Abstract
OBJECTIVE
To compare total retinal blood flow in diabetic patients with no or mild nonproliferative diabetic retinopathy and healthy control subjects and to investigate in patients whether there is a difference between retinal blood flow before morning insulin and under normoglycemic conditions using a glucose clamp.
RESEARCH DESIGN AND METHODS
Twenty patients with type 1 diabetes with no or mild diabetic retinopathy were included in this open parallel-group study, and 20 healthy age- and sex-matched subjects were included as control subjects. Retinal blood flow was assessed by combining velocity measurements using laser Doppler velocimetry and diameter measurements using a commercially available dynamic vessel analyzer. Measurements were performed before and during a euglycemic clamp.
RESULTS
Total retinal blood flow was higher in diabetic patients (53 ± 16 μl/min) than in healthy subjects (43 ± 16 μl/min; P = 0.034 between groups). When plasma glucose in diabetic patients was reduced from 9.3 ± 1.7 to 5.3 ± 0.5 mmol/l (P < 0.001) retinal blood flow decreased to 49 ± 15 μl/min (P = 0.0003 vs. baseline). Total retinal blood flow during the glucose clamp was not significantly different from blood flow in normal control subjects (P = 0.161).
CONCLUSIONS
Type 1 diabetic patients with no or only mild diabetic retinopathy have increased retinal blood flow before their morning insulin dosage. Blood flow is reduced toward normal during euglycemic conditions. Retinal blood flow may fluctuate significantly with fluctuating plasma glucose levels, which may contribute to the microvascular changes seen in diabetic retinopathy.
The mechanisms underlying the development of diabetic retinopathy are still not fully understood. Elevated glucose levels may be the initial factor leading to alterations of vessel architecture in the retina, perfusion abnormalities, and progression of the disease. Early microstructural changes of retinal vessels preceding clinically visible retinopathy include thickening of the capillary basement membrane, pericyte loss, retinal capillary nonperfusion, and capillary loss (1). Capillary hypoperfusion is accompanied by the development of shunt vessels and decreased tissue oxygenation, finally representing a stimulus for neovascularization in proliferative diabetic retinopathy (2).
Retinal blood flow in patients with diabetes has been investigated by various research groups and techniques in the last few years, but the results have not been conclusive for a variety of reasons. For example, with use of combined analysis of vessel diameters and bidirectional laser Doppler velocimetry in patients with type 1 diabetes, it has been shown that total retinal blood flow and blood velocity are increased in early diabetic retinopathy (3). This was also seen even before the clinical onset of diabetic retinopathy (4). Other investigators have, however, reported that blood flow velocities in the retrobulbar central retinal artery (5) and in branch retinal arteries are reduced (6) or that blood flow is not different compared with that in healthy control subjects (7). Results from the various studies are often not directly comparable because of their different assessment methods in the various sections of the ocular vasculature and differences in the study population concerning the patient disease type, duration, and stage. A detailed overview of results from ocular blood flow studies in diabetes is available elsewhere (2).
Looking at these partially inconsistent results of blood flow alterations in diabetes, it has to be noted that glucose and insulin plasma levels may have a considerable influence on retinal blood flow. Grunwald et al. (8) reported that a pronounced reduction in plasma glucose levels in type 2 diabetic patients, induced by administration of insulin, leads to a significant reduction in retinal blood flow associated with a normalization of vessel reactivity in response to hyperoxia. Reduced retinal blood flow after glucose reduction was also found in patients with type 1 diabetes (9). In keeping with these results several studies identified a positive correlation between blood glucose levels and retinal blood flow parameters in diabetic patients (5,10). A recent study, however, reported unchanged ocular blood flow parameters after acute elevation of blood glucose in diabetic patients and healthy control subjects (11). It has to be noted that in these interventional studies differences in methodology also limit comparability of the results.
The situation is further complicated by the vasodilator effects of insulin in the eye (12) and by the additive effects of insulin and glucose on ocular blood flow (13). Taken together these results indicate that findings from blood flow studies in diabetic patients have to be interpreted with caution, because fluctuations in blood glucose and insulin levels in these patients may affect blood flow considerably and need to be taken into account to assess blood flow changes due to diabetes correctly. In the present study we addressed this problem by investigating retinal blood flow in diabetic patients during normalized glucose levels using the glucose clamp technique. The hypothesis that retinal blood flow is altered in patients with type 1 diabetes with no or mild diabetic retinopathy at elevated compared with physiological plasma levels was tested.
RESEARCH DESIGN AND METHODS
The study was started after the approval of the study protocol by the competent authorities and the positive judgment of the responsible ethics committee. It was performed in adherence to the guidelines of the Declaration of Helsinki and Good Clinical Practice guidelines. Twenty patients with type 1 diabetes with no or mild nonproliferative retinopathy were included in this open parallel group study. The patients were classified according to the criteria established in the Early Treatment Diabetic Retinopathy Study (14) using seven standard field color fundus photographs. As a control group, 20 age- and sex-matched healthy subjects were included. All subjects passed a screening examination including physical examination, blood pressure measurement, and ophthalmic examination. Exclusion criteria were age <19 years, ametropia >6 diopters, best corrected visual acuity <0.8, the presence of ocular pathological conditions other than diabetic retinopathy in diabetic patients, previous laser photocoagulation treatment, A1C >8%, and systemic hypertension (defined as either systolic blood pressure >145 mmHg or diastolic blood pressure >90 mmHg, or a diagnosis of systemic hypertension in the medical history). Other clinically relevant illness before the study, pregnancy, or lactation also excluded participation in the study as well as drug intake in the healthy subjects in the 3 weeks before the study.
Experimental paradigm
All participants received 1 drop of 0.5% tropicamide (Mydriaticum Agepha; Agepha, Vienna, Austria) to achieve pupil dilation. Measurements of all outcome variables were done in the morning between 8:00 a.m. and 9:00 a.m. before the morning insulin dose. After a resting period of 20 min to achieve hemodynamic stabilization, baseline measurements of retinal blood flow parameters were performed in the right eye. Thereafter, all measurements were repeated during normoglycemic conditions. To achieve glycemic control in diabetic patients, the glucose clamp technique was applied as described earlier (15). For this purpose two indwelling intravenous cannulas were inserted into antecubital veins for simultaneous monitoring of glucose plasma concentrations via venous blood sampling on one arm and for insulin and glucose administration on the other arm. Diabetic patients received insulin (Insulin Huminsulin Normal; Lilly, Fegersheim, France) intravenously over 60 min with a continuous dose of 6 pmol/kg/min. Simultaneously, 20% glucose solution (Glucose 20% Leopold Infusionsflaschen; Leopold Pharma, Linz, Austria) was administrated intravenously at a rate necessary to maintain blood glucose levels at 5.6 ± 1.1 mg/dl. Blood glucose levels were measured from venous blood samples every 5 min using a Beckman glucose analyzer (Beckman Coulter, Brea, CA). Blood flow measurements were repeated 30 min after the start of the infusion. Blood pressure, pulse rate, and electrocardiogram were monitored throughout the trial.
Assessment of retinal blood flow
Measurement of retinal blood flow velocity was performed with a fundus camera–based laser Doppler velocimeter (LDV-5000; Oculix, Arbaz, Switzerland). All major retinal veins around the optic nerve head were investigated to achieve measurement of total retinal blood flow. The principle of laser Doppler velocimetry (LDV) is based on the optical Doppler effect. Laser light of a single-mode laser diode with a wavelength of 670 nm is scattered and reflected by moving erythrocytes, leading to a broadened and shifted frequency spectrum. This frequency shift is proportional to the blood flow velocity in the retinal vessel whereupon the maximum Doppler shift corresponds to the centerline erythrocyte velocity (Vmax). In bidirectional LDV, two Doppler shift power spectra are recorded simultaneously for two directions of the scattered light, which enables absolute velocity measurements (16). From Vmax mean blood velocity in retinal vessels (Vmean) may be calculated as Vmean = Vmax/2. Vessel diameters at all measurement sites were obtained with the dynamic vessel analyzer (DVA) (IMEDOS Systems, Jena, Germany). The DVA assesses retinal vessel diameters (D) by analyzing the brightness profile of the vessel using a fundus camera (FF 450; Carl Zeiss Meditec, Jena, Germany), a high-resolution digital video camera, and a personal computer with analyzing software (17). Images are recorded, digitized, and analyzed in real time with a frequency of 25 Hz. After selection of the measurement location, the DVA is able to track the vessels during eye movements within the measurement window. The system provides excellent reproducibility and sensitivity (18).
Blood flow (Q) was measured in all retinal veins i that were entering the optic nerve head as Qi = Vmean, i × Di2π/4 (16). Total retinal blood flow was obtained using the equation
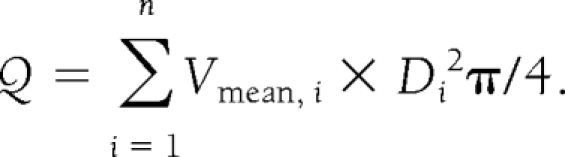 |
The number of veins that were used for calculation of total retinal blood flow varied from four to five depending on the individual retinal angioarchitecture. In addition, the total venous diameter was calculated as the sum of all Di values as well as the mean vessel diameter and mean venous blood velocity.
Measurement of systemic hemodynamics
Systolic (SBP), diastolic (DBP), and mean arterial blood pressure (MAP) were measured repeatedly before and after blood flow measurements on the upper arm by an automated oscillometric device (HP-CMS patient monitor; Hewlett Packard, Palo Alto, CA). Pulse rate was automatically recorded by the same unit from a finger pulse oximetric device.
Statistical analysis
Data are presented as means ± SD. To assess differences between the diabetic patients and the healthy control subjects, an unpaired t test was performed. Repeated-measures ANOVA was applied to data of the diabetic group to compare values before and during the glucose clamp. P < 0.05 was considered as the level of significance. To control for associations between blood pressure and retinal blood flow a Pearson product-moment correlation coefficient was calculated.
RESULTS
The baseline characteristics of both groups are presented in Table 1. All diabetic patients either presented with no (n = 17) or mild (n = 3) diabetic retinopathy with a mean ± SD disease duration of 9 ± 3 years. There were no significant differences in age and pulse rate observed between the two groups. SBP, DBP, and MAP were higher in diabetic patients. Baseline plasma levels of glucose and A1C were elevated in diabetic patients, whereas normoglycemia was found in healthy subjects. As expected, insulin and C-peptide values were significantly diminished in the diabetic patients compared with the healthy subjects. Total retinal blood flow in diabetic patients was increased at baseline compared with that in control subjects (53 ± 16 vs. 43 ± 16 μl/min; P = 0.035, unpaired t test). There was no significant correlation observed between blood pressure and retinal blood flow (r = 0.150, P = 0.355). Retinal vein diameters were slightly larger in diabetic patients than in healthy control subjects, but the difference was only of borderline significance (P = 0.054). Similarly, total and mean retinal vein diameter as well as mean venous blood velocity tended to be increased in patients with diabetes, but this effect also did not reach the level of significance.
Table 1.
Characteristics of participating patients and matched healthy subjects and parameter changes in diabetic patients during euglycemic clamp
| Healthy subjects (n = 20) | P value (between groups) | Diabetic patients (n = 20) |
P value (change from baseline) | ||
|---|---|---|---|---|---|
| Before clamp | During clamp | ||||
| Age (years) | 33 ± 7 | — | 34 ± 6 | — | — |
| Sex (male/female) | 12/8 | — | 12/8 | — | — |
| A1C (%) | — | — | 6.9 ± 0.4 | — | — |
| Blood glucose (mmol/l) | 4.5 ± 0.5 | <0.001 | 9.3 ± 1.7* | 5.3 ± 0.5† | <0.001 |
| Insulin (pmol/l) | 319 ± 49 | <0.001 | 104 ± 41* | 1,155 ± 278† | <0.001 |
| C-Peptide (nmol/l) | 2.4 ± 0.4 | <0.001 | 0.002 ± 0.002* | — | |
| Systolic blood pressure (mmHg) | 119 ± 10 | 0.024 | 126 ± 7* | 127 ± 7 | 0.447 |
| Diastolic blood pressure (mmHg) | 61 ± 8 | <0.001 | 72 ± 7* | 72 ± 7 | 0.899 |
| Mean arterial pressure (mmHg) | 81 ± 8 | <0.001 | 90 ± 6* | 90 ± 6 | 0.651 |
| Pulse rate (bpm) | 62 ± 9 | 0.077 | 67 ± 7 | 66 ± 8 | 0.091 |
| Mean retinal vein diameter (μm) | 124 ± 10 | 0.054 | 131 ± 12 | 132 ± 12 | 0.152 |
| Total retinal vein diameter (μm) | 537 ± 56 | 0.062 | 573 ± 60 | 578 ± 69 | 0.122 |
| Mean venous blood velocity (cm/s) | 1.21 ± 0.14 | 0.106 | 1.30 ± 0.19 | 1.17 ± 0.17† | < 0.001 |
| Total volumetric blood flow (μl/min) | 43 ± 12 | 0.035 | 53 ± 16* | 49 ± 15† | 0.0003 |
Data are means ± SD, except for sex (n).
*Significant difference between the two groups at baseline (unpaired t test).
†Significant changes during glucose clamp in diabetic patients (repeated-measures ANOVA). P < 0.05 was considered the level of significance.
Parameters in diabetic patients during the euglycemic clamp are also presented in Table 1. Blood glucose levels of diabetic patients were reduced from 9.3 ± 1.7 to 5.3 ± 0.5 mmol/l (P < 0.001, ANOVA), whereas insulin was increased during the clamp. Total volumetric blood flow was reduced from 53 ± 16 to 49 ± 15 μl/min (P = 0.0003, ANOVA). At the same time, mean venous blood velocity was also significantly reduced. In contrast, total and mean retinal venous diameters as well as SBP, DBP, MAP, and pulse rate did not change during the glucose clamp. Although retinal blood flow tended to be higher in patients with diabetes during the euglycemic clamp compared with the values for the healthy control subjects, this difference was no longer significant (P = 0.161, unpaired t test).
CONCLUSIONS
Using various techniques to study retinal blood flow in patients with diabetes, researchers have drawn different conclusions regarding whether blood flow is increased or decreased (2–10,19,20). The present study indicates that retinal blood flow in diabetic patients with no or mild retinopathy is slightly elevated before the patients take their insulin but is reduced toward normal when blood glucose levels are normalized.
Compared with previous studies addressing the question of retinal blood flow in diabetes, the present approach offers several methodological advantages. Most importantly only a few studies have measured total retinal blood flow (3,4). The technique applied in this study, combining velocity data with diameter data is currently the only approach to study this in humans. As such, our data are in good agreement with a previous study indicating increased retinal blood flow in diabetes before the onset of diabetic retinopathy (4). Compared with this previous study, though, the method used for the measurement of retinal venous diameters in the present study provides a much better resolution. In the present study, a tendency toward increased retinal venous diameter in diabetes was found, although this effect did not reach the level of significance, most likely because of the relatively small sample size. This result is in keeping with a number of large-scale studies clearly indicating that patients with diabetes have larger retinal arteriolar and venial vessel calibers and that increased retinal vessel diameters are associated with diabetic retinopathy (21,22).
As mentioned earlier, one advantage of this study using LDV and DVA is that total volumetric retinal blood flow rate was assessed. Most results from previous studies presented results from a single vessel only (7,11,20). The main point to be considered in this approach is that because of the large variability in the retinal angioarchitecture among subjects, a conclusion on the entire retinal circulation cannot necessarily be drawn from results for a single vessel.
The present study focused on patients with type 1 diabetes only. Whether our results can be extrapolated to patients with type 2 diabetes is unclear, because this disease might present other retinal vascular characteristics due to older age and concomitant diseases such as hypertension and hyperlipidemia. In addition, we focused on a homogeneous group of patients with relatively good glycemic control who were taking no vasoactive medications to reduce the influence of these confounding factors on our results. Blood pressure in the diabetic patients was found to be higher compared with that in the healthy control group but did not reach abnormal levels. There was no correlation found between blood pressure and retinal blood flow. In a previous study in 200 young, healthy subjects we showed that there is a weak association between retinal blood flow and blood pressure (23). The sample size of the present study may be too small to determine a correlation of such a dimension. A considerable influence of the observed blood pressure values on ocular blood flow seems, however, unlikely.
Only very few studies used glucose clamps to control for blood glucose when retinal perfusion in diabetes was measured. In keeping with a study using fluorescein angiography to examine retinal blood velocities in early type 1 diabetes (9), our study indicates that normalizing blood glucose leads to a reduction of retinal blood velocities. This result compatible with a variety of studies in diabetes indicating that glucose increases blood flow in the ocular vasculature (5,10). In healthy subjects, a hyperglycemic-euinsulinemic clamp leads to an increase in ocular blood flow parameters (13). A reduction in elevated blood glucose by administration of insulin reduces retinal blood flow (8) in keeping with the results of the present study. One study, however, found no change in retinal blood flow after an acute glucose load (11), although such experiments did not control for plasma insulin, which has vasoactive properties in the ocular vasculature as well. We have shown previously that insulin increases ocular blood flow (12,13). In the present study insulin reached levels highly above normal to achieve normoglycemia in diabetic patients. However, retinal blood flow was found to be decreased compared with baseline, and retinal vein diameter was not changed during the glucose clamp. Therefore, it seems likely that the possible vasoactive effects of insulin were exceeded by the blood flow changes due to glucose reduction. Besides the effects of glucose and insulin, altered tissue oxygenation due to perfusion changes during the experiment could have an effect on blood flow in the diabetic retina (4).
Measurement of blood velocities in all vessels entering the optic nerve head using LDV is a time-consuming procedure and requires a high degree of subject cooperation. Therefore, this procedure cannot be applied either in the clinical routine or in large-scale epidemiological studies. Only recently, however, retinal blood velocity measurements in human eyes based on optical coherence tomography were introduced (24,25). With the further development of this technology instruments that allow for measurement of total retinal blood flow in a fast and reliable way may become available commercially.
In summary, the present study indicates that patients with early type 1 diabetes have increased retinal blood flow that is reduced toward normal under euglycemic conditions. Hence, retinal blood flow may fluctuate significantly with fluctuating plasma glucose levels. This fluctuation may contribute to the microvascular damage seen early in diabetic retinopathy.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
B.P. wrote the manuscript and reviewed/edited the manuscript. E.P. researched data. G.G., A.K.-W., and L.S. contributed to discussion and reviewed/edited the manuscript. M.B.-E. contributed to discussion.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Cai J, Boulton M: The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye 2002;16:242–260 [DOI] [PubMed] [Google Scholar]
- 2. Schmetterer L, Wolzt M: Ocular blood flow and associated functional deviations in diabetic retinopathy. Diabetologia 1999;42:387–405 [DOI] [PubMed] [Google Scholar]
- 3. Grunwald JE, Riva CE, Baine J, Brucker AJ: Total retinal volumetric blood flow rate in diabetic patients with poor glycemic control. Invest Ophthalmol Vis Sci 1992;33:356–363 [PubMed] [Google Scholar]
- 4. Grunwald JE, DuPont J, Riva CE: Retinal haemodynamics in patients with early diabetes mellitus. Br J Ophthalmol 1996;80:327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawagishi T, Nishizawa Y, Emoto M, Konishi T, Maekawa K, Hagiwara S, Okuno Y, Inada H, Isshiki G, Morii H: Impaired retinal artery blood flow in IDDM patients before clinical manifestations of diabetic retinopathy. Diabetes Care 1995;18:1544–1549 [DOI] [PubMed] [Google Scholar]
- 6. Feke GT, Buzney SM, Ogasawara H, Fujio N, Goger DG, Spack NP, Gabbay KH: Retinal circulatory abnormalities in type 1 diabetes. Invest Ophthalmol Vis Sci 1994;35:2968–2975 [PubMed] [Google Scholar]
- 7. Lorenzi M, Feke GT, Cagliero E, Pitler L, Schaumberg DA, Berisha F, Nathan DM, McMeel JW: Retinal haemodynamics in individuals with well-controlled type 1 diabetes. Diabetologia 2008;51:361–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grunwald JE, Riva CE, Martin DB, Quint AR, Epstein PA: Effect of an insulin-induced decrease in blood glucose on the human diabetic retinal circulation. Ophthalmology 1987;94:1614–1620 [DOI] [PubMed] [Google Scholar]
- 9. Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA: Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci 1996;37:886–897 [PubMed] [Google Scholar]
- 10. Findl O, Dallinger S, Rami B, Polak K, Schober E, Wedrich A, Ries E, Eichler HG, Wolzt M, Schmetterer L: Ocular haemodynamics and colour contrast sensitivity in patients with type 1 diabetes. Br J Ophthalmol 2000;84:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilmore ED, Hudson C, Nrusimhadevara RK, Ridout R, Harvey PT, Mandelcorn M, Lam WC, Devenyi RG: Retinal arteriolar hemodynamic response to an acute hyperglycemic provocation in early and sight-threatening diabetic retinopathy. Microvasc Res 2007;73:191–197 [DOI] [PubMed] [Google Scholar]
- 12. Schmetterer L, Müller M, Fasching P, Diepolder C, Gallenkamp A, Zanaschka G, Findl O, Strenn K, Mensik C, Tschernko E, Eichler HG, Wolzt M: Renal and ocular hemodynamic effects of insulin. Diabetes 1997;46:1868–1874 [DOI] [PubMed] [Google Scholar]
- 13. Luksch A, Polak K, Matulla B, Dallinger S, Kapiotis S, Rainer G, Wolzt M, Schmetterer L: Glucose and insulin exert additive ocular and renal vasodilator effects on healthy humans. Diabetologia 2001;44:95–103 [DOI] [PubMed] [Google Scholar]
- 14. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98:823–833 [PubMed] [Google Scholar]
- 15. DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E233 [DOI] [PubMed] [Google Scholar]
- 16. Riva CE, Grunwald JE, Sinclair SH, Petrig BL: Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci 1985;26:1124–1132 [PubMed] [Google Scholar]
- 17. Garhofer G, Bek T, Boehm AG, Gherghel D, Grunwald J, Jeppesen P, Kergoat H, Kotliar K, Lanzl I, Lovasik JV, Nagel E, Vilser W, Orgul S, Schmetterer L: Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol. 14 August 2009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18. Polak K, Dorner G, Kiss B, Polska E, Findl O, Rainer G, Eichler HG, Schmetterer L: Evaluation of the Zeiss retinal vessel analyser. Br J Ophthalmol 2000;84:1285–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arend O, Wolf S, Remky A, Sponsel WE, Harris A, Bertram B, Reim M: Perifoveal microcirculation with non-insulin-dependent diabetes mellitus. Graefes Arch Clin Exp Ophthalmol 1994;232:225–231 [DOI] [PubMed] [Google Scholar]
- 20. Guan K, Hudson C, Wong T, Kisilevsky M, Nrusimhadevara RK, Lam WC, Mandelcorn M, Devenyi RG, Flanagan JG: Retinal hemodynamics in early diabetic macular edema. Diabetes 2006;55:813–818 [DOI] [PubMed] [Google Scholar]
- 21. Rogers SL, Tikellis G, Cheung N, Tapp R, Shaw J, Zimmet PZ, Mitchell P, Wang JJ, Wong TY: Retinal arteriolar caliber predicts incident retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetes Care 2008;31:761–763 [DOI] [PubMed] [Google Scholar]
- 22. Nguyen TT, Wang JJ, Sharrett AR, Islam FM, Klein R, Klein BE, Cotch MF, Wong TY: Relationship of retinal vascular caliber with diabetes and retinopathy: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2008;31:544–549 [DOI] [PubMed] [Google Scholar]
- 23. Fuchsjäger-Mayrl G, Polak K, Luksch A, Polska E, Dorner GT, Rainer G, Eichler HG, Schmetterer L: Retinal blood flow and systemic blood pressure in healthy young subjects. Graefes Arch Clin Exp Ophthalmol 2001;239:673–677 [DOI] [PubMed] [Google Scholar]
- 24. Werkmeister RM, Dragostinoff N, Pircher M, Götzinger E, Hitzenberger CK, Leitgeb RA, Schmetterer L: Bidirectional Doppler Fourier-domain optical coherence tomography for measurement of absolute flow velocities in human retinal vessels. Opt Lett. 2008;33:2967–2969 [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Lu A, Gil-Flamer J, Tan O, Izatt JA, Huang D: Measurement of total blood flow in the normal human retina using Doppler Fourier-domain optical coherence tomography. Br J Ophthalmol 2009;93:634–637 [DOI] [PMC free article] [PubMed] [Google Scholar]


