Abstract
OBJECTIVE
To evaluate the optimal interval for rechecking A1C levels below the diagnostic threshold of 6.5% for healthy adults.
RESEARCH DESIGN AND METHODS
This was a retrospective cohort study. Participants were 16,313 apparently healthy Japanese adults not taking glucose-lowering medications at baseline. Annual A1C measures from 2005 to 2008 at the Center for Preventive Medicine, a community teaching hospital in Japan, estimated cumulative incidence of diabetes.
RESULTS
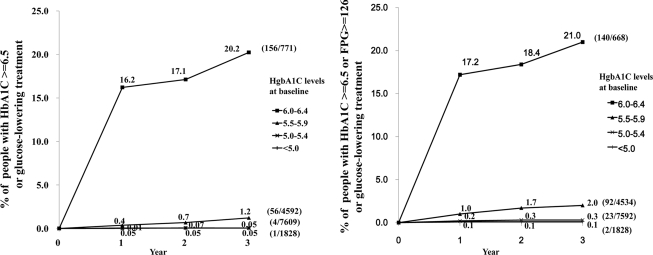
Mean age (±SD) of participants was 49.7 ± 12.3 years, and 53% were male. Mean A1C at baseline was 5.4 ± 0.5%. At 3 years, for those with A1C at baseline of <5.0%, 5.0–5.4%, 5.5–5.9%, and 6.0–6.4%, cumulative incidence (95% CI) was 0.05% (0.001–0.3), 0.05% (0.01–0.11), 1.2% (0.9–1.6), and 20% (18–23), respectively.
CONCLUSIONS
In those with an A1C <6.0%, rescreening at intervals shorter than 3 years identifies few individuals (∼≤1%) with an A1C ≥6.5%.
Since fasting and post-glucose challenge blood glucose levels were found to predict the risk of diabetic retinopathy, they have been the international standard for diagnosis (1). Recently, a shift to A1C for diagnosis has been proposed because A1C integrates longer-term glucose levels and has better preanalytic stability (2). The proposed diagnostic threshold of 6.5% was based on retinopathy risk at different levels of A1C (2). However, optimal frequency for repeating A1C has not been determined (3). We used a large, longitudinal dataset to explore the value of repeating A1C at different intervals to identify subjects who might progress above the threshold (≥6.5%).
RESEARCH DESIGN AND METHODS
Between January and December 2005, we consecutively enrolled all adults (aged >20 years) attending the Center for Preventive Medicine at St. Luke's International Hospital in Tokyo, Japan, for the health check program. The program promotes early detection of chronic diseases and disease risk factors. We excluded people who took glucose-lowering medications at baseline.
Data collection
We extracted data from records of people undergoing annual health checks from January 2005 to December 2008. We excluded those without health checks in years 1, 2, or 3. St. Luke's International Hospital Institutional Review Board approved the study.
Measurements
The annual health check collected demographic information and medical history with an initial evaluation (vital signs and laboratory data). Laboratory data included A1C, fasting plasma glucose (FPG), and lipids (total cholesterol, LDL cholesterol, and HDL cholesterol). Venous blood was drafter an overnight fast and analyzed at the Central Laboratory at St. Luke's International Hospital. A1C assays were performed by an automated glycohemoglobin analyzer (HLC-723G; Tosoh, Tokyo, Japan), with a coefficient of variation (CV) of <1.34%, and certified by the National Glycohemoglobin Standardization Program (4). We classified subjects with either a single measured A1C ≥6.5% (5,6) or self-reported commencement of glucose-lowering treatment as diabetic. As a sensitivity analysis, we used FPG ≥126 mg/dl as one of the diagnostic criteria.
Statistical methods
For the analyses, we used SPSS software 15.0J (SPSS Japan, Tokyo, Japan), except with 95% CIs, which were based on an exact binominal (7) using Stata version 10 (Stata, College Station, TX).
RESULTS
From January 2005 to July 2008, 16,313 people of the enrolled population of 39,284 underwent annual checks. Mean age (±SD) of participants was 49.7 ± 12.3 years, and 53% were male. Mean BMI was 22.5 ± 3.2 kg/m2; fasting plasma glucose was 99.2 ± 12.7 mg/dl; A1C at baseline was 5.4 ± 0.5%; total cholesterol, LDL cholesterol, and HDL cholesterol levels at baseline were 204.3 ± 33.8 mg/dl, 117.6 ± 29.7 mg/dl, and 62.4 ± 15.8 mg/dl, respectively; and systolic and diastolic blood pressure was 119 ± 18 mmHg and 73 ± 11 mmHg, respectively. The trends of mean A1C levels for the entire cohort from 2005 to 2008 slightly increased over 3 years (0.05% per year). The demographic characteristics of nonparticipants and participants were similar.
At 3 years, the cumulative incidence of diabetes was 3.2% (95% CI 3.0–3.4). However, this varies greatly by initial level of A1C. At 3 years, for those with A1C of <5.0%, 5.0–5.4%, 5.5–5.9%, and 6.0–6.4% at baseline, cumulative incidence (95% CI) was 0.05% (0.001–0.3), 0.05% (0.01–0.11), 1.2% (0.9–1.6), and 20% (18–23), respectively, and adding FPG ≥126 mg/dl to the diagnostic criteria showed similar results (Fig. 1). Logistic regression suggested that only BMI (odds ratio 1.14/kg/m2) and FPG (1.06/mg/dl) added to the baseline A1C; age, sex, systolic blood pressure, and LDL were not significant. The average CV of A1C stratified by baseline A1C was 2.7% and did not differ among these subgroups.
Figure 1.
Percent of patients at annual rechecks with A1C >6.5% or FPG >126 mg/dl (by baseline A1C).
CONCLUSIONS
This study confirms that the rise in A1C in a nondiabetic population is slow. Participants who are well under the diagnostic threshold of A1C 6.5% are unlikely to exceed this within several years of follow-up.
Much of the increased detection of diabetes in those with a higher baseline A1C was at 1 year and may be attributable to measurement error and short-term variation in A1C. The CV (including within-subject variation) varies between 2 and 5% (8); a CV of 5% would mean that a 95% measurement interval of a single A1C in this range would be ±0.6%. This degree of variation would lead to some individuals having sequential tests from just below to just above 6.5%. Although the variation could occur at all time points, this is much less likely in the 5.0–5.9% range.
Our findings echo the slow rise of A1C found in trials with diabetic patients. For example, in the UK Prospective Diabetes Study, the patients on diet alone had a rise of <0.2% per year (9). Our nondiabetic cohort had an even lower average change in A1C of 0.05% per year.
This study has several limitations. First, the follow-up is incomplete because not all participants came back every year. This could be addressed by other analysis, such as a linear mixed model. However, any bias would be likely to favor those developing diabetes to reattend. Second, a few participants (1.1%) began taking glucose-lowering drugs, but this is unlikely to make a large difference to our conclusions. Third, our data are from one institution in Tokyo, Japan, and might not generalize to other populations. For example, adult mean BMI levels of 22–23 kg/m2 are found in Africa and Asia, while levels of 25–27 kg/m2 are prevalent across North America and Europe, and then BMI level could be related to the cumulative incidence of diabetes. Finally, although the American Diabetes Association criteria recommend a repeat A1C test to confirm the diagnosis of type 2 diabetes (2), our study included only a single measurement of A1C.
In conclusion, for the purpose of detecting new cases of diabetes, in those with an initial A1C <6.0%, rescreening at intervals shorter than 3 years identifies few individuals (∼≤1%) with an A1C ≥6.5%. At A1C ≥6%, rescreening even at a 1-year interval would be reasonable strategy to identify disease.
Acknowledgments
This work was supported in part by a U.K. National Institute for Health Research Program Grant and by a grant from the Ministry of Health, Labour, and Welfare of Japan.
No potential conflicts of interest relevant to this article were reported.
O.T. wrote the manuscript, A.J.F. wrote the manuscript, P.P.G. wrote and reviewed the manuscript, and T.S. and T.F. contributed to the discussion.
We thank the following people: Sachiko Ohde, St. Luke's Life Science Institute, and Gautam A. Deshpande, St. Luke's Life Science Institute and the University of Hawaii, for their helpful comments, and Jiro Suwa and Sonoe Hiramatsu, the Center for Preventive Medicine at St. Luke's International Hospital, for the data collection.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 2. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kahn R, Alperin P, Eddy D, Borch-Johnsen K, Buse J, Feigelman J, Gregg E, Holman RR, Kirkman MS, Stern M, Tuomilehto J, Wareham NJ: Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet 2010;375:1365–1374 [DOI] [PubMed] [Google Scholar]
- 4. Terreni A, Paleari R, Caldini A, Ognibene A, Mosca A, Messeri G: Evaluation of the analytic performances of the new HPLC system HLC-723 G7 for the measurement of hemoglobin A1c. Clin Biochem 2003;36:607–610 [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bloomgarden ZT: A1C: recommendations, debates, and questions. Diabetes Care 2009;32:e141–e147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clopper SJ, Pearson ES: The use of confidence of fiducial limits illustrated in the case of the binominal. Biometrika 1932;26:404–413 [Google Scholar]
- 8. Schwartz KL, Monsur JC, Bartoces MG, West PA, Neale AV: Correlation of same-visit HbA1c test with laboratory-based measurements: a MetroNet study. BMC Fam Pract 2005;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. UK Prospective Diabetes Study (UKPDS). Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ 1995;310:83–88 [PMC free article] [PubMed] [Google Scholar]