Abstract
Studies of ERK1/2 generally focus on the regulation of nuclear ERK1/2 function mainly related to proliferation, whereas less attention has been drawn to the role ERK1/2 plays in the cytosol. Scaffolding proteins for ERK1/2 have been shown to control the time point and also the intracellular location of ERK1/2 activation. Hence, by concentrating ERK1/2 within subcellular compartments, scaffolding proteins restrict the substrate specificity of ERK1/2 and thus optimize the cell response for specific signal transduction programs in order to manipulate specific cellular functions. We have presented evidence that the F-actin binding protein calponin represents a new type of ERK1/2 scaffold, controlling the activation of a subfraction of ERK1/2 which is connected solely to contractile and/or migratory events in a cell.
Key words: ERK1/2 signaling, calponin, SmAV, cytoskeleton, cell migration
Extracellular Signal-Regulated Kinase ERK1/2
The serine/threonine extracellular signal-regulated kinase ERK1/2 can be activated in response to diverse stimuli such as activated receptor tyrosine kinases or integrin receptors.1,2 The kinase is then able to regulate various cellular functions including cell proliferation, cell death, migration, cell shape and differentiation. Phosphorylation and activation of ERK1/2 generally takes place at the cell membrane in a signaling module which includes Ras, Raf and MEK.3 In its phosphorylated form, ERK1/2 is reported to dimerize and enter the nucleus where it phosphorylates transcription factors like c-Myc, c-Fos and members of the Ets family.4 This nuclear function of ERK1/2 has been examined extensively in the past and the vast majority of studies focus on the role of ERK1/2 in regulating cell proliferation. However, cytosolic ERK1/2 is known to control other cell functions, e.g., cytoskeleton reorganization and cell contractile events.5,6 To be able to control when and where ERK1/2 is activated and to which substrates the kinase binds, cells contain multiple ERK1/2 scaffolding proteins. Scaffolds can be defined as proteins without any enzyme activity, but providing a platform on which to connect kinases with their upstream regulators or substrates. Moreover, scaffolds can tether their binding partners to distinct cellular sub-compartments/locations; thereby regulating their radius of operation.7 Many scaffolding proteins for ERK1/2 have been identified and have been reviewed in Ramos (2008).3
Calponin as a New ERK1/2 Scaffold
The F-actin binding protein calponin, which we have shown functions as an ERK1/2 scaffold that regulates ERK1/2 function related to contractile/migratory events, exists as three different isoforms.8 The basic isoform of calponin (h1 calponin), a marker of differentiated smooth muscle cells,9 was the first of the calponin proteins linked to the function of ERK1/2. Via its calponin homology domain in the N-terminus, h1 calponin has been shown to bind ERK1/2 directly.10 In differentiated vascular smooth muscle cells, a knockdown of endogenous h1 calponin decreases agonist-induced ERK1/2 activation and ERK-dependent contractility.11 In this cell type the data indicate that h1 calponin binds ERK1/2 at the contractile filaments, translocates to the cell membrane where it binds to a newly identified interaction partner Smooth muscle ArchVillin (SmAV). SmAV, which provides a platform for ERK1/2 activation due to binding of the ERK1/2 upstream regulators Raf and MEK1/2, was therefore also considered as an ERK1/2 scaffold itself.12,13
The more widely expressed h2 or neutral isoform of calponin has been shown to regulate ERK1/2 signaling upstream of MEK as well and in response to stimulation with the basic fibroblast growth factor. A knockdown of h2 calponin in the HUVEC cell line results in decreased ERK1/2 activation upon basic fibroblast growth factor stimulation. Moreover, overexpression of h2 calponin promotes migration of HUVEC cells in a wound healing assay, whereas effects on cell proliferation have not been documented. A MEK inhibitor, U0126, downregulates h2 calponin induced ERK1/2 activation and HUVEC migration; suggesting that h2 calponin controls cell motility, but not cell proliferation, via ERK1/2 signaling.14
Our recent findings show that the h3/acidic isoform of calponin is another ERK1/2 activator, since a knockdown impairs translocation of ERK1/2 to the cell cortex upon stimulation and hence, impairs phospho-ERK1/2 levels in a fibroblast cell line. Moreover, knockdown of h3/acidic calponin inhibits cell motility in a wound healing assay. Interestingly, a knockdown of h3/acidic calponin in these cells does not affect nuclear ERK1/2 levels, neither does it slow down cell proliferation (unpublished data). Therefore, it appears that h3/acidic calponin affects only a subfraction of ERK1/2, linked to motile events but not cell proliferation. ERK1/2 localizes at filamentous actin in the fibroblast cell line. This moreover indicates that the kinase plays an important role in cytoskeletal functions in the cell.
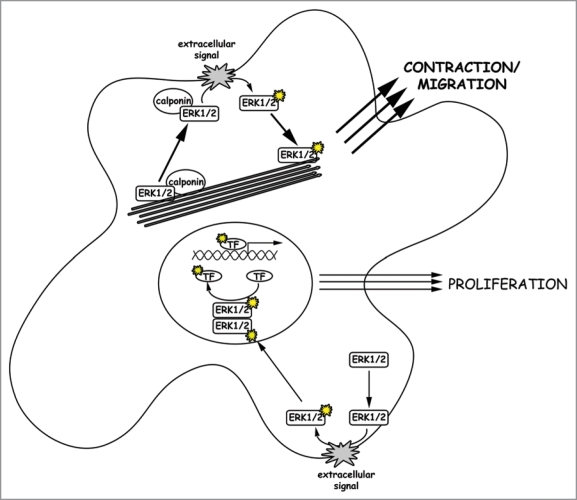
One might suggest that the actin binding properties of calponin sequester ERK1/2 in the cytoplasmic compartment and thus direct it toward contractile functions rather than proliferative functions. However, h3/acidic calponin is not involved in F-actin binding of ERK1/2, since a knockdown of the protein does not alter ERK1/2 localization in unstimulated cells. Together with results from previous studies10 we conclude that ERK1/2 itself binds F-actin, and hence will intrinsically tend to act on motile functions in cells with larger populations of actin filaments. Alternatively, the degree to which dimerization of ERK1/2 occurs has been questioned.15 Perhaps calponin scaffolding of ERK1/2 promotes a monomeric cytosolic form of ERK1/2 which only acts on cytosolic but not nuclear substrates (see Fig. 1).
Figure 1.
Activation of different ERK1/2 populations. ERK1/2 translocation to the cell cortex, where ERK1/2 gets activated via the Raf/Ras/MEK signaling cascade, is initiated by an extracellular signal. We assume that two populations of ERK1/2 molecules exist in a cell. First there is free cytosolic ERK1/2, which translocates upon activation and dimerization into the nucleus to activate proliferation in response to extracellular growth signals. Secondly, there is an ERK1/2 population bound to both calponin and F-actin. Upon a migratory or contractile stimulus, this ERK1/2 population translocates together with its scaffold calponin to the cell cortex where it is activated. Upon activation, monomeric active ERK1/2 molecules move back to actin filaments where they trigger cell migration or other contractile events of the cell. In cells with a pronounced actin cytoskeleton, the latter pathway might be dominant due to attracting more ERK1/2 molecules to the actin filaments and thereby depleting the kinase from the nucleus.
In summary, all three calponin isoforms seem to represent a new family of ERK1/2 scaffolds, promoting ERK1/2 activation connected to contractile, but not proliferative events in a cell.
Acknowledgements
We are supported by grants NIH R01 HL31704 and P01 HL86655.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/11832
References
- 1.Cabodi S, Moro L, Bergatto E, Boeri Erba E, Di Stefano P, Turco E, et al. Integrin regulation of epidermal growth factor (EGF) receptor and of EGF-dependent responses. Biochem Soc Trans. 2004;32:438–442. doi: 10.1042/BST0320438. [DOI] [PubMed] [Google Scholar]
- 2.Goldsmith ZG, Dhanasekaran DN. Gprotein regulation of MAPK networks. Oncogene. 2007;26:3122–3142. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- 3.Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, et al. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 5.English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, et al. New insights into the control of MAP kinase pathways. Experimental Cell Research. 1999;253:255–270. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]
- 6.Hai CM, Gu Z. Caldesmon phosphorylation in actin cytoskeletal remodeling. Eur J Cell Biol. 2006;85:305–309. doi: 10.1016/j.ejcb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26:3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- 8.Jin JP, Zhang Z, Bautista JA. Isoform diversity, regulation and functional adaptation of troponin and calponin. Crit RevEukaryotGene Expr. 2008;18:93–124. doi: 10.1615/critreveukargeneexpr.v18.i2.10. [DOI] [PubMed] [Google Scholar]
- 9.Winder SJ, Walsh MP. Calponin: thin filamentlinked regulation of smooth muscle contraction. Cell Signal. 1993;5:677–686. doi: 10.1016/0898-6568(93)90029-l. [DOI] [PubMed] [Google Scholar]
- 10.Leinweber BD, Leavis PC, Grabarek Z, Wang CL, Morgan KG. Extracellular regulated kinase (ERK) interaction with actin and the calponin homology (CH) domain of actin-binding proteins. BiochemJ. 1999;344:117–123. [PMC free article] [PubMed] [Google Scholar]
- 11.Je HD, Gangopadhyay SS, Ashworth TD, Morgan KG. Calponin is required for agonist-induced signal transduction—evidence from an antisense approach in ferret smooth muscle. JPhysiol. 2001;537:567–577. doi: 10.1111/j.1469-7793.2001.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangopadhyay SS, Kengni E, Appel S, Gallant C, Kim HR, Leavis P, et al. Smooth muscle archvillin is an ERK scaffolding protein. J Biol Chem. 2009;284:17607–17615. doi: 10.1074/jbc.M109.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med. 2008;12:2165–2180. doi: 10.1111/j.1582-4934.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang J, Hu G, Hanai J, Yadlapalli G, Lin Y, Zhang B, et al. A critical role for calponin 2 in vascular development. JBiolChem. 2006;281:6664–6672. doi: 10.1074/jbc.M506991200. [DOI] [PubMed] [Google Scholar]
- 15.Casar B, Pinto A, Crespo P. Essential role of ERK dimers in the activation of cytoplasmic but not nuclear substrates by ERK-scaffold complexes. Mol Cell. 2008;31:708–721. doi: 10.1016/j.molcel.2008.07.024. [DOI] [PubMed] [Google Scholar]