-Mazoyer and colleagues.41
Results
Participants
Five patients had recurrent depression, and 8 had a first depressed episode (Table 1). The patients with recurrent depression had either received medication or psychotherapy during a previous episode (1 patient received mirtazapine, which was stopped 1 year before the scan; 1 patient received doxepine, which was stopped 2 years before the scan; 1 patient had taken an antidepressant 15 years before but could not remember its name; and 2 patients received psychotherapeutic treatment during their last episode). A clear positive family history of affective disorders was found in 3 patients.
Table 1.
Anamnestic and behavioural characteristics of patients and healthy controls
| Group; mean (SD) |
||||
|---|---|---|---|---|
| Characteristic | Patients | Healthy controls | p value | t26 |
| Age, yr | 37.9 (10.1) | 35.5 (10.9) | 0.56 | 0.60 |
| Sex, male:female | 10:3 | 10:5 | 0.7* | |
| No. of right-handed participants | 13 | 15 | ||
| Illness duration, mo | 52.3 (71.5) | — | ||
| No. of episodes, mean | 1.45 (0.68) | — | ||
| DSM-IV diagnosis, no. of participants | — | |||
| 296.21 | 2 | |||
| 296.22 | 5 | |||
| 296.23 | 1 | |||
| 296.31 | 1 | |||
| 296.32 | 2 | |||
| 296.33 | 2 | |||
| Hamilton Rating Scale for Depression32 score | 20.5 (4.7) | — | ||
| Explicit task | ||||
| Reaction time, ms | 2671 (549) | 2514 (565) | 0.48 | 0.73 |
| No. of correct answers | 37 (7) | 38 (7) | 0.67 | −0.44 |
| Implicit task | ||||
| Reaction time, ms | 1463 (508) | 1628 (657) | 0.49 | −0.71 |
| No. of correct answers | 43 (7) | 44 (5) | 0.82 | −0.24 |
SD = standard deviation.
The χ2 test was used to test the difference in sex distribution.
Patients and healthy controls did not differ significantly with regard to age or sex (Table 1). Their reaction times for the explicit and implicit conditions and the number of correct answers did not differ significantly.
Functional differences
Intragroup analysis for the healthy control sample showed activity in the left hippocampus and insula, frontal lobe including the orbitofrontal cortex, right temporal and occipital gyri and parietal cortex (inferior parietal gyrus and precuneus; Table 2). Intragroup analysis for the patients showed an activity pattern similar to that in the healthy controls, but it also included the right thalamus, lingual gyri, left precentral gyrus and cerebellum.
Table 2.
Between-group results (p < 0.001) for healthy controls and patients for structural and functional differences
| Comparison; difference | Region | Maxima MNI coordinates |
t | Cluster size, k | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Patients > healthy controls | ||||||
| Functional difference | Left middle frontal gyrus | −20 | −16 | 46 | 4.43 | 276 |
| Left precentral gyrus | −56 | 12 | 36 | 4.22 | 63 | |
| −44 | −2 | 42 | 3.51 | 5 | ||
| Left caudate nucleus | −24 | 14 | 26 | 3.74 | 23 | |
| Left supplementary motor area | −16 | −10 | 52 | 4.25 | 276 | |
| −10 | 2 | 50 | 3.68 | 8 | ||
| Left precuneus | −22 | −46 | 16 | 3.58 | 10 | |
| Left lingual gyrus | −30 | −50 | 0 | 3.57 | 13 | |
| Structural difference | Right cerebellum* | 13 | −50 | −24 | 4.47* | 540 |
| 42 | −82 | −31 | 3.68 | 75 | ||
| Right rolandic operculum | 53 | −5 | 17 | 4.27 | 209 | |
| Right superior frontal gyrus | 21 | −9 | 56 | 3.60 | 58 | |
| Right precuneus | 18 | −61 | 47 | 3.35 | 27 | |
| Left inferior frontal gyrus | −35 | 39 | 11 | 3.33 | 20 | |
| Right amygdala | 23 | 1 | −17 | 3.29 | 32 | |
| Healthy controls > patients | ||||||
| Structural difference | Left inferior frontal gyrus pars orbitalis* | −22 | 28 | −21 | 5.09* | 677 |
| Left inferior frontal operculum | −47 | 14 | 13 | 4.82 | 338 | |
| Left middle temporal gyrus | −63 | −50 | −4 | 4.72 | 401 | |
| Right postcentral gyrus | 64 | −15 | 17 | 4.29 | 183 | |
| Left inferior parietal gyrus | −35 | −76 | 40 | 4.08 | 198 | |
| Left superior temporal gyrus | −65 | −44 | 19 | 4.05 | 77 | |
| Left postcentral gyrus | −49 | −11 | 38 | 3.97 | 142 | |
| Left rolandic operculum | −51 | −24 | 14 | 3.94 | 70 | |
| Right inferior occipital | 44 | −80 | −11 | 3.70 | 80 | |
| Left inferior frontal | −51 | 24 | −1 | 3.67 | 30 | |
| Left inferior temporal | −58 | −63 | −14 | 3.65 | 23 | |
MNI = Montreal Neurological Institute.
Structural differences with the highest t values.
Relative to the healthy controls, the patients showed increased activity in the left hemisphere, namely in the middle frontal gyrus, supplementary motor area, precentral gyrus, caudate nucleus, precuneus and lingual gyrus. We did not observe increased activity in the healthy controls compared with patients (Table 2).
Structural differences
The most pronounced structural reductions in patients relative to controls were detected in the left inferior orbitofrontal cortex (Table 2). Patients showed reductions in the left inferior frontal operculum, left temporal lobe, postcentral gyri, right gyrus angularis, left inferior parietal gyrus, left rolandic operculum and right occipital lobe.
Patients also showed also increased volumes relative to controls; the increased volume was most pronounced in the right cerebellum. The intergroup comparison showed volumetric increases in patients in the right rolandic operculum, right superior frontal gyrus, right precuneus, left inferior frontal gyrus and right amygdala.
Functional and structural data
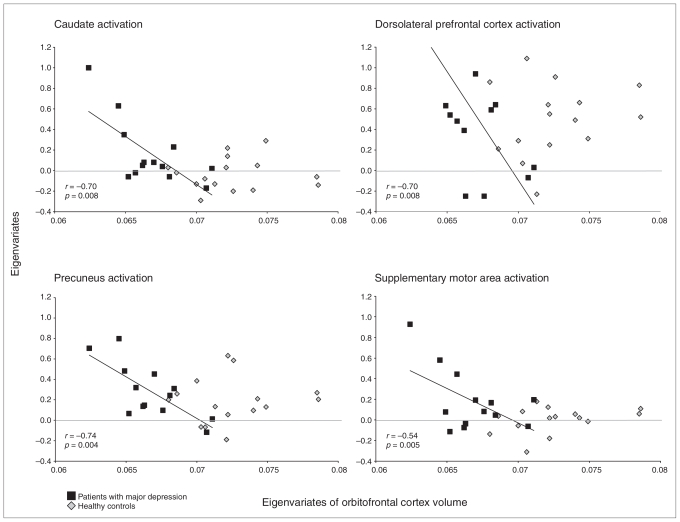
Healthy controls showed no correlations between functional activity and the extracted eigenvariates from the structural between-group analysis (from the left inferior orbitofrontal cortex and the right cerebellum; Table 3). The patients showed a significant negative correlation between the extracted eigenvariates of the orbitofrontal cortex and functional activity in the left caudate nucleus, left precuneus, left middle frontal gyrus, left supplementary motor area and left precentral gyrus (Fig. 2). The negative correlation remained significant in the direct comparison of structural–functional associations between patients and controls for all regions except the left precentral gyrus (Fig. 3).
Table 3.
Regression analysis between structural volume in the left orbitofrontal cortex and right cerebellum and cerebral activity in patients
| Comparison; region | Region of activity | Maxima MNI coordinates |
t | Cluster size, k | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Patients | ||||||
| Left orbitofrontal cortex | −22 | 28 | −21 | |||
| Left caudate nucleus | −16 | 26 | 4 | 5.09 | 53 | |
| Left precuneus | −12 | −52 | 46 | 4.70 | 414 | |
| −12 | −66 | −40 | 4.56 | 414 | ||
| −16 | −56 | 36 | 3.71 | 2 | ||
| Left middle frontal gyrus | −42 | 24 | 36 | 4.51 | 224 | |
| −20 | 6 | 56 | 3.89 | 15 | ||
| Left supplementary motor area | −12 | 2 | 60 | 4.12 | 80 | |
| −4 | −2 | 66 | 3.78 | 80 | ||
| −12 | −8 | 52 | 3.82 | 2 | ||
| −12 | 6 | 46 | 3.76 | 2 | ||
| Left precentral | −34 | −12 | 58 | 3.85 | 30 | |
| Right cerebellum | 13 | −50 | −24 | |||
| Left supplementary motor area | −4 | −2 | 66 | 3.90 | 1 | |
| Left precuneus | −12 | −50 | 46 | 3.56 | 1 | |
| Healthy controls > patients* | ||||||
| Left orbitofrontal cortex | −22 | 28 | −21 | |||
| Left precuneus | −12 | −52 | 48 | 4.78 | 227 | |
| Left supplementary motor area | −4 | −2 | 66 | 3.82 | 13 | |
| −12 | 4 | 60 | 3.66 | 13 | ||
| Left caudate nucleus | −16 | 26 | 4 | 3.78 | 7 | |
| Left middle frontal gyrus | −42 | 22 | 38 | 3.75 | 19 | |
MNI = Montreal Neurological Institute.
puncorrected < 0.001.
Fig. 3.
Scatterplots and correlation coefficients (r) for the association between orbitofrontal cortex volume and cerebral activity of the dorsolateral prefrontal cortex, caudatus, precuneus and supplementary motor area for patients with major depression and healthy controls. Note that the regression slopes were significantly different between patients with major depression and healthy controls in the statistical parametric mapping analysis. The regression slope is shown only for the patients with major depression, because the slope was not significant for the controls.
Patients showed a negative correlation between the extracted eigenvariates of the right cerebellum and functional activity in the left supplementary motor area and left precuneus. This result was not significant for the intergroup comparison.
Correlation with depression severity
No significant correlation was detected in the patient group for cerebral activity and HAM-D score. There was no significant correlation between the extracted eigenvariates of the orbitofrontal cortex and the HAM-D score in the patient group (r = −0.28, p = 0.35).
Discussion
The current perspective about pathophysiology of major depression assumes that the connections between brain regions and their functional interplay are more relevant than changes within isolated single regions. To the best of our knowledge, this is one of the first studies within the field of major depression to address this issue. We found that reductions in orbitofrontal cortex volume in major depression are associated with functional alterations in the network of affect regulation. This functional consequence was specific to patients with major depression and was not seen in healthy controls. The orbitofrontal cortex, therefore, seems to be a key area for major depression with major connectivity to other areas of the mood regulation network. In particular, dysfunctional frontosubcortical circuits may play an essential role in major depression.
These results are specific to the areas involved in processing of the face-matching task used in our study. The use of a working memory task might have shown stronger associations between orbitofrontal cortex volume and prefrontal cortical regions.
The frontal cortex is crucial for human behaviour. Five frontosubcortical circuits have been described.42 Their basis is a common origin in the prefrontal cortex, a subsequent projection to the striatum, a pursued connection to the globus pallidus and substantia nigra, a final connection to the thalamus and ending with a final loop to the frontal cortex to close the circuit. The orbitofrontal cortex plays a major role in the lateral orbitofrontal circuit, which originates in Brodmann areas (BAs) 10 and 11. Subsequently, there are connections to the caudate nucleus, globus pallidus, thalamus and finally back to the orbitofrontal cortex. It is assumed that this circuit connects the frontal system to the limbic structures.43 Therefore, disturbances might cause changes in social behaviour and emotional experiences.
Our main focus was to study the association between structural and functional alterations in major depressive disorders. The analysis of structural data showed the greatest reduction in volume in patients relative to controls in BA 11, a part of the orbitofrontal cortex. Depressive disorders are associated with reductions of the orbitofrontal cortex, as shown by our data and demonstrated in previous investigations.7 Decreases in grey matter in the orbitofrontal cortex with 32% volume reduction have been demonstrated by Bremner and colleagues7 and region-of-interest studies.5,44 These results are also supported by a voxel-based morphometry analyses in elderly patients with depression,6 which revealed prominent bilateral reductions in the orbitofrontal cortex.
With regard to functional disturbances, we found in-creased activity during emotional processing in patients compared with healthy controls in the middle frontal cortex, caudate nucleus, precuneus and supplementary motor area. No significant differences were observed in task performance, indicating that these differences were because of changes in brain function rather than because of different motivation of participants to carry out the task. The lack of differences in task performance (with respect to errors) might be because of the low level of difficulty of the presented task. Thus, participants made zero or few mistakes. Additionally, the instructions did not indicate that the participants should react as fast as possible, which might explain the similar reaction times between the healthy controls and patients.
Although there is a broad consensus that depressive disorders are associated with altered cerebral functioning during emotional processing, previous results are not consistent.24,25,28 This can be explained by the use of different emotional stimuli (happy, neutral, angry, fearful or sad faces; fearful or neutral scenes). In contrast, it has been clearly shown that antidepressant treatment leads to significant changes in cerebral functioning.45,46
Lee and colleagues47 demonstrated reduced activation in depressed patients compared with healthy controls in the dorsolateral prefrontal cortex and orbitofrontal cortex, hippocampus and caudate nucleus when sad faces were presented and reduced activity in the orbitofrontal cortex when angry faces were presented. But if only patients receiving antidepressive treatment are included, it is impossible to rule out the effects of medication in connection with cerebral activity. Anand and colleagues48 observed increased activity of the anterior cingulate cortex, insula, parahippocampus, amygdala and anteromedial prefrontal cortex when only unmedicated depressed patients were presented with negative pictures from the International Affective Picture System. She-line and colleagues24 detected increased cerebral activity in the amygdala by performing a region-of-interest analysis in unmedicated patients, which normalized after antidepressant treatment, by presenting masked happy and fearful faces. A further study25 reported increased signals in the fusiform gyrus, parahippocampal gyrus and basal ganglia when depressed patients receiving pharmacologic treatment were compared with healthy controls during the processing of sad facial expressions.
In contrast to the findings of other studies, we found that BOLD responses in the amygdala were not greater in patients than in controls. Increased responses in the amygdala to masked fearful faces,49 sad faces50 and sad pictures51 have been reported. In our tasks, the participants probably used more visual and cognitive strategies to solve the task so that amygdala activation may have been inhibited by the anterior cingulate cortex and prefrontal cortices. Another explanation is that angry faces do not activate the amygdala to the same extent as fearful faces, as shown in a recent study.52
To the best of our knowledge, there have been no studies of emotional processing that combine structural and functional data, although an interdependency of structural and functional changes in psychiatric disorders seems obvious. Depressive disorders are associated with volume reductions of the orbitofrontal cortex, a critical region in emotional processing. Our study provides a clear link between structural reductions and altered cerebral functioning during the processing of emotional stimuli. One previous study that investigated cognitive control processes also found a significant link between structural reductions of the orbitofrontal cortex and altered cerebral function.30 Using the Stroop task to investigate cognitive control, the authors found that the activity of the ACC was not correlated with orbitofrontal cortex volume in healthy controls. In contrast, they found a negative correlation between orbitofrontal cortex volume and anterior cingulate cortex activation in medication-free patients. Our data also provide evidence for a negative correlation between orbitofrontal cortex volume and cerebral activity, namely in the left hemisphere in the precuneus, supplementary motor area, caudate nucleus and middle frontal gyrus.
Heterogeneous laterality effects have been reported in patients with major depression depending on the task used. For example, fMRI studies showed different results with respect to dorsolateral prefrontal cortex activation. Decreased right activity in the dorsolateral prefrontal cortex was detected in a study using an attentional interference task in 27 patients with major depression compared with 24 healthy controls.53 Activity in the left dorsolateral prefrontal cortex was reduced during cognitive (digit sorting) and emotional (personal relevance rating of words) tasks in 30 patients with major depression compared with 28 healthy controls.54 Increased right dorsolateral prefrontal cortex activity was found in response to painful stimuli in 13 patients with major depression compared with 13 healthy controls55 and during a Tower of London task and an n-back task in 13 patients with major depression compared with controls.56 In contrast, increased left prefrontal activation was detected in a working memory task in 12 patients with major depression compared with 17 healthy volunteers57 and in the face-matching task in the present study.
The association between orbitofrontal cortex and caudate nucleus, both of which are part of the lateral orbitofrontal circuit that connects the frontal system with the limbic structures,43 seems plausible within the context of altered emotional experiences. However, the contiguity between orbitofrontal cortex volume and activity in the precuneus and supplementary motor area is more surprising.
The precuneus, which is part of the medial parietal cortices and has various connections with other cortical and subcortical areas, facilitates the integration between external and internal information that marks human mental activity.58 As shown in animal studies, there is a strong connection of the precuneus with the frontal lobes (mainly BA 8, 9 and 46) as well as with the supplementary motor area.59,60 Additionally, there are reciprocal connections between the orbitofrontal cortex and other prefrontal areas (BA 9 and 46).61 Dense connections of the precuneus and the caudate nucleus have been described.60 Functional activity of the precuneus has been observed in the context of visuospatial imagery, episodic memory, self-processing and consciousness.58 This broad functionality underlines the role of the precuneus in many highly integrated mental functions that are not limited to simple visuospatial processing. This assumption is supported by fMRI studies investigating social cognition62 and emotional state attribution.63 Therefore, previous research with regard to anatomic connectivity has helped to clarify the nature of structural reductions of the orbitofrontal cortex in depressed patients and the association with increased cerebral activity in the reported structures in the context of emotional processing.
Limitations
There are several limitations of our study that need to be addressed. First the sample size was relatively small, and we found the effects with a statistical threshold of p < 0.001 uncorrected for multiple comparisons. Additionally, there might be task-order effects because the explicit and implicit trials were presented in a random order for each participant. Unfortunately, our results were not significant after correction for multiple comparison (e.g., false discovery rate or family-wise error). Therefore, the results have to be considered as being preliminary, and replication is warranted. Other analysis techniques such as dynamic causal modelling might be helpful for this in a further step, since we now have a hypothesis about the association between orbitofrontal cortex structure and function.
Different fMRI tasks (e.g., cognitive or emotional paradigms) and differences in patient samples (e.g., medication use, comorbidity, depression severity) may have contributed to the heterogeneity of findings in the field. In our study, we used medication-free patients and an adjusted emotional face matching task, which may have also contributed to differences shown in our study compared with previous studies. Nevertheless, the patients included in the present study, including those with recurrent depression and first-episode depression, had limited clinical homogeneity. In general, fMRI studies investigating emotional functioning represent a limited aspect of real emotional processes, because static emotional pictures and the artificial environment of MRI investigations cannot consider dynamic and reciprocal interactions that are an important part of emotional and social action and communication in every day life. This result raises questions about the neuroanatomical connections of the orbitofrontal cortex and cerebral regions as detected by the correlation analysis.
Conclusion
Dysfunctional emotional processing (e.g., a negative bias in perceptional or mnestic function) represents a therapeutically relevant aspect in the treatment of major depression. Merging the assumptions of a key role of the orbitofrontal cortex in emotional processing and the fact that the experience and processing of emotions is one of the central aspects for all kinds of psychotherapies,64 raises the question as to whether cerebral alterations represent a biological long-term vulnerability or whether they are the consequence of depressive symptoms. A recent fMRI study found functional differences during emotional categorization in people at high risk of major depression with no personal history of depressive episodes,65 suggesting that cerebral changes represent a vulnerability marker. Nevertheless, further studies are needed to clarify if psychotherapeutic interventions such as emotion-focused therapy, which is based on the integration and differentiation of emotional processes (emotional awareness and arousal, emotion regulation, reflection on emotion, and emotion transformation66), could provide a promising tool to influence structural and functional neurobiological changes within the context of emotional processing in patients with major depression.
We conclude that the orbitofrontal cortex is a key region within the neurobiological framework of depressive disorders. Structural abnormalities within the orbitofrontal cortex may be related to persistent emotional dysfunction. Emotional dysfunction is related to vulnerability to major depression.65,67 Further studies should investigate how brain structure and function as well as how clinical and epidemiological characteristics of this disease are associated.
Acknowledgement
This study was supported by the Eli Lilly International Foundation.
Footnotes
Competing interests: None declared for Drs. Frodl, Linn, Meisen-zahl, Koutsouleris, Brückmann, Schöpf, Wiesmann and Scheuerecker and Mr. Roesner. Dr. Möller has received research grants or support from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Eisai, Glaxo-SmithKline, Janssen Cilag, Lundbeck, Merck, Novartis, Organon, Pfizer, Sanofi Aventis, Sepracor, Servier, Wyeth. He has served as a consultant or on advisory boards for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen Cilag, Lundbeck, Organon, Pfizer, Sepracor, Servier and Wyeth, and he is a member of the speaker bureau for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Eisai, GlaxoSmithKline, Janssen Cilag, Lundbeck, Organon, Pfizer, Sanofi Aventis and Sepracor.
Previously published at www.jpn.ca
Contributors: Drs. Koutsouleris, Meisenzahl and Frodl conceived the study and its design. Drs. Frodl, Scheuerecker, Schöpf, Wiesmann, and Brückmann and Mr. Roesner acquired the data, which was analyzed by Drs. Linn, Frodl and Scheuerecker. The article was written by Drs. Frodl, Scheuerecker and Koutsouleris; it was critically revised by Drs. Frodl, Meisenzahl, Koutsouleris, Wiesmann, Brück-mann, Schöpf and Linn and Mr. Roesner. All authors approved the final version submitted for publication.
References
- 1.Gotlib IH, Krasnoperova E, Yue DN, et al. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:121–35. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 2.Persad SM, Polivy J. Differences between depressed and nondepressed individuals in the recognition of and response to facial emotional cues. J Abnorm Psychol. 1993;102:358–68. doi: 10.1037//0021-843x.102.3.358. [DOI] [PubMed] [Google Scholar]
- 3.Frodl T, Moller HJ, Meisenzahl E. Neuroimaging genetics: New perspectives in research on major depression? Acta Psychiatr Scand. 2008;118:363–72. doi: 10.1111/j.1600-0447.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.Coffey CE, Wilkinson WE, Weiner RD, et al. Quantitative cerebral anatomy in depression. A controlled magnetic resonance imaging study. Arch Gen Psychiatry. 1993;50:7–16. doi: 10.1001/archpsyc.1993.01820130009002. [DOI] [PubMed] [Google Scholar]
- 5.Lacerda ALT, Keshavan MS, Hardan AY, et al. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol Psychiatry. 2004;55:353–8. doi: 10.1016/j.biopsych.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Ballmaier M, Toga AW, Blanton RE, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- 7.Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–9. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 8.Botteron KN, Raichle ME, Drevets WC, et al. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–4. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 9.Hastings RS, Parsey RV, Oquendo MA, et al. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–9. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- 10.Caetano SC, Kaur S, Brambilla P, et al. Smaller cingulate volumes in unipolar depressed patients. Biol Psychiatry. 2006;59:702–6. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–8. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 12.Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caetano SC, Hatch JP, Brambilla P, et al. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 2004;132:141–7. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan KR, McDonald WM, Escalona PR, et al. Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Arch Gen Psychiatry. 1992;49:553–7. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- 15.Husain MM, McDonald WM, Doraiswamy PM, et al. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40:95–9. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- 16.Shah PJ, Ebmeier KP, Glabus MF, et al. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry. 1998;172:527–32. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- 17.Shah PJ, Glabus MF, Goodwin GM, et al. Chronic, treatment-resistant depression and right frontostriatal atrophy. Br J Psychiatry. 2002;180:434–40. doi: 10.1192/bjp.180.5.434. [DOI] [PubMed] [Google Scholar]
- 18.Bell-McGinty S, Butters MA, Meltzer CC, et al. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159:1424–7. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 19.Vasic N, Walter H, Höse A, et al. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord. 2008;109:107–16. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Frodl TS, Koutsouleris N, Bottlender R, et al. Depression-related variation in brain morphology over 3 years: Effects of stress? Arch Gen Psychiatry. 2008;65:1156–65. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- 21.Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 22.Frodl T, Schaub A, Banac S, et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci. 2006;31:316–23. [PMC free article] [PubMed] [Google Scholar]
- 23.Hickie I, Naismith S, Ward PB, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- 24.Sheline YI, Barch DM, Donnelly JM, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 25.Surguladze S, Brammer MJ, Keedwell P, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–9. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Fu CHY, Williams SCR, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–89. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 27.Chen C-H, Lennox B, Jacob R, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–9. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Frodl T, Scheuerecker J, Albrecht J, et al. Neuronal correlates of emotional processing in patients with major depression. World J Biol Psychiatry. 2009;10:202–8. doi: 10.1080/15622970701624603. [DOI] [PubMed] [Google Scholar]
- 29.Frodl T, Bokde AL, Scheuerecker J, et al. Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol Psychiatry. 2010;67:161–7. doi: 10.1016/j.biopsych.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Wagner G, Koch K, Schachtzabel C, et al. Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. J Psychiatry Neurosci. 2008;33:199–208. [PMC free article] [PubMed] [Google Scholar]
- 31.First Michael B, Spitzer Robert L, Miriam Gibbon, Williams Janet BW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington (DC): American Psychiatric Press, Inc; 1996. [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 34.Gur RC, Schroeder L, Turner T, et al. Brain activation during facial emotion processing. Neuroimage. 2002;16:651–62. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- 35.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 36.Scheuerecker J, Frodl T, Koutsouleris N, et al. Cerebral differences in explicit and implicit emotional processing — an fMRI study. Neuropsychobiology. 2007;56:32–9. doi: 10.1159/000110726. [DOI] [PubMed] [Google Scholar]
- 37.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 38.Cuadra MB, Cammoun L, Butz T, et al. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging. 2005;24:1548–65. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- 39.Friston KJ, Frith CD, Turner R, et al. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2:157–65. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- 40.Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 41.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 42.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 43.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–54. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 44.Lai T, Payne ME, Byrum CE, et al. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–5. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- 45.Fu CHY, Williams SCR, Brammer MJ, et al. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry. 2007;164:599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- 46.Keedwell P, Drapier D, Surguladze S, et al. Neural markers of symptomatic improvement during antidepressant therapy in severe depression: subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. J Psychopharmacol. 2009;23:775–88. doi: 10.1177/0269881108093589. [DOI] [PubMed] [Google Scholar]
- 47.Lee B-T, Seok J-H, Lee B-C, et al. Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:778–85. doi: 10.1016/j.pnpbp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Anand A, Li Y, Wang Y, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an fMRI study. Neuropsychopharmacology. 2005;30:1334–44. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- 49.Sheline YI, Barch DM, Donnelly JM, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 50.Fu CH, Williams SC, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–89. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 51.Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–88. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 52.Fitzgerald DA, Angstadt M, Jelsone LM, et al. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–8. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siegle GJ, Thompson W, Carter CS, et al. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 55.Bar KJ, Wagner G, Koschke M, et al. Increased prefrontal activation during pain perception in major depression. Biol Psychiatry. 2007;62:1281–7. doi: 10.1016/j.biopsych.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Fitzgerald PB, Srithiran A, Benitez J, et al. An fMRI study of pre-frontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp. 2008;29:490–501. doi: 10.1002/hbm.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walter H, Wolf RC, Spitzer M, et al. Increased left prefrontal activation in patients with unipolar depression: an event-related, parametric, performance-controlled fMRI study. J Affect Disord. 2007;101:175–85. doi: 10.1016/j.jad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 58.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 59.Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–16. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- 60.Leichnetz GR. Connections of the medial posterior parietal cortex (area 7m) in the monkey. Anat Rec. 2001;263:215–36. doi: 10.1002/ar.1082. [DOI] [PubMed] [Google Scholar]
- 61.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Farrow TF, Zheng Y, Wilkinson ID, et al. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12:2433–8. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- 63.Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 64.Lambert ML. Bergin and Garfield’s handbook of psychotherapy and behavior change. 5th ed. New York (NY): Wiley; 2004. [Google Scholar]
- 65.Chan SWY, Harmer CJ, Goodwin GM, et al. Risk for depression is associated with neural biases in emotional categorisation. Neuropsychologia. 2008;46:2896–903. doi: 10.1016/j.neuropsychologia.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 66.Bischkopf J, Auszra L, Herrmann I. Emotionsfokussierte therapie. Psychodynam Psychother. 2008;3:171–6. [Google Scholar]
- 67.Chan SW, Goodwin GM, Harmer CJ. Highly neurotic never-depressed students have negative biases in information processing. Psychol Med. 2007;37:1281–91. doi: 10.1017/S0033291707000669. [DOI] [PubMed] [Google Scholar]