Abstract
OBJECTIVE
Diabetes is a predictor of both coronary artery disease (CAD) and heart failure. It is unknown to what extent the association between diabetes and heart failure is influenced by other risk factors for heart failure.
RESEARCH DESIGN AND METHODS
We evaluated the association of diabetes and A1C with incident heart failure in outpatients with stable CAD and no history of heart failure (average follow-up 4.1 years).
RESULTS
Of 839 participants, 200 had diabetes (23.8%). Compared with patients who did not have diabetes, those with diabetes had an increased risk of heart failure (hazard ratio [HR] 2.17 [95% CI 1.37–3.44]). Adjustment for risk factors for CAD (age, sex, race, smoking, physical inactivity, obesity, blood pressure, and LDL cholesterol), interim myocardial infarction, and myocardial ischemia did not alter the strength of the association between diabetes and heart failure. After inclusion also of other risk factors for heart failure (left ventricular ejection fraction, diastolic dysfunction, and C-reactive protein) and medication use, diabetes remained an independent predictor of heart failure (HR 3.34 [95% CI 1.65–6.76]; P = 0.001). Each 1% increase in A1C concentration was associated with a 36% increased HR of heart failure hospitalization (HR 1.36 [95% CI 1.17–1.58]).
CONCLUSIONS
In patients with stable CAD who are free from heart failure at baseline, diabetes and glycemic control are independent risk factors for new-onset heart failure. The mechanisms by which diabetes and hyperglycemia lead to heart failure deserve further study, as the association is independent of baseline functional assessment of ischemia, systolic and diastolic function, and interim myocardial infarction.
Heart failure is an enormous burden of disease, leading to substantial health care costs. Despite advances in treatment, the number of heart failure hospitalizations has increased steadily. The 2005 Heart Failure Guidelines of the American College of Cardiology/American Heart Association (1) and European Society of Cardiology (2) emphasized the importance of identification and treatment of risk factors. Among the patients classified in the highest risk group are patients with diabetes. Diabetes is associated with incident heart failure in the general population (3,4) and with adverse outcomes among patients with already existing heart failure (5). Diabetes also predicts heart failure in patients with acute coronary syndromes (6). Whether diabetes predicts heart failure in patients with stable coronary artery disease (CAD) has not been evaluated in detail.
The precise underlying mechanism by which diabetes portends heart failure is unclear. In fact, it remains to be elucidated whether in this context the diagnosis of diabetes per se is more important than just the presence of inadequate glycemic control. CAD is the number one risk factor for heart failure in the developed world (1,2). Because diabetes is strongly associated with CAD, it is plausible to attribute the risk of heart failure associated with diabetes to the effects of CAD. However, although it is known that hyperglycemia predicts heart failure among diabetic patients with CAD (7), it is not known whether this risk is independent of CAD severity, CAD progression, or the presence of myocardial ischemia. Even in the absence of CAD, patients with diabetes show changes in myocardial performance that put them at risk for heart failure (diabetic cardiomyopathy).
To determine to what extent the association between diabetes and heart failure is influenced by other risk factors for heart failure (including interim myocardial infarction and the presence of baseline myocardial ischemia), we evaluated the risk of heart failure associated with diabetes in a cohort of outpatients with stable CAD. The cohort is derived from the Heart and Soul Study (8), which allows thorough investigation of the strength of the association between diabetes (both the diagnosis per se and the level of glycemic control) and future heart failure episodes, while taking into account the above-mentioned established and presumed risk factors.
RESEARCH DESIGN AND METHODS
The Heart and Soul Study is a prospective cohort study of psychosocial factors and health outcomes in patients with stable CAD. Design of the study has been published previously (8). In summary, patients were recruited from outpatient clinics of 12 different centers in the San Francisco Bay area if they met one or more of the following inclusion criteria: prior myocardial infarction, angiographic evidence of ≥50% stenosis in one of the coronary arteries, prior coronary revascularization, and exercise-induced ischemia (treadmill or nuclear scintigraphy). Exclusion criteria were acute coronary syndrome within the past 6 months, the inability to walk one block, and plans to move out of the area within 3 years.
Between September 2000 and December 2002, 1,024 patients were enrolled in the study. For the current investigation, 185 patients (18.1%) were excluded because they had a history of heart failure (n = 179) or heart failure status was unknown (n = 6).
Baseline study variables
All patients completed a daylong baseline study visit that included a medical history interview, physical examination, questionnaire, laboratory analysis, exercise test, and echocardiogram. Diabetes was defined as self-reported diabetes or the use of antidiabetes medication. Alcohol use was determined by questionnaire. Participants rated their physical activity during the previous month using a 6-point Likert scale. Those responding “not at all active” or “a little active” were classified as physically inactive. An estimate of chronic glycemia was provided by serum A1C measurement. Serum glucose level, A1C, LDL cholesterol, and C-reactive protein (CRP) were assessed by standard routine biochemistry analysis after an overnight fast (except for taking their regularly prescribed medication with water) using a venous blood sample, drawn via a 21-gauge butterfly needle. Subjects were considered to have metabolic syndrome if they met the criteria of the National Cholesterol Education Program (9). Echocardiography was performed with an Acuson Sequoia Ultrasound System (Siemens Medical Solutions USA, Malvern, PA), with a 3.5-MHz transducer. Left ventricular ejection fraction (LVEF) was calculated using the modified Simpson rule as recommended by the American Society of Echocardiography (10). In addition, a full description of diastolic function was performed according to predefined established criteria (normal, impaired, pseudonormal, or restrictive diastolic function). An exercise treadmill test (standard Bruce protocol) was performed. Immediately after exercise, echocardiographic analysis was performed to investigate exercise-induced wall motion abnormalities, which served as an indicator of myocardial ischemia (11). Details pertaining to acquisition and analyses of echocardiographic data were reported elsewhere (12). The institutional review board at each of the sites approved the study protocol, and all participants provided written informed consent.
End points
The main study outcome was time to hospitalization for heart failure, as was previously reported for the whole cohort in detail (13). Heart failure was diagnosed according to established criteria (1,2) using clinical and radiological evaluation. Potential events were recorded annually by telephonic interviews. Additional information (e.g., medical records and death certificates) was collected and reviewed by two independent and blinded adjudicators. Discrepancies were discussed, and decisions were made by unanimity. In case of disagreement, a third blinded adjudicator was consulted. Follow-up was completed for all patients.
Statistical analysis
The study sample comprised 839 patients. Baseline differences between participants with diabetes and without were compared using t tests for continuous variables and χ2 tests for dichotomous variables.
In addition to the association of diabetes per se with hospitalization for heart failure, we investigated the role of glycemic control in the development of heart failure. A1C was used as a proxy measure for glycemic control (both dichotomized and continuous, per 1% change). For the former categorization, a cutoff of ≥6.5% and <6.5% was used because this cutoff was recently used to redefine the diagnosis of diabetes (14). The following analyses were conducted with diabetes and A1C as independent variables.
First, Kaplan-Meier analysis was used to estimate the time from baseline to heart failure hospitalization in patients with or without diabetes and in patients with low or high A1C. The log-rank test was used for bivariate significance testing. In addition, given the influence of antidiabetes medication on A1C levels, we compared the effect of glycohemoglobin on heart failure in patients taking antidiabetes medication.
Second, Cox proportional hazard regression analyses were performed to investigate the impact of diabetes and A1C level, respectively, on the time to first hospitalization for heart failure. To study the impact of diabetes and A1C on heart failure in the context of several potential confounders, we made a selection of the most important risk factors for heart failure based on recent guidelines (1,2). We then applied the following series of a priori determined Cox regression models in which we sequentially controlled for the following groups of confounders: model 1: age, sex, and race; model 2: smoking, physical inactivity, BMI, LDL cholesterol, and systolic blood pressure; model 3: myocardial infarction during follow-up; model 4: LVEF; model 5: exercise-induced wall motion abnormalities (i.e., ischemia); model 6: diastolic dysfunction; model 7: logCRP; and model 8: ACE inhibitor/angiotensin receptor blocker (ARB) and β-blocker-use. All models included age, sex, and race. In the final model (model 9) we include all the variables that were used in models 1–8. Finally, in sensitivity analyses, the relationship between several other definitions of diabetes and time to onset of heart failure were tested. These definitions were 1) self-reported diagnosis of diabetes (irrespective of antidiabetes medication use), 2) fasting blood glucose >126 mg/dl, and 3) fasting blood glucose >126 mg/dl or use of antidiabetes medication. Moreover, the presence of metabolic syndrome was tested in sensitivity analyses. P < 0.05 was used for all tests to indicate statistical significance. Hazard ratios (HRs) with 95% CIs are reported. All statistical analyses were performed using SPSS (version 17.0 for Windows; SPSS, Chicago, IL).
RESULTS
Baseline characteristics of the study population (n = 839) are outlined in Table 1. Mean age was 67 years, and 200 patients (23.8%) had diabetes.
Table 1.
Baseline characteristics
| No diabetes | Diabetes | P value | |
|---|---|---|---|
| n | 639 | 200 | |
| Age (years) | 67.4 ± 11.0 | 65.3 ± 10.3 | 0.02 |
| Sex, male (%) | 528 (82.6) | 161 (80.5) | 0.49 |
| Race, white (%) | 416 (65.2) | 88 (44.0) | <0.001 |
| BMI (kg/m2) | 27.9 ± 4.7 | 29.9 ± 6.0 | <0.001 |
| Smoking (%) | 124 (19.4) | 34 (17.0) | 0.44 |
| Heavy alcohol use (%) | 215 (33.8) | 38 (19.0) | <0.001 |
| Physical inactivity (%) | 221 (33.1) | 85 (42.5) | 0.02 |
| LDL cholesterol (mg/dl) | 106 (34) | 100 (32) | 0.03 |
| A1C (%) | 5.5 ± 0.5 | 7.1 ± 1.4 | <0.001 |
| Systolic blood pressure (mmHg) | 132 ± 20 | 137 ± 23 | 0.003 |
| Medical history | |||
| Myocardial infarction (%) | 313 (49.1) | 106 (53.8) | 0.25 |
| Revascularization (%) | 367 (57.4) | 104 (52.3) | 0.20 |
| Medication use | |||
| ACE inhibitor/ARB (%) | 261 (40.8) | 136 (68.0) | <0.001 |
| β-Blocker (%) | 343 (53.7) | 129 (64.5) | 0.007 |
| Baseline LVEF (%) | 62.7 ± 8.6 | 63.1 ± 8.8 | 0.59 |
| Diastolic function (%) | 0.22 | ||
| Normal | 361 (63.2) | 116 (63.7) | |
| Impaired | 151 (26.4) | 40 (22.0) | |
| Pseudo/restricted | 59 (10.3) | 26 (14.3) | |
| Exercise-induced wall motion abnormalities (%) | 128 (21.5) | 44 (24.4) | 0.40 |
| Creatinine clearance (ml/min) | 82.4 ± 26.9 | 82.0 ± 31.2 | 0.87 |
| CRP (mg/l) | 4.0 ± 6.8 | 4.8 ± 7.0 | 0.11 |
Data are means ± SD unless otherwise indicated. CRP was log-transformed for statistical analysis.
Diabetes as a predictor of heart failure hospitalizations
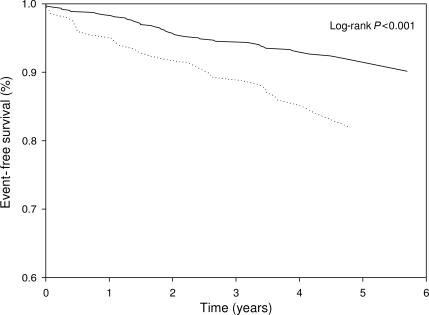
During a mean ± SD follow-up of 4.1 ± 1.2 years, 30 (15.0%) patients with diabetes and 47 (7.4%) patients without diabetes developed heart failure. Between baseline and end of follow-up (either heart failure event or end of study), 52 patients (6.2%) had a myocardial infarction. In Fig. 1, Kaplan-Meier analysis shows the proportion of patients with hospitalizations for heart failure divided into patients with and without diabetes. In Table 2, results of the Cox regression models are presented. Diabetes was a significant predictor of heart failure hospitalization (HR 2.17 [95% CI 1.37–3.44]; P = 0.001). Diabetes remained a strong predictor of heart failure while adjustments were made for other predefined predictors of heart failure. Thus, adjustment for age, sex, race, smoking, physical inactivity, BMI, LDL cholesterol, systolic blood pressure, myocardial infarction during follow-up, LVEF, exercise-induced wall motion abnormalities (i.e., ischemia), diastolic dysfunction, or CRP did not attenuate the strength of the relationship between diabetes and heart failure. In the fully adjusted model, diabetes was associated with an increased HR for hospitalization because of heart failure (3.34 [1.65–6.76]; P = 0.001). Other significant multivariable predictors were age (years, HR 1.06), smoking status (3.01), physical inactivity (2.18), LVEF (percent, 0.94), exercise-induced wall motion abnormalities (2.34), diastolic dysfunction (1.26–4.97, depending on the grade of diastolic dysfunction), and logCRP (2.10).
Figure 1.
Proportions of patients free of hospitalization for heart failure divided into patients with diabetes (· · · ·) and patients without diabetes (——).
Table 2.
Diabetes and A1C as risk factors for heart failure hospitalization (multivariable Cox regression)
| Diabetes as predictor for heart failure | P value | A1C ≥6.5% as predictor for heart failure | P value | A1C (%) as predictor for heart failure | P value | |
|---|---|---|---|---|---|---|
| n | 839 | 832 | 832 | |||
| Univariable analysis | 2.17 (1.37–3.44) | 0.001 | 1.61 (0.96–2.71) | 0.071 | 1.36 (1.17–1.58) | <0.001 |
| Model 1 | 2.50 (1.57–4.01) | <0.001 | 1.72 (1.02–2.92) | 0.043 | 1.46 (1.24–1.73) | <0.001 |
| Model 2 | 2.65 (1.61–4.36) | <0.001 | 1.58 (0.90–2.78) | 0.114 | 1.50 (1.26–1.79) | <0.001 |
| Model 3 | 2.53 (1.58–4.07) | <0.001 | 1.72 (1.02–2.92) | 0.043 | 1.48 (1.25–1.76) | <0.001 |
| Model 4 | 2.79 (1.74–4.50) | <0.001 | 2.03 (1.18–3.47) | 0.010 | 1.46 (1.24–1.71) | <0.001 |
| Model 5 | 2.19 (1.29–3.71) | 0.003 | 1.50 (0.82–2.73) | 0.189 | 1.33 (1.09–1.61) | 0.004 |
| Model 6 | 2.60 (1.55–4.36) | <0.001 | 2.02 (1.16–3.52) | 0.014 | 1.48 (1.24–1.75) | <0.001 |
| Model 7 | 2.42 (1.50–3.90) | <0.001 | 1.71 (1.01–2.92) | 0.047 | 1.39 (1.17–1.64) | <0.001 |
| Model 8 | 2.49 (1.52–4.08) | <0.001 | 1.67 (0.98–2.84) | 0.061 | 1.45 (1.22–1.72) | <0.001 |
| Model 9 (full) | 3.34 (1.65–6.76) | 0.001 | 2.27 (1.06–4.87) | 0.036 | 1.40 (1.13–1.74) | 0.003 |
Data are HR (95% CI) unless otherwise indicated. Model 1: age, sex, and race. Model 2: age, sex, race, smoking, BMI, physical inactivity, LDL cholesterol, and systolic blood pressure. Model 3: age, sex, race, and myocardial infarction during follow-up. Model 4: age, sex, race, and LVEF. Model 5: age, sex, race, and exercise-induced wall motion abnormalities. Model 6: age, sex, race, and diastolic dysfunction. Model 7: age, sex, race, and logCRP. Model 8: age, sex, race, ACE inhibitor/ARB and β-blocker use. Model 9: age, sex, race, smoking, BMI, physical inactivity, LDL cholesterol, systolic blood pressure, myocardial infarction during follow-up, LVEF, exercise-induced wall motion abnormalities, diastolic dysfunction, logCRP, and ACE inhibitor/ARB and β-blocker use.
Glycemic control
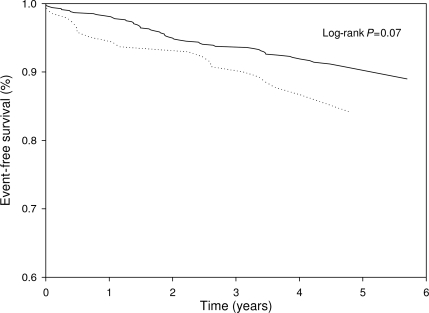
Diabetes was correlated with the level of A1C (correlation coefficient r = 0.635, P < 0.001). Patients who were hospitalized for heart failure had significantly higher A1C levels than those who remained free from hospitalization (6.4 ± 1.4 vs. 5.9 ± 1.0%; P = 0.003). Patients who developed heart failure had borderline significantly more often a high level of A1C (≥6.5%), compared with patients who remained free of heart failure (24.7 vs. 16.6%; P = 0.07). In Fig. 2, Kaplan-Meier analysis shows the proportion of patients with hospitalization for heart failure divided into patients with high and low A1C levels (log-rank test P = 0.07). Subgroup analysis revealed that, in patients who used antidiabetes medications (n = 156), the level of A1C was not related to new-onset heart failure (P = 0.21).
Figure 2.
Proportions of patients free of hospitalization for heart failure divided into patients with high A1C levels (≥6.5%; · · · ·) and patients with low A1C levels (<6.5%; ——).
In Cox regression analysis, A1C≥6.5% was borderline significantly related to incidence of new onset of heart failure (HR 1.61 [95% CI 0.96–2.71]; P = 0.07) (Table 2). With adjustment for the predefined predictors, on average, A1C remained a borderline significant predictor for heart failure.
When A1C was entered into Cox regression analysis as a continuous variable, A1C was strongly related to heart failure outcome. Each percent increase in A1C was associated with a HR of 1.36 ([95% CI 1.17–1.58]; P < 0.001) for the incidence of heart failure hospitalization. Model-like adjustments did not decrease HRs significantly (Table 2). As shown also in Table 2, diabetes was associated with a higher HR for heart failure hospitalization than A1C (dichotomized with a cutoff of 6.5%).
Sensitivity analyses
Sensitivity analyses using different diabetes definitions showed similar statistically significant outcomes with respect to heart failure development (data not shown). All HRs were between 1.95 and 2.23 for univariable analyses and 1.87 and 3.81 for multivariable analyses. Metabolic syndrome was not significantly related to heart failure in unadjusted analyses (HR 1.40 [95% CI 0.89–2.19]; P = 0.143). However, after adjustment for age and sex, metabolic syndrome was borderline statistically significant related to heart failure (1.56 [95% CI 0.99–2.45]; P = 0.054).
CONCLUSIONS
In the present study, we established that diabetes and glycemic control are independent risk factors for the development of heart failure in patients with stable CAD.
Diabetes as a risk factor for heart failure
The first observation that diabetes was linked to the development of heart failure was demonstrated 30 years ago (15). Nevertheless, only recently has heart failure been acknowledged as a serious and often fatal complication for patients with diabetes (16). Other studies (3,17) reported that, in the general population, diabetes increases the risk for heart failure approximately twofold. Studies in elderly individuals (18), patients with high-risk vascular disease (19), patients with myocardial infarctions (6) or patients with chronic heart failure (20) had comparable observations, although some of them failed to adjust for accepted confounding factors such as diastolic dysfunction (6,18–20) or LVEF (19). Our data extend this knowledge as we performed several important assessments.
First, we assessed the strength of the association between diabetes and heart failure in a population with stable CAD. In this patient group, we found a more than twofold elevated risk for heart failure, attributable to diabetes (HR 2.17). This result is in line with recent outcomes from a placebo-controlled ACE inhibitor trial (21). The association persisted after adjustment for age, sex, race, smoking, physical inactivity, BMI, LDL cholesterol, and systolic blood pressure. Because interim myocardial infarction (as a surrogate measure for progression of CAD) and exercise-induced wall motion abnormalities also did not attenuate the strength of the association between diabetes and heart failure, our data suggest that diabetes is a risk factor for heart failure, independent of CAD risk factors, CAD progression, and the baseline presence of myocardial ischemia.
Adequate adjustment is important because diabetes is a disorder that frequently is clustered with other risk factors for CAD and heart failure. Without this adjustment, an estimation of the real strength of the association between diabetes and heart failure remains a subject of discussion, making the causality issue a matter of speculation. Yet, as far as we know, no previous studies took into account the severity of the underlying cardiac disease as extensively as we did herein. This strategy confirmed not only that diabetes is an independent risk factor for heart failure but it also showed also that diabetes, compared with other established risk factors (e.g., age, smoking status, physical inactivity, LVEF, exercise-induced wall motion abnormalities, diastolic dysfunction, and CRP), is among the key players in heart failure development.
Second, because diabetes emerged as a risk factor for heart failure, we investigated whether the level of glycemic control is related to the incidence of heart failure hospitalizations. Previous studies have been inconsistent on this point. Although the majority of studies point to a relation between glucose levels and heart failure (4,19,22), a report of the Cardiovascular Health Study (18) showed no association after adjustment for demographic data and baseline disease. Importantly, these studies measured glycemia on a single occasion. However, the widely used clinical test, A1C, which reflects mean blood glucose over the previous 8–12 weeks, may be more informative in terms of a patient's glucose control. In our study, A1C was strongly associated with new heart failure episodes as a continuous variable, whereas it was borderline significant as a dichotomized variable. This finding is in line with a study by Nichols et al. (23) and remained robust in analyses incorporating other important covariates of heart failure development. The HR of heart failure hospitalization was larger for diabetes than for A1C, suggesting that the level of glycemic control may not be the crucial factor when one is unraveling causality between diabetes and heart failure. However, it should be emphasized that this result was based on a single measurement of A1C at baseline and did not necessarily reflect glycemic control during the follow-up period. Nevertheless, this idea is supported by our observation that in patients who used antidiabetes medications (n = 156), those with adequate glucose control did not differ from those with poorly regulated diabetes in terms of heart failure outcome. Another study (24) also failed to find a relationship between effective antidiabetes treatment (in terms of glycemic control) and improvement in heart failure outcome.
The reason that diabetes (or the lack of glycemic control) is related to heart failure has been intensively investigated. Both preclinical and clinical studies resulted in the identification of potential pathways leading to “diabetic cardiomyopathy” (25,26): facilitation of atherogenesis, autonomic dysfunction, interstitial fibrosis, glycation of interstitial proteins, impaired calcium homeostasis, upregulation of the renin-angiotensin system, increased oxidative stress, altered substrate metabolism, and mitochondrial dysfunction. In our study, the strength of the association between diabetes and heart failure did not attenuate after adjustment for established risk factors for heart failure, suggesting that traditional risk factors are not responsible for the detrimental association, giving fuel to the diabetic cardiomyopathy hypothesis.
Study limitations
Our results must be interpreted in the light of several limitations. First, no catheterization was performed at baseline to ascertain the extent of CAD. However, we think that in the context of the development of heart failure, a functional assessment (i.e., inducible myocardial ischemia) is even more important. In our study, a method with high accuracy was used to detect myocardial ischemia (i.e., exercise-induced wall motion abnormalities) (11). Second, because our study population consisted only of patients with stable CAD, conclusions cannot be extrapolated to populations with unstable coronary syndromes.
In summary, our study endorses once more the detrimental influence of diabetes on heart failure prognosis. In the context of heart failure development, the level of A1C, as a measure of cumulative glycemic burden, is an important marker of increased risk. Because prevention of heart failure is an important public health goal and no clear improvement in event rates was noted during the past few years, future studies should focus on the mechanism by which diabetes leads to heart failure and how these effects can be prevented.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying editorial, p. 2120.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW: 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009;53:e1–e90 [DOI] [PubMed] [Google Scholar]
- 2.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Tendera M, Auricchio A, Bax J, Böhm M, Corrà U, della Bella P, Elliott PM, Follath F, Gheorghiade M, Hasin Y, Hernborg A, Jaarsma T, Komajda M, Kornowski R, Piepoli M, Prendergast B, Tavazzi L, Vachiery JL, Verheugt FW, Zamorano JL, Zannad F: ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008;10:933–989 [DOI] [PubMed] [Google Scholar]
- 3.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK: Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 2001;161:996–1002 [DOI] [PubMed] [Google Scholar]
- 4.Thrainsdottir IS, Aspelund T, Gudnason V, Malmberg K, Sigurdsson G, Thorgeirsson G, Hardarson T, Rydén L: Increasing glucose levels and BMI predict future heart failure experience from the Reykjavík Study. Eur J Heart Fail 2007;9:1051–1057 [DOI] [PubMed] [Google Scholar]
- 5.Varela-Roman A, Grigorian Shamagian L, Barge Caballero E, Mazon Ramos P, Rigueiro Veloso P, Gonzalez-Juanatey JR: Influence of diabetes on the survival of patients hospitalized with heart failure: a 12-year study. Eur J Heart Fail 2005;7:859–864 [DOI] [PubMed] [Google Scholar]
- 6.Stone PH, Muller JE, Hartwell T, York BJ, Rutherford JD, Parker CB, Turi ZG, Strauss HW, Willerson JT, Robertson T: The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol 1989;14:49–57 [DOI] [PubMed] [Google Scholar]
- 7.Pazin-Filho A, Kottgen A, Bertoni AG, Russell SD, Selvin E, Rosamond WD, Coresh J: HbA 1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 2008;51:2197–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA: Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA 2003;290:215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ: Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–1463 [DOI] [PubMed] [Google Scholar]
- 11.Quiñones MA, Verani MS, Haichin RM, Mahmarian JJ, Suarez J, Zoghbi WA: Exercise echocardiography versus 201Tl single-photon emission computed tomography in evaluation of coronary artery disease. Analysis of 292 patients. Circulation 1992;85:1026–1031 [DOI] [PubMed] [Google Scholar]
- 12.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA: Is B-type natriuretic peptide a useful screening test for systolic or diastolic dysfunction in patients with coronary disease? Data from the Heart and Soul Study. Am J Med 2004;116:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA: N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA 2007;297:169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannel WB, McGee DL: Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035–2038 [DOI] [PubMed] [Google Scholar]
- 16.Bell DS: Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 2003;26:2433–2441 [DOI] [PubMed] [Google Scholar]
- 17.Thrainsdottir IS, Aspelund T, Thorgeirsson G, Gudnason V, Hardarson T, Malmberg K, Sigurdsson G, Rydén L: The association between glucose abnormalities and heart failure in the population-based Reykjavík study. Diabetes Care 2005;28:612–616 [DOI] [PubMed] [Google Scholar]
- 18.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC: Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol 2000;35:1628–1637 [DOI] [PubMed] [Google Scholar]
- 19.Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, Sleight P, Teo K: ONTARGET/TRANSCEND Investigators Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation 2007;115:1371–1375 [DOI] [PubMed] [Google Scholar]
- 20.Gerstein HC, Swedberg K, Carlsson J, McMurray JJ, Michelson EL, Olofsson B, Pfeffer MA, Yusuf S: CHARM Program Investigators The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med 2008;168:1699–1704 [DOI] [PubMed] [Google Scholar]
- 21.Lewis EF, Solomon SD, Jablonski KA, Rice MM, Clemenza F, Hsia J, Maggioni AP, Zabalgoitia M, Huynh T, Cuddy TE, Gersh BJ, Rouleau J, Braunwald E, Pfeffer MA: PEACE Investigators Predictors of heart failure in patients with stable coronary artery disease: a PEACE study. Circ Heart Fail 2009;2:209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barzilay JI, Kronmal RA, Gottdiener JS, Smith NL, Burke GL, Tracy R, Savage PJ, Carlson M: The association of fasting glucose levels with congestive heart failure in diabetic adults > or =65 years: the Cardiovascular Health Study. J Am Coll Cardiol 2004;43:2236–2241 [DOI] [PubMed] [Google Scholar]
- 23.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB: The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004;27:1879–1884 [DOI] [PubMed] [Google Scholar]
- 24.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 25.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A: New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 1972;30:595–602 [DOI] [PubMed] [Google Scholar]
- 26.Boudina S, Abel ED: Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–3223 [DOI] [PubMed] [Google Scholar]