Abstract
OBJECTIVE
Some individuals with normal glucose tolerance (NGT) exhibit a 1-h excursion of plasma glucose during oral glucose tolerance testing as high as that of individuals with impaired glucose tolerance (IGT). The aim of this study was to characterize their metabolic phenotype.
RESEARCH DESIGN AND METHODS
A total of 1,205 healthy volunteers (aged 29–61 years) underwent assessment of 1) oral glucose tolerance and 2) insulin sensitivity (standardized euglycemic-hyperinsulinemic clamp), as part of the Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) study.
RESULTS
One-hour plasma glucose correlated better than 2-h plasma glucose with total insulin secretion (r = 0.43), β-cell glucose sensitivity (r = −0.46), and β-cell rate sensitivity (r = −0.18). Receiver operating characteristic analysis identified 8.95 mmol/l as the best cutoff value for prediction of IGT from 1-h plasma glucose (sensitivity 77% and specificity 80%). Participants with NGT with 1-h plasma glucose >8.95 mmol/l had larger waist circumference, higher BMI, lower insulin sensitivity, higher fasting glucose, and higher insulin secretion than their counterparts with 1-h plasma glucose ≤8.95 mmol/l (P < 0.001 for all comparisons). Moreover, they exhibited lower β-cell glucose sensitivity (P < 0.001), β-cell rate sensitivity (P < 0.001), and potentiation factor (P = 0.026). When compared with conventionally defined IGT, they were not different in waist circumference and BMI, hepatic insulin extraction, β-cell glucose sensitivity, β-cell rate sensitivity, and potentiation factor but did have greater insulin sensitivity along with reduced basal (P = 0.001) and total insulin secretion (P = 0.002).
CONCLUSIONS
Higher values of 1-h plasma glucose may identify an intermediate condition between NGT and IGT characterized by greater insulin resistance, reduced β-cell glucose sensitivity, and reduced β-cell rate sensitivity.
Impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) are states of carbohydrate metabolism intermediate between normal glucose tolerance (NGT) and type 2 diabetes, which represent two partially overlapping conditions with distinct metabolic characteristics (1,2). In IFG, there is marked hepatic insulin resistance with near-normal muscle insulin sensitivity, whereas this pattern is reversed in IGT (2). Although both conditions are characterized by reduced early-phase insulin secretion, there is an additional impairment of late-phase insulin secretion in IGT. Accordingly, individuals with IGT have a rapid early (30 min) rise in plasma glucose during an oral glucose tolerance test (OGTT) which continues to rise until 60 min (1-h plasma glucose) and thereafter remains ≥7.8 mmol/l (140 mg/dl) at 120 min (2-h plasma glucose).
As longitudinal studies have demonstrated that 40% of patients who develop type 2 diabetes after 10 years have NGT at baseline (1), there may be additional information beyond conventional IFG/IGT categories that may better discriminate future progression to type 2 diabetes (3). We have noted a subset of individuals with NGT who have early glucose excursions during an OGTT as high as those observed in individuals with IGT. However, because plasma glucose concentrations decline adequately by 2 h, due to preservation of late-phase insulin secretion, these individuals do not have, by current definitions, any form of disordered carbohydrate metabolism (4). Data from the San Antonio Study have shown that β-cell glucose sensitivity and insulin sensitivity contribute to values of 2-h plasma glucose independently of each other (5); thus, we hypothesized that individuals with NGT with 1-h plasma glucose levels as high as in those with IGT might represent an intermediate phenotype of abnormal carbohydrate metabolism with either impaired insulin sensitivity or β-cell glucose sensitivity, who are potentially at increased risk of progression to type 2 diabetes.
To investigate this hypothesis we analyzed cross-sectional data from the European Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) study (6), examining the metabolic phenotype of individuals with NGT who had high 1-h plasma glucose excursions. We aimed to identify a new glucose tolerance subgroup who might benefit from targeted lifestyle advice and/or pharmacological intervention.
RESEARCH DESIGN AND METHODS
The RISC study is a prospective (3- and 10-year follow-up), observational, cohort study. Primary objectives include 1) establishing whether insulin resistance predicts deterioration of cardiovascular risk markers, diabetes, obesity, atherosclerosis, and cardiovascular disease and 2) developing methods based on mathematical modeling to identify insulin-resistant participants in clinical practice.
Healthy adults, aged 29–61, with no history of diabetes, hypertension, or hyperlipidemia were recruited from 18 centers across Europe between 2002 and 2004. Specific inclusion and exclusion criteria are reported elsewhere (6). Participants gave written informed consent and local ethics committee approval was obtained in each center. Every volunteer had a clinical examination, including body composition estimated by bioimpedance (TBF-300 body composition analyzer; Tanita, Hoofddorp, the Netherlands), and underwent a 75-g OGTT and euglycemic-hyperinsulinemic clamp (EHC).
OGTT
After an overnight fast, a 75-g OGTT was performed in the morning, with sampling at baseline and at 30, 60, 90, and 120 min after glucose ingestion, with measurement of glucose, insulin, and C-peptide. Data from the OGTT were used for assessment of β-cell function and calculation of mean hepatic insulin extraction [(HIEOGTT = 1 − (clearance from the clamp)/(endogenous clearance during the OGTT)].
EHC
One to 3 weeks after the OGTT, an EHC was performed in all participants. Exogenous insulin was administered as primed-continuous infusion at a rate of 240 pmol · min−1 · m−2, with a simultaneous variable infusion of 20% dextrose adjusted every 5 min to maintain plasma glucose within 0.8 mmol/l (±15%) of the target glucose level (4.5–5.5 mmol/l). The clamp procedure was standardized across centers (6). An initial fasting blood sample and two samples during the last 40 min of the clamp were taken for measurement of glucose, insulin, and C-peptide concentrations. Insulin sensitivity was assessed as the mean glucose infusion rate over the last 40 min of the clamp, corrected for mean plasma insulin levels achieved during the same period (M/I, in micromoles per minute · per kilogram of free fat mass [FFM] per nanomoles per liter). The peripheral clearance of insulin during the clamp (liters per minute per meter squared) was computed as follows: Iclclamp = (240 pmol · min−1 · m−2 insulin infusion)/(steady-state insulin).
Assessment of β-cell function
β-Cell function was assessed from the OGTT using a well-validated model that describes the relationship between insulin secretion and glucose concentration (7,8). The model expresses insulin secretion (in picomoles per minute per meter) as the sum of two components. The first is β-cell glucose sensitivity, which represents the dependence of insulin secretion on absolute glucose concentration at any time point during the OGTT through a dose-response function relating the two variables (expressed as the mean slope of dose response over the observed glucose range). This dose response is modulated by a potentiation factor, which accounts for higher insulin secretion on the descending phase of OGTT hyperglycemia than at the same glucose concentration on the ascending phase. The potentiation factor is a positive function of time and is constrained to average unity during the experiment (9). The second insulin secretion component represents the dependence of insulin secretion on the rate of change of glucose concentration. This component is determined by a single parameter, denoted as rate sensitivity, and is related to early insulin release (9).
The model parameters were estimated from glucose and C-peptide concentrations by regularized least squares (7,8). Insulin secretion rates were calculated from the model every 5 min. The integral of insulin secretion during the 2-h OGTT was denoted as total insulin output.
Analytical methods
Local laboratory data were used for study inclusion criteria. Blood collected was stored at −20°C and centrally analyzed in Odense, Denmark. Plasma glucose was measured by the glucose oxidase technique (Cobas Integra; Roche, Basel, Switzerland); serum insulin and C-peptide were measured by a specific time-resolved fluoroimmunoassay (AutoDELFIA insulin kit; Wallac Oy, Turku, Finland).
Statistical analysis
Continuous data are reported as mean ± SD, with categorical data as counts and percentages. χ2 tests was used for comparing association between categorical variables, and ANOVA was performed for comparison among groups and for repeated measures. Stepwise logistic regression analysis was used to predict insulin resistance from age, sex, 1-h plasma glucose, 2-h plasma glucose, BMI, and waist circumference. Receiver operating characteristic curve analysis was used to evaluate specificity and sensitivity of plasma glucose at 60 min to identify individuals with IGT. Odds ratios (ORs) were computed to estimate risk of insulin resistance or impaired insulin secretion according to 1-h plasma glucose levels.
Insulin sensitivity was expressed as the natural logarithm of the M/I ratio. Insulin resistance was defined categorically as the lowest decile of the ln(M/I) as done previously (10) in those participants who were nonobese (BMI <25 kg/m2), with normal values of fasting and 2-h glucose and no family history for diabetes. Individuals satisfying these criteria served as the control subjects. Categories of “insulin hypersecretion” and “insulin hyposecretion” were defined as the upper and the lowest deciles of insulin secretion (basal or total), in those control subjects presenting with insulin sensitivity between the lowest and the upper deciles of ln(M/I). The same approach (upper and lower deciles) was also used for hepatic insulin extraction, peripheral insulin clearance, β-cell glucose sensitivity, and rate sensitivity. IFG and IGT were defined according to the criteria of the American Diabetes Association (4).
P < 0.05 was considered as statistically significant. Data analysis was performed with SPSS statistical software (version 12.0; SPSS, Chicago, IL).
RESULTS
After we excluded individuals from the initial sample (n = 1,566) with IFG (n = 72), with type 2 diabetes (n = 30), or with missing OGTT and clamp data (n = 309), 1,205 (56.1% women) individuals (aged 44 ± 8 years) with complete EHC data were included in the analysis. Of these, 509 participants (42.2%) were overweight or obese (BMI ≥25 kg/m2) and 105 met the criteria for IGT (8.7%).
Prevalence of insulin resistance
Thirty-two overweight/obese participants (mean BMI 28.45 ± 3.04 kg/m2) were insulin resistant [ln(M/I) <4.48). Among these insulin-resistant individuals, 41% had total cholesterol ≥5.2 mmol/l (P < 0.001, in comparison with insulin-sensitive individuals), 42% had LDL cholesterol ≥3.3 mmol/l (P < 0.002), 37% had HDL cholesterol ≤1.03 mmol/l (men) or ≤1.3 (women) (P < 0.001), 24% had circulating triglycerides ≥1.7 mmol/l (P < 0.001), and 42% had a waist circumference ≥102 cm (men) or ≥88 cm (women) (P < 0.001).
Prevalence of insulin hyper- and hyposecretion
There were 341 (28.3%) “hypersecretors” and 80 (6.6%) “hyposecretors,” defined as >86.42 and <38.13 pmol · min−1 · m−2, respectively (equivalent figures were 43.12 and 3.05%, respectively, for overweight/obese participants). For total insulin secretion, there were 230 (19.1%) hypersecretors and 110 (9.1%) hyposecretors, defined as >51.97 and >25.21 nmol · m−2 (equivalent figures were 26.1 and 7.1% for overweight/obese participants).
Insulin resistance, 1-h plasma glucose, and 2-h plasma glucose
Both 1-h plasma glucose and 2-h plasma glucose values were inversely related to insulin sensitivity (1-h plasma glucose 6.74 ± 1.9 mmol/l in insulin-sensitive individuals, 7.3 ± 2.1 mmol/l in subjects with intermediate insulin sensitivity, and 8.6 ± 2.1 mmol/l in insulin-resistant subjects, P < 0.001; 2-h plasma glucose 5.0 ± 1.2, 5.5 ± 1.3, and 6.4 ± 1.5 mmol/l, respectively, P < 0.001). Glucose concentrations of insulin-resistant participants during the OGTT were higher than those of other participants at all time points (P < 0.0001, repeated-measures ANOVA, time points, group, and time per group), the greatest difference being at 1 h (7.2 ± 2.1 vs. 8.6 ± 2.1 mmol/l, corresponding to a difference of ∼25 mg/dl). In contrast, 2-h plasma glucose values were 5.5 ± 1.3 vs. 6.4 ± 1.5 mmol/l (a difference of ∼17 mg/dl).
Insulin sensitivity, hepatic insulin extraction, basal and total insulin secretion, β-cell glucose sensitivity, rate sensitivity, and potentiation factor correlated significantly with both 1-h plasma glucose and 2-h plasma glucose (supplementary Table 1S, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-2261/DC1), although higher r values were noted for 1-h plasma glucose than for 2-h plasma glucose in the correlations with total insulin secretion, β-cell glucose sensitivity, and rate sensitivity.
Independent and significant predictors of insulin resistance determined by stepwise logistic regression, using the variables of sex, age, 1-h plasma glucose, 2-h plasma glucose, BMI, and waist circumference, were 1-h plasma glucose (OR 1.10 [95% CI 1.01–1.20]), 2-h plasma glucose (1.41 [1.24–1.60]), BMI (1.23 [1.18–1.29]), and female sex (0.41 [0.29–0.57]).
Insulin secretion, 1-h plasma glucose, and 2-h plasma glucose
Whether considering total or basal insulin secretion, we found that hypersecretors had higher 1-h plasma glucose and 2-h plasma glucose values (P < 0.001 by ANOVA for all Bonferroni pairwise comparisons) (Table 1), although no difference in 1-h plasma glucose or 2-h plasma glucose was detected between hyposecretors and participants with normal secretion. No difference in 1-h plasma glucose or 2-h plasma glucose was detected when participants were categorized according to insulin clearance (P = 0.09) or hepatic insulin extraction (P = 0.2), respectively.
Table 1.
One-hour plasma glucose and 2-h plasma glucose after OGTT in subjects stratified according to parameters deriving from the clamp and from the OGTT
| Classification | % | 1-h plasma glucose | P value | 2-h plasma glucose | P value |
|---|---|---|---|---|---|
| Insulin clearance (l/min/m2) | |||||
| Low clearance (<0.47) | 15.5 | 7.82 ± 2.22 | 0.096 | 5.9 ± 1.41 | 0.003 |
| Normal clearance (0.470–0.82) | 72.9 | 7.48 ± 2.14 | 5.67 ± 1.42 | ||
| High clearance (>0.82) | 11.6 | 7.34 ± 2.54 | 5.37 ± 1.53 | ||
| Insulin sensitivity [log(μmol · min−1 · kgFFM−1 · nmol/l−1)] | |||||
| Hypersensitivity [ln(M/I) >5.53] | 6.4 | 6.74 ± 1.90 | <0.001 | 5.01 ± 1.17 | <0.001 |
| Normal sensitivity [4.47 ≤ ln(M/I) ≤ 5.53] | 72.9 | 7.27 ± 2.15 | 5.51 ± 1.35 | ||
| Insulin resistance [ln(M/I) <4.47] | 20.7 | 8.63 ± 2.12 | 6.4 ± 1.51 | ||
| Total insulin secretion (nmol · m−2) | |||||
| Low secretion (<24.60) | 9.1 | 6.15 ± 1.90 | <0.001 | 4.87 ± 1.22 | <0.001 |
| Normal secretion (24.60–54.54) | 71.8 | 7.3 ± 2.05 | 5.57 ± 1.36 | ||
| High secretion (>54.54) | 19.1 | 8.98 ± 2.20 | 6.41 ± 1.50 | ||
| Basal insulin secretion (pmol · min−1 · m−2) | |||||
| Low secretion (<37.98) | 6.6 | 6.78 ± 1.94 | <0.001 | 5.18 ± 1.21 | <0.001 |
| Normal secretion (37.98–92.10) | 65.1 | 7.20 ± 2.04 | 5.51 ± 1.38 | ||
| High secretion (>92.10) | 28.3 | 8.41 ± 2.37 | 6.14 ± 1.49 | ||
| Insulin extraction (adimensional) | |||||
| Low extraction (<0.44) | 16.43 | 8.07 ± 2.46 | <0.001 | 5.82 ± 1.54 | 0.182 |
| Normal extraction (0.44–0.76) | 78.09 | 7.44 ± 2.14 | 5.65 ± 1.41 | ||
| High extraction (>0.76) | 5.48 | 6.98 ± 2.15 | 5.49 ± 1.38 | ||
| Glucose sensitivity (pmol · min−1 · m−2 · mmol/l−1) | |||||
| Low sensitivity (<54.76) | 11.62 | 8.86 ± 2.89 | <0.001 | 6.38 ± 1.74 | <0.001 |
| Normal sensitivity (54.76–247.37) | 80.41 | 7.54 ± 2.01 | 5.64 ± 1.38 | ||
| High sensitivity (>247.37) | 7.97 | 5.30 ± 0.99 | 4.89 ± 0.89 | ||
| Rate sensitivity (pmol · m−2 · mmol/l−1) | |||||
| Low sensitivity (<3.34 × 10−12) | 8.30 | 6.51 ± 1.91 | <0.001 | 5.31 ± 1.28 | <0.001 |
| Normal sensitivity (3.34 × 10−12–2,494.27) | 81.83 | 7.83 ± 2.18 | 5.79 ± 1.42 | ||
| High sensitivity (>2,494.27) | 9.88 | 5.75 ± 1.52 | 4.96 ± 1.39 |
Data are means ± SD or % individuals within each group. P < 0.001 from ANOVA. All Bonferroni comparisons were significant except the comparisons between participants with 1) normal and low basal insulin secretion for both 1-h plasma glucose and 2-h plasma glucose, 2) normal and high hepatic insulin extraction for 1-h plasma glucose, 3) low and high rate sensitivity for 2-h plasma glucose, 4) normal and low as well as normal and high insulin clearance for 2-h plasma glucose, and 5) normal insulin sensitivity and hypersensitivity for 1-h plasma glucose.
Insulin resistance and insulin secretion in participants with NGT categorized by IGT-based threshold of 1-h plasma glucose
Mean 1-h plasma glucose in the 105 individuals with IGT was 10.11 ± 1.67 mmol/l (upper and lower deciles 12.04 and 7.86 mmol/l). Receiver operating characteristic curve analysis for sensitivity and specificity of 1-h plasma glucose to predict IGT provided an area of 0.86 ± 0.02 (P < 0.001) with a glucose level of 8.95 mmol/l (sensitivity 77% and specificity 80%), maximizing sensitivity and specificity. When this 1-h plasma glucose threshold was used to categorize individuals with NGT (Table 2), 222 participants had 1-h plasma glucose >8.95 mmol/l and 878 participants with NGT had 1-h plasma glucose ≤8.95 mmol/l.
Table 2.
Comparison among participants with NGT with 1-h plasma glucose ≤8.95 mmol/l (group 0), participants with NGT with 1-h plasma glucose >8.95 mmol/l (group 1), and participants with IGT (group 2)
| Classification | n | Value | P value |
P value for group comparison |
||
|---|---|---|---|---|---|---|
| 0 vs. 1 | 1 vs. 2 | 0 vs. 2 | ||||
| Age (years) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 43.01 ± 8.27 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 45.39 ± 8.26 | <0.001 | <0.001 | NS | <0.001 |
| IGT | 105 | 46.01 ± 7.96 | ||||
| Waist (cm) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 863 | 84.28 ± 11.88 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 220 | 90.61 ± 12.34 | <0.001 | <0.001 | NS | <0.001 |
| IGT | 105 | 91.38 ± 13.47 | ||||
| BMI (kg/m2) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 24.86 ± 3.77 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 26.25 ± 3.95 | <0.001 | <0.001 | NS | <0.001 |
| IGT | 105 | 27.27 ± 4.39 | ||||
| Insulin sensitivity [log(μmol · min−1 · kgFFM−1 · nmol/l−1)] | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 4.93 ± 0.45 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 4.73 ± 0.51 | <0.001 | <0.001 | <0.001 | <0.001 |
| IGT | 105 | 4.49 ± 0.48 | ||||
| Fasting plasma glucose (mmol/l) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 4.94 ± 0.49 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 5.25 ± 0.47 | <0.001 | <0.001 | NS | <0.001 |
| IGT | 105 | 5.17 ± 0.48 | ||||
| Basal insulin secretion (pmol · min−1 · m−2) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 68.94 ± 27.52 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 82.87 ± 31.66 | <0.001 | <0.001 | 0.001 | <0.001 |
| IGT | 105 | 95.52 ± 33.42 | ||||
| Total insulin secretion (nmol · m−2) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 37.79 ± 12.00 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 47.86 ± 13.90 | <0.001 | <0.001 | 0.002 | <0.001 |
| IGT | 105 | 53.05 ± 17.82 | ||||
| Insulin clearance (l/min/m2) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 0.63 ± 0.25 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 0.62 ± 0.18 | NS | |||
| IGT | 105 | 0.62 ± 0.23 | ||||
| Insulin extraction | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 0.59 ± 0.24 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 0.52 ± 0.48 | 0.003 | NS | NS | 0.003 |
| IGT | 105 | 0.37 ± 1.90 | ||||
| Glucose sensitivity (pmol · min−1 · m−2 · mmol/l−1) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 153.12 ± 97.34 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 86.88 ± 30.28 | <0.001 | <0.001 | NS | <0.001 |
| IGT | 105 | 80.38 ± 44.26 | ||||
| Rate sensitivity (pmol · m−2 · mmol/l−1) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 1,150.22 ± 1,431.50 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 781.78 ± 561.72 | <0.001 | <0.001 | NS | NS |
| IGT | 105 | 987.14 ± 859.81 | ||||
| Potentiation factor | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 878 | 2.17 ± 1.55 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 222 | 1.89 ± 1.04 | <0.001 | 0.026 | NS | <0.001 |
| IGT | 105 | 1.50 ± 0.57 | ||||
| Total cholesterol (mmol/l) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 874 | 4.75 ± 0.85 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 220 | 5.04 ± 0.94 | <0.001 | <0.001 | NS | NS |
| IGT | 104 | 4.92 ± 0.84 | ||||
| HDL cholesterol (mmol/l) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 875 | 1.47 ± 0.38 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 220 | 1.35 ± 0.36 | <0.001 | <0.001 | NS | 0.001 |
| IGT | 104 | 1.33 ± 0.35 | ||||
| LDL cholesterol (mmol/l) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 870 | 2.83 ± 0.78 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 213 | 3.10 ± 0.83 | <0.001 | <0.001 | NS | NS |
| IGT | 104 | 2.99 ± 0.81 | ||||
| Triglycerides (mmol/l) | ||||||
| NGT with 1-h plasma glucose ≤8.95 mmol/l | 875 | 0.99 ± 0.52 | ||||
| NGT with 1-h plasma glucose >8.95 mmol/l | 220 | 1.28 ± 0.88 | <0.001 | <0.001 | NS | <0.001 |
| IGT | 104 | 1.32 ± 0.64 | ||||
Data are means ± SD unless otherwise indicated. n represents the absolute frequency and varies according to missing data for each variable. P values are reported for statistical significance from ANOVA and Bonferroni post hoc comparisons.
Comparison of participants with NGT, NGT with 1-h plasma glucose >8.95 mmol/l, and IGT
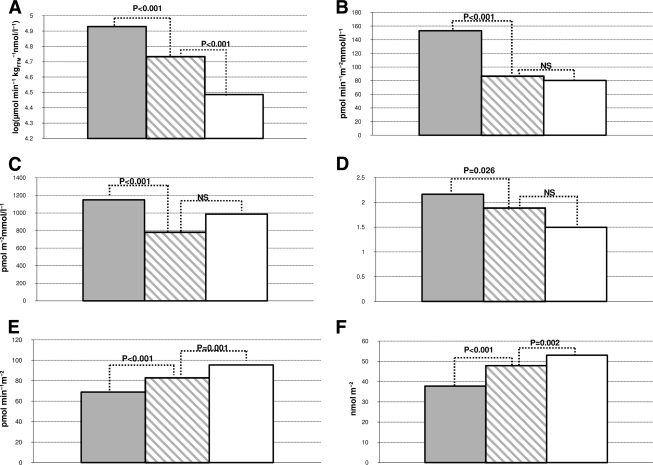
ANOVA was significant for all variables considered except insulin clearance during the EHC. Post hoc comparisons of participants with NGT with 1-h plasma glucose >8.95 mmol/l demonstrated significantly larger waist circumference (90.6 ± 12.3 vs. 84.3 ± 11.9 cm), higher BMI (26.2 ± 3.9 vs. 24.9 ± 3.8 kg/m2), lower ln(M/I) (4.7 ± 0.5 vs. 4.9 ± 0.5, corresponding to ∼110 vs. 134 μmol · min−1 · kgFFM−1 · nmol/l−1), higher fasting glucose (5.2 ± 0.5 vs. 4.9 ± 0.5 mmol/l), and higher basal (82.9 ± 31.7 vs. 68.9 ± 27.5 pmol · min−1 · m−2) and total insulin secretion (47.9 ± 13.9 vs. 37.8 ± 12 nmol · m−2) (P < 0.001 for all comparisons) than their counterparts with NGT with 1-h plasma glucose ≤8.95 mmol/l (Table 2). Moreover, they exhibited lower glucose sensitivity (86.9 ± 30.3 vs. 153.1 ± 97.3 pmol · min−1 · m−2 · mmol/l−1; P < 0.001), lower rate sensitivity (781.8 ± 561.7 vs. 1150.2 ± 1431.5 pmol · m−2 · mmol/l−1; P < 0.001), lower potentiation factor (1.9 ± 1.0 vs. 2.2 ± 1.6; P = 0.026), higher total cholesterol (5.04 ± 0.9 vs. 4.8 ± 0.9 mmol/l; P < 0.001), lower HDL cholesterol (1.35 ± 0.4 vs. 1.47 ± 0.4 mmol/l; P < 0.001), higher LDL cholesterol (3.1 ± 0.8 vs. 2.8 ± 0.8 mmol/l; P < 0.001), and higher triglycerides (1.28 ± 0.9 vs. 1 ± 0.5 mmol/l; P < 0.001) (Fig. 1).
Figure 1.
Comparison of insulin sensitivity (A), β-cell glucose sensitivity (B), rate sensitivity (C), potentiation factor (D), basal insulin secretion (E), and total insulin secretion (F) among the following groups: NGT with 1-h plasma glucose ≤8.95 mmol/l (gray bars); NGT with 1-h plasma glucose >8.95 mmol/l (hatched bars), and IGT individuals (white bars). Significance is reported using the Bonferroni post hoc test.
Post hoc comparison of subjects with NGT and 1-h plasma glucose >8.95 mmol/l versus those with IGT revealed no difference in age, waist circumference, fasting glucose, hepatic insulin extraction, β-cell glucose sensitivity, rate sensitivity, potentiation factor, total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides. For subjects with IGT, those with NGT with 1-h plasma glucose >8.95 mmol/l had higher FFM (56.7 ± 11.03 vs. 51.8 ± 11.0 kg; P < 0.001), insulin sensitivity [4.7 ± 0.5 vs. 4.5 ± 0.5 log(μmol · min−1 · kgFFM−1 · nmol/l−1); P < 0.001) in the presence of lower basal (82.9 ± 31.6 vs. 95.5 ± 33.4 pmol · min−1 · m−2; P = 0.001) and total insulin secretion (47.9 ± 13.9 vs. 53 ± 17.8 nmol · m−2; P = 0.002) (Fig. 1).
Sixty-eight (30.6%) of 222 participants with NGT with 1-h plasma glucose >8.95 mmol/l were insulin resistant compared with 131 (14.9%) of 878 participants with 1-h plasma glucose ≤8.95 mmol/l (P < 0.001). The OR for insulin resistance was 2.52 (95% CI% 1.8–3.5). Moreover, in terms of both basal and total insulin secretion, a higher percentage of participants with 1-h plasma glucose >8.95 mmol/l were hypersecretors as defined above: (basal) 95 (42.8%) of 222 vs. 191 (21.75%) of 878 (P < 0.001; OR 2.7 [1.8–3.7]); and (total) 82 (36.9%) of 222 vs. 102 (11.62%) of 878 (P < 0.001; 4.46 [3.2–6.3]).
CONCLUSIONS
Our analysis of cross-sectional data from the RISC study suggests that increased 1-h plasma glucose (>8.95 mmol/l) on an OGTT identifies a subgroup of individuals with increased insulin resistance and β-cell dysfunction (reduced β-cell rate sensitivity, glucose sensitivity, and potentiation factor) (Fig. 1) who would otherwise be classified as having NGT by current definitions (4).
Not unexpectedly, these differences in insulin sensitivity may relate to increased visceral and total body fat. Thus, participants with NGT with 1-h plasma glucose >8.95 mmol/l do not differ from individuals with IGT in waist circumference, insulin clearance, hepatic insulin extraction, β-cell rate sensitivity, glucose sensitivity, and potentiation factor. However, participants with NGT with 1-h plasma glucose >8.95 mmol/l are more insulin sensitive than those with IGT albeit they present already impaired dynamic β-cell function.
Individuals with NGT but IGT-level excursions of plasma glucose at 1-h during the glucose challenge may therefore represent an intermediate state of glucose intolerance. It is known that insulin sensitivity varies over a six-to sevenfold range in individuals with NGT (10) as β-cell compensation can preserve NGT. Four factors influence this dynamic relationship: 1) β-cell glucose sensitivity, i.e., the ability of β-cells to respond to changes in plasma glucose concentration; 2) rate sensitivity, i.e., the ability to respond to changes in the rate of variations of glucose concentration; 3) potentiation, which depends on glucose potentiation per se, incretin potentiation and neural modulation; and 4) the degree of insulin resistance. In the spectrum from glucose tolerance to intolerance, β-cell glucose sensitivity progressively decreases, whereas insulin secretion classically exhibits an inverted U-shape. Rate sensitivity is impaired in individuals with NGT with high 1-h plasma glucose to the same extent as in individuals with IGT.
A higher level of 1-h plasma glucose might represent a surrogate marker of impaired β-cell function (β-cell glucose sensitivity and rate sensitivity) in individuals otherwise regarded as having NGT who are insulin resistant. In the RISC cohort, according to this analysis, NGT participants with 1-h plasma glucose >8.95 mmol/l had an OR of 2.5 for insulin resistance.
The concept that hyperglycemia (even within the normal range of glucose tolerance) may arise from an intrinsic β-cell defect in the presence of increased insulin resistance has been reinforced in recent years by a number of studies in both obese youngsters (11,12) and adults (3,13). However, these studies have focused on the association between dynamic parameters of β-cell function and 2-h plasma glucose. Our study is unique in that we have focused on 1-h plasma glucose.
In clinical practice, neither IFG nor IGT is considered a clinical entity but rather both are risk categories for development of type 2 diabetes and cardiovascular disease. Glycemic thresholds are based on a consensus interpretation of evidence, with cutoff values representing points at which risk is deemed excessive (14,15). Nevertheless, studies have shown that targeted treatment of IGT can reduce progression to type 2 diabetes, and the risk of cardiovascular disease or future diabetes seems to be continuous across the glucose range (16).
There are examples in the literature of 1-h plasma glucose being predictive of the risk of myocardial infarction (17) and coronary heart disease (18,19) in type 2 diabetes. One-hour plasma glucose was a strong predictor of development of type 2 diabetes in a large cohort of 1,611 participants without diabetes in the San Antonio Heart Study (20,21). Furthermore, 1-h plasma glucose values improved the accuracy of a model to predict development of type 2 diabetes incorporating components of the metabolic syndrome (21), with a 1-h plasma glucose concentration of 155 mg/dl (8.5 mmol/l) identified to stratify three classes of risk. In this study 16.7% of participants with NGT with 1-h plasma glucose concentration >155 mg/dl developed type 2 diabetes over a 7- to 8-year period, whereas those with NGT and 1-h plasma glucose >155 mg/l, plus the metabolic syndrome, were at much higher risk for the development of diabetes, exceeding that of those with IGT (21). One-hour plasma glucose was also shown to correlate more strongly with surrogate measures of hepatic and muscle insulin resistance and β-cell dysfunction than 2-h plasma glucose (20). Analysis of cohort data from the San Antonio Heart and the Botnia studies (22) demonstrated that 1-h plasma glucose is a better predictor of future type 2 diabetes than 2-h plasma glucose with a 13.1-fold increased OR for developing the disease in subjects with higher 1-h plasma glucose (>155 mg/dl).
These observations are consistent with the results of the present analysis, supporting the concept that the 1-h plasma glucose concentration can identify participants with NGT according to current definitions who have an adverse metabolic profile at an earlier stage. We speculate that they too may benefit from lifestyle (and possibly pharmacological) interventions analogous to the state of IGT (16).
A limitation of the present study is lack of longitudinal data. However, such data will be available through ongoing follow-up of the RISC cohort. In contrast, a major strength of our study is the consistent methodology used to evaluate insulin sensitivity across centers in a large European population.
In summary, our data suggest that 1-h plasma glucose may represent an index of metabolic impairment useful in clinical practice to identify individuals with more severe insulin resistance and impaired β-cell glucose sensitivity and rate sensitivity. These individuals might benefit from an intervention program (with diet, exercise, and/or pharmacotherapy). Future longitudinal studies are necessary to evaluate the association between 1-h plasma glucose and the risk of development of type 2 diabetes and/or cardiovascular disease.
Supplementary Material
Acknowledgments
The RISC study is partly supported by the European Union (grant QLG1-CT-2001-01252). Additional support for the RISC Study has been provided by AstraZeneca (Sweden). The European Group for the Study of Insulin Resistance (EGIR) is supported by Merck Santé (Lyon, France).
No other potential conflicts of interest relevant to this article were reported.
M.M. conceived study and wrote the manuscript. S.P. performed data analysis. D.P.M. reviewed/edited the manuscript. A.G., O.M., and T.K. research data and reviewed/edited the manuscript. J.RP. contributed to discussion, research data, and reviewed/edited the manuscript. G.M. conceived study, researched data, contributed to discussion, and reviewed/edited the manuscript.
Parts of this study were presented in abstract form at the 70th annual meeting of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Unwin N, Shaw J, Zimmet P, Alberti KG: Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708–723 [DOI] [PubMed] [Google Scholar]
- 2. Abdul-Ghani MA, Tripathy D, DeFronzo RA: Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 3. Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B: American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 4. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 5. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: β-Cell function in participants spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 6. Hills SA, Balkau B, Coppack SW, Dekker JM, Mari A, Natali A, Walker M, Ferrannini E: EGIR-RISC Study Group. The EGIR-RISC STUDY (the European Group for the Study of Insulin Resistance: Relationship between Insulin Sensitivity and Risk of Cardiovascular Disease): I. Methodology and objectives. Diabetologia 2004;47:566–570 [DOI] [PubMed] [Google Scholar]
- 7. Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E: Meal and oral glucose tests for assessment of β-cell function: modeling analysis in normal participants. Am J Physiol Endocrinol Metab 2002;283:E1159–E1166 [DOI] [PubMed] [Google Scholar]
- 8. Mari A, Tura A, Gastaldelli A, Ferrannini E: Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 2002;51(Suppl. 1):S221–S226 [DOI] [PubMed] [Google Scholar]
- 9. Mari A, Ferrannini E: β-Cell function assessment from modelling of oral tests: an effective approach. Diabetes Obes Metab 2008;10:77–87 [DOI] [PubMed] [Google Scholar]
- 10. Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G: Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest 1997;100:1166–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coutinho M, Gerstein HC, Wang Y, Yusuf S: The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999;22:233–240 [DOI] [PubMed] [Google Scholar]
- 12. Yeckel CW, Taksali SE, Dziura J, Weiss R, Burgert TS, Sherwin RS, Tamborlane WV, Caprio S: The normal glucose tolerance continuum in obese youth: evidence for impairment in β-cell function independent of insulin resistance. J Clin Endocrinol Metab 2005;90:747–754 [DOI] [PubMed] [Google Scholar]
- 13. Cali AM, Man CD, Cobelli C, Dziura J, Seyal A, Shaw M, Allen K, Chen S, Caprio S: Primary defects in β-cell function further exacerbated by worsening of insulin resistance mark the development of impaired glucose tolerance in obese adolescents. Diabetes Care 2009;32:456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA: San Antonio metabolism study. β-Cell dysfunction and glucose intolerance: results from the San Antonio Metabolism (SAM) study. Diabetologia 2004;47:31–39 [DOI] [PubMed] [Google Scholar]
- 15. DECODE Study Group, European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 2003;26:688–696 [DOI] [PubMed] [Google Scholar]
- 16. Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF: Diabetes Prevention Program Research Group. Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care 2009;32:1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanefeld M, Fischer S, Julius U, Schulze J, Schwanebeck U, Schmechel H, Ziegelasch HJ, Lindner J: Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia 1996;39:1577–1583 [DOI] [PubMed] [Google Scholar]
- 18. Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, Anfossi G, Costa G, Trovati M: Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006;91:813–819 [DOI] [PubMed] [Google Scholar]
- 19. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M: What is the best predictor of future type 2 diabetes? Diabetes Care 2007;30:1544–1548 [DOI] [PubMed] [Google Scholar]
- 21. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA: One-hour plasma glucose concentration and the metabolic syndrome identify participants at high risk for future type 2 diabetes. Diabetes Care 2008;31:1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abdul-Ghani MA, Stern MP, Lyssenko V, Tuomi T, Groop L, Defronzo RA: Minimal contribution of fasting hyperglycemia to the incidence of type 2 diabetes in subjects with normal 2-h plasma glucose. Diabetes Care 2010;33:557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.