Abstract
OBJECTIVE
In adults, higher fasting plasma glucose (FPG) levels, even within the normoglycemic range, are associated with increased diabetes risk. This investigation tested the hypothesis that β-cell function relative to insulin sensitivity decreases with increasing FPG in youth.
RESEARCH DESIGN AND METHODS
A total of 223 youth with FPG <126 mg/dl underwent evaluation of first- and second-phase insulin secretion during a 2-h hyperglycemic (∼225 mg/dl) clamp, insulin sensitivity during a 3-h hyperinsulinemic-euglycemic clamp, body composition, and abdominal adiposity with dual-energy X-ray absorptiometry and computed tomographic scan. β-Cell function relative to insulin sensitivity was calculated as the product of first-phase insulin and insulin sensitivity, i.e., glucose disposition index (GDI). The subjects were divided into three FPG categories: ≤90, >90–<100, and ≥100–<126 mg/dl.
RESULTS
GDI decreased significantly across the three categories as FPG increased (1,086 ± 192 vs. 814 ± 67 and 454 ± 57 mg/kg/min, P = 0.002). This decline remained significant after adjustment for race, sex, BMI, and percent body fat or visceral fat. Within each FPG category, GDI declined with increasing BMI percentiles.
CONCLUSIONS
The impairment in β-cell function relative to insulin sensitivity is apparent even within the nondiabetic FPG range in children. At the current cutoff of 100 mg/dl for impaired fasting glucose (IFG), there is an ∼49% decline in the GDI independent of obesity and race. This observation may reflect a heightened risk of β-cell dysfunction and progression to diabetes in these children. Considering the near doubling of IFG prevalence among youth between National Health and Nutrition Examination Survey 1999–2000 and 2005–2006, our findings have important public health implications.
Higher fasting plasma glucose (FPG) levels within the currently accepted normoglycemic range seem to have an impact on diabetes risk in adults (1–3). FPG levels >125 mg/dl indicate a provisional diagnosis of diabetes (4). FPG levels below this cutoff but above normal increase the risk of developing diabetes (5) and are associated with a higher cardiovascular disease risk (6,7). The American Diabetes Association classifies this intermediate state of FPG as impaired fasting glucose (IFG) (4) and initially defined it as fasting glucose of 110–125 mg/dl (8), which was lowered to 100 mg/dl in 2003 to better identify subjects at risk of diabetes development (9). Several studies in adults suggested that future diabetes risk increases continually with increasing FPG even below this lower cutoff for normoglycemia (1,2). In adults, fasting glucose levels of 90–94 mg/dl conferred a 49% greater risk of developing diabetes compared with levels <85 mg/dl (2). FPG levels in the top quintiles (95–99 mg/dl) of the normoglycemic range constituted an independent risk factor for type 2 diabetes among young men in the Israeli army after adjustment for several risk factors (1). In a Mauritius population-based study, parameters related to diabetes and cardiovascular disease such as higher BMI, cholesterol, triglycerides, and hypertension were positively correlated with higher FPG values in an approximately linear relationship (3). In addition, a meta-analysis of prospective studies showed a continuous relationship between baseline fasting glucose and subsequent cardiovascular risk (10). These observations raise a fundamental question: are insulin secretion and sensitivity impaired at higher levels of FPG but within the normal range? Few studies in adults have investigated this question, and results are conflicting (11–13). However, none of these studies used robust methodologies of assessing in vivo insulin secretion and sensitivity to derive a glucose disposition index (GDI), which is accepted to be the best indicator of β-cell dysfunction (14). Currently there are no published data assessing the relationship between high-normal fasting glucose levels and insulin sensitivity and secretion in children. The pre-diabetes cutoff levels for FPG in pediatrics are based on adult data and not on data generated in pediatric populations.
In this study, we investigated the relationship between levels of fasting glucose and insulin secretion relative to insulin sensitivity, i.e., GDI, in children. Based on adult observations we hypothesized that β-cell function relative to insulin sensitivity decreases as FPG concentrations increase within the currently accepted nondiabetic range in children.
RESEARCH DESIGN AND METHODS
Youth 8–<19 years old with FPG concentrations <126 mg/dl who participated in our National Institutes of Health–funded studies to evaluate childhood metabolic markers of adult morbidity were identified. We included 223 participants who had undergone both a hyperglycemic clamp and a hyperinsulinemic-euglycemic clamp, 181 of whom were reported previously in a different context (15). Participants for our studies are recruited from the community through local newspaper advertisements, flyers posted at various locations including public bus routes, children's recreational centers, the medical campus, and the Children's Hospital of Pittsburgh as before (15). A complete medical history was obtained from each participant, and all underwent a thorough physical examination including pubertal Tanner staging by a pediatric endocrinologist and/or a certified nurse practitioner. For girls, Tanner stage was determined based on pubic hair and breast development and for boys it was based on pubic hair and testicular volume, using the Prader orchidometer. In case of a discrepancy between pubic hair stage (which is mainly driven by adrenarche) and breast development in girls or testicular volume in boys (which are driven by gonadal activation of puberty), the latter staging was assigned. All studies were approved by the Institutional Review Board of the University of Pittsburgh. Informed consent and assent were obtained from each participant and their legal guardians.
Clamp studies
Participants were admitted twice within a 1- to 4-week period to the Pediatric Clinical and Translational Research Center the day before the clamp studies, once for a hyperinsulinemic-euglycemic clamp and then for a hyperglycemic clamp in random order. Each clamp evaluation was performed after a 10- to 12-h overnight fast. A fasting blood sample was obtained for total cholesterol, HDL, LDL, VLDL, and triglyceride measurements. For each clamp study, two intravenous catheters were inserted after the skin and subcutaneous tissues were anesthetized with EMLA cream (Astra Pharmaceutical Products, West Borough, MA). One catheter was placed in a vein on the forearm for administration of glucose and insulin; the second catheter was placed in a vein in the dorsum of the contralateral heated hand for sampling of arterialized venous blood as reported (16).
Hyperinsulinemic-euglycemic clamp
A 3-h hyperinsulinemic-euglycemic clamp was performed with plasma glucose clamped at 100 mg/dl with a variable rate infusion of 20% dextrose based on arterialized plasma glucose determinations every 5 min as described before (16). Continuous indirect calorimetry by a ventilated hood system (Deltatrac Metabolic Monitor; Sensormedics, Anaheim, CA) was performed to measure CO2 production, O2 consumption, and respiratory quotient for 30 min at baseline and at the end of the clamp (16).
Hyperglycemic clamp
First- and second-phase insulin secretion were assessed during a 2-h hyperglycemic clamp (225 mg/dl) as described before (16). Glucose and insulin concentrations were measured every 2.5 min (at 2.5, 5, 7.5, 10, and 12.5 min, first phase) and thereafter every 5 min for glucose and every 15 min for insulin.
Body composition
Body composition was determined by dual energy X-ray absorptiometry. Subcutaneous abdominal adipose tissue (SAT) and visceral adipose tissue (VAT) were examined by a single slice computed tomographic scan at L4–L5 as described before (16).
Biochemical measurements
Plasma glucose was measured with a glucose analyzer (YSI, Yellow Springs, OH), and insulin was measured by radioimmunoassay as before (16). A1C was measured by high-performance liquid chromatography (Tosoh Medics), and lipids were measured using the standards of the Centers for Disease Control and Prevention (16).
Calculations
Insulin-stimulated Rd was calculated during the last 30 min of the 3-h hyperinsulinemic-euglycemic clamp to be equal to the rate of exogenous glucose infusion. Peripheral insulin sensitivity was calculated by dividing the Rd by the steady-state clamp insulin level as reported by us (17). Insulin-stimulated carbohydrate and fat oxidation rates were calculated according to the formulas of Frayn (18) from the indirect calorimetry data. Nonoxidative glucose disposal was estimated by subtracting the rate of glucose oxidation from the total Rd. During the hyperglycemic clamp, the first- and second-phase insulin concentrations were calculated as described previously (16). GDI was calculated as the product of insulin sensitivity × first-phase insulin (17).
Statistical analysis
Differences in continuous variables among the three groups were tested with either a one-way ANOVA or the nonparametric Kruskall-Wallis test, based on the nonviolation of statistical assumptions. Differences in categorical variables were tested using the χ2 test or the Fisher exact test. The univariate general linear model (ANCOVA) was used to compare the main outcome measures among the three categories of FPG. Each outcome measure/dependent variable was entered separately into the model, with FPG category as the fixed factor. Potential confounders adjusted for were entered as covariates in each model and are specified with the reported results. All statistical assumptions were met. Data are presented as means ± SEM unless otherwise indicated. Statistical significance was set at P ≤ 0.05. All statistical analyses were performed using SPSS software (SPSS, Chicago, IL).
RESULTS
The group consisted of 86 boys and 137 girls (38.6 and 61.4%, respectively). Ninety-eight subjects (43.9%) self-identified as African Americans, 120 (53.8%) as Caucasians, and 5 (2.2%) as biracial. Of the female subjects, 52 had polycystic ovary syndrome (PCOS) (Table 1). None of the participants, including the girls with PCOS, were taking medications that affected blood glucose metabolism.
Table 1.
Physical and metabolic characteristics of participants categorized according to fasting glucose levels
| FPG |
ANOVA P value | |||
|---|---|---|---|---|
| ≤90 mg/dl | >90–<100 mg/dl | ≥100–<126 mg/dl | ||
| n | 40 | 136 | 47 | |
| Age (years) | 12.8 ± 0.4 | 12.9 ± 0.2 | 13.2 ± 0.3 | 0.69 |
| Sex* | 0.18* | |||
| Male | 11 (27.5) | 53 (39) | 22 (47) | |
| Female | 29 (72.5) | 83 (61) | 25 (53) | |
| Race* | 0.98* | |||
| African American | 16 (40) | 60 (44) | 22 (47) | |
| Caucasian | 23 (57.5) | 73 (54) | 24 (51) | |
| Biracial | 1 (2.5) | 3 (2.2) | 1 (2) | |
| Tanner stage* | 0.052* | |||
| I | 14 (35) | 25 (18.4) | 4 (8.5) | |
| II | 3 (7.5) | 19 (14) | 4 (8.5) | |
| III | 6 (15) | 28 (20.6) | 10 (21) | |
| IV | 4 (10) | 19 (14) | 13 (28) | |
| V | 13 (32.5) | 45 (33) | 16 (34) | |
| Weight (kg) | 68.2 ± 5.7 | 73.0 ± 2.7 | 82.9 ± 3.9 | 0.08 |
| Height (cm) | 154.2 ± 2.2 | 156.4 ± 1.1 | 160.5 ± 1.6 | 0.07 |
| BMI (kg/m2) | 27.1 ± 1.7 | 28.6 ± 0.8 | 31.6 ± 1.2 | 0.07 |
| BMI % | 76.2 ± 4.4 | 82.2 ± 2.1 | 92.5 ± 2.3 | 0.005 |
| Fat mass (kg) | 24.1 ± 3.2 | 26.1 ± 1.6 | 32.2 ± 2.4 | 0.07 |
| % body fat | 33.6 ± 2.5 | 34.0 ± 1.2 | 38.0 ± 1.8 | 0.21 |
| Waist circumference (cm) | 85.9 ± 5.0 | 86.1 ± 2.4 | 94.7 ± 3.2 | 0.14 |
| Waist circumference percentile | 0.16* | |||
| <75th | 12 (40) | 34 (37.8) | 7 (20) | |
| 75th–90th | 6 (20) | 12 (13.3) | 4 (11.4) | |
| >90th | 12 (40) | 44 (48.9) | 24 (68.6) | |
| SAT (cm2) | 311.7 ± 44.3 | 337.1 ± 22.3 | 398.0 ± 32.8 | 0.25 |
| VAT (cm2) | 49.8 ± 6.7 | 48.5 ± 3.6 | 56.9 ± 5.1 | 0.47 |
| Glucose (mg/dl) | 87.6 ± 0.3 | 94.9 ± 0.2 | 105.0 ± 0.6 | <0.001 |
| Insulin (μU/ml) | 28.1 ± 4.2 | 30.5 ± 1.7 | 40.2 ± 3.6 | 0.02 |
| A1C (%) | 5.3 ± 0.08 | 5.3 ± 0.04 | 5.4 ± 0.07 | 0.56 |
| Cholesterol (mg/dl) | 160.8 ± 5.7 | 164.8 ± 2.4 | 165.3 ± 5.2 | 0.75 |
| LDL (mg/dl) | 90.1 ± 5.7 | 98.6 ± 2.1 | 100.4 ± 5.2 | 0.2 |
| HDL (mg/dl) | 47.0 ± 1.7 | 45.8 ± 0.9 | 42.4 ± 1.8 | 0.11 |
| Triglycerides (mg/dl) | 116.9 ± 11.5 | 102.4 ± 4.6 | 114.8 ± 10.8 | 0.29 |
| VLDL (mg/dl) | 23.4 ± 2.3 | 20.3 ± 0.9 | 21.6 ± 1.8 | 0.3 |
Data are unadjusted means ± SEM or n (%). Ten subjects with FPG ≤90 mg/dl, 46 subjects with FPG >90–<100 mg/dl, and 12 subjects with FPG >100–<126 mg/dl did not have waist circumference data because this measurement was implemented late in the course of the grant project.
*χ2 analysis.
The study participants were divided into three groups based on the mean of four FPG levels obtained 10 min apart before the start of the hyperinsulinemic-euglycemic clamp: ≤90 mg/dl (n = 40), >90–<100 mg/dl (n = 136), and ≥100–<126 mg/dl (n = 47). There was 85% concordance between the FPG levels obtained at the time of the hyperinsulinemic-euglycemic clamp and the mean of three FPG levels, 15 min apart, at the time of the hyperglycemic clamp. Age, sex, race, and Tanner stage distributions were similar among the three groups (Table 1). The numbers of girls with PCOS were similar in the three FPG groups: 12 (30%), 29 (21.3%), and 11 (23.4%) (P = 0.5), respectively. BMI tended to be higher with higher FPG categories (P = 0.07); this difference became significant after adjustment for race, sex, and age (ANCOVA; P = 0.025). BMI percentiles were significantly higher with higher FPG categories. Fat mass tended to be higher with higher FPG categories (P = 0.07). This difference reached significance after adjustment for race, age, and sex (ANCOVA; P = 0.019). Waist circumference, waist circumference percentiles, VAT, SAT, and percent body fat were not significantly different among the three groups (Table 1) and remained nonsignificant after adjustment (ANCOVA) for age, race, and sex, waist circumference (P = 0.21), VAT (P = 0.49), and SAT (P = 0.11) but became significant for percent body fat (P = 0.04).
Fasting metabolic profile
Besides glucose, there were no significant differences among the groups except for fasting insulin levels (Table 1). However, after adjustment for BMI or BMI percent the difference disappeared (P = 0.17 and 0.27, respectively).
In vivo insulin sensitivity and insulin secretion
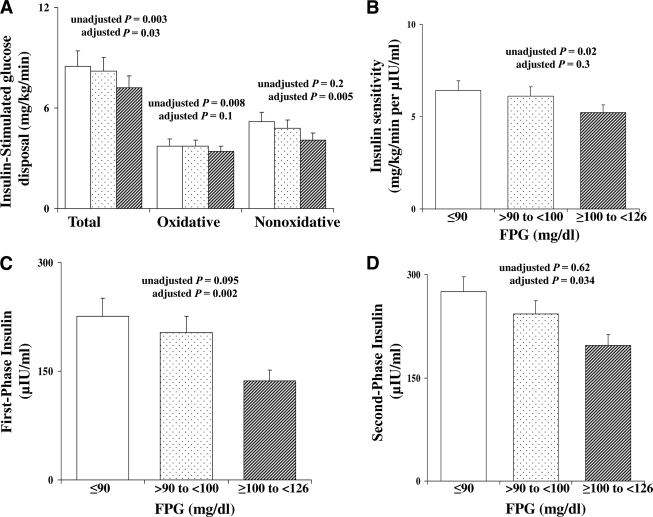
Insulin-stimulated total, oxidative, and nonoxidative glucose disposal was significantly lower in the highest fasting glucose category (Fig. 1A). Similarly, insulin sensitivity was significantly lower in the categories with higher FPG (Fig. 1B). The difference in insulin sensitivity did not persist after adjustment for BMI (P = 0.3). First- and second-phase insulin secretion were not significantly different among the three categories of FPG, but became significantly lower with increasing FPG after adjustment for BMI (Fig. 1C and D).
Figure 1.
Insulin-stimulated glucose disposal (total, oxidative, and nonoxidative) (A) and insulin sensitivity (B) during the hyperinsulinemic-euglycemic clamp and first- and second-phase insulin levels (C and D) during the hyperglycemic clamp in youth with FPG ≤90 mg/dl (□), >90–<100 mg/dl ( ), and ≥100–<126 mg/dl (▨). Data represent the estimated marginal means from the ANCOVA model corrected for BMI. P values unadjusted and adjusted for BMI are shown.
), and ≥100–<126 mg/dl (▨). Data represent the estimated marginal means from the ANCOVA model corrected for BMI. P values unadjusted and adjusted for BMI are shown.
GDI
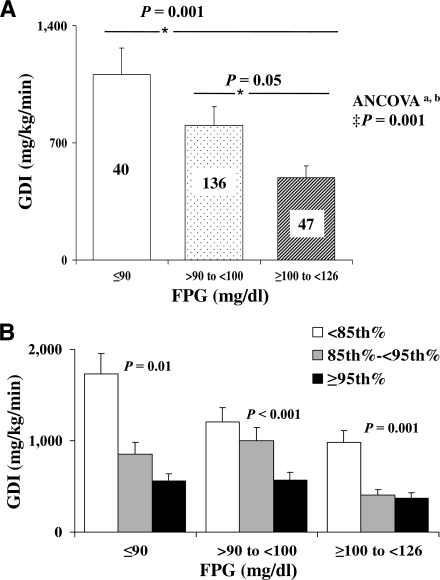
The GDI decreased significantly with increasing FPG categories (Fig. 2A). Using ANCOVA with BMI, VAT, age, race, sex, and Tanner stage as the covariates, the difference in GDI remained significant (P = 0.001). Likewise, the difference remained significant when fat mass was added to the model (P = 0.002). Furthermore, within each glucose category participants with BMI ≥95th percentile had significantly lower GDI than those with BMI <85th percentile (Fig. 2B). In addition, GDI remained significantly lower in the higher categories of FPG when the subjects with PCOS were excluded from the analysis. When the data were analyzed separately for prepubertal (Tanner stage I, n = 43) and pubertal (Tanner stages II–V, n = 180) subjects, GDI in the pubertal group but not in the prepubertal group was consistent with the whole-group analysis. Among pubertal youth, GDI was lowest in the higher FPG category (383.4 ± 40.6 vs. 743.6 ± 77.2 and 712.5 ± 115.9 mg/kg/min, P = 0.02, in FPG ≥100–<126 mg/dl [n = 43], >90–<100 mg/dl [n = 110], and ≤90 mg/dl [n = 26], respectively). This trend in prepubertal children did not reach significance; however, the numbers of subjects were limited, especially in the highest glucose category (1779.8 ± 459.4, 1121.4 ± 106.1, and 1216.7 ± 359.7 mg/kg/min, P = 0.2, in FPG ≤90 mg/dl [n = 14], >90–<100 mg/dl [n = 25], and ≥100–<126 mg/dl [n = 4], respectively).
Figure 2.
A: GDI (milligrams per kilogram per minute) in youth with FPG ≤90 mg/dl (□), >90–<100 mg/dl ( ), and ≥100–<126 mg/dl (▨). Data represent the estimated marginal means from the ANCOVA model. ‡Covariates appearing in the model are age, sex, race, Tanner stage, BMI, and VAT. aP = 0.002 when fat mass is added as a covariate to the model; bP = 0.003 when fat mass, lean mass, and height are added as covariates to the model. *P represents post hoc Bonferroni correction. B: GDI (milligrams per kilogram per minute) in youth with FPG ≤90 mg/dl, >90–<100 mg/dl, and ≥100–<126 mg/dl according to BMI <85th percentile (□), between the 85th and 95th percentiles (
), and ≥100–<126 mg/dl (▨). Data represent the estimated marginal means from the ANCOVA model. ‡Covariates appearing in the model are age, sex, race, Tanner stage, BMI, and VAT. aP = 0.002 when fat mass is added as a covariate to the model; bP = 0.003 when fat mass, lean mass, and height are added as covariates to the model. *P represents post hoc Bonferroni correction. B: GDI (milligrams per kilogram per minute) in youth with FPG ≤90 mg/dl, >90–<100 mg/dl, and ≥100–<126 mg/dl according to BMI <85th percentile (□), between the 85th and 95th percentiles ( ), and ≥95th percentile (■). P represents ANOVA.
), and ≥95th percentile (■). P represents ANOVA.
CONCLUSIONS
In the present study, we investigated in children the relationship between β-cell function relative to insulin sensitivity and fasting plasma glucose levels across the normoglycemic and IFG ranges. Our findings demonstrate that in the IFG category, ≥100–<126 mg/dl, there is a significant decline of ∼49% in β-cell function relative to insulin sensitivity independent of obesity and race, whereas a decline of ∼23% is present in the >90–<100 mg/dl category compared with that in the lower FPG of ≤90 mg/dl. Furthermore, within each fasting glucose category BMI ≥95th percentile is associated with an additional decline of ∼50–70% in GDI. Under normal physiologic conditions glucose concentrations remain within a narrow range in the fasting as well as in the fed state. This tight regulation is maintained by a delicate balance between insulin sensitivity and secretion (19). A hyperbolic relationship governs this balance, such that the product of insulin sensitivity and insulin secretion is a constant in any given individual (20). This product is known as the GDI and is a more accurate reflection of β-cell function than absolute insulin secretion, because it normalizes the pancreatic response to the degree of insulin sensitivity (21). In this study, insulin sensitivity decreased as FPG increased even within the normal range, but insulin secretion did not increase sufficiently to maintain the GDI. These findings suggest that the impairments in insulin sensitivity and β-cell response leading to diabetes may be evident at plasma glucose levels well within the normoglycemic range.
Our findings with respect to insulin sensitivity are consistent with data from the adult literature, which have used different FPG categories. We elected to categorize the study participants according to the above FPG levels to show relevance with respect to clinically recommended diagnostic categories. In a recent large study of 6,414 adult Finnish men, insulin sensitivity derived from an oral glucose tolerance test (OGTT) (Matsuda index) was 17% lower within the normal range of FPG 90–97.2 mg/dl compared with that for FPG <90 mg/dl independent of obesity (13). Likewise, another study reported decreased insulin sensitivity in the highest quartile of FPG (94–110 mg/dl) among 148 postmenopausal women using a 2-h hyperinsulinemic-euglycemic clamp (11). An earlier study in adults reported a 20% decline in insulin sensitivity, measured by the euglycemic clamp, among 62 adults with normal glucose tolerance and a fasting glucose ranging from 84.6 to 108 mg/dl (22). Our data revealed a decline in total, oxidative, and nonoxidative insulin-stimulated glucose disposal and a decline in in vivo clamp-measured insulin sensitivity as FPG increased. This decline in insulin sensitivity was associated with an increase in BMI.
For insulin secretion and FPG, studies from the adult literature have conflicting results. A Finnish study reported only a slight decline in early-phase insulin secretion during the OGTT in the normoglycemic range among adult men (13). Another study in adult men, using the intravenous glucose tolerance test showed a marked decline in first-phase insulin secretion at FPG levels between 90 and 97.2 mg/dl and a loss of first-phase β-cell function at a rate of 3.8 to 4.5% per 1.8 mg/dl increase in FPG. In the same study a decline in late-phase insulin secretion was detected at FPG levels >108 mg/dl (12). On the other hand, a study in postmenopausal women reported an increase in baseline and maximal arginine-induced insulin secretion in the highest quartiles of FPG (94–110 mg/dl) (11). An earlier study in adults of both sexes, using the C-peptide deconvolution during the OGTT for β-cell function, reported a decline in β-cell glucose sensitivity by 50–70% within the normal glucose tolerance range (22). However, the study included subjects with impaired glucose tolerance and with overt diabetes and reported a detectable decline in GDI only in the subjects with type 2 diabetes (22). In our young subjects, there was no significant difference in absolute first- and second-phase insulin secretion during the hyperglycemic clamp as FPG increased. This finding could potentially be explained by the younger age of our participants, which might have precluded the manifestation of an overt decline in the absolute β-cell response during the hyperglycemic clamp. However, when insulin secretion was expressed relative to insulin sensitivity (GDI), the impairment in β-cell function and insulin secretion became apparent as FPG levels increased within the normal and IFG ranges. Use of ANCOVA for age, Tanner stage, sex, and race did not alter this observation. The decline in GDI was significant in pubertal youth but not in prepubertal youth when the data were analyzed separately. However, the number of prepubertal children in the IFG category was very limited (n = 4) and did not allow for any statistical significance, especially because the study was not designed to test this. It would be interesting however, to test whether the observation of declining GDI with increasing FPG holds true in a larger group of prepubertal children.
Two large longitudinal studies in adults, one in the U.S. (2) and one in Israel (1), have reported an increased risk of diabetes incidence as FPG increases within the normoglycemic range. This risk was potentiated by an increase in BMI (2). Although our study is cross-sectional, the findings of ∼23% lower GDI with higher FPG in the normoglycemic range and ∼49% in the IFG range is in concordance with the increased risk of type 2 diabetes development in the aforementioned studies. Considering the near doubling of IFG prevalence among youth between the National Health and Nutrition Examination Survey (NHANES) 1999–2000 (7%) and 2005–2006 (13.1%) (23), our current findings bear important public health implications for the ∼4,381,711 U.S. adolescents with IFG. In adults ∼22% of subjects with IFG progress to diabetes over an observational period of 3–5 years (3). There are no longitudinal studies assessing the progression from IFG to overt diabetes in children and adolescents. However, if the conversion rate is similar to that in adults, our findings together with the increased prevalence of IFG paint a bleak picture for the future rates of diabetes among older adolescents and young adults. Furthermore, in our study GDI was significantly lower (∼50–70%) in children with BMI ≥95th percentile versus those with BMI <85th percentile within each category of FPG (Fig. 2B). The reported NHANES 2003–2006 prevalence rate of children 2–19 years with BMI ≥95th percentile is 16.3% (24), which translates to ∼5 million children in the overweight category. Recent reports show that young adults who were overweight/obese at age 5 years had an increased odds ratio of 2.6 of having diabetes by age 21 years (25). Our findings of ∼50% lower GDI in obese youths, even within the normoglycemic range suggest that within the same fasting glucose category obese individuals have twice the impairment in β-cell function potentially increasing their risk of future diabetes. Of interest is the 8.8% prevalence of IFG among the participants with BMI <85th percentile in our study. Our subjects were recruited from the community with no specific targeting for offspring of parents with diabetes or high-risk children with abnormal fasting glucose levels. Furthermore, they were tested in a Pediatric Clinical Research Center under very controlled conditions with close supervision. Considering the latter and also considering that the current rate of IFG is lower than that observed in other studies (23), we trust that these are representative data. One limitation of our study is the lack of oral glucose tolerance data, mainly in normal-weight youth. Some of our subjects with or without IFG could have impaired glucose tolerance and represent a different category with further impairment in GDI. However, all of the participants had normal A1C levels. Another limitation is the discrepancy in the sample sizes in the different FPG categories. The relatively smaller numbers in the FPG ≤90 mg/dl category may have precluded detection of significant differences in GDI between subjects in this group and those in the FPG >90–<100 mg/dl category.
In summary, our findings demonstrate that the impairment in β-cell function and insulin secretion relative to insulin sensitivity is already evident at fasting plasma glucose levels well within the normoglycemic range in children. This stepwise decline in β-cell function with increasing FPG seems to be potentiated by higher BMI, a finding of particular importance in the context of the ever-escalating trajectory in childhood obesity. It remains to be seen in longitudinal studies whether those children with higher FPG levels will be at higher risk to develop overt diabetes.
Acknowledgments
This work was supported by the U.S Public Health Service (grants R01-HD-27503 and K24-HD-01357 to S.A., the Richard L. Day Endowed Chair to S.A., grant M01-RR-00084 to the General Clinical Research Center, and Clinical and Translational Science Award UL1-RR -024153).
No potential conflicts of interest relevant to this article were reported.
H.T. analyzed data and wrote the manuscript. S.L. contributed laboratory/analytical tools. S.A. provided the study concept and design, acquired data, obtained funding, provided administrative technical and material support, supervised the study, reviewed/edited the manuscript.
Parts of this study were presented in abstract form at the 70th annual meeting of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
These studies would not have been possible without the nurses and the staff of the Pediatric Clinical and Translational Research Center, the devotion of Sabrina Kadri and Nancy Guerra, CRNP (project coordinators), the laboratory expertise of Resa Stauffer and Katie McDowell, and the assistance of all past endocrine fellows with some of the clamp experiments, but most importantly, the commitment of the study participants and their parents.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A: Israeli Diabetes Research Group. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 2005;353:1454–1462 [DOI] [PubMed] [Google Scholar]
- 2. Nichols GA, Hillier TA, Brown JB: Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med 2008;121:519–524 [DOI] [PubMed] [Google Scholar]
- 3. Shaw JE, Zimmet PZ, Hodge AM, de Courten M, Dowse GK, Chitson P, Tuomilehto J, Alberti KG: Impaired fasting glucose: how low should it go? Diabetes Care 2000;23:34–39 [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009;32(Suppl. 1):S62–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B: Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 6. Charles MA, Fontbonne A, Thibult N, Warnet JM, Rosselin GE, Eschwege E: Risk factors for NIDDM in white population. Paris prospective study. Diabetes 1991;40:796–799 [DOI] [PubMed] [Google Scholar]
- 7. Charles MA, Balkau B, Vauzelle-Kervroedan F, Thibult N, Eschwege E: Revision of diagnostic criteria for diabetes. Lancet 1996;348:1657–1658 [DOI] [PubMed] [Google Scholar]
- 8. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 9. Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P: Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 10. Coutinho M, Gerstein HC, Wang Y, Yusuf S: The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999;22:233–240 [DOI] [PubMed] [Google Scholar]
- 11. Ahrén B: Insulin secretion and insulin sensitivity in relation to fasting glucose in healthy subjects. Diabetes Care 2007;30:644–648 [DOI] [PubMed] [Google Scholar]
- 12. Godsland IF, Jeffs JA, Johnston DG: Loss of β cell function as fasting glucose increases in the non-diabetic range. Diabetologia 2004;47:1157–1166 [DOI] [PubMed] [Google Scholar]
- 13. Stancáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M: Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 2009;58:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kahn SE: Clinical review 135: The importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 2001;86:4047–4058 [DOI] [PubMed] [Google Scholar]
- 15. Burns SF, Lee S, Arslanian SA: In vivo insulin sensitivity and lipoprotein particle size and concentration in black and white children. Diabetes Care 2009;32:2087–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gungor N, Bacha F, Saad R, Janosky J, Arslanian S: Youth type 2 diabetes: insulin resistance, β-cell failure, or both? Diabetes Care 2005;28:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J: Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 2002;51:3014–3019 [DOI] [PubMed] [Google Scholar]
- 18. Frayn KN: Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 19. Kahn SE: The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 20. Arslanian SA: Clamp techniques in paediatrics: what have we learned? Horm Res 2005;64(Suppl. 3):16–24 [DOI] [PubMed] [Google Scholar]
- 21. Bergman RN, Ader M, Huecking K, Van Citters G: Accurate assessment of β-cell function: the hyperbolic correction. Diabetes 2002;51:S212–S220 [DOI] [PubMed] [Google Scholar]
- 22. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 23. Li C, Ford ES, Zhao G, Mokdad AH: Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care 2009;32:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogden CL, Carroll MD, Flegal KM: High body mass index for age among US children and adolescents, 2003–2006. JAMA 2008;299:2401–2405 [DOI] [PubMed] [Google Scholar]
- 25. Al Mamun A, Cramb SM, O'Callaghan MJ, Williams GM, Najman JM: Childhood overweight status predicts diabetes at age 21 years: a follow-up study. Obesity (Silver Spring) 2009;17:1255–1261 [DOI] [PubMed] [Google Scholar]