Abstract
OBJECTIVE
Paraoxonase (PON) exhibits esterase activity (PON-AREase) and lactonase activity (PON-HCTLase), which prevent LDL oxidation and detoxify homocysteine thiolactone (HCTL). The role of HCTL and PON-HCTLase as a risk factor for the microvascular complication in diabetic retinopathy at the level of vitreous has not been investigated.
RESEARCH DESIGN AND METHODS
Undiluted vitreous from patients with proliferative diabetic retinopathy (PDR) (n = 13) and macular hole (MH) (n = 8) was used to determine PON-HCTLase and PON-AREase activity spectrophotometrically. HCTL levels were detected by liquid chromatography–tandem mass spectrometry. In vitro studies were done in primary cultures of bovine retinal capillary endothelial cells (BRECs) to determine the dose- and time-dependent effect of HCTL and homocysteine (Hcys) on PON-HCTLase activity, as well as to determine mRNA expression of PON by RT-PCR.
RESULTS
A significant increase in HCTL and PON-HCTLase activity was observed in PDR compared with MH (P = 0.036, P = 0.001), with a significant positive correlation between them (r = 0.77, P = 0.03). The in vitro studies on BRECs showed a dose- and time-dependent increase in the PON-HCTLase activity and mRNA expression of PON2 when exposed to HCTL and Hcys.
CONCLUSIONS
This is the first study showing elevated levels of vitreous HCTL and PON-HCTLase activity in PDR. These elevations are probably a protective effect to eliminate HCTL, which mediates endothelial cell dysfunction. Thus, vitreous levels of HCTL and PON activity can be markers of diabetic retinopathy. The bioinformatics analysis reveals that the structure and function of PON that can be modulated by hyperhomocysteinemia in PDR can affect the dual-enzyme activity of PON.
Hyperhomocysteinemia is a well-established independent risk factor for the development of macrovascular and microvascular diseases (1). Recent reports show that increased homocysteine thiolactone (HCTL) levels are associated with diabetic macrovasculopathy (2). HCTL is formed in all cell types as a result of error-editing met-tRNA synthetase when there is excess homocysteine (Hcys). The interaction of HCTL with proteins leads to protein homocysteinylation and loss of function (3). Therefore, detoxification of HCTL is crucial. This is possible by the lactonase (HCTLase) activity of paraoxonase (PON) (4). The enzyme PON is a calcium-dependent 45-kDa protein coded by chromosome 7q21-22. The PON gene family in humans has three members: PON1, PON2, and PON3. Whereas PON1 and PON3 are associated with serum HDL (5), PON2 is ubiquitously expressed in tissues (6). PON1 exhibits antioxidant properties, thereby preventing the accumulation of oxidized LDL, and PON2 acts mainly at the cellular level (7). Lipid oxidation plays a role not only in macrovascular diseases but also in microvascular dysfunction, and serum PON1 activity was decreased in patients with diabetic retinopathy (8). While elevated Hcys in the vitreous of patients with proliferative diabetic retinopathy (PDR) was reported by us and others (9,10), there are no reports on HCTL levels and PON activity. This study aims to detect the vitreous levels of HCTL, PON-HCTLase, and esterase (PON-AREase) activity in PDR case subjects and in in vitro studies in bovine retinal capillary endothelial cells (BRECs).
RESEARCH DESIGN AND METHODS
All experiments involving human subjects adhered to the tenets of the Declaration of Helsinki. In patients with PDR, the clinical ocular findings were graded at the time of vitrectomy for the presence of hemorrhage, tractional retinal detachment, and presence or absence of patent new vessels in the retina or optic disc. Macular hole (MH) patients with an idiopathic full-thickness retinal defect of more than 400 μm with posterior vitreous detachment were included as disease control subjects. Clinical details of the patients with PDR and MH are given in Tables I and II in the online appendix, available at http://care.diabetesjournals.org/cgi/content/full/dc10-0132/DC1. Undiluted vitreous samples from 13 patients with PDR (mean age 52 ± 7 years; 7 male and 6 female) and 8 patients with MH (mean age 56 ± 10 years; 5 male and 3 female) were collected during vitreoretinal surgery, centrifuged, and frozen at −80°C. Vitreous HCTL levels, PON-AREase activity, PON-HCTLase activity, total protein, thiobarbituric acid reacting substances (TBARS), total antioxidant capacity (TAC), and total thiols were measured.
In vitro experiments in BRECs
BRECs were cultured and characterized as endothelial cells using factor VIII and vascular-endothelial cadherin (VE-cadherin). The cells were exposed to various concentrations (25, 50, 100, and 200 μmol/l) of Hcys and HCTL at different time points (3, 6, 12, 24, and 48 h) in Dulbecco's modified Eagle's medium: nutrient mixture F-12 (DMEM/F12). The activity of PON-HCTLase and PON-AREase were estimated in the cell lysates.
DL-homocysteine and homocysteinethiolactone-HCl were obtained from Sigma, and L-homatropine was from Boehringer Ingelheim, Germany. Mercaptoethanol (MS grade), acetonitrile (MS grade), formic acid (MS grade), phenylacetate (PA), γ-thiobutyrolactone (γ-TBL), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), thiobarbituric acid (TBA), iron (Fe), EDTA, benzoic acid, trichloroacetic acid, acetic acid, and dimethyl sulfoxide (DMSO) were obtained from Sigma. Other materials used were DMEM/F12 (GIBCO), endopan media (Genex), FBS (GIBCO), factor VIII antibody (Dako), VE-cadherin (Chemicon), GenElute mammalian total RNA miniprep kit (Sigma), and cDNA conversion (ThermoScript; Invitrogen).
Cytotoxic effect of HCTL and Hcys in BRECs
BRECs were grown in a 96-well plate (1,000 cells per well) and exposed to varying concentrations of Hcys (25, 50, 100, and 200 μmol/l) for 3, 6, 12, 24, and 48 h, respectively, in DMEM/F12. The formazan, formed after treatment with MTT, was dissolved in DMSO and read at 570 nm to assess the cell viability.
HCTL estimated using liquid chromatography–tandem mass spectrometry (LC-MS/MS)
The liquid chromatography separation of HCTL in the vitreous was done by gradient elution using acetonitrile with 0.1% formic acid and water with 0.1% formic acid in a 70:30 ratio, pumped at a flow rate of 0.5 ml/min in a Thermo Surveyor quaternary HPLC pump (Thermo Electron, Waltham, MA) coupled with a 4000 Q Trap (Applied Biosystems, Foster City, CA) and positive electron spray ionization (ESI) mode. The analytical separation was achieved by using Chromolith SpeedROD RP-18e (50 × 4.6 mm) (Merck, Darmstadt, Germany) within the run time of 5 min, with homatropine was the internal standard. Analyst software version 1.4.2 was used to control all the parameters of mass spectrometry. Quantification was performed using the multiple reaction monitoring (MRM) mode, on the basis of parent → product ion transitions for HCTL (118.2 → 56) and homatropine (276.1 → 142). Source-dependent parameters optimized were gas 1 (40 psi), gas 2 (40 psi), curtain gas (10 psi), ion spray voltage (5,500 V), and temperature (300°C). Compound-dependent parameters, declustering potential, entrance potential, collision energy and cell exit potential, and dwell time were set at 75, 10, 35, 10, and 200 for both analyte and the internal standard. Then 20 μl of either standard or sample was mixed with 200 μl of extraction solvent (70:30 ratio of acetonitrile to water with 1% zinc sulfate) containing homatropine at a concentration of 250 ng as the internal standard (11,12).
Determination of PON-AREase activity
PON-AREase activity was measured using the method of Cabana et al. (13). The rate of hydrolysis of substrate PA was measured spectrophotometrically in the kinetic mode by detecting the increase in phenol concentration at 270 nm. Undiluted vitreous was added to the buffer, consisting of 10 mmol/l Tris and 1 mmol/l CaCl2, pH 8.0. Enzyme activity was expressed as micromoles PA hydrolyzed per milliliter per minute.
Determination of PON-HCTLase activity
The PON-HCTLase–activity assay was standardized in house using γ-TBL as substrate, and the rate of hydrolysis was measured spectrophotometrically in the kinetic mode at 450 nm (main wavelength) and 546 nm (subwavelength), suitably modifying the method of Koubaa et al. (14). Then 5 μl of vitreous sample was used for the assay with DTNB as chromogen at pH 7.2, using 100 mmol/l of phosphate buffer. Enzyme activity was expressed in U/l.
Activity stain for PON using PA as substrate
The AREase activity of PON protein in the vitreous was observed by doing an activity stain using PA as substrate. The liberated phenol couples with the hexazotized pararosaniline solution to give an insoluble, brightly colored azo dye seen as pink bands in the gel. Briefly, 50 μg of the vitreous protein from patients with either MH or PDR was run on a native page (12%). The gel was then immersed in a staining solution (pararosaniline 0.125 mol/l, sodium nitrite 4%, phenylacetate 1 mmol/l in phosphate buffer pH 6.8–7.2.) for 1 h at 37°C. Once the bands were visualized, the gel was destained with 0.33% sodium meta-bisulphate in phosphate buffer (15).
Determination of TBARS
Estimation of vitreous TBARS was done spectrophotometrically on the basis of absorbance of the chromophore at 530 nm. The results were expressed as nanomoles of malondialdehyde released per milliliter per milligram of protein (16).
Determination of TAC
Estimation of vitreous TAC was done spectrophotometrically by a Fenton-type reaction. Antioxidants from the added sample of human fluid suppress the production of TBARS and were measured at 532 nm (17).
Determination of total thiols
Estimation of total thiols (a disulphide compound that is readily reduced) was measured spectrophotometrically using DTNB as a chromogen at 412 nm (18).
Statistical analysis
Student t test was used to compare the continuous variables between groups. Pearson correlation was used to calculate the r value. Statistical significance was defined as P < 0.05. The statistical analysis was done using SPSS version 14.0.
RESULTS
PON-HCTLase and PON-AREase activity in vitreous
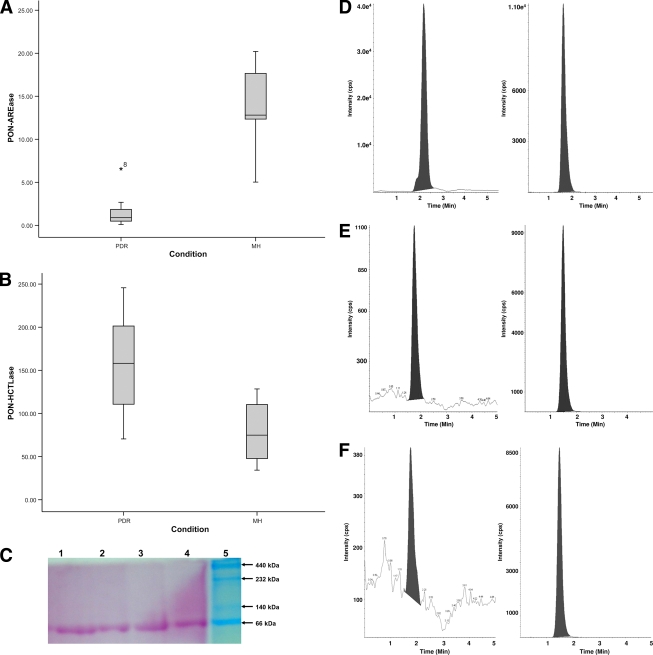
The PON-HCTLase activity in the vitreous of PDR case subjects was found to be significantly elevated (mean 175.17 ± 16.4 units/l) compared with athat of MH case subjects (78.5 ± 12.7 units/l) (P = 0.00). Correspondingly, a significant decrease in PON-AREase activity was observed in PDR (1.5 ± 1.7 μmol/ml/min) compared with MH (3.8 ± 1.6 μmol/ml/min) (P = 0.00) (Table 1). Distribution of PON-AREase and PON-HCTLase activity shows a shift in the median, with a 9-fold drop in the PON-AREase activity and a 2.2-fold increase in PON-HCTLase in PDR compared with MH case subjects (Fig. 1A and B). There were six case subjects with PDR who were on atorvostatin treatment, and there was no significant difference in the activity of PON-HCTLase and PON-AREase in this group. Activity staining for PON-AREase revealed the presence of PON protein in the vitreous (Fig. 1C). To see if this increase in PON-HCTLase is associated with an increase in HCTL levels, PON-HCTLase levels were detected in the vitreous by mass spectrometry (Fig. 1D–F). There was a significant increase in HCTL levels in the vitreous of PDR case subjects (1.37 ± 0.51 nmol/l) compared with MH case subjects (0.65 ± 0.18 nmol/l) (Fig. 1G).
Table 1.
Homocysteine thiolactonase, arylesterase activity, and oxidative stress parameters in the vitreous of PDR compared with MH case subjects
| MH | PDR | P | |
|---|---|---|---|
| n | 8 | 13 | |
| Homocysteine thiolactonase (U/l) | 78.5 ± 12.7 | 175.1 ± 16.4 | 0.000 |
| Arylesterase activity (μmol/ml/min) | 13.8 ± 1.6 | 1.5 ± 1.7 | 0.000 |
| TBARS (nmol/ml/mg protein) | 24.42 ± 5.2, 26.05 (2.7) | 17.1 ± 15.2, 11.6 (22.3) | 0.090 |
| TAC (mmol) | 0.22 ± 0.03 | 0.319 ± 0.24 | 0.0001 |
| Total thiols (mmol) | 43.88 ± 6.3 | 28.7 ± 12.9 | 0.000 |
| Protein (mg/ml) | 1.29 ± 0.2 | 2.83 ± 2.5 | 0.002 |
Data are means ± SD and median (interquartile range).
Figure 1.
PON activity and HCTL levels in the vitreous of PDR and MH case subjects. Distribution graphs show the reciprocal relationship of HCTLase and AREase in PDR (n = 13) and MH (n = 8). A: PON-AREase activity. B: PON-HCTLase activity. C: Activity staining for PON protein in the vitreous using phenylacetate as substrate and parasoaniline as chromogen. The band was observed at 66 kDa (lane 1: MH; lanes 2–4: PDR; lane 5: high–molecular weight marker). Representative liquid chromatography–tandem mass spectrometry chromatogram showing the HCTL (left) and the corresponding internal standard, namely homatropine (right). D: Standard vitreous. E: MH vitreous. F: PDR vitreous. The m/z of HCTL is 118.2 and homatroprine is 276.1 (seen as the peak). G: Distribution of HCTL levels in PDR (n = 9) and MH (n = 3) case subjects. Correlation between HCTL and PON-HCTLase is shown. H: PDR (n = 9), ♦; MH (n = 3), ●. (A high-quality color representation of this figure is available in the online issue.)
Correlation between PON-HCTLase and PON-AREase with HCTL
A significant positive correlation was observed between vitreous HCTL levels and PON-HCTLase activity in PDR and MH (n = 3) (r = 0.88; P = 0.03) (Fig. 1H). However, no significant correlation was observed with PON-AREase and HCTL.
Oxidative stress parameters in the vitreous
There was a significant decrease in total thiols in PDR compared with MH case subjects (P = 0.00), with significant increase in the TAC levels (P = 0.0001). This increase in TAC value in spite of reduced thiol status can be attributed to the cumulative effect of small molecule antioxidants, such as vitamins E and C. Izuta et al. (19) suggest that the thioredoxin and Nrf2/ARE pathways can also mediate the redox status in the vitreous body of PDR cases and have reported increased antioxidant potential in the vitreous of PDR cases. However, the alteration in the TBARS levels was not significant, and the median showed 11.6 in PDR (interquartile [IQ] range 22.3) and 26.05 in MH (IQ range 2.7). The total protein level was found to be significantly elevated in PDR (P = 0.002) (Table 1).
In vitro studies on BRECs
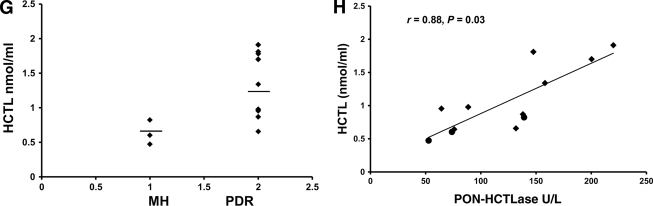
The MTT assay revealed that the cells were viable at all the concentrations of HCTL and Hcys tested until 48 h. The in vitro experiments showed a dose- and time-dependent increase in PON-HCTLase activity when exposed to both HCTL and Hcys (Fig. 2A and B). PON-HCTLase activity was found to be significantly increased and maximal at 200 μmol/l at 24 h for both HCTL and Hcys exposure compared with untreated control (P = 0.000). Correspondingly, the PON-AREase activity was significantly decreased (P = 0.000) (Fig. 2C). In the same experimental condition, the mRNA expression of PON enzyme was tested and found to be increased for both HCTL and Hcys, and the effect was very pronounced in Hcys (Fig. 2D).
Figure 2.
In vitro experiments. PON-HCTLase activity in BRECs exposed to Hcys and HCTL. Graphs showing the dose- and time-dependent increase in PON-HCTLase activity after treatment with HCTL (A) or Hcys (B). C: PON-AREase and PON-HCTLase activity in BRECs exposed to Hcys and HCTL at 200 μmol/l compared with the baseline control activity. D: mRNA expression of PON2 in BRECs exposed to Hcys and HCTL (200 μmol/l at 24 h). The PCR was carried out using the following primers for bovine glyceraldehyde 3-phosphate dehydrogenase (GAPDH): forward primer 5′-TGTTCCAGTATGATTCCACCC-3′ and reverse primer 5′-GTCTTCTGGGTGGCAGTGAT-3′ corresponding to 424 bp, and for PON2: forward primer 5′-CCT TCC TAA TTG CCA CCT GA-3′ and reverse primer 5′-TGG AGG CCT GGA CAT TTT AG-3′, corresponding to ∼150 bp. The bands obtained were quantified using National Institutes of Health ImageJ software after normalization to GAPDH. (A high-quality color representation of this figure is available in the online issue.)
CONCLUSIONS
Homocysteinylated protein mediates the development and progression of both diabetic macrovasculopathy and microvascular complications (20,21). However, its impact on the microvascular endothelial cell is still not well understood. Increased levels of Hcys have been reported to increase the concentration of HCTL levels (3). Serum PON-HCTLase and PON-AREase activity of PON were reported to be significantly lowered in diabetic patients (8). In this study, we observed for the first time that the vitreous HCTL and PON-HCTLase activity is significantly increased in the vitreous of PDR case subjects compared with MH case subjects. The increase of PON in vitreous could be contributed to by the inner retinal barrier permeability and proliferating endothelial cells, which are characteristic of PDR. PON2, which is ubiquitously present in all tissues, has the highest lactonase activity, though all three isoforms exhibit it (6). The in vitro study supports this fact, wherein exposure of Hcys and HCTL increases the PON2 mRNA expression. Phylogenetic analysis suggests that PON2 is the oldest member of the family. It is reportedly present intracellularly in three major vascular cell types, namely cultured human umbilical vein endothelial cells, smooth muscle vascular cells, and aortic adventitial fibroblasts, with the major function of reducing reactive oxygen species–mediated endothelial cell dysfunction (22).
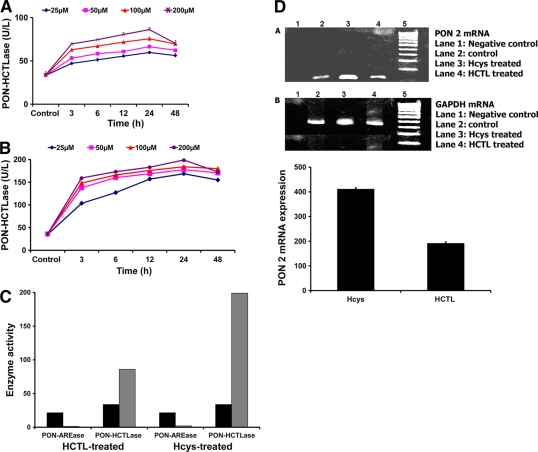
In this study, the BRECs were found to increase PON-HCTLase activity upon exposure to HCTL. Comparatively, PON-HCTLase activity was two- to threefold higher upon exposure to Hcys. The mRNA expression of PON2 was similarly increased. Concomitantly, there was a significant drop in the AREase activity both in the HCTL- and Hcys-treated BRECs. The differential effect seen in Hcys- and HCTL-treated cells may be explained by the differential uptake of the molecules and characteristics of the cell type. The retinal capillary endothelial cell uptake of Hcys and HCTL has not been examined. However, Hcys transport is differentially regulated in vascular cells. In endothelial cells, Hcys transport is predominantly mediated by a sodium-lysosome–dependent system ASC with low transport activity (23). To see if the binding characteristics of PON with Hcys alters the PON-HCTLase and AREase activity, we took a bioinformatics approach. PON2 bound to two Ca2+ ions was modeled using MODELER 9V7 (24) on the basis of the crystal structure of PON1 (1V04) as template, given by Hasel et al. (25). Because the binding of Hcys to PON2 protein is not known, blind docking was done (Fig. 3A and B). The docking study reveals that Hcys binds to the same pocket to which HCTL and PA dock (Table 2). Therefore, it is quite possible that Hcys can affect the PON-HCTLase and PON-AREase activity of PON2.
Figure 3.
Bioinformatic analysis of PON2 interaction with Hcys. A: Residues of the PON2 protein that have hydrogen bonding with the ligand Hcys. B: Residues of the PON2 protein that have hydrophobic interaction with the ligand Hcys.
Table 2.
Binding kinetics of the substrates HCTL and PA and the ligand Hcys with PON2 protein
| Ligands | Binding energy (kcal/mol) | Residues involved in hydrogen bonding | Residues involved in hydrophobic interactions | Inhibitory constant (Ki) |
|---|---|---|---|---|
| Hcys | −5.08 | Asp168, Ile169, Ile225, Thr170, Ile55 | Ile116, Leu270, Asn226, Asp56, Ca355 | 188.88 μmol/l |
| HCTL | −6.63 | Asp168, Ile169, Ile225, Thr170 | Ser117, Ile116, Asn226, Leu270, Ca355 | 13.87 μmol/l |
| PA | −4.73 | Thr118, Ala171 | Ser117, Ile225, Ile116, Ile169, Leu270, Thr170, Asn226, Ca355 | 338.45 μmol/l |
This is the first report showing that the increased activity of PON-HCTLase at the level of vitreous may be a protective effect to eliminate HCTL, which mediates endothelial cell dysfunction through N-homocysteinylation of the lysine residues in the proteins (26), while PON-AREase is lowered, probably due to the increased Hcys levels. Vitreous levels of HCTL and PON activities can be markers of diabetic retinopathy. However the structure function that determines the dual-enzyme activity of PON, which is altered by molecules like Hcys, such as in PDR, warrants further attention.
Supplementary Material
Acknowledgments
Financial support for this project was provided by the Indian Council of Medical Research (52/18/2002-BMS).
No potential conflicts of interest relevant to this article were reported.
S.B. and N.A. researched data, contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript. A.P. researched data. S.K.N. researched data and contributed to the discussion. R.P. researched clinical data, contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript. M.D. researched clinical data. T.V. researched mass spectrophotometer data. C.M. and M.S. researched bioinformatics data.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I: Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med 1991;324:1149–1155 [DOI] [PubMed] [Google Scholar]
- 2. Gu W, Lu J, Yang G, Dou J, Mu Y, Meng J, Pan C: Plasma homocysteine thiolactone associated with risk of macrovasculopathy in Chinese patients with type 2 diabetes mellitus. Adv Ther 2008;25:914–924 [DOI] [PubMed] [Google Scholar]
- 3. Jakubowski H, Zhang L, Bardeguez A, Aviv A: Homocysteine thiolactone and protein homocysteinylation in human endothelial cells: implications for atherosclerosis. Circ Res 2000;87:45–51 [DOI] [PubMed] [Google Scholar]
- 4. Domagała TB, Łacinski M, Trzeciak WH, Mackness B, Mackness MI, Jakubowski H: The correlation of homocysteine-thiolactonase activity of the paraoxonase (PON1) protein with coronary heart disease status. Cell Mol Biol 2006;52:4–10 [PubMed] [Google Scholar]
- 5. Reddy ST, Wadleigh DJ, Grijalva V, Ng C, Hama S, Gangopadhyay A, Shih DM, Lusis AJ, Navab M, Fogelman AM: Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol 2001;21:542–547 [DOI] [PubMed] [Google Scholar]
- 6. Stoltz DA, Ozer EA, Recker TJ, Estin M, Yang X, Shih DM, Lusis AJ, Zabner J: A common mutation in paraoxonase-2 results in impaired lactonase activity. J Biol Chem 2009;284:35564–35571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shiner M, Fuhrman B, Aviram M: Paraoxonase 2 (PON2) expression is upregulated via a reduced-nicotinamide-adenine-dinucleotide-phosphate (NADPH)-oxidase-dependent mechanism during monocytes differentiation into macrophages. Free Radic Biol Med 2004;37:2052–2063 [DOI] [PubMed] [Google Scholar]
- 8. Sonoki K, Iwase M, Sasaki N, Ohdo S, Higuchi S, Matsuyama N, Iida M: Relations of lysophosphatidylcholine in low-density lipoprotein with serum lipoprotein-associated phospholipase A2, paraoxonase and homocysteine thiolactonase activities in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2009;86:117–123 [DOI] [PubMed] [Google Scholar]
- 9. Coral K, Angayarkanni N, Gomathy N, Bharathselvi M, Pukhraj R, Rupak R: Homocysteine levels in the vitreous of proliferative diabetic retinopathy and rhegmatogenous retinal detachment: its modulating role on lysyl oxidase. Invest Ophthalmol Vis Sci 2009;50:3607–3612 [DOI] [PubMed] [Google Scholar]
- 10. Aydemir O, Türkçüoğlu P, Güler M, Celiker U, Ustündağ B, Yilmaz T, Metin K: Plasma and vitreous homocysteine concentrations in patients with proliferative diabetic retinopathy. Retina 2008;28:741–743 [DOI] [PubMed] [Google Scholar]
- 11. Velpandian T, Angayarkanni N, Nirmal J, Ravi AK, Arora B: Development and validation of a simple LC-MS/MS method for the quantification of homocysteine and homocysteine thiolactone in plasma. (in commun) [Google Scholar]
- 12. Scholl HP, Fleckenstein M, Charbel Issa P, Keilhauer C, Holz FG, Weber BH: An update on the genetics of age-related macular degeneration. Mol Vis 2007;13:196–205 [PMC free article] [PubMed] [Google Scholar]
- 13. Cabana VG, Reardon CA, Feng N, Neath S, Lukens J, Getz GS: Serum paraoxonase: effect of the apolipoprotein composition of HDL and the acute phase response. J Lipid Res 2003;44:780–792 [DOI] [PubMed] [Google Scholar]
- 14. Koubaa N, Hammami S, Nakbi A, Ben Hamda K, Mahjoub S, Kosaka T, Hammami M: Relationship between thiolactonase activity and hyperhomocysteinemia according to MTHFR gene polymorphism in Tunisian Behçet's disease patients. Clin Chem Lab Med 2008;46:187–192 [DOI] [PubMed] [Google Scholar]
- 15. Thiersch M, Raffelsberger W, Frigg R, Samardzija M, Wenzel A, Poch O, Grimm C: Analysis of the retinal gene expression profile after hypoxic preconditioning identifies candidate genes for neuroprotection. BMC Genomics 2008;9:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devasagayam TP, Tarachand U: Decreased lipid peroxidation in the rat kidney during gestation. Biochem Biophys Res Commun 1987;145:134–138 [DOI] [PubMed] [Google Scholar]
- 17. Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V: Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 2001;54:356–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coral K, Raman R, Rathi S, Rajesh M, Sulochana KN, Angayarkanni N, Paul PG, Ramakrishnan S: Plasma homocysteine and total thiol content in patients with exudative age-related macular degeneration. Eye 2006;20:203–207 [DOI] [PubMed] [Google Scholar]
- 19. Izuta H, Matsunaga N, Shimazawa M, Sugiyama T, Ikeda T, Hara H: Proliferative diabetic retinopathy and relations among antioxidant activity, oxidative stress, and VEGF in the vitreous body. Mol Vis 2010;16:130–136 [PMC free article] [PubMed] [Google Scholar]
- 20. Joseph AJ, Friedman EA: Diabetic nephropathy in the elderly. Clin Geriatr Med 2009;25:373–389 [DOI] [PubMed] [Google Scholar]
- 21. Brazionis L, Rowley K, Sr, Itsiopoulos C, Harper CA, O'Dea K: Homocysteine and diabetic retinopathy. Diabetes Care 2008;31:50–56 [DOI] [PubMed] [Google Scholar]
- 22. Mackness B, Hunt R, Durrington PN, Mackness MI: Increased immunolocalization of paraoxonase, clusterin, and apolipoprotein A-I in the human artery wall with the progression of atherosclerosis. Arterioscler Thromb Vasc Biol 1997;17:1233–1238 [DOI] [PubMed] [Google Scholar]
- 23. Jiang X, Yang F, Brailoiu E, Jakubowski H, Dun NJ, Schafer AI, Yang X, Durante W, Wang H: Differential regulation of homocysteine transport in vascular endothelial and smooth muscle cells. Arterioscler Thromb Vasc Biol 2007;27:1976–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barathi S, Charanya M, Muthukumaran S, Angayarkanni N, Umashankar V: Comparative modeling of PON2 and analysis of its substrate binding interactions using computational methods. (in commun) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R, Dvir H, Ravelli RB, McCarthy A, Toker L, Silman I, Sussman JL, Tawfik DS: Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol 2004;11:412–419 [DOI] [PubMed] [Google Scholar]
- 26. Mendes RH, Sirvente RA, Candido GO, Mostarda C, Salemi VM, D'Almeida V, Jacob MH, Ribeiro MF, Belló-Klein A, Rigatto K, Irigoyen MC: Homocysteine thiolactone induces cardiac dysfunction: role of oxidative stress. J Cardiovasc Pharmacol 2010;55:198–202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.