Abstract
Background
Although there is considerable evidence that patients with schizophrenia have impaired executive functions, the neural mechanisms underlying these deficits are unclear. Generation and selection is one of the basic mechanisms of executive functioning. We investigated the neural correlates of this mechanism by means of functional magnetic resonance imaging (fMRI) in patients with schizophrenia and healthy controls.
Methods
We used the Wisconsin Card Sorting Test (WCST) in an event-related fMRI study to analyze neural activation patterns during the distinct components of the WCST in 36 patients with schizophrenia and 28 controls. We focused our analyses on the process of set-shifting. After participants received negative feedback, they had to generate and decide on a new sorting rule.
Results
A widespread activation pattern encompassing the inferior and middle frontal gyrus, parietal, temporal and occipital cortices, anterior cingulate cortex (ACC), supplementary motor area, insula, caudate, thalamus and brainstem was observed in patients with schizophrenia after negative versus positive feedback, whereas in healthy controls, significant activation clusters were more confined to the cortical areas. Significantly increased activation in the rostral ACC after negative feedback and in the dorsal ACC during matching after negative feedback were observed in schizophrenia patients compared with controls. Controls showed activation in the bilateral dorsolateral prefrontal cortex (Brodmann area 46), whereas schizophrenia patients showed activation in the right dorsolateral pre-frontal cortex only.
Limitations
All patients were taking neuroleptic medication, which has an impact on cognitive function as well as on dopaminergic and serotonergic prefrontal metabolism.
Conclusion
Our data suggest that, in patients with schizophrenia, set-shifting is associated with increased activation in the rostral and dorsal ACC, reflecting higher emotional and cognitive demands, respectively.
Introduction
In a constantly changing environment, executive abilities allow individuals to shift their minds quickly and to adapt to diverse situations while simultaneously repressing inappropriate behaviours. Thus, executive functions encompass a series of high-level processes (e.g., set-shifting, inhibitory processes and feedback management).1 In schizophrenia, impaired executive functioning is the basis of a number of general theories of cognitive dysfunction,2 and performance on executive tests has been correlated with functional outcomes.3,4 The Wisconsin Card Sorting Test (WCST)5 is one of the most widely used tests to evaluate executive functioning, and deficits in WCST performance have most consistently been reported in patients with schizophrenia.6,7 Performance on the WCST is related most strongly to shifting functions. Prentice and colleagues8 hypothesized that specific deficits in the processing of negative feedback are responsible for impaired WCST performance in patients with schizophrenia.
Early studies on the neural correlates of executive functioning stemmed from observations of patients with frontal lesions. More recently, neuroimaging studies have shown the involvement of the prefrontal cortex in executive functions. In a meta-analysis of WCST neuroimaging studies in healthy participants,9 there was a cross-study trend of bilaterally clustered activity in the inferior frontal gyrus, anterior cingulate cortex (ACC) and inferior parietal lobe. Using event-related new imaging techniques, neural activation associated with different stages of WCST performance has been further scrutinized. As one of the prominent mental subcomponents, set-shifting was found to be related to a frontoparietal activation pattern involving the inferior frontal gyrus.10 In contrast, when participants received negative feedback indicating the need for a cognitive shift, neuronal activity was observed in a cortical–subcortical loop comprising the prefrontal and parietal cortices and basal ganglia.11 In participants with schizophrenia, functional magnetic resonance imaging (fMRI) studies reported reduced activation of the prefrontal cortex during WCST performance,12,13 and hypofrontality was described as the most salient activation difference in patients with schizophrenia compared with controls in single-photon emission computed tomography studies.14
To the best of our knowledge, this study is the first to use event-related fMRI to investigate activation patterns during different subcomponents of the WCST in patients with schizophrenia and healthy controls. Rule generation and selection have been proposed as elements of executive functioning with a well-founded basis in cognitive neuroscience research.1 Thus, we focused our analyses on the set-shifting component of the WCST. During this period, participants received negative feedback, indicating that they needed to reconsider their choice of category and that they needed to match the next card according to a new dimension. Separate analyses of the feedback and matching periods allowed the simultaneous assessment of 2 different aspects of set-shifting.
We predicted that distinctive activation patterns related to set-shifting would be observed in a cortical–subcortical network comprising the prefrontal, temporal and parietal cortical areas, basal ganglia and thalamus in schizophrenia patients and controls. In view of the imaging literature involving healthy participants,10,11,15–17 we believed that that the ACC, inferior frontal and superior temporal gyrus would be most explicitly related to set-shifting and that differential activations between patients and controls would be most prominent in these areas. On the basis of increasing evidence of complex prefrontal hyper- and hypoactivation patterns related to cognitive performance in patients with schizophrenia and our finding of a significant positive correlation between N-acetylaspartate concentration in the ACC and WCST performance,18 we expected stronger activations in the ACC in patients compared with controls. Because the dorsolateral prefrontal cortex is involved in set-shifting,15,16 we also focused on this brain area and expected to observe hypofrontality in patients with schizophrenia.
Methods
Participants
We recruited 39 patients with schizophrenia from the Department of Psychiatry of the University of Muenster, and we recruited 31 healthy controls by advertisements in local newspapers. All participants had normal vision, were right-handed (Edinburgh Handedness Inventory19) and were native German speakers. We excluded controls with any lifetime history of a psychiatric disorder (Structured Clinical Interview for DSM Disorders [SCID-I]20) or any first-degree relative with a psychiatric disorder. We excluded from both groups people with neurologic disorders, brain damage, serious head injury, substance abuse or dependence, and any contraindication for MRI scanning. We excluded 1 patient and 1 control because of diagnostic reasons, 2 patients because of intolerance of the scanner and 2 controls because they did not perform the task correctly. We confirmed the diagnoses of schizophrenia by chart review and consultation with the treating clinician, and we excluded patients with any history of other psychiatric disorders. At the time of testing, all patients were stabilized after an acute psychotic episode with low psychopathology scores (Positive and Negative Syndrome Scale [PANSS]21 ). The final sample consisted of 36 patients who met the DSM-IV criteria for schizophrenia (SCID-I) and 28 controls. Neuropsychologic testing included the vocabulary component of the Wechsler Adult Intelligence Scale revised,22 the Auditory Verbal Learning Test23 and the Trail Making Test A and B.24
Participants gave written informed consent before participating in the study, which was approved by the local ethics committee (Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Medizinischen Fakultät der Westfälischen Wilhelms Universität Münster). To ensure that patients with schizophrenia were able to give informed consent, comprehend the study design and successfully complete the WCST in the scanner, we consulted the treating psychiatrist about the performance of patients in, for example, psychoeducational classes.
Wisconsin Card Sorting Test
Participants performed a computerized version of the WCST that was displayed on a projector–mirror system during the scan (Sharp XG-PC10XE with additional high-frequency shielding). The stimuli consisted of the 4 standard reference cards (1 red triangle, 2 green stars, 3 yellow crosses and 4 blue circles) displayed throughout the complete task in the upper section of the screen, with test cards presented in a random order in the lower section of the screen. According to the standard WCST procedure,6 participants were instructed to match the test card to 1 of the 4 reference cards. By trial and error, participants had to identify the correct sorting rule (colour, number of objects or shape). After 8 subsequent correct responses, the matching dimension changed without notice, and participants had to change their sorting dimension (set shift). Before scanning, participants had been trained on a 64-card version of the WCST. The session in the scanner started with 15 practice trials, in which the test cards displaying the letters A, B, C or D had to be matched to the identical reference card (identity matching task). Bilateral keypads with 2 keys each were used for responses. The experiment ended after 6 successfully completed dimensional changes.
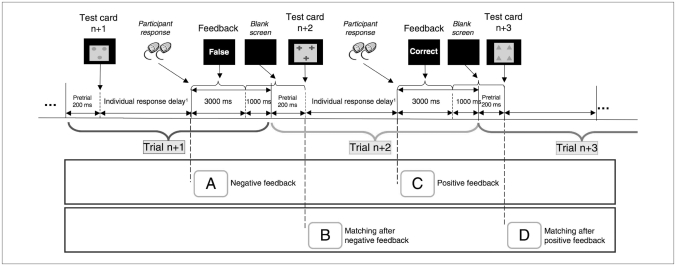
Each trial consisted of 2 periods (Fig. 1). The first period started with the presentation of a new test card. The length of this matching period depended on the participant’s response time (group mean of individual mean response times, patients: mean 1802.6 [SD 484.3] ms; controls: mean 1602.7 [SD 440.4] ms; t62 = −1.704, p = 0.09). As soon as the participants had made a decision, the second period started, and written feedback (“correct” or “false”) appeared for 3000 ms followed by a blank screen (1200 ms). The average total inter-stimulus interval was 4200 ms + 1802.6 ms (mean response delay) in patients and 4200 ms + 1602.7 ms (mean response delay) in controls (i.e., 6.0 s in patients and 5.8 s in controls). Two successive feedback or 2 successive matching periods were separated by an average of 2 repetition times (repetition time 3.0 s), thereby allowing separation of the hemodynamic response. Variable lengths of individual response delays result in a temporal jitter, which optimizes the analyses of event-related fMRI designs.
Fig. 1.
Wisconsin Card Sorting Test paradigm. Each trial starts with the presentation of a new card followed by an individual response period during which participants decide on the sorting rule. Directly after their responses, participants are informed if the response was correct or incorrect. For group analyses, 4 different events were defined: (A) receiving negative feedback, indicating that a shift is required; (B) matching after negative feedback (i.e., the execution after the required set shift); (C) receiving positive feedback, indicating that the current matching criterion is still adequate; and (D) matching after positive feedback (i.e., the execution of matching according to the current rule).
To investigate activation patterns during the different stages of the WCST, we defined 4 events:25 receiving negative feedback, indicating that a shift was required (event A); matching after negative feedback (i.e., the execution after the required set shift; event B); receiving positive feedback, indicating that the current matching criterion was still adequate (event C); and matching after positive feedback (i.e., the execution of matching according to the current rule; event D).
Owing to the overall low number of errors, we did not perform functional analyses separately for the different error types. We defined events C and D as starting at the onset of the third correctly matched card, thus eliminating randomly correct responses and forming a continuous epoch of recalling and maintaining the previous sorting rule. Because of the individual response delay, timing of the WCST was manually controlled.
Image acquisition and analysis
We acquired T2* functional data by use of a 3-T scanner (Gyroscan Intera 3T, Philips Medical Systems). Functional scans were obtained by use of a single-shot echo-planar sequence with parameters selected to minimize distortion while retaining adequate signal-to-noise ratios and T2* sensitivity (repetition time 3000 ms, echo time 35 ms, flip angle 90°, field of view 224 mm). Forty contiguous axial slices (slice thickness 3.5 mm, matrix 642, resolution 3.5 mm3 voxels) were obtained parallel to the anterior commissure–posterior commissure line. To avoid T1-effects, we applied 4 “dummy” scans before running the experimental set. We obtained structural images by use of a high-resolution T1-weighted 3-dimensional sequence (isotropic voxel 0.5 mm3).
Statistical analyses
We realigned the functional imaging data to minimize motion-related artifacts, implementing a set of 6 rigid body transformations determined for each image, using Statistical Parametric Mapping (SPM5; Wellcome Trust Centre for Neuroimaging, Institute of Neurology, University College of London). The images were spatially normalized to the standard Montreal Neurological Institute space (MNI) and smoothed (Gaussian kernel, 6-mm full-width at half-maximum).
We performed statistical analyses by modelling the different response conditions as variables within the context of a fixed-effects single-subject general linear model convolved with a standard hemodynamic response function.26,27 Individual events were identified by retrospectively analyzing and relating stimulus-generating personal-computer protocols to the fMRI data. We calculated activation data (t maps) for each participant for the different events. The analysis contrasted activation related to negative feedback versus positive feedback (event A v. event C) and to matching after negative feedback versus matching after positive feedback (event B v. event D). The between-group differences in the number of scans was not statistically significant (total number of scans, patients: 143.9 [SD 27.6]; controls: 129.8 [SD 31.3]; t62 = 1.918, p = 0.06; correct trials, patients: 57.0 [SD 7.0]; controls: 55.8 [SD 7.4]; t62 = 0.668, p = 0.51; incorrect trials, patients: 14.9 [SD 9.1]; controls: 11.0 [SD 6.2]; t62 = 1.981, p = 0.05). The percentage of scans of incorrect trials was entered as covariate in the second-level analyses. Analyses with this covariate revealed no effect on the results (i.e., the same brain areas had significant between-group activation differences with only marginal differences regarding cluster sizes and Z values).
We entered subject-specific contrasts, reflecting either the degree of feedback-related activation or matching-related activation, into a random-effects analysis, and within-group activation patterns were assessed by 1-sample t tests. For all 1-sample t tests, the significance level was set at p < 0.05 for corrected p values based on family-wise error (FWE) control, with clusters defined by at least 8 contiguous voxels of significant response. All 2-sample t tests were performed at a significance level of p < 0.001 (uncorrected), with clusters defined by at least 8 contiguous voxels. For additional illustrations, regions of interest (ROIs) were defined of all brain areas revealing significant activation differences between patients and controls. The voxel values of these regions were extracted and summarized by mean using the MarsBaR toolbox.28
Because reduced activity in the dorsolateral prefrontal cortex (DLPFC) has been repeatedly shown in patients with schizophrenia,29 we applied an additional hypothesis-driven ROI analysis in the DLPFC (Brodmann area [BA] 46 and 9, Wake Forest University PickAtlas; p < 0.05, FWE-corrected was considered significant).
Anatomic labels of reported coordinates (transformed from MNI to Talairach space) of peak clusters were retrieved from the Talairach Daemon database.30 We analyzed demographic and performance data for between-group differences using Student t tests, Pearson χ2 tests and univariate analyses of variance (ANOVA; SPSS 15.0). We considered p < 0.05, 2-tailed, to be significant.
Results
Participants
The clinical characteristics and neuropsychologic measures of the participants are shown in Table 1. All but 2 patients were taking atypical antipsychotic medications at a stable dose for at least 2 weeks before scanning (chlorpromazine equivalent dose: mean 545.9 mg/d, standard deviation [SD] 400.5 mg/d; atypical neuroleptics: quetiapine n = 16, risperidone n = 8, clozapine n = 6, amisulpride n = 6, aripiprazole n = 5, olanzapine n = 4, ziprasidone n = 2; combination of atypical neuroleptics n = 15; and typical neuroleptics: perazine n = 1, flupenthixol n = 1).
Table 1.
Clinical characteristics and neuropsychologic measures in patients with schizophrenia and healthy controls
| Group; mean (SD)* |
||||
|---|---|---|---|---|
| Characteristic or measure | Schizophrenia, n = 36 | Control, n = 28 | Statistic | p value |
| Age, yr | 27.6 (7.4) | 30.7 (8.0) | ||
| Sex, male:female | 20:16 | 15:13 | χ12 = 0.025 | 0.87 |
| Education, yr | 12.0 (1.6) | 12.2 (1.3) | t62 = 0.659 | 0.51 |
| Duration of illness, mo | 42.9 (61.0) | — | — | — |
| Positive and Negative Syndrome Scale21 score | ||||
| Sum | 59.3 (13.7) | — | — | — |
| Positive scale | 11.6 (4.1) | — | — | — |
| Negative scale | 16.0 (4.7) | — | — | — |
| General psychopathology scale | 31.4 (8.4) | — | — | — |
| WAIS-R vocabulary, percentile rank | 62.6 (32.1) | 72.3 (21.5) | t62 = 1.373 | 0.18 |
| Auditory Verbal Learning Test23 score | ||||
| Immediate recall | 45.4 (9.8) | 56.5 (7.9) | t62 = 4.870 | 0.001 |
| Delayed recall | 8.8 (3.0) | 11.7 (2.1) | t62 = 4.389 | 0.001 |
| Recognition | 11.3 (3.4) | 13.3 (3.0) | t62 = 2.443 | 0.001 |
| Trail Making Test A,24 s | 36.5 (12.1) | 26.9 (8.1) | t62 = −3.556 | 0.001 |
| Trail Making Test B,24 s | 78.7 (30.8) | 54.2 (17.3) | t62 = −3.708 | 0.001 |
SD = standard deviation; WAIS-R = Wechsler Adult Intelligence Scale.22
Unless otherwise indicated.
Activation after negative versus positive feedback
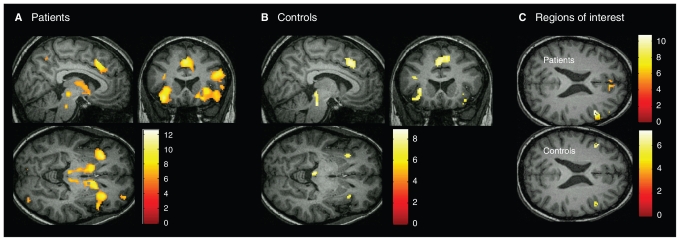
Within-group analyses showed that brain activation after negative feedback, indicating that a shift was required, was contrasted with activation after positive feedback in each group. Patients with schizophrenia showed extensive right-lateralized activation patterns in the inferior and middle frontal gyrus (BA 44/45), ACC (BA 32), temporal, parietal and occipital cortices, insula, left supplementary motor area (BA 6), bilateral caudate nucleus, left thalamus, cerebellum, midbrain brainstem and pons (Fig. 2A). In controls, increased activation was more confined and encompassed bilateral inferior and middle frontal gyrus (BA 44/45), insula, supplementary motor area (BA 6), parietal cortex and midbrain brainstem (Fig. 2B). Additional ROI analysis showed a significant activation in the right BA 46 in both groups (schizophrenia: x, y, z = 46, 36, 21; 250 voxels, Z = 6.95; controls: x, y, z = 48, 24, 29; 34 voxels, Z = 4.73; FWE-corrected). In the left BA 46, a significant activation was found in the controls only (x, y, z = −42, 28, 23; 45 voxel, Z = 5.33; FWE-corrected; Fig. 2C). Brodmann area 9 was bilaterally activated in both groups.
Fig. 2.
Regions of increased brain activation after negative feedback (set-shifting) versus positive feedback (1-sample t tests, p < 0.05, family-wise error–corrected). All activations were rendered on a single subject template in Montreal Neurological Institute space. (A) Schizophrenia patients (n = 36) had activation clusters in the inferior and medial frontal gyrus, anterior cingulate cortex, supplementary motor area, insula, basal ganglia and thalamus. (B) Controls (n = 28) had more confined activation clusters in the inferior and medial frontal gyrus, supplementary motor area, insula and superior and inferior parietal lobes. (C) Region-of-interest analysis of the dorsolateral prefrontal cortex (DLPFC; Brodmann areas 46, 9) in patients and controls showed activation of the right DLPFC in patients and activation of the bilateral DLPFC in controls.
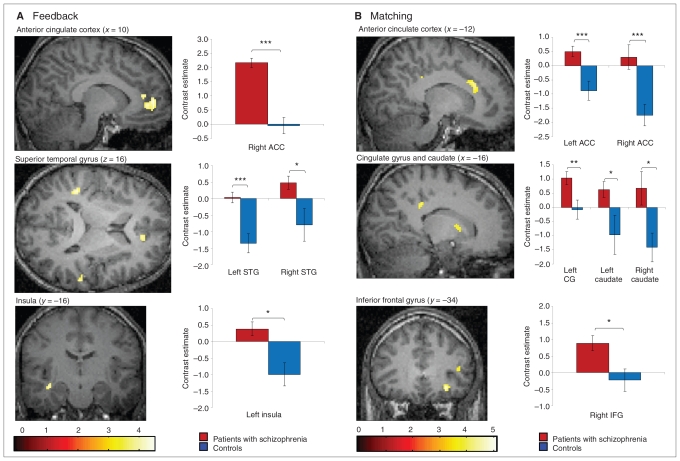
A between-group comparison revealed significantly increased activation in the right ACC and left insula and bilaterally in the superior temporal gyrus in patients with schizophrenia compared with controls (Table 2, Fig. 3A). Controls did not show any areas of increased brain activation compared with schizophrenia patients.
Table 2.
Brain areas with significantly* increased activation in patients with schizophrenia compared with controls in the contrast of trials after negative feedback (set shift) versus positive feedback
| Brain region | Talairach coordinates |
Brodmann area | Z score† | No. of voxels | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right hemisphere | ||||||
| Anterior cingulate gyrus | 10 | 41 | 11 | 32 | 3.60 | 23 |
| Anterior cingulate gyrus | 10 | 45 | 1 | 32 | 4.00 | 91‡ |
| Superior temporal gyrus | 61 | −32 | 15 | 22 | 3.62 | 10 |
| Left hemisphere | ||||||
| Superior temporal gyrus | −44 | −36 | 17 | 41 | 4.18 | 53§ |
| Insula | −38 | −18 | −8 | 13 | 3.73 | 25 |
Determined by 2-sample t test, p < 0.001, uncorrected. Controls revealed no areas of increased brain activation compared with schizophrenia patients.
Expressed as the maximum within each area. Local maxima are separated by a minimum of 8 mm.
Cluster-level correction (p < 0.005) for multiple comparisons.
Cluster-level correction (p < 0.05) for multiple comparisons.
Fig. 3.
Regions of increased brain activation in schizophrenia patients compared with controls (2-sample t tests, p < 0.001, uncorrected) and bar graphs of contrast estimates (MarsBaR28) of all regions with significant activation differences between patients and controls. All activations were rendered on a single subject template in Montreal Neurological Institute space. (A) Significantly increased activation after negative feedback (set-shifting) versus activation after positive feedback in the right rostral anterior cingulate cortex (ACC) in schizophrenia patients. (B) Significantly increased activation during matching after negative feedback (execution of set-shifting) versus activation during matching after positive feedback in the left dorsal ACC in schizophrenia patients. CG = Cingulate gyrus, IFG = inferior frontal gyrus, STG = superior temporal gyrus.
Activation during matching after negative versus positive feedback
Within-group analyses showed that patients with schizophrenia showed increased brain activation in the left and right cerebellum only. In controls, there were no areas of increased activation during matching after negative versus positive feedback. Also, ROI analysis revealed no significant activations.
In the between-group comparison, increased activation was found in the bilateral ACC, right inferior frontal gyrus and bilateral caudate nucleus in patients with schizophrenia compared with controls (Table 3, Fig. 3B). Again, no increased activation was observed in the control compared with the patient group.
Table 3.
Brain areas with significantly increased activation in patients with schizophrenia compared with controls in the analysis of matching after negative feedback (set-shifting) versus positive feedback
| Brain region | Talairach coordinates |
Brodmann area | Z score* | No. of voxels | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right hemisphere | ||||||
| Anterior cingulate gyrus | 12 | 44 | −10 | 32 | 3.52 | 18 |
| Inferior frontal gyrus | 28 | 31 | −12 | 47 | 4.69 | 48† |
| Inferior frontal gyrus | 42 | 37 | 7 | 46 | 3.55 | 26 |
| Caudate nucleus | 18 | 23 | 1 | — | 3.50 | 34 |
| Left hemisphere | ||||||
| Anterior cingulate gyrus | −12 | 27 | 28 | 32 | 3.92 | 56‡ |
| Cingulate gyrus | −16 | −31 | 37 | 31 | 3.78 | 20 |
| Caudate nucleus | −18 | 8 | 11 | — | 3.60 | 26 |
Expressed as the maximum within each area. Local maxima are separated by a minimum of 8 mm.
Cluster-level correction (p < 0.05) for multiple comparisons.
Determined by 2-sample t test, p < 0.001, uncorrected. Controls revealed no areas of increased brain activation compared with schizophrenia patients.
Behavioural data
There was no significant group difference in the mean number of trials needed to complete 6 dimensional changes (patients: mean 71.9 [SD 12.4, range 52–102] trials; controls: mean 66.7 [SD 13.0, range 50–105] trials; t62 = 1.625, p = 0.11). There was a group effect for the mean percentage of errors, with inferior performance in patients (patients: 19.9% [SD 8.4%]; controls: 15.5% [SD 5.9%]; t62 = −2.358, p = 0.022). The mean percentage of perseverative errors did not differ significantly (patients: 13.7% [SD 5.9%]; controls: 11.5% [SD 4.0%]; t62 = 1.765, p = 0.08). Controls performed worse than expected with regard to the percentage of perseverative errors (normative data for 30-year-old participants with 12 years of education, 7.8% perseverative errors (50th percentile; adapted to a 64-card version6), which might have been because of the unfamiliar magnetic resonance surroundings.
Among patients with schizophrenia, none of the WCST performance measures (mean number of trials, percentage of errors, percentage of perseverative errors) correlated significantly with psychopathology (Table 4). Univariate ANOVAs that included the WCST performance measures revealed no significant main effect of sex and no significant interaction between sex and group (mean number of trials: sex F1,60 = 2.189, p = 0.14, sex × group F1,60 = 0.677, p = 0.41; mean percentage of errors: sex F1,60 = 1.130, p = 0.29, sex × group F1,60 = 0.408, p = 0.53; mean percentage of perseverative errors: sex F1,60 = 1.895, p = 0.17, sex × group F1,60 = 0.095, p = 0.76).
Table 4.
Pearson correlation coefficients between PANSS scores and WCST performance in patients with schizophrenia (n = 36)
| Variable | Pearson correlation coefficient, mean (p value) |
|||
|---|---|---|---|---|
| Sum | Positive scale | Negative scale | General psychopathology scale | |
| No. of trials | −0.286 (0.09) | −0.146 (0.39) | −0.092 (0.59) | −0.220 (0.20) |
| Percentage of errors | −0.150 (0.38) | −0.216 (0.21) | 0.067 (0.70) | −0.110 (0.52) |
| Percentage of perseverative errors | −0.161 (0.35) | −0.219 (0.20) | 0.093 (0.59) | −0.143 (0.41) |
Mean accuracy on the identity matching task was nearly perfect for both groups (patients: 99.1% [SD 4.6%]; controls: 99.5% [SD 1.7%]; t62 = 0.508, p = 0.61), indicating that deficient WCST performance in patients with schizophrenia did not reflect fundamental difficulties in sorting a test card according to 4 reference cards.
Discussion
We used an event-related fMRI study to investigate neural activity during set-shifting components of the WCST in patients with schizophrenia. All patients recruited a widespread cortical and subcortical neural network during set-shifting, and the patients showed significantly increased activation in the ACC compared with the controls. Interestingly, directly after negative feedback, increased activity was located in the rostral part of the ACC, whereas, during matching after negative feedback, increased activity was observed in the dorsal ACC.
Neuroanatomically, the ACC can be divided into a dorsal region that connects with the striatum to oversee motor and cognitive processes, and a rostral region that interacts with other paralimbic and limbic regions to mediate emotional processes.31 Both regions of the ACC show increased activation in response to errors; rostral ACC activity has been proposed to reflect appraisal of the affective or motivational significance of errors, and dorsal ACC activity has been understood as training signals used to implement reinforcement learning in response to error feedback.32
Thus, in our study, increased activation of the rostral ACC is related to a stronger emotional response to negative feedback. Increased activation in the dorsal ACC during matching reflects a higher cognitive demand for this decision process in schizophrenia patients compared with controls. Interestingly, data from early single-photon emission computed tomography studies support our results of increased ACC activation in patients with schizophrenia. Kawasaki and colleagues33 observed a significant increase in blood flow in the ACC during the WCST in patients compared with controls, and Toone and colleagues34 reported a positive relation between ACC activity and performance on the WCST.
Directly after receiving negative feedback, we observed increased activity in the bilateral superior temporal gyri and the left insula in patients with schizophrenia. Superior temporal brain regions have been found to be activated in odd-ball tasks and in association with preparation for potential action, which is likely to be strong during switch trials.35 Activation in the insula has been related to unsuccessful inhibition and negative emotional states.36 During matching, significant activation differences were also discerned in the inferior frontal gyrus, the area most explicitly linked to set-shifting,10,11,17 and in the bilateral caudate nucleus. In the basal ganglia, the caudate nucleus has been shown to play the greatest role in executive processing.11
Previous studies on executive functioning in schizophrenia using disparate cognitive approaches, including a modified Sternberg paradigm,37 the Hayling38 or a modified Stroop task,39 also revealed increased medial prefrontal activation in patients with schizophrenia, although some studies reported no activation differences between patients and controls.40,41
The distribution of executive functions over a wide cerebral network that encompasses subcortical structures and thalamic pathways has been established by extensive imaging research, which included the generation of tasks involving only 1 specific executive process (e.g., set-shifting1,42) and event-related functional MRI studies deconstructing the different stages or elements of complex executive tasks. For example, using modified versions of the WCST, Monchi and colleagues11 observed a cortical-basal ganglia-thalamic loop after negative feedback, and Lie and colleagues15 found a neural network including frontoparietal regions and the striatum underlying WCST performance. Overall, widespread activation clusters in our patients and controls are in agreement with these and other data from imaging studies on set-shifting.9,16,43 The increased circumscribed activations in our healthy participants compared with the patient group must be interpreted in the context of our subtraction design, which emphasized set-shifting by subtracting activation after positive feedback from activation after negative feedback. Thus, fewer or smaller activation clusters in healthy participants are caused by fewer activation differences between the 2 cognitive tasks and most likely imply a stronger cognitive load required by the set-shifting task in the patient group.
Working memory, which is closely related to activity in the DLPFC, has been regarded as essential for successful performance on the WCST because participants have to compare current and previous feedback information, update working memory content while searching for the correct sorting dimension and maintain sorting rules. Although our subtraction design deliberately diminished activation differences related to working memory and between-group comparisons did not reveal significant activation differences in the DLPFC, we also scrutinized this area by a within-group ROI analysis. In line with previous studies, we found significant activation in the bilateral DLPFC in the controls, whereas schizophrenia patients showed activation of the right DLPFC (BA 46) only. In a meta-analysis of working memory studies,29 similar activation patterns (bilateral activation in healthy participants and activation only in the right DLPFC in patients with schizophrenia) were reported for BA 9 of the DLPFC.
Limitations
An important caveat to our results is that all patients were taking neuroleptics; dopamine-blocking drugs may affect the functioning of the ACC.44 Atypical neuroleptics, as used by most of our patients, are expected to positively influence cognitive abilities on a behavioural level. These assumptions are related to the effects of atypical neuroleptics on cortical 5-HT2 receptors in combination with lower affinities and faster dissociation time-courses on striatal dopaminergic receptors.45,46 Still, neither the large Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study nor other investigations using the CATIE neuropsychologic test battery found any differential neuroleptic effect on WCST performance.47 In our study, type and dosage of medication was prescribed by the treating clinician to achieve clinical improvement, and almost half of the patients received a combination of atypical neuroleptics, so we were unable to investigate the effect of specific types of atypical medications. Interestingly, structural imaging studies in patients with schizophrenia have suggested ameliorative effects of atypical neuroleptics on grey matter volume reductions in the prefrontal cortex, the medial and superior temporal gyrus and the anterior cingulate gyrus.48,49
Another limitation might be the small number of errors, which did not allow a separate analysis of different error types. However, we would expect that emotional and cognitive processes related to the generation of a new sorting rule should be comparable for both error types. One constraint in the interpretation of our BOLD signals for feedback and matching is that these 2 conditions, which are not directly contrasted in our design, are not independent of each other because of the hemodynamic response function. However, the 2 feedback or 2 matching periods were separated by an average of 6 seconds, thereby allowing separation of the hemodynamic response
Additionally, the included patients were young, with a short duration of illness, low psychotic symptomatology and high educational levels; therefore, they may not be representative of chronic schizophrenia. Because age and chronicity of illness are most likely confounding variables that influence functional imaging data, we believe that the homogeneity of the sociodemographic and clinical characteristics in our patient group is an advantage of our study. Still, studies involving patients with chronic schizophrenia as well as unmedicated patients are needed to obtain a complete picture of impaired set-shifting in schizophrenia.
Conclusion
Our results support the hypothesis that, in patients with schizophrenia, the higher emotional and cognitive demand of set-shifting is related to increased neural activity in the ACC. Because impaired executive functioning forms the basis of general theories of cognitive dysfunction, elucidating the neural activation patterns underlying the cognitive processes of set-shifting will further aid in our understanding of the pathophysiology of schizophrenia. Our data also suggest that cognitive flexibility, an important component in executive functioning in patients with schizophrenia, is most strongly related to neural functionality of the ACC. Targeting the neurobiology of the ACC (e.g., the glutamatergic neurotransmitter system) might thus be a promising approach to improve cognition in schizophrenia.
Footnotes
Competing interests: None declared.
Contributors: Drs. Wilmsmeier, Ohrmann and Pedersen and Mr. Bauer conceived the study and its design. They, along with Drs. Siegmund, Koelkebeck, Rothermudt and Kugel acquired the data, which was analyzed by Drs. Wilmsmeier, Ohrmann, Kugel and Pedersen and Mr. Bauer. Drs. Wilmsmeier, Ohrmann and Pedersen and Mr. Bauer wrote the manuscript, which was critically revised by all other authors. All of the authors approved the final version submitted for publication.
References
- 1.Kerns JG, Nuechterlein KH, Braver TS, et al. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JD, Barch DM, Carter CS, et al. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–33. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 3.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Arán A, Penadés R, Vieta E, et al. Executive function in patients with remitted bipolar disorder and schizophrenia and its relationship with functional outcome. Psychother Psychosom. 2002;71:39–46. doi: 10.1159/000049342. [DOI] [PubMed] [Google Scholar]
- 5.Heaton RK, Chelune GJ, Talley JL, et al. Wisconsin Card Sorting Test. Odessa (FL): Psychological Assessment Resources; 1993. [Google Scholar]
- 6.Braff DL, Heaton RK, Kuck J, et al. The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry. 1991;48:891–8. doi: 10.1001/archpsyc.1991.01810340023003. [DOI] [PubMed] [Google Scholar]
- 7.Weickert TW, Goldberg TE, Gold JM, et al. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–13. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 8.Prentice KJ, Gold JM, Buchanan RW. The Wisconsin Card Sorting impairment in schizophrenia is evident in the first four trials. Schizophr Res. 2008;106:81–7. doi: 10.1016/j.schres.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchsbaum BR, Greer S, Chang WL, et al. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting Task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konishi S, Nakajima K, Uchida I, et al. Transient activation of inferior prefrontal cortex during cognitive set-shifting. Nat Neurosci. 1998;1:80–3. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- 11.Monchi O, Petrides M, Petre V, et al. Wisconsin Card Sorting Test revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–41. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volz HP, Gaser C, Häger F, et al. Brain activation during cognitive stimulation with the Wisconsin Card Sorting Test — a functional MRI study on healthy volunteers and schizophrenics. Psychiatry Res. 1997;75:145–57. doi: 10.1016/s0925-4927(97)00053-x. [DOI] [PubMed] [Google Scholar]
- 13.Riehemann S, Volz HP, Stutzer P, et al. Hypofrontality in neuroleptic-naive schizophrenic patients during the Wisconsin Card Sorting Test — a fMRI study. Eur Arch Psychiatry Clin Neurosci. 2001;251:66–71. doi: 10.1007/s004060170055. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Tam WC, Xie Y, et al. The relationship between regional cerebral blood flow and the Wisconsin Card Sorting Test in negative schizophrenia. Psychiatry Clin Neurosci. 2002;56:3–7. doi: 10.1046/j.1440-1819.2002.00924.x. [DOI] [PubMed] [Google Scholar]
- 15.Lie CH, Specht K, Marshall JC, et al. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage. 2006;30:1038–49. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Smith AB, Taylor E, Brammer M, et al. Neural correlates of switching set as measured in fast, event-related functional magnetic resonance imaging. Hum Brain Mapp. 2004;21:247–56. doi: 10.1002/hbm.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–32. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohrmann P, Kugel H, Bauer J, et al. Learning potential on the WCST in schizophrenia is related to the neuronal integrity of the anterior cingulate cortex as measured by proton magnetic resonance spectroscopy. Schizophr Res. 2008;106:156–63. doi: 10.1016/j.schres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Oldfield RC. The assessment of analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 20.Wittchen HU, Zaudig M, Fydrich T. Structured Clinical Interview (SCID-I) for DSM-IV, research version — German modified version. Göttingen, Germany: Hogrefe; 1997. [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Tewes U. Wechsler Adult Intelligence Scale — revised German version. Bern (Germany): Hans Huber; 1991. [Google Scholar]
- 23.Helmstaedter C, Lendt M, Lux S. Auditory Verbal Learning Test —German modified version. Göttingen (Germany): Beltz; 2001. [Google Scholar]
- 24.Reitan RM. The validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 25.Monchi O, Petrides M, Strafella AP, et al. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006;59:257–64. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- 26.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited —again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 27.Miezin FM, Maccotta L, Ollinger JM, et al. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–59. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- 28.Brett M, Anton JL, Valabregue R, et al. Region of interest analysis using an SPM toolbox [abstract] Neuroimage. 2002;16:497. [Google Scholar]
- 29.Glahn DC, Ragland DJ, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–9. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bush G, Luu P, Posner MI. Cognitive and emotional influence in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 32.Taylor SF, Stern E, Gehring WJ. Neural systems of error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–72. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki Y, Maeda Y, Suzuki M, et al. SPECT analysis of regional cerebral blood flow changes in patients with schizophrenia during the Wisconsin Card Sorting Test. Schizophr Res. 1993;10:109–16. doi: 10.1016/0920-9964(93)90045-k. [DOI] [PubMed] [Google Scholar]
- 34.Toone BK, Okocha CI, Sivakumar K, et al. Changes in regional cerebral blood flow due to cognitive activation among patients with schizophrenia. Br J Psychiatry. 2000;177:222–8. doi: 10.1192/bjp.177.3.222. [DOI] [PubMed] [Google Scholar]
- 35.Thoenissen D, Zilles K, Toni I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci. 2002;22:9024–34. doi: 10.1523/JNEUROSCI.22-20-09024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramautar JR, Slagter HA, Kok A, et al. Probability effects in the stop-signal paradigm: the insula and the significance of inhibition. Brain Res. 2006;1105:143–54. doi: 10.1016/j.brainres.2006.02.091. [DOI] [PubMed] [Google Scholar]
- 37.Schlösser RG, Koch K, Wagner G, et al. Inefficient executive cognitive control in schizophrenia is preceded by altered functional activation during information encoding: an fMRI study. Neuropsychologia. 2008;46:336–47. doi: 10.1016/j.neuropsychologia.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Royer A, Schneider FC, Grosselin A, et al. Brain activation during executive processes in schizophrenia. Psychiatry Res. 2009;173:170–6. doi: 10.1016/j.pscychresns.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Weiss EM, Golaszewski S, Mottaghy FM, et al. Brain activation patterns during a selective attention test — a functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res. 2003;123:1–15. doi: 10.1016/s0925-4927(03)00019-2. [DOI] [PubMed] [Google Scholar]
- 40.Harrison BJ, Yücel M, Fornito A, et al. Characterizing anterior cingulate activation in chronic schizophrenia: a group and single subject fMRI study. Acta Psychiatr Scand. 2007;116:271–9. doi: 10.1111/j.1600-0447.2007.01002.x. [DOI] [PubMed] [Google Scholar]
- 41.Yücel M, Brewer WJ, Harrison BJ, et al. Anterior cingulate activation in antipsychotic-naïve first-episode schizophrenia. Acta Psychiatr Scand. 2007;115:155–8. doi: 10.1111/j.1600-0447.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 42.Collette F, Hogge M, Salmon E, et al. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–21. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 43.Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–93. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 44.Suhara T, Okubo Y, Yasuno F, et al. Decreased dopamine D2 receptor binding in the anterior cingulate cortex in schizophrenia. Arch Gen Psychiatry. 2002;59:25–30. doi: 10.1001/archpsyc.59.1.25. [DOI] [PubMed] [Google Scholar]
- 45.Beninger RJ, Wasserman J, Zanibbi K, et al. Typical and atypical antipsychotic medications differentially affect two nondeclarative memory tasks in schizophrenic patients: a double dissociation. Schizophr Res. 2003;61:281–92. doi: 10.1016/s0920-9964(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 46.Kuroki T, Nagaoa N, Nakaharaa T. Neuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin–dopamine hypothesis. Prog Brain Res. 2008;172:199–212. doi: 10.1016/S0079-6123(08)00910-2. [DOI] [PubMed] [Google Scholar]
- 47.Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch Gen Psychiatry. 2007;64:633–47. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 48.Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763–77. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- 49.Smieskova R, Fusar-Poli P, Allen P, et al. The effects of antipsychotics on the brain: What have we learnt from structural imaging of schizophrenia? A systematic review. Curr Pharm Des. 2009;15:2535–49. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]