Abstract
OBJECTIVE
Recent epidemiological studies suggested that some insulin analogues could be associated with increased risk of cancer. The present study is aimed at assessing the long-term association of different insulin analogues with cancer incidence.
RESEARCH DESIGN AND METHODS
A nested case-control study dataset was generated from the cohort study dataset (n = 1,340 insulin-treated diabetic outpatients) by sampling control subjects from the risk sets. For each case subject, the control subjects (up to five) were chosen randomly from those members of the cohort who are at risk for the same follow-up time of the case subject. Five-year age classes, sex, and BMI classes (<18.5, 18.5–24.9, 25–29.9, and ≥30 kg/m2) were considered as additional categorical matching variables.
RESULTS
During a median follow-up of 75.9 months (interquartile range 27.4–133.7), 112 case subjects of incident cancer were compared with 370 matched control subjects. A significantly higher mean daily dose of glargine was observed in case subjects than in control subjects (0.24 IU/kg/day [0.10–0.39] versus 0.16 IU/kg/day [0.12–0.24], P = 0.036). Incident cancer was associated with a dose of glargine ≥0.3 IU/kg/day even after adjusting for Charlson comorbidity score, other types of insulin administration, and metformin exposure (odds ratio 5.43 [95% CI 2.18–13.53], P < 0.001). No association between incident cancer and insulin doses was found for human insulin or other analogues.
CONCLUSIONS
The possibility of association between cancer and higher glargine doses suggests that dosages should always be considered when assessing the possible association of insulin and its analogues with cancer.
Long-acting insulin analogues, glargine and detemir, were introduced for providing basal insulinization with a lower hypoglycemic risk than NPH insulin (1). Recent epidemiological studies suggested an association of glargine with malignancies (2–4) and particularly with breast cancer (2,4), possibly in a dose-dependent fashion (3). These results, not confirmed by other investigators (5), have been widely criticized for some methodological limitations (6,7) either in the quality of administrative data used for analyses (6) or their statistical management (7). Moreover, patients receiving prescriptions for different analogues might differ for clinical characteristics, potentially accounting for diversities in cancer incidence, such a prescription bias could be particularly relevant in register-based studies, which allow adjustments for a limited number of confounders. Furthermore, the comparison of the basal insulin glargine with human insulin, which includes both basal and prandial formulations, could reflect diversities between treatment regimens rather than actual differences between human insulin and its analogues. The increased risk of malignancies observed in patients using glargine only was not confirmed in those treated with combinations of glargine and other insulins (2–4). The short duration of observation in the above-cited studies represents a further limitation, considering the long incubation period characterizing most malignancies.
The present investigation is aimed at assessing the long-term association between incidence of cancer and use of different insulin analogues, considering insulin doses and a larger number of confounders than those included in previous studies. A main problem of previous studies was the management of variations of insulin therapy during follow-up, which becomes more relevant with a longer observation; some studies (2,4) analyzed baseline therapy, missing relevant information, while another (3) applied questionable statistical models, using therapies during follow-up as if they were baseline variables. A nested case-control design, using a multiple conditional logistic regression model, was used in order to overcome those limitations. Furthermore, doses for each insulin treatment were considered as a possible moderator of the effect of each insulin type (1).
RESEARCH DESIGN AND METHODS
Within a cohort of insulin-treated type 2 diabetic patients, those with incident cancer during a longitudinal follow-up were identified as case subjects and compared for treatments received with matched control subjects from the same cohort.
Of a consecutive series of 1,533 diabetic outpatients, referred to the diabetes clinics of the University of Florence, Italy, and starting insulin therapy between 1 January 1998 and 31 December 2007, those free of previous malignancies (n = 1,340) were enrolled in the study, provided that they met the following criteria:
They were living in the city of Florence at the date of enrolment;
They had a clinical diagnosis of type 2 diabetes;
They had no reported insulin therapy during the previous 5 years; and
They had no reported previous malignancy or hospital admission for malignancy after 1 January 1998 and before initiation of insulin therapy.
The participating hospital-based clinics provided outpatient care for diabetes; a large majority of patients initiating insulin therapy are referred to specialist clinics, which also provided subsequent follow-ups, at least yearly. All patients were treated by specialists who did not receive incentives for the use of less expensive drugs.
Demographic and clinical information was obtained from clinical records, including a detailed medical history, self-reported smoking habits and alcohol intake, prescribed medications, A1C (measured every 3–4 months with high-perfomance liquid chromatography [Menarini Diagnostics, Florence, Italy], upper normal limit 5.9%), lipid profile, and serum creatinine, and assessed as a part of routine follow-up. Weight, height, and blood pressure were routinely measured at each visit following World Health Organization recommendations (8). The nearest-available measure within a 3-month interval from enrollment was used as the baseline value for each parameter. Alcohol abuse was defined as a consumption exceeding two drinks per day. Comorbidity was assessed with the Charlson comorbidity score (CCS), which includes diabetes and its complications, malignancies, arthritis/arthrosis, HIV-infections, chronic cardiovascular disease, skin ulcers, renal insufficiency, liver diseases, and obstructive pulmonary disease (9).
Patients were observed until 31 December 2008 or to the date in which they moved to another city. Cancer incident case subjects were identified as first hospital admission or death with ICD-9 codes 140–209. Information on hospital admission was obtained through the Regional Hospital Discharge System, an administrative database containing ICD codes of current diagnoses, which collects all reimbursed admissions (in public or private hospitals), with no possibility of loss at follow-up. Deaths from cancer were obtained from the Mortality Registry of Tuscany. Patients with previous malignancies at first visit (n = 193) were excluded. Case finding was therefore performed on 1,340 patients (746 women and 594 men), aged (means ± SD) 63.1 ± 14.9 years, with a median duration of diabetes of 7.5 years (interquartile range 0.5–19.2), A1C 8.7 ± 1.9%, and BMI 27.9 ± 5.4 kg/m2.
A nested case-control study dataset was generated from the cohort study dataset by sampling control subjects from the risk sets. For each case subject, the control subjects (up to five) were chosen randomly from those members of the cohort who are at risk for the same follow-up time of the case subject. The resulting case-control sample is therefore matched with respect to analysis time. Five-year age classes, sex, and BMI classes (<18.5, 18.5–24.9, 25–29.9, and ≥30 kg/m2) were considered as additional categorical variables for matching control subjects to case subjects. We used the STATA 9.0 software and the procedure “sttocc,” which takes into account survival times, to implement this step.
Hypoglycemic treatments
Exposure to hypoglycemic drugs was assessed from enrollment to incident cancer in case subjects and during the corresponding time from initiation of insulin therapy in matched control subjects. To compare case and control subjects for differences in exposure to each insulin type, the mean daily dose (MDD) was calculated, dividing total insulin units prescribed for duration of observation (days) and for weight (kg), thus accounting for both time of treatment and daily dose during treatment.
MDD was calculated separately for basal and prandial formulations of each insulin. For premixed insulin, the fraction of the dose attributed to basal or prandial therapy was calculated and used in subsequent analyses. For oral hypoglycemic drugs, the length of exposure during the 10 years preceding the end of observation was considered. Drug exposure was obtained from clinical records containing a self-reported history of hypoglycemic treatment before the first contact and all drug prescriptions afterward. Information retrieval for each case and control subject was performed independently by two investigators, and conflicts were resolved by a third investigator (E.M.). If the last available visit had occurred >3 months before the event (or the matching index date), a telephone contact was attempted to collect further information on subsequent drug use; if unsuccessful, the patient was assumed to have continued the last reported therapy. Three cases with a last visit occurring >2 years from the end of follow-up were excluded from analysis.
Statistical analysis
The main predefined analysis was the comparison of case and control subject for proportion of patients exposed and MDD (units/kg/day) for each insulin. Further multivariate analyses were designed on the basis of those results. Unpaired Student t test and Mann-Whitney test were used to compare continuous variables whenever appropriate. χ2 Test was used for between-group comparisons of categorical variables. The statistical analysis was done by conditional logistic regression, which takes into account the matching structure. This was especially important because for some case subjects, less than five control subjects were available in the risk set, so that an unequal number of control subjects were sampled by case subject. In all models, total insulin MDD, CCS, and metformin exposure were used as covariates, along with MDD and proportion of subjects with MDD ≥0.3 IU/kg/day for each insulin type. Alternative models in which MDD for basal and prandial insulins were entered separately were applied. Furthermore, an analysis with CCS, metformin exposure, total insulin MDD, glargine exposure time, and mean glargine dose during exposure was performed. All analyses were carried out with SPSS 15.0 statistical package and STATA 9.0, and a P < 0.05 was considered statistically significant.
RESULTS
During a median follow-up of 75.9 months (interquartile range 27.4–133.7), 112 patients had new diagnoses of cancer (incidence 1.9/100 person-years); in 1 case subject, 2 different cancers were observed, for a total number of 113 registered cancers (11 colorectal, 18 other gastrointestinal/hepatic, 14 pancreatic, 16 lung, 10 leukemia/lymphoma, 7 breast, 5 female urogenital, 9 male urinary, 4 prostate, and 20 other cancers).
The demographic and clinical characteristics of case and control subjects are summarized in Table 1. Case subjects had a greater comorbidity and a nonsignificant trend toward less exposure to metformin in the previous 10 years, whereas the mean daily metformin dose (median [interquartile range]) was not significantly different between case and control subjects (16.0 mg/kg/day [11.8–21.4] vs. 18.2 mg/kg/day [10.0–20.9], P = 0.63). After adjusting for CCS and mean daily total insulin dose, time of exposure to metformin (hazard ratio [HR] 0.993 [95% CI 0.986–0.999], P = 0.046, for each month) and metformin doses (0.943 [0.915–0.971], P < 0.01, for each mg/kg/day) were both inversely associated with incident cancer. The proportion of patients who discontinued insulin treatment during follow-up was similar in case and control subjects (34.3 vs. 41.2%, P = 0.54).
Table 1.
Main demographic and clinical characteristics of enrolled sample
| Case subjects | Control subjects | P | |
|---|---|---|---|
| n (male/female) | 112 (60/52) | 370 (189/181) | 0.64 |
| Age (years) | 68.9 ± 9.9 | 68.0 ± 10.0 | 0.41 |
| Duration of diabetes (years) | 8.4 (0.3–20.9) | 10.0 (0.6–21.0) | 0.28 |
| BMI (kg/m2) | 28.1 ± 5.3 | 28.2 ± 5.1 | 0.78 |
| Weight (kg) | 75.5 ± 14.2 | 75.8 ± 16.5 | 0.47 |
| Systolic blood pressure (mmHg) | 142.5 ± 20.9 | 141.5 ± 21.6 | 0.71 |
| Diastolic blood pressure (mmHg) | 80.4 ± 10.0 | 79.8 ± 11.2 | 0.42 |
| Alcohol abuse | 15 (13.4) | 40 (10.8) | 0.61 |
| Current smokers | 25 (22.5) | 66 (17.8) | 0.39 |
| CCS | 2.4 ± 1.4 | 2.1 ± 1.1 | 0.035 |
| Laboratory parameters | |||
| A1C (%) | 8.5 ± 1.9 | 8.5 ± 1.8 | 0.99 |
| Total cholesterol (mg/dl) | 195.8 ± 47.3 | 208.9 ± 44.3 | 0.029 |
| HDL cholesterol (mg/dl) | 47.1 ± 15.3 | 52.6 ± 15.5 | 0.031 |
| Triglycerides (mg/dl) | 143.0 (108.0–93.0) | 138.0 (95.7–196.2) | 0.61 |
| Creatinine (mg/dl) | 1.1 ± 0.4 | 1.1 ± 0.5 | 0.99 |
| Microalbuminuria (μg/ml) | 13.0 (5.4–44.8) | 11.0 (5.7–31.0) | 0.82 |
| Medical history | |||
| Ischemic heart disease | 30 (26.8) | 79 (21.4) | 0.23 |
| Cerebrovascular disease | 5 (4.5) | 25 (6.8) | 0.38 |
| Renal failure | 9 (8.0) | 26 (7.0) | 0.72 |
| Retinopathy | 10 (8.9) | 75 (20.3) | 0.006 |
| Neuropathy | 31 (27.7) | 103 (27.8) | 0.97 |
| Foot ulcers | 3 (2.7) | 22 (5.9) | 0.17 |
| Chronic obstructive pulmonary disease | 7 (6.3) | 23 (6.2) | 0.99 |
| Any liver disease | 16 (14.3) | 27 (7.3) | 0.10 |
| Microalbuminuria | 18 (16.1) | 68 (18.4) | 0.58 |
| Pharmacological therapy at enrollment | |||
| Antihypertensive drugs | 60 (53.6) | 212 (57.3) | 0.58 |
| Statins | 30 (26.7) | 86 (23.2) | 0.62 |
| Antiaggregants | 46 (41.0) | 155 (41.9) | 0.81 |
| Oral hypoglycaemic drugs | |||
| Any exposure during the previous 10 years | |||
| Metformin | 70 (62.5) | 228 (61.6) | 0.86 |
| Insulin secretagogues | 65 (58.0) | 199 (53.8) | 0.48 |
| Thiazolidinediones | 1 (0.9) | 11 (3.0) | 0.21 |
| Length of exposure during the previous 10 years (months)* | |||
| Metformin | 27.5 (9.0–61.0) | 40.0 (13.2–73.76) | 0.08 |
| Insulin secretagogues | 20.2 (5.5–77.5) | 50.0 (10.5–67.5) | 0.59 |
| Patients exposed during follow-up | |||
| Any exposure | |||
| Human insulin | 82 (73.2) | 245 (66.2) | 0.16 |
| Basal | 26 (23.2) | 74 (20.0) | 0.46 |
| Prandial | 78 (69.6) | 231 (62.4) | 0.16 |
| Lispro | 53 (47.3) | 184 (49.7) | 0.65 |
| Basal | 1 (0.9) | 3 (0.8) | 0.93 |
| Prandial | 53 (47.3) | 180 (48.6) | 0.80 |
| Aspart | 9 (8.0) | 34 (9.2) | 0.71 |
| Basal | 0 (0.0) | 0 (0.0) | — |
| Detemir | 1 (0.9) | 13 (3.5) | 0.14 |
| Glargine | 29 (26.0) | 105 (28.4) | 0.61 |
| MDD (IU/day)* | |||
| Human insulin | 13.9 (8.0–29.9) | 17.7 (8.0–29.9) | 0.40 |
| Basal | 11.0 (6.2–19.0) | 12.1 (6.9–20.9) | 0.45 |
| Prandial | 14.2 (9.1–24.8) | 16.1 (8.0–33.0) | 0.83 |
| Lispro | 20.1 (10.2–30.2) | 22.0 (10.5–30.0) | 0.93 |
| Basal | 7.0 (7.0–7.0) | 3.9 (2.4–33.7) | 0.71 |
| Prandial | 20.1 (8.7–30.2) | 22.0 (10.5–30.0) | 0.91 |
| Aspart | 16.0 (9.2–24.0) | 25.2 (15.0–31.4) | 0.11 |
| Glargine | 17.1 (7.0–30.6) | 12.1 (8.0–18.0) | 0.08 |
| Total | 27.1 (14.0–42.0) | 27.9 (14.7–43.2) | 0.71 |
| MDD (IU/kg/day)* | |||
| Human insulin | 0.19 (0.10–0.38) | 0.24 (0.11–0.40) | 0.40 |
| Basal | 0.15 (0.09–0.24) | 0.17 (0.09–0.28) | 0.86 |
| Prandial | 0.21 (0.10–0.36) | 0.23 (0.11–0.37) | 0.61 |
| Lispro | 0.26 (0.13–0.46) | 0.30 (0.15–0.43) | 0.48 |
| Basal | 0.12 (0.12–0.12) | 0.05 (0.03–0.51) | 0.37 |
| Prandial | 0.26 (0.13–0.46) | 0.30 (0.15–0.43) | 0.91 |
| Aspart | 0.25 (0.11–0.29) | 0.30 (0.20–0.44) | 0.33 |
| Glargine | 0.24 (0.10–0.39) | 0.16 (0.12–0.24) | 0.036 |
| Total | 0.35 (0.20–0.59) | 0.39 (0.20–0.59) | 0.75 |
Data are expressed as means ± SD, median (interquartile range), or n (%).
*In exposed patients.
No significant difference was observed between case and control subjects in the proportion of patients exposed to each insulin. Among those exposed, a significantly higher MDD (IU/kg/day) was observed in case subjects than in control subjects for glargine only. In those receiving glargine, median duration of glargine treatment was 20.0 months (9.0–28.0) and 14.5 months (4.7–26.0) in case and control subjects, respectively (P = 0.16); corresponding figures for human basal insulin were 19.4 months (5.7–48.2) and 21.0 months (7.0–45.7) (P = 0.86). The proportion of patients receiving ≥0.3 IU/kg/day of glargine was higher in case subjects (13/112 [11.6%] vs. 14/370 [3.8%], P = 0.002), whereas no such difference was observed for human insulin (27/112 [24.1%] vs. 107/370 [28.8%], P = 0.33), lispro (19/112 [17.0%] vs. 92/370 [24.9%], P = 0.08), or aspart (2/112 [1.8%] vs. 2/370 [4.2%], P = 0.24). Median doses of basal insulin did not differ between case and control subjects (0.16 [0.08–0.31] vs. 0.16 [0.07–027], P = 0.59). Mean total insulin dose was not significantly different between case and control subjects, even after adjusting for metformin exposure and CCS (odds ratio 1.024 [95% CI 0.946–1.108], P = 0.56, for each 0.1 IU/kg/day increment in MDD). After adjusting for the same confounders, exposure to a mean daily total insulin dose ≥0.3 IU/kg/day was not significantly associated with incident cancer (HR 0.75 [95% CI 0.50–1.17], P = 0.21).
A majority of patients were treated with combinations of basal and prandial insulin at some time during follow-up, in both case and control subjects. In particular, 82.9 and 86.2% of patients receiving glargine were also exposed to prandial insulin among case and control subjects, respectively; the corresponding figures for human basal insulin were 58.9 and 55.1%. Of those treated with glargine, 23.8 and 37.9% in control and case subjects, respectively, also received NPH at some time during follow-up; a large majority (94.5%) of those were treated initially with NPH and with glargine afterward.
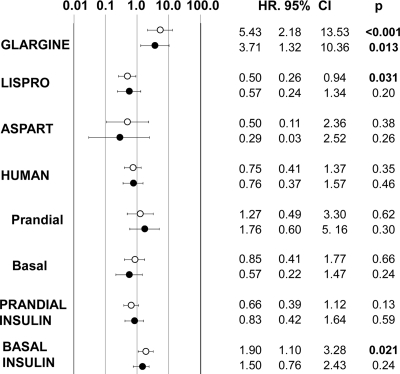
The association of ≥0.3 IU/kg/day insulin doses, adjusted for CCS, metformin exposure, and mean daily total insulin dose, is reported in Fig. 1 for each preparation, with the exception of detemir and basal lispro, which could not be analyzed for insufficient case numbers. At total of ≥0.3 IU/kg/day basal insulin was associated with incident cancer; this phenomenon was due to the association of cancer with high-dose glargine, which was not observed with basal (NPH) human insulin. In fact, ≥0.3 IU/kg/day glargine was associated with increased risk of cancer, while no effect was observed for human insulin or aspart; this association was confirmed after exclusion from analysis of cases with cancer occurring within the first 12 months of observation and of their corresponding matched control subjects (Fig. 1). After adjusting for confounders, lispro was associated with a marginally lower risk of cancer, which was not confirmed after exclusion of cases occurring within the first 12 months of observation. An alternative model, adjusting for metformin exposure, CCS, exposure to prandial insulin, and doses of other basal insulins, provided similar results (odds ratio 4.76 [95% CI 1.99–11.40], P < 0.001, and 0.73 [0.34–1.53], P = 0.40, for glargine and NPH, respectively). In a further model, adjusting for the same confounders plus any exposure to other basal insulins during follow-up, use of ≥0.3 IU/kg/day glargine was still associated with cancer (5.83 [2.34–14.30], P < 0.001).
Figure 1.
Risk of cancer associated with doses of each insulin type ≥0.3 IU/kg/day, adjusted for comorbidity, exposure to metformin, and doses of other types of insulin. ○, all case subjects; ●, after exclusion of case subjects with cancer occurring within the first 12 months of observation and of their matched control subjects.
The association of ≥0.3 IU/kg/day glargine with incident cancer was evident in younger, but not in older, subjects, while it was similar in men and women and in leaner and overweight/obese patients (Fig. A-1 in the online appendix, available at http://care.diabetesjournals.org/cgi/content/full/dc10-0476/DC1). Considering different cancer types, the odds ratio for breast cancer (n = 7) was 5.46 (95% CI 0.45–66.1) (P = 0.18); corresponding figures for gastrointestinal/hepatic (n = 29), pulmonary (n = 16), pancreatic (n = 14), and leukemia/lymphoma (n = 10) were 0.58 [0.06–5.87], 2.89 [0.37–22.23], 4.00 [0.25–63.95], and 2.83 [0.17–47.15], respectively.
When insulin doses (IU/kg/day) were considered as a continuous variable, no significant unadjusted association between MDDs and risk of incident cancer was found for any insulin preparation, although a nonsignificant trend could be observed for glargine. When adjusting for total insulin dose, comorbidity, and exposure to metformin, glargine (adjusted odds ratio 1.33 [95% CI 1.07–1.65], P = 0.011, for each 0.1 IU/kg/day increment), but not basal human insulin (1.06 [0.91–1.24], P = 0.11), was associated with a greater risk of incident cancer; similar results were obtained when adding current smoking as a covariate (1.35 [1.04–1.76], P = 0.020, and 1.04 [0.87–1.29], P = 0.18, for each 0.1 IU/kg/day increment of glargine and human basal insulin, respectively). This association was maintained after exclusion of case subjects occurring in the first 12 months of observation and of their matching control subejects (1.34 [1.04–1.73], P = 0.024, for each 0.1 IU/kg/day increment). To better discriminate the effect of duration of treatment with glargine and daily dose during treatment, an alternative analysis was performed entering those two variables together with CCS, metformin exposure, and total insulin dose; incident cancer was associated with higher mean glargine dose during treatment (1.21[1.01–1.47], P = 0.047, for each 0.1 IU/kg/day) but not with duration of glargine exposure (0.99 [0.97–1.01], P = 0.41, for each month).
CONCLUSIONS
The proportion of case subjects exposed to each insulin was not different from that of control subjects, whereas higher MDDs of glargine, but not of other types of insulin, were associated with cancer after adjusting for confounders.
Several hypoglycemic agents have been reported to modify the risk of malignancies in type 2 diabetic patients (10–14). The link between hypoglycemic treatment and cancer has been suggested only by observational studies; however, randomized clinical trials, designed to assess the effects of drugs on metabolic and/or cardiovascular outcomes, cannot have an adequate sample size and length of follow-up to provide relevant information on incident malignancies.
In observational studies, different agents are prescribed to patients with different characteristics, and those diversities cannot be entirely eliminated by statistical adjustments (6,7). Previous studies suggesting an association between glargine and cancer (2–4) suffered from further limitations, including limited information on comorbidities (2–4), short duration of observation, the inclusion of probably pre-existing cases of cancer diagnosed shortly after the initiation of insulin (2,3,5), and the failure to discriminate between basal and prandial human insulin used as comparator (2–4). In one study (3), the inclusion of time-dependent covariates within a traditional Cox model (7) could have interfered with results (2). On the other hand, because of the frequent change of insulin regimens in clinical practice, the use of baseline therapy for analysis (2,4,5) is a limitation in studies with longer follow-up.
The present study was designed to overcome, at least partly, those limitations. The prolonged follow-up allowed to identify relevant numbers of incident case subjects whose onset reasonably occurred after the initiation of insulin therapy. The persistent association of high-dose glargine with cancer after exclusion of cases occurring within the first 12 months of observation suggests that differences cannot be attributed to pre-existing, but still subclinical, malignancies. The adjustment for a comorbidity index confirms that treatment-related differences in cancer incidence cannot be considered the effect of comorbid conditions. Moreover, the association of high-dose glargine with incident cancer cannot be regarded as a “class effect” of any formulation of basal insulin or as a consequence of the characteristics of patients prescribed any type of basal insulin, since it was not observed for basal (NPH) human insulin.
Several observational studies have shown that oral hypoglycemic treatments can be associated either with increased or reduced incidence of cancer (10–14). In particular, a dose-dependent inverse association of metformin with incident cancer has been reported (11,12) and confirmed in our sample. Notably, the association of high-dose glargine with cancer was confirmed even after adjusting for metformin exposure.
A recent meta-analysis of randomized clinical trials failed to show any association between glargine and cancer (15), but the limited number of cases produced wide CIs in risk estimates. The short duration of most randomized trials is a further limitation to that meta-analysis. These considerations also apply to another meta-analysis showing a lower incidence of cancer with detemir than with human NPH insulin (16). The two meta-analyses enrolled both type 1 and type 2 diabetic patients, limiting their interpretation for each of those two individual conditions.
Short-acting insulin analogues, lispro and aspart, did not appear to be associated with a higher risk than prandial (regular) human insulin. The limited proportion of patients treated with aspart suggests a special caution in the interpretation of results on this analog, while data on detemir were too limited to provide any valuable information.
Two previous studies (2,4) reported an association of glargine with breast cancer but not with other types of malignancy. The present sample does not have an adequate size to provide detailed information on specific types of tumors; although it is possible that glargine treatment has a greater association with breast cancer, the significant association between high-dose glargine and cancer in men suggests that malignancies other than breast cancer could be involved.
Discrepancies between the present results and some previous data (2,4,5) could have been determined, at least in part, by differences in duration of follow-up. It should be recognized that even the longer follow-up of this study could be insufficient to detect differences with respect to cancer incidence. Furthermore, we observed a dose-dependent association of glargine with incident malignancies, consistent with one previous observation (3).
The mechanisms that could account for differences in cancer risk in type 2 diabetic patients treated with different types of insulin are uncertain. The mitogenic properties of glargine observed in some preclinical models (17–19), but not consistently confirmed in others (20,21), have been reported to be either specific for glargine (18,19) or shared with other insulin analogues (19). Compared with human insulin, glargine has a higher affinity for the IGF-1 receptor, which could translate into a greater stimulation of cell growth and transformation, leading to increased cancer risk (17); however, the impact of the IGF-1 axis on malignancies is still controversial (22). Furthermore, insulin, particularly when at high doses, has a mitogenic and transforming effect independent of its interaction with the IGF-1 receptor (23). In the present study, higher doses of insulin other than glargine were not associated with incident cancer, but this result could be due to an insufficient sample size.
Some further limitations of our study should be acknowledged. No detailed information on reproductive history in women, potentially affecting the incidence of breast cancer, was available. Information on treatments was based on drug prescription, rather than output from pharmacies, as in previous studies (2–5); in the case of reduced compliance, this should lead to overestimating actual insulin consumption. However, assessment of insulin delivered from pharmacies could also overestimate actual exposure, because a fraction of the insulin bought in pharmacies is wasted for several reasons. It is possible that patients receiving higher basal insulin doses were at greater risk and that they had a greater chance of receiving glargine instead of NPH, although median basal insulin doses did not differ between case and control subjects. It should also be considered that cases of incident cancer were identified through hospital admissions and death certificates so that few cases of nonfatal malignancies not receiving treatment in public hospitals could have been missed. Furthermore, the choice of 0.3 IU/kg/day as a threshold for high-dose treatment is somewhat arbitrary; a larger sample size is needed for exploring more thoroughly the dose-response relationship, identifying a more reliable cutoff. Another relevant limitation is represented by the observational nature of our study, which prevents the entire elimination of prescription biases, although the multiple adjustments performed attenuate the problem. Notably, local guidelines (www.siditalia.it) recommend either NPH or analogues as basal insulins, recognizing that the former is associated with higher hypoglycemic risk. Some potentially relevant confounders affecting treatment choices, such as insulin resistance, are not available. Further, glargine insulin could have been used as a rescue therapy in cases not adequately controlled with other, less expensive, insulin preparations, such as NPH, making glargine a marker of severity of disease. This is a relevant issue, considering that diabetes itself is associated with increased risk of cancer. It should also be considered that available evidence shows that glargine reduces the risk of hypoglycemia without improving A1C (1), as stated also in Italian national guidelines (www.siditalia.it); therefore, the shift from NPH to glargine is likely to be motivated by hypoglycemic episodes, rather than to inadequate control of fasting glucose. Furthermore, the association between use of higher glargine doses and incident cancer was confirmed after adjusting for previous therapy with NPH insulin.
We should all be aware that the epidemiological approach cannot provide definitive conclusions on the effects of any pharmacological treatment. In fact, not all the motivations underlying a therapeutic decision can be formally explored and adjusted for in multivariate analyses. For example, patients treated with insulin analogues (either basal or prandial) could be different for some clinical or sociocultural characteristics not reported in clinical records; however, only high-dose glargine, and not other (rapid-acting) analogues, was associated with incident cancer. The only fully reliable instrument to assess the effects of glargine on the incidence of cancer would be an adequately sized, long-term, randomized trial with other basal insulins as active comparators and the onset of malignancies as the predefined, primary end point. Unfortunately, such a trial is not likely to be performed, so that information on safety of glargine, and of other insulin analogues, will inevitably derive from observational studies, such as the present one.
Clinical decisions should be based on a careful evaluation of risk/benefit (and cost/benefit) ratios. Glargine has some advantages over NPH human insulin, such as a lower risk of nocturnal hypoglycemia in head-to-head comparisons (1). The only other available long-acting insulin analog, detemir, is absorbed more rapidly after subcutaneous injection and it has therefore a shorter duration of action (24); as a consequence, many patients treated with detemir need a twice-daily administration, while glargine usually is effective with once-a-day administration (25). Conversely, long-acting insulin analogues have a higher cost than NPH insulin. The potentially greater risk for cancer with glargine should be weighed against those advantages. Further, larger-scale observational studies on the incidence of malignancies in patients treated with glargine should be performed taking into account insulin doses, in order to quantify the actual impact of different insulin analogues on the onset of new cases of cancer and identify patients at higher risk.
Supplementary Material
Acknowledgments
E.M. has received consultancy fees from Eli Lilly and Novo Nordisk; speaking fees from Eli Lilly, Novo Nordisk, and sanofi-aventis; and research grants from Eli Lilly, Novo Nordisk, and sanofi-aventis. M.M. has received speaking fees from Eli Lilly and sanofi-aventis. B.C. has received speaking fees from Eli Lilly and sanofi-aventis. L.P. has received speaking fees from Eli Lilly and sanofi-aventis. A.B. has received speaking fees and research grants from Novo Nordisk. N.M. has received speaking fees from Eli Lilly, Novo Nordisk, and sanofi-aventis; and research grants from Eli Lilly, Novo Nordisk, and sanofi-aventis. C.M.R. has received consultancy fees from Eli Lilly, research grants from Eli Lilly, and speaking fees from Eli Lilly, Novo Nordisk, and sanofi-aventis.
No other potential conflicts of interest relevant to this article were reported.
E.M. designed the study, prepared and revised the manuscript, and took part in data analysis. M.M. organized the collection of clinical data, prepared and revised the manuscript, and performed data analysis. D.B. collected administrative data and assisted in study design and data analysis. B.C. collected clinical data. L.P. collected clinical data. C.M. collected administrative data. C.L. collected clinical data. I.B. collected clinical data. M.B. collected clinical data. A.B. assisted in study design and data analysis. N.M. reviewed/edited manuscript. C.M.R. contributed to discussion and reviewed/edited manuscript.
The authors thank the following individuals who were involved in the organization of the study and in the collection and management of data: Alessandro Antenore, Claudia Colombi, Rossella Del Bianco, Romano Desidero, Mauro Di Bari, Iacopo Iacomelli, Giulio Ippolito, Cristina Marchi, Alberto Marsilii, Daniele Martelli, Maria Vivarelli, and Valentina Vitale, all from the Section of Geriatric Cardiology and Medicine, Department of Cardiovascular Medicine, University of Florence and Careggi Teaching Hospital, Florence, Italy.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Monami M, Marchionni N, Mannucci E: Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract 2008;81:184–189 [DOI] [PubMed] [Google Scholar]
- 2. The SDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Res Network Epidemiology Group. Diabetologia 2009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, Sawicki PT: Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009;52:1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jonasson JM, Ljung R, Talback M, Haglund B, Gudbjornsdottir S, Steineck G: Insulin glargine use and short-term incidence of malignancies—a population-based follow-up study in Sweden. Diabetologia 2009;52:1745–1754 [DOI] [PubMed] [Google Scholar]
- 5. Currie CJ, Poole CD, Gale EA: The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–1777 [DOI] [PubMed] [Google Scholar]
- 6. Nagel JM, Mansmann U, Wegscheider K, Rohmel J: Insulin resistance and increased risk for malignant neoplasms: confounding of the data on insulin glargine. Diabetologia 2010;53:206–208 [DOI] [PubMed] [Google Scholar]
- 7. Pocock SJ, Smeeth L: Insulin glargine and malignancy: an unwarranted alarm. Lancet 2009;374:511–513 [DOI] [PubMed] [Google Scholar]
- 8. Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, Neal B, Rodgers A, Ni Mhurchu C, Clark T: 1999 World Health Organization–International Society of Hypertension Guidelines for the management of hypertension: Guidelines Subcommittee of the World Health Organization. Clin Exp Hypertens 1999;21:1009–1060 [DOI] [PubMed] [Google Scholar]
- 9. Charlson M, Szatrowski T, Peterson T, Gold J: Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–1251 [DOI] [PubMed] [Google Scholar]
- 10. Bowker SL, Majumdar SR, Veugelers P, Johnson JA: Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006;29:254–258 [DOI] [PubMed] [Google Scholar]
- 11. Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD: Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM: New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monami M, Balzi D, Lamanna C, Barchielli A, Masotti G, Buiatti E, Marchionni N, Mannucci E: Are sulphonylureas all the same? A cohort study on cardiovascular and cancer-related mortality. Diabetes Metab Res Rev 2007;23:479–484 [DOI] [PubMed] [Google Scholar]
- 14. Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E: Sulphonylureas and cancer: a case-control study. Acta Diabetol 2009;46:279–284 [DOI] [PubMed] [Google Scholar]
- 15. Home PD, Lagarenne P: Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Diabetologia 2009;52:2499–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dejgaard A, Lynggaard H, Rastam J, Krogsgaard TM: No evidence of increased risk of malignancies in patients with diabetes treated with insulin detemir: a meta-analysis. Diabetologia 2009;52:2507–2512 [DOI] [PubMed] [Google Scholar]
- 17. Kurtzhals P, Schaffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, Trub T: Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000;49:999–1005 [DOI] [PubMed] [Google Scholar]
- 18. Mayer D, Chantelau E: Treatment with insulin glargine (Lantus) increases the proliferative potency of the serum of patients with type-1 diabetes: a pilot study on MCF-7 breast cancer cells. Arch Physiol Biochem 2010;116:73–78 [DOI] [PubMed] [Google Scholar]
- 19. Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H: Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009;25:41–49 [DOI] [PubMed] [Google Scholar]
- 20. Erbel S, Reers C, Eckstein VW, Kleeff J, Buchler MW, Nawroth PP, Ritzel RA: Proliferation of colo-357 pancreatic carcinoma cells and survival of patients with pancreatic carcinoma are not altered by insulin glargine. Diabetes Care 2008;31:1105–1111 [DOI] [PubMed] [Google Scholar]
- 21. Liefvendahl E, Arnqvist HJ: Mitogenic effect of the insulin analogue glargine in malignant cells in comparison with insulin and IGF-I. Horm Metab Res 2008;40:369–374 [DOI] [PubMed] [Google Scholar]
- 22. Renehan AG, Harvie M, Howell A: Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocr Relat Cancer 2006;13:273–278 [DOI] [PubMed] [Google Scholar]
- 23. Milazzo G, Sciacca L, Papa V, Goldfine ID, Vigneri R: ASPB10 insulin induction of increased mitogenic responses and phenotypic changes in human breast epithelial cells: evidence for enhanced interactions with the insulin-like growth factor-I receptor. Mol Carcinog 1997;18:19–25 [DOI] [PubMed] [Google Scholar]
- 24. Plank J, Bodenlenz M, Sinner F, Magnes C, Gorzer E, Regittnig W, Endahl LA, Draeger E, Zdravkovic M, Pieber TR: A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care 2005;28:1107–1112 [DOI] [PubMed] [Google Scholar]
- 25. Rosskamp RH, Park G: Long-acting insulin analogs. Diabetes Care 1999;22(Suppl. 2):B109–B113 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.