Abstract
OBJECTIVE
Reduction in LDL and high sensitivity (hs) C-reactive protein (CRP) are independent indicators of successful cardiovascular risk reduction with statins. This study compared the effect of equivalent LDL-lowering doses of simvastatin and atorvastatin on hsCRP in type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
A crossover study of 26 patients with type 2 diabetes taking either 40 mg simvastatin or 10 mg atorvastatin was undertaken. After 3 months on one statin, lipids and hsCRP were measured on 10 occasions over a 5-week period. The same procedure was then followed taking the other statin.
RESULTS
LDL was comparable on either treatment: atorvastatin 2.2 ± 0.2 vs. 2.1 ± 0.3 mmol/l (mean ± SD; P = 0.19). CRP of individuals taking atorvastatin was significantly lower than when they were taking simvastatin (median 1.08 vs. 1.47 mg/l, P = 0.0002) and was less variable (median SD of logCRP 0.0036 vs. 0.178, P = 0.0001).
CONCLUSIONS
Compared with simvastatin, atorvastatin reduced hsCRP and its variability in type 2 diabetic patients. This enhanced anti-inflammatory effect may prove beneficial if lower CRP is associated with improved cardiovascular risk.
The ability of high sensitivity (hs) C-reactive protein (CRP) to predict cardiovascular risk has been confirmed in diverse population cohorts including type 2 diabetic patients (1). Patients who have lower hsCRP levels after statin therapy have better clinical outcomes regardless of the resultant level of LDL (2,3). Reduction in LDL and hsCRP are independent indicators of the success of statins in reducing cardiovascular risk (4). To assess any difference between the effect of long and short half-life statins on hsCRP and its variability, we conducted a crossover study with equivalent lipid-lowering doses of simvastatin and atorvastatin.
RESEARCH DESIGN AND METHODS
All subjects gave their informed consent, which had been approved by the Research Ethics Committee. A total of 30 consecutive Caucasian patients with type 2 diabetes and A1C between 6 and 9% were recruited. There were 19 patients taking 10 mg atorvastatin and 11 patients on 40 mg simvastatin before bed. The biological variation of hsCRP was assessed by measuring fasting blood samples at 4-day intervals on 10 consecutive occasions. Thereafter, the patients on simvastatin were changed to the equivalent dose of atorvastatin and vice versa (5). After 3 months, the biological variation was again assessed by measuring fasting blood samples at 4-day intervals on 10 consecutive occasions. Duplicate samples were randomized and then analyzed using a single batch (6,7). Serum CRP was measured by the high-sensitivity method on a Beckman DXC analyzer. The intra-assay CV was 4% using the study samples.
Statistical analysis
The distribution of hsCRP concentrations within and between individuals is non-Gaussian, so the Wilcoxon signed-rank test was used to compare the median hsCRP values of individual patients taking simvastatin compared with atorvastatin. To compare the hsCRP variability while on the statins, each hsCRP measurement was log-transformed. Biological variability of CRP on each drug was analyzed by calculating the analytical and within-subject variability (8). The distribution of these standard deviation (SDI) values within the study population was non-Gaussian, so the Wilcoxon signed-rank test was used to compare the individual SDs of patients when they were taking each statin.
RESULTS
The baseline demographics, duration of diabetes, and glycemic control were comparable in both groups (9), as was the baseline hsCRP in patients who were taking atorvastatin or simvastatin initially (median [interquartile range] 1.18 [0.78–3.88] vs. 1.37 [0.70–5.21] mg/l, P = 0.52). LDL was comparable on either treatment (atorvastatin 2.2 ± 0.2 vs. 2.1 ± 0.3 mmol/l [mean ± SD], P = 0.19); however, the degree of lowering with each statin was not assessed, since the patients were already established on a statin at study entry. One patient from each group dropped out after completing one arm because of poor compliance, one patient withdrew because of developing myalgia without any rise in creatinine kinase when changed to simvastatin, and another withdrew because of developing lethargy on starting simvastatin.
The median hsCRP when taking atorvastatin was significantly lower than when the same patients took simvastatin (median [interquartile range] 1.08 [0.70–4.06] vs. 1.47 [0.87–4.88] mg/l, P = 0.0002) (Fig. 1). The median hsCRP rose significantly when patients who were initially taking atorvastatin were changed over to simvastatin (1.18 [0.78–3.88] vs. 1.62 [1.03–5.01], P = 0.0002), whereas the median hsCRP fell after patients who were initially taking simvastatin were changed (1.37 [0.70–5.21] vs. 1.04 [0.68–5.49], P = 0.049). In 14 of 16 patients who changed from atorvastatin to simvastatin, the hsCRP rose, and in 9 of 10 patients who changed from simvastatin to atorvastatin, the hsCRP fell.
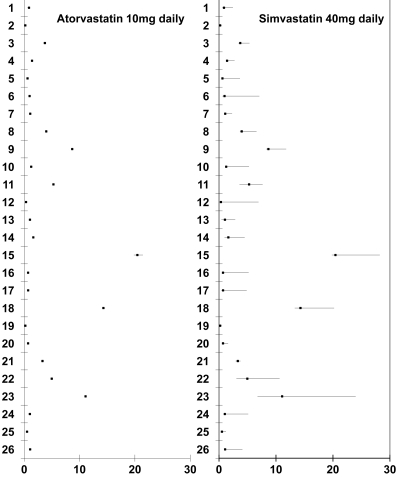
Figure 1.
Mean (range) hsCRP concentrations in 26 patients while taking 10 mg atorvastatin and 40 mg simvastatin. Patients 1–16 took atorvastatin initially and then changed to simvastatin. Patients 17–26 took simvastatin initially and then atorvastatin.
The variability of hsCRP was also much lower when taking atorvastatin compared with simvastatin (median [interquartile range] of SD of logCRP 0.0036 [0–0.014] vs. 0.178 [0.091–0.280], P = 0.0001) (Fig. 1). As with the median concentrations, the variability of hsCRP became higher when changed from atorvastatin to simvastatin (0.0053 [0–0.014] vs. 0.148 [0.110–0.200], P = 0.002), with a change from simvastatin to atorvastatin having the opposite effect (0.210 [0.080–0.297] vs. 0.0069 [0–0.017], P = 0.0052). In 15 of 16 patients who changed from atorvastatin to simvastatin, their hsCRP variability increased, and in 10 of 10 patients who changed from simvastatin to atorvastatin, the hsCRP fluctuations reduced.
CONCLUSIONS
This study shows that among patients with type 2 diabetes, there is a greater reduction of hsCRP with atorvastatin compared with equivalent doses of simvastatin, even though their lipid profiles were similar. The variability of hsCRP was also less with atorvastatin compared with simvastatin.
The best clinical outcomes for patients on statin treatment seem to occur when there is a reduction in hsCRP in addition to the reduction of LDL (3,4,10). It is therefore hypothesized that among very-high-risk patients undergoing statin therapy, the dual goals of LDL and hsCRP reduction should be considered a new clinical target for therapy (2). From the current study findings, it seems that patients are more likely to attain these two goals if they are treated with atorvastatin rather than simvastatin.
One of the features of hsCRP measurement that complicates interpretation of results is its wide intra-individual variability (11,12). Our data have shown that patients taking atorvastatin not only had lower hsCRP concentrations, but had markedly less biological variability than when taking equivalent doses of simvastatin. A single value on atorvastatin treatment is therefore more likely to reflect the true hsCRP concentration of the patient. However, the potential clinical benefits of this reduced variability are less clear than that of purely lowering average hsCRP values.
Looking for existing evidence that atorvastatin leads to fewer cardiovascular events than simvastatin at the same degree of LDL lowering is hampered by the fact that most clinical trials involving the two agents have deliberately aimed to show a difference in LDL. Nonetheless, in these comparative studies, event rates have been more favorable with atorvastatin in both randomized controlled studies (13) and observational studies (14).
There was a significant deterioration of median hsCRP and its variability when patients on atorvastatin were changed over to simvastatin. On the other hand, median hsCRP and its variability improved when patients on simvastatin were changed over to atorvastatin. The difference in variability of hsCRP and direct LDL could be due to the difference in stability of lipids while taking a relatively short half-life statin such as simvastatin (2–3 h) compared with that of a longer half-life statin such as atorvastatin (24 h) (15). These changes may need to be taken into account when considering switching patients between the two statins on any grounds.
In conclusion, an equivalent lipid-lowering dose of atorvastatin improves hsCRP as well as reduces the variability compared with simvastatin in type 2 diabetic patients.
Acknowledgments
This study was funded by an unrestricted educational grant from Pfizer.
No other potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial registry no. ISRCTN73479504; http://www.ISRCTN.org.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Best LG, Zhang Y, Lee ET, Yeh JL, Cowan L, Palmieri V, Roman M, Devereux RB, Fabsitz RR, Tracy RP, Robbins D, Davidson M, Ahmed A, Howard BV: C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: the Strong Heart Study. Circulation 2005;112:1289–1295 [DOI] [PubMed] [Google Scholar]
- 2. Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E: Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol 2005;45:1644–1648 [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E: Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005;352:20–28 [DOI] [PubMed] [Google Scholar]
- 4. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ: JUPITER Trial Study Group. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 2009;373:1175–1182 [DOI] [PubMed] [Google Scholar]
- 5. Law MR, Wald NJ, Rudnicka AR: Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jayagopal V, Kilpatrick ES, Jennings PE, Hepburn DA, Atkin SL: The biological variation of testosterone and sex hormone-binding globulin (SHBG) in polycystic ovarian syndrome: implications for SHBG as a surrogate marker of insulin resistance. J Clin Endocrinol Metab 2003;88:1528–1533 [DOI] [PubMed] [Google Scholar]
- 7. Jayagopal V, Kilpatrick ES, Jennings PE, Holding S, Hepburn DA, Atkin SL: The biological variation of sex hormone-binding globulin in type 2 diabetes: implications for sex hormone-binding globulin as a surrogate marker of insulin resistance. Diabetes Care 2004;27:278–280 [DOI] [PubMed] [Google Scholar]
- 8. Fraser CG, Harris EK: Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 1989;27:409–437 [DOI] [PubMed] [Google Scholar]
- 9. Sathyapalan T, Atkin SL, Kilpatrick ES: Variability of lipids in patients with type 2 diabetes taking statin treatment: implications for target setting. Diabet Med 2008;25:909–915 [DOI] [PubMed] [Google Scholar]
- 10. Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, Rifai N, Califf RM, Braunwald E: Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation 2006;114:281–288 [DOI] [PubMed] [Google Scholar]
- 11. Cho LW, Jayagopal V, Kilpatrick ES, Atkin SL: The biological variation of C-reactive protein in polycystic ovarian syndrome. Clin Chem 2005;51:1905–1907 [DOI] [PubMed] [Google Scholar]
- 12. Campbell B, Badrick T, Flatman R, Kanowski D: Limited clinical utility of high-sensitivity plasma C-reactive protein assays. Ann Clin Biochem 2002;39:85–88 [DOI] [PubMed] [Google Scholar]
- 13. Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J: Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005;294:2437–2445 [DOI] [PubMed] [Google Scholar]
- 14. Jacobson TA, Wertz DA, Hoy T, Kuznik A, Grochulski D, Cziraky M: Comparison of cardiovascular event rates in patients without cardiovascular disease in whom atorvastatin or simvastatin was newly initiated. Mayo Clin Proc 2008;83:1316–1325 [DOI] [PubMed] [Google Scholar]
- 15. Furberg CD: Natural statins and stroke risk. Circulation 1999;99:185–188 [DOI] [PubMed] [Google Scholar]