Abstract
A mutant pyrrolysyl-tRNA synthetase/tRNA pair was used to genetically encode allylcarbamoyl methyllysine in bacteria. This amino acid can be converted to methyllysine with a ruthenium catalyst, providing a straightforward approach for site-specifically introducing methyllysine residues into proteins.
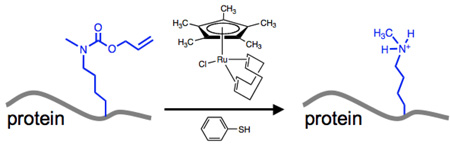
The posttranslational modification (PTM) of specific lysine residues regulates many biological processes.1 For example, the sumoylation, ubiquitylation, acetylation, and methylation of specific lysine residues in histones lead to changes in interactions between histones and DNA, and provide unique docking sites for protein complexes that control the transcription of DNA or regulate protein half-life.2 Specifically, histone lysine methylation has been shown to correlate with gene silencing or activation,3 and aberrant gene silencing induced by histone hypermethylation has often been observed in cancer cells.4 Therefore, the ability to site-specifically control lysine methylation in proteins provides a useful tool for investigating the role of PTM in regulating protein function.5, 6 Here we report a new method to site-specifically introduce methyllysine into proteins by genetically encoding Nε-allyloxycarbonyl-Nε-methyl-L-lysine (2) and then converting it to methyllysine with a ruthenium catalyst in neutral aqueous solution. This method is based on the use of the pyrrolysyl-tRNA synthetase/tRNA (PylRS/tRNA) pair, which naturally aminoacylates pyrrolysyl-tRNA with pyrrolysine and a range of structurally related unnatural amino acids (UAAs), resulting in their site-specific incorporation into proteins in response to the amber nonsense codon (TAG).7–10
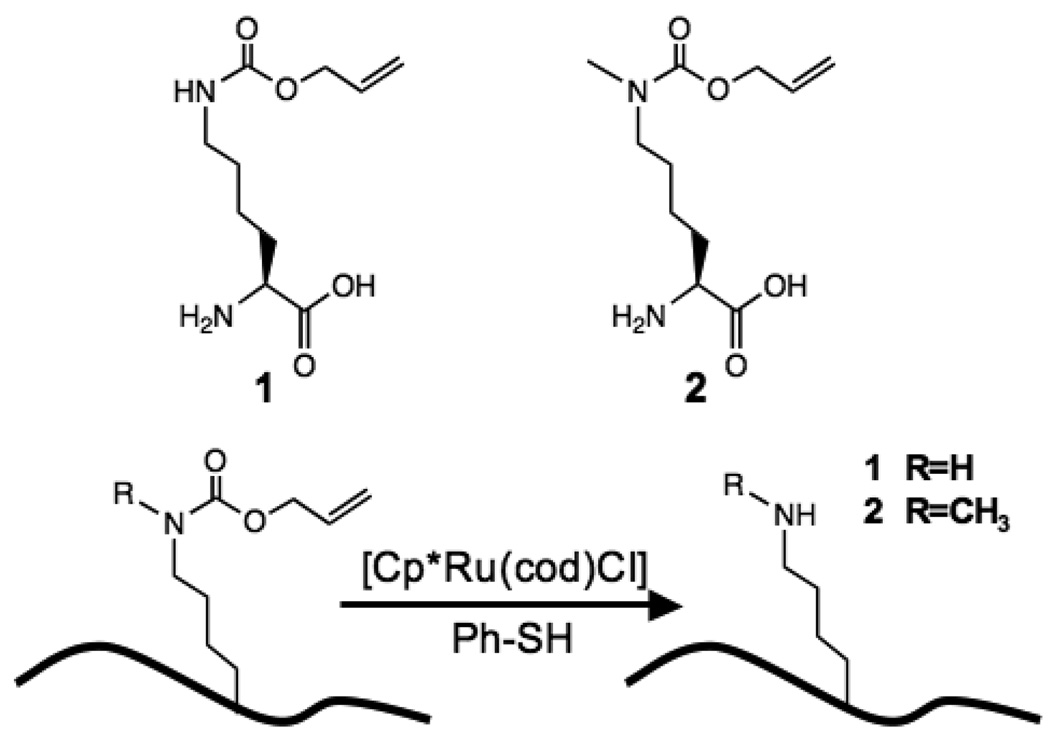
Previously, direct enzymatic modification11 and solid-phase synthesis coupled with native chemical ligation12 have been used to site-specifically modify lysines to generate methylated and acetylated histone proteins. Other chemical methods have also been developed to generate mimetic lysine modifications from either the cysteine side chain,5 or dehydroalanine.13 In addition, the PylRS/tRNA pair has been engineered to encode lysine analogues. Examples include acetyllysine,14 a photocaged lysine,15–17 and Nε-t-butyloxycarbonyl-Nε-methyl-L-lysine (which can subsequently be converted to methyllysine with trifluoroacetic acid).18 We decided to take advantage of transition metal-catalyzed reactions that are orthogonal to the native chemical functionalities of biological systems,19–21 and were specifically interested in the ruthenium-catalyzed elimination of the allylcarbamoyl protecting group from the amine group.22 We reasoned that since wild-type PylRS and the PylRS-Tyr384Phe mutant charge a variety of lysine carbamate derivatives (including 1) onto their cognate tRNA,7 2 could also be incorporated using these synthetases, thus providing a direct route to the site-specific incorporation of methyllysine via ruthenium-catalyzed allylcarbamoyl elimination (Scheme 1).
Scheme 1.
Molecular structures of the allylcarbamoyl derivatives of lysine (1) and methyllysine (2), and ruthenium-catalyzed conversion to lysine or methyllysine.
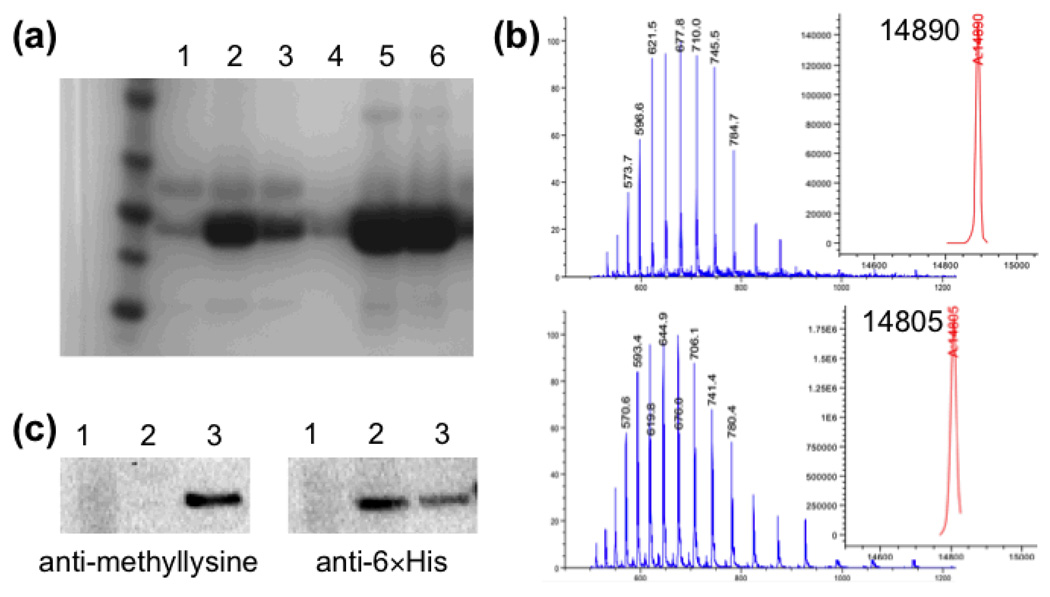
Plasmids encoding the M. barkeri PylRS or M. barkeri PylRS-Tyr384Phe were used to cotransform E. coli cells with plasmid pMyo-Lys99TAG (harboring a 6×His tagged myoglobin gene with an amber codon at position 99, and a copy of pyrrolysyl-tRNA gene). Protein expression was induced in the presence of 1 mM 2, and Ni-affinity chromatography was used to purify full-length myoglobin. In parallel, protein expression was tested in the absence of UAAs, or in the presence of 1 mM 1. Eluted proteins from Ni-NTA columns were further analyzed by SDS-PAGE (Fig. 1a). Full-length myoglobin was observed when either PylRS or PylRS-Tyr384Phe was coexpressed in the presence of 1 or 2. SDS-PAGE showed that PylRS-Tyr384Phe afforded more efficient amber codon suppression than PylRS (14.3 ± 3.4 mg or 10.2 ± 2.5 mg myoglobin per liter of culture with PylRS-Tyr384Phe in the presence of 1 mM 1 or 2, respectively). Myoglobin proteins were characterized by electrospray ionization mass spectrometry (ESI-MS). The observed average molecular masses confirmed that 1 or 2 were site-specifically incorporated at residue 99 of myoglobin (Fig. S1a and S1c).
Fig. 1.
(a) SDS-PAGE of Ni-NTA purified myoglobin. Lanes 1, 2 and 3 show proteins expressed with the wild-type PylRS/tRNA pair in the absence of UAAs or in the presence of 1 mM 1 or 2, respectively. Lanes 4, 5 and 6 show proteins expressed with the PylRS-Tyr384Phe/tRNA pair in the absence of UAAs or in the presence of 1 mM 1 or 2, respectively. (b) ESI-MS of histone protein expressed in the presence of 1 mM 1 (top) and treated with the ruthenium catalyst (bottom). (c) Western blots of Ni-NTA purified histone protein. Lanes 1 and 2 show eluted proteins expressed with the PylRS-Tyr384Phe/tRNA pair in the absence of UAAs or in the presence of 1 mM 2, respectively. Lane 3 shows the same protein in lane 2 following treatment with the ruthenium catalyst. The membranes were blotted with anti-methyllysine antibody (left) or anti-6×His antibody (right).
Chloro-pentamethylcyclopentadienyl-cyclooctadiene-ruthenium(II) ([Cp*Ru(cod)Cl]) was recently reported to effectively cleave allylcarbamate groups under conditions compatible with live cells.22 We therefore investigated ruthenium-catalyzed allylcarbamoyl elimination in proteins containing 1 or 2 in neutral aqueous solution. Myoglobin mutants (0.43 mg/ml) were treated with 0.8 mM [Cp*Ru(cod)Cl] and 20 mM thiophenol in phosphate buffered saline (PBS, pH 7.8). Reaction mixtures were incubated at 37 °C for 3 hours, and directly analyzed by ESI-MS (Fig. S1b and S1d). This analysis revealed the stoichiometric conversion of 1 or 2 at residue 99 of myoglobin to lysine or methyllysine, respectively, as indicated by single ESI-MS peaks consistent with the mass loss after allylcarbamoyl elimination.
We then used the above method to synthesize site-specifically methylated histone proteins. A plasmid encoding PylRS-Tyr384Phe and its cognate tRNA (designated as pDule-PylRSY384F) was used to transform E. coli along with a second plasmid encoding a histone H2B-Lys27TAG gene. Protein expression of the transformed cells was induced in the presence of 1 mM 2. The cells were lysed and the histone mutant was purified from inclusion bodies by Ni-affinity chromatography. The resulting protein (3.3 ± 1.5 mg per liter of culture) was refolded by dialysis against a buffer containing 20 mM sodium phosphate (pH 7.8) and 300 mM NaCl and then treated with [Cp*Ru(cod)Cl]. Efficient conversion of 2 to methyllysine was observed by ESI-MS after 3-hour incubation at room temperature (Fig. 1b). The conversion was further confirmed by western blot using anti-6×His antibody and anti-methyllysine antibody (Fig. 1c). Only the protein expressed in the presense of 2 and treated with the ruthenium catalyst could be specifically recognized by both anti-methyllysine and anti-6×His antibodies.
In conclusion, Nε-allyloxycarbonyl-Nε-methyl-L-lysine has been genetically encoded in E. coli by a mutant pyrrolysyl-tRNA synthetase/tRNA pair. The subsequent ruthenium-catalyzed allylcarbamoyl elimination reaction can be used to convert this UAA to methyllysine in neutral aqueous solution to afford the site-specific incorporation of methyllysine in proteins. This mild conversion should be especially valuable when soluble histone proteins are obtained by co-expressing multiple histone components in bacteria.23 This method should further facilitate investigation of the role of lysine methylation in signaling, protein structure, and epigenetics.
Supplementary Material
Acknowledgments
This work was supported by the US Department of Energy, Division of Materials Sciences, under Award No. DE-FG03-00ER46051 and the Skaggs Institute for Chemical Biology. We thank Dr. Chang Liu, Dr. Jonathan Day, Francis Peters and Emily Remba for manuscript preparation.
Footnotes
Electronic Supplementary Information (ESI) available: synthesis and characterization of coumpound 1 and 2; methods for protein expression, protein mass spectrometry, ruthenium-induced allylcarbamoyl elimination and western blot. See DOI: 10.1039/b000000x
Notes and references
- 1.Benayoun BA, Veitia RA. Trends Cell Biol. 2009;19:189–197. doi: 10.1016/j.tcb.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Cheung P, Lau P. Mol. Endocrinol. 2005;19:563–573. doi: 10.1210/me.2004-0496. [DOI] [PubMed] [Google Scholar]
- 3.Martin C, Zhang Y. Nat. Rev. Mol. Cell Biol. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 4.Kondo Y, Shen L, Issa JP. Mol. Cell. Biol. 2003;23:206–215. doi: 10.1128/MCB.23.1.206-215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon MD, Chu F, Racki LR, de la Cruz CC, Burlingame AL, Panning B, Narlikar GJ, Shokat KM. Cell. 2007;128:1003–1012. doi: 10.1016/j.cell.2006.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekirnik R, Rose NR, Thalhammer A, Seden PT, Mecinovic J, Schofield CJ. Chem. Commun. 2009:6376–6378. doi: 10.1039/b916357c. [DOI] [PubMed] [Google Scholar]
- 7.Yanagisawa T, Ishii R, Fukunaga R, Kobayashi T, Sakamoto K, Yokoyama S. Chem. Biol. 2008;15:1187–1197. doi: 10.1016/j.chembiol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Fekner T, Li X, Lee MM, Chan MK. Angew. Chem. Int. Ed. 2009;48:1633–1635. doi: 10.1002/anie.200805420. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DP, Lusic H, Neumann H, Kapadnis PB, Deiters A, Chin JW. J. Am. Chem. Soc. 2009;131:8720–8721. doi: 10.1021/ja900553w. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Fekner T, Ottesen J, Chan M. Angew. Chem. Int. Ed. 2009;48:9184–9187. doi: 10.1002/anie.200904472. [DOI] [PubMed] [Google Scholar]
- 11.Martino F, Kueng S, Robinson P, Tsai-Pflugfelder M, van Leeuwen F, Ziegler M, Cubizolles F, Cockell MM, Rhodes D, Gasser SM. Mol. Cell. 2009;33:323–334. doi: 10.1016/j.molcel.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 12.He S, Bauman D, Davis JS, Loyola A, Nishioka K, Gronlund JL, Reinberg D, Meng F, Kelleher N, McCafferty DG. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12033–12038. doi: 10.1073/pnas.2035256100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo J, Wang J, Lee J, Schultz P. Angew. Chem. Int. Ed. 2008;47:6399–6401. doi: 10.1002/anie.200802336. [DOI] [PubMed] [Google Scholar]
- 14.Neumann H, Peak-Chew SY, Chin JW. Nat. Chem. Biol. 2008;4:232–234. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]
- 15.Chen P, Groff D, Guo J, Ou W, Cellitti S, Geierstanger B, Schultz P. Angew. Chem. Int. Ed. 2009;48:4052–4055. doi: 10.1002/anie.200900683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautier A, Nguyen DP, Lusic H, An W, Deiters A, Chin JW. J. Am. Chem. Soc. 2010;132:4086–4088. doi: 10.1021/ja910688s. [DOI] [PubMed] [Google Scholar]
- 17.While the manuscript was under review and revision, Liu et al. (Mol. BioSyst., 2010, DOI: 10.1039/c002155e) and our own group (ChemBioChem, 11, 1066–1068) published work on the genetic incorporation of photocaged methyllysine into proteins; in the same paper, Liu et al. also reported the genetic incorporation of carboxybenzyl (Cbz) group protected methyllysine.
- 18.Nguyen DP, Garcia Alai MM, Kapadnis PB, Neumann H, Chin JW. J. Am. Chem. Soc. 2009;131:14194–14195. doi: 10.1021/ja906603s. [DOI] [PubMed] [Google Scholar]
- 19.Antos JM, Francis MB. Curr. Opin. Chem. Biol. 2006;10:253–262. doi: 10.1016/j.cbpa.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 20.van Maarseveen JH, Reek JN, Back JW. Angew. Chem. Int. Ed. 2006;45:1841–1843. doi: 10.1002/anie.200504352. [DOI] [PubMed] [Google Scholar]
- 21.Chalker JM, Lin YA, Boutureira O, Davis BG. Chem. Commun. 2009:3714–3716. doi: 10.1039/b908004j. [DOI] [PubMed] [Google Scholar]
- 22.Streu C, Meggers E. Angew. Chem. Int. Ed. 2006;45:5645–5648. doi: 10.1002/anie.200601752. [DOI] [PubMed] [Google Scholar]
- 23.Anderson M, Huh JH, Ngo T, Lee A, Hernandez G, Pang J, Perkins J, Dutnall RN. Protein Expr. Purif. 2010;72:194–204. doi: 10.1016/j.pep.2010.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.