Abstract
OBJECTIVE
Disposition index (DI) and glucose effectiveness (SG) are risk factors for diabetes. However, the effect of DI and SG on future diabetes has not been examined in large epidemiological studies using direct measures.
RESEARCH DESIGN AND METHODS
Insulin sensitivity index (SI), acute insulin response (AIR), and SG were measured in 826 participants (aged 40–69 years) in the Insulin Resistance Atherosclerosis Study (IRAS) by the frequently sampled intravenous glucose tolerance test. DI was expressed as SI × AIR. At the 5-year follow-up examination, 128 individuals (15.5%) had developed diabetes.
RESULTS
The area under the receiver operating characteristic curve of a model with SI and AIR was similar to that of DI (0.767 vs. 0.774, P = 0.543). In a multivariate logistic regression model that included both DI and SG, conversion to diabetes was predicted by both SG (odds ratio × 1 SD, 0.61 [0.47–0.80]) and DI (0.68 [0.54–0.85]) after adjusting for demographic variables, fasting and 2-h glucose concentrations, family history of diabetes, and measures of obesity. Age, sex, race/ethnicity, glucose tolerance status, obesity, and family history of diabetes did not have a significant modifying impact on the relation of SG and DI to incident diabetes.
CONCLUSIONS
The predictive power of DI is comparable to that of its components, SI and AIR. SG and DI independently predict conversion to diabetes similarly across race/ethnic groups, varying states of glucose tolerance, family history of diabetes, and obesity.
Both insulin sensitivity and first-phase insulin secretion are independent determinants of conversion to diabetes in different ethnic groups and varying states of glucose tolerance, family history of diabetes, and obesity (1). First-phase insulin secretion compensates for the worsening of insulin sensitivity (2). In studies using direct methods, such as the frequently sampled intravenous glucose tolerance test (FSIGTT) with minimal model analysis, this relationship is hyperbolic (2) and similar across glucose tolerance categories (3). Known as the disposition index (DI), the product of measures of insulin sensitivity and first-phase insulin secretion, it has been shown to predict conversion to diabetes (4–6). The evidence, however, comes from studies that have enrolled relatively few participants or targeted persons from a single ethnic group. Since direct methods have demanding technical requirements, the product of measures of insulin sensitivity and insulin secretion derived from the oral glucose tolerance test has attracted interest. This product has been shown to have a modest correlation with minimal model–derived DI, to decrease as glucose tolerance status deteriorates, and to predict the development of diabetes independent of other risk factors including fasting and 2-h glucose concentrations (7,8). It includes the incretin effect and therefore may not always follow the hyperbolic law (7–9). The hyperbolic paradigm of the minimal model–derived DI has also been criticized (10). Furthermore, its predictive power has not been tested for large epidemiological studies.
In addition to the insulin-dependent component of glucose tolerance (or DI), the insulin-independent component (glucose effectiveness [SG]) has already been explored in mice (11) and humans (12). SG is the capacity of glucose to enhance its own cellular uptake and to suppress endogenous glucose production. Although reduced in individuals with impaired glucose tolerance (IGT) and diabetes (4,13), SG contributes to glucose tolerance even in conditions of significant insulin resistance, including diabetes (13). Reduced SG has also been described in healthy individuals following the infusion of cortisol or glucagon, individuals in states of very low caloric intake, women with polycystic ovary syndrome, and the elderly (13–15). Contrary to the insulin sensitivity index (SI), SG may not be influenced by exercise (16) or weight loss interventions (17). In relatively small studies, SG has been shown to predict future diabetes (4,5,18), but its contribution to the development of diabetes remains largely unknown.
Since the hyperbolic paradigm has not been tested in large epidemiological studies, our first objective was to analyze the risk of future diabetes associated with minimal model-derived DI relative to its components, SI, and acute insulin response (AIR). The second objective was to assess the relative contribution of the insulin-independent component of glucose tolerance, SG, to the development of diabetes. To meet these aims, we used data from the Insulin Resistance Atherosclerosis Study (IRAS), a multicenter observational epidemiological study of different ethnic groups and varying states of glucose tolerance (19).
RESEARCH DESIGN AND METHODS
The design and methods of the IRAS have been described elsewhere (19). Briefly, the study was conducted at four clinical centers. Centers in Oakland and Los Angeles, California, recruited non-Hispanic whites and African Americans from Kaiser Permanente, a nonprofit HMO. Two other centers in San Antonio, Texas, and San Luis Valley, Colorado, recruited non-Hispanic whites and Hispanics from two ongoing population–based studies, the San Antonio Heart Study and the San Luis Valley Diabetes Study. A total of 1,625 individuals were enrolled (56% women) between October 1992 and April 1994. A follow-up examination was performed 5 years after the baseline examination (range 4.5–6.6 years). The response rate was 81%. Protocols were approved by local institutional review committees. All the participants gave written informed consent.
The present report includes information on 826 (79.2%) participants (332 non-Hispanic whites, 206 African Americans, and 288 Hispanics) after excluding 153 individuals who failed to return to the follow-up visit and 64 individuals with missing information on relevant variables. Baseline characteristics were similar in the participants included in this analysis and those who were excluded (e.g., age, ethnicity, sex, glucose tolerance status, BMI, waist circumference, and SI [all comparisons, P ≥ 0.2]) except for AIR (higher in eligible participants, P = 0.005). Protocols were identical at the baseline and follow-up examinations. Each examination required two visits, one week apart, of ∼4 h each. During the first visit, a 75-g oral glucose tolerance test was administered to assess glucose tolerance status.
During the second visit, insulin sensitivity and first-phase insulin secretion were measured by FSIGTT with two modifications to the original protocol. First, an injection of regular insulin was used to ensure adequate plasma insulin levels for the accurate computation of insulin sensitivity across a broad range of glucose tolerance (20). Second, the reduced sampling protocol (12 samples) was used because of the large number of subjects. SI and SG were calculated using mathematical modeling methods (MINMOD, version 3.0 [1994], Los Angeles, California, courtesy of Richard Bergman, PhD) (21). AIR was calculated as the mean of 2- and 4-min insulin concentrations after glucose administration and DI as the product of SI and AIR.
Race/ethnicity was assessed by self-report. Family history of diabetes was defined as diabetes in parents or siblings. Anthropometric variables and blood pressure were obtained by trained personnel. Plasma glucose and insulin concentrations were determined by the glucose oxidase and dextran-charcoal radioimmunoassay methods, respectively. This last assay displays a high degree of cross-reactivity with proinsulin. The 1999 World Health Organization criteria were used to define diabetes (fasting glucose concentration ≥7.0 mmol/l, 2-h plasma glucose concentration ≥11.1 mmol/l, or treatment with hypoglycemic medications) and IGT (2-h plasma glucose level between ≥7.8 and <11.1 mmol/l).
Statistical analyses
The analysis was carried out using the SAS statistical software (version 9.1, SAS Institute, Cary, NC). One-way ANCOVA or logistic regression analysis was used to compare the differences for continuous or dichotomous variables between groups, respectively. Linear regression analyses were used to examine the relation of SG to SI, AIR, and DI. In separate models, we introduced the interaction term SG × family history of diabetes. Results were adjusted for demographic variables. Risk of future diabetes associated with SI, AIR, DI, and SG was assessed by logistic regression analysis. Odds ratios (ORs) were expressed for binary traits or per SD increase for continuous traits. Demographic variables, family history of diabetes, measures of obesity, and plasma glucose concentrations were also included as covariates. We assessed the impact of these covariates on the relation of DI and SG to conversion to diabetes by including appropriate interaction terms in separate logistic regression models. In both linear and logistic regression models, log-transformed values of SI, AIR, and DI were used to improve discrimination and calibration of the models and to minimize the influence of extreme observations. Given that some individuals had SI and DI equal to zero, we used the natural logarithms of SI + 1 and DI + 1 as the transformation for SI and DI, respectively. Accuracy for the prediction of diabetes was determined by the area under the receiver operating characteristic curve (AUC).
RESULTS
During the 5-year follow-up period, 128 (15.5%) of 826 IRAS participants developed type 2 diabetes: 44 (7.9%) of 557 participants with normal glucose tolerance at baseline, and 84 (31.2%) of 269 participants with IGT at baseline. Those participants converting to type 2 diabetes did not differ from nonconverters in terms of sex or ethnicity (Table 1). However, older age and family history of diabetes were more common among converters. Converters also had higher BMI and waist circumference, higher glucose and insulin levels, and lower SI, AIR, DI, and SG.
Table 1.
Baseline characteristics by diabetes status at follow-up
| Nonconvertors | Convertors | P | |
|---|---|---|---|
| n | 698 | 128 | |
| Age (years)* | 54.4 ± 0.3 | 56.5 ± 0.7 | 0.009 |
| Female (%)* | 55.4 (51.7–59.1) | 59.4 (50.7–67.5) | 0.410 |
| Ethnicity (%)* | 0.647 | ||
| African Americans | 25.4 (22.3–28.7) | 22.7 (16.2–30.7) | |
| Mexican Americans | 34.2 (30.8–37.8) | 38.3 (30.3–47.0) | |
| Non-Hispanic whites | 40.4 (36.8–44.1) | 39.1 (31.0–47.8) | |
| Family history of diabetes (%) | 37.6 (34.0–41.4) | 50.6 (41.7–59.6) | <0.001 |
| BMI (kg/m2) | 27.8 ± 0.2 | 30.8 ± 0.5 | <0.001 |
| Waist circumference (cm) | 89.2 ± 0.4 | 95.5 ± 1.0 | <0.001 |
| Fasting glucose (mmol/l) | 5.35 ± 0.02 | 5.83 ± 0.05 | <0.001 |
| 2-h glucose (mmol/l) | 6.61 ± 0.06 | 8.37 ± 0.15 | <0.001 |
| Impaired glucose tolerance (%) | 25.6 (22.6–29.2) | 64.3 (55.3–72.4) | <0.001 |
| Fasting insulin (pmol/l)† | 70.8 ± 1.4 | 106.7 ± 5.5 | <0.001 |
| Insulin sensitivity index (SI) (× 10−4 min−1 · μU−1 · ml−1)† | 1.92 ± 0.06 | 1.03 ± 0.13 | <0.001 |
| Acute insulin response (AIR) (μU/ml)† | 52.5 ± 1.6 | 38.9 ± 2.8 | <0.001 |
| Disposition index (SI × AIR)† | 96.5 ± 2.9 | 41.2 ± 3.4 | <0.001 |
| Glucose effectiveness (× 100 min−1) | 2.03 ± 0.03 | 1.58 ± 0.07 | <0.001 |
Data are n (%) with 95% CI or means ± SD. Results are adjusted for age, sex, race/ethnicity, and research center.
*Unadjusted results;
†log-transformed variables. These variables were then back-transformed to their units for presentation in the table.
In linear regression models, SG was directly related to SI (parameter estimate × 1 SD, 0.11 ± 0.02, P < 0.001), AIR (parameter estimate × 1 SD, 0.22 ± 0.03, P < 0.001), and DI (parameter estimate × 1 SD, 0.29 ± 0.05, P < 0.001) after accounting for the effects of age, sex, race/ethnicity, and research center. There was no interaction effect of family history of diabetes on the relation of SG to SI (P = 0.534), AIR (P = 0.139), and DI (P = 0.990).
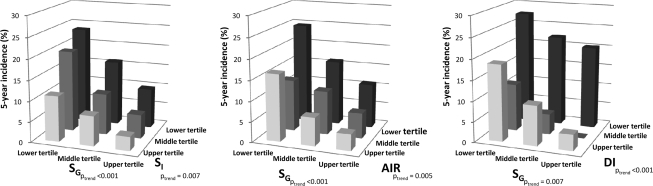
We used the AUC to quantify the ability of DI to predict conversion to diabetes relative to that of a model with both SI and AIR (found in the supplemental figure, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0165/DC1). The AUC of the model with SI and AIR was similar to that of DI (0.767 vs. 0.774, P = 0.543). The 5-year incidence of diabetes by baseline tertiles of SG and each one of the other measures (SI, AIR, or DI) are presented in Fig. 1.
Figure 1.
Five-year incidence of diabetes by tertiles of SI, AIR, DI, and SG. Results were adjusted for age, sex, race/ethnicity, research center, IGT, family history of diabetes, and BMI. Cut points for tertiles of SI (× 10−4 min−1 · μU−1 · ml−1) were ≤1.16 (lower), 1.17–2.38 (middle), and ≥2.39 (upper). Corresponding cut points for tertiles of AIR (μU/ml) were ≤37.5, 38.0–75.0, and ≥75.5; DI (× 10−4 min−1 · μU−1· ml−1 per μU · ml−1) ≤55.8, 55.9–120.0, and ≥120.1; SG (× 10−2 ml−1), ≤1.58, 1.59–2.22, and ≥2.23.
SG predicted future development of diabetes after adjusting for age, sex, race/ethnicity, and research center (OR × 1 SD, 0.50 [0.40–0.64]), as did DI (OR × 1 SD, 0.47 [0.40–0.56]). In a multivariate logistic regression model that included SG and DI as independent variables, the odds remained statistically significant for both SG (OR × 1 SD, 0.61 [0.47–0.80]) and DI (OR × 1 SD, 0.68 [0.54–0.85]) (Table 2). Age, sex, race/ethnicity, research center, family history of diabetes, fasting glucose, 2-h glucose, BMI, and waist circumference were also included as covariates. In a different model that included SI, AIR, and SG, these three measures were all independent predictors of diabetes.
Table 2.
Predictors of conversion to type 2 diabetes by multiple logistic regression analysis
| OR (95% CI) | P | |
|---|---|---|
| Model 1* | ||
| Age (× 1 SD) | 1.09 (0.87–1.38) | 0.449 |
| Female vs. male | 0.93 (0.52–1.69) | 0.824 |
| Family history of diabetes (yes vs. no) | 1.25 (0.79–1.96) | 0.340 |
| BMI (× 1 SD) | 1.33 (0.87–2.05) | 0.187 |
| Waist circumference (× 1 SD) | 0.74 (0.47–1.18) | 0.206 |
| Fasting glucose concentration (× 1 SD) | 1.60 (1.24–2.06) | <0.001 |
| 2-h glucose concentration (× 1 SD) | 1.95 (1.48–2.57) | <0.001 |
| Disposition index (× 1 SD)† | 0.68 (0.54–0.85) | 0.001 |
| Glucose effectiveness (× 1 SD) | 0.61 (0.47–0.80) | <0.001 |
| Model 2‡ | ||
| Age (× 1 SD) | 1.10 (0.87–1.39) | 0.422 |
| Female vs. male | 0.91 (0.50–1.65) | 0.754 |
| Family history of diabetes (yes vs. no) | 1.25 (0.80–1.96) | 0.328 |
| BMI (× 1 SD) | 1.39 (0.90–2.14) | 0.137 |
| Waist circumference (× 1 SD) | 0.72 (0.45–1.17) | 0.190 |
| Fasting glucose concentration (× 1 SD) | 1.54 (1.18–2.00) | 0.001 |
| 2-h glucose concentration (× 1 SD) | 1.91 (1.44–2.53) | <0.001 |
| Insulin sensitivity index (× 1 SD)† | 0.61 (0.44–0.86) | 0.004 |
| Acute insulin response (× 1 SD)† | 0.73 (0.55–0.95) | 0.022 |
| Glucose effectiveness (× 1 SD) | 0.67 (0.50–0.88) | 0.005 |
ORs expressed for binary traits or per 1 SD unit change for continuous traits.
*Results in Model 1 adjusted also for race/ethnicity (P = 0.494) and research center (P = 0.006);
†log-transformed variables;
‡results in Model 2 also adjusted for race/ethnicity (P = 0.446) and research center (P = 0.006).
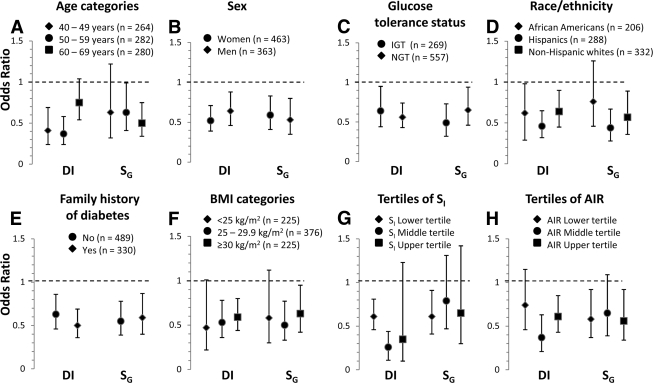
In separate logistic regression models, we examined the impact of age, sex, race/ethnicity, research center, BMI, glucose tolerance status, family history of diabetes on the relationship between SG (or DI), and incident diabetes. None of these variables had a significant impact on the relation of SG and DI to conversion to diabetes (P ≥ 0.16 for all potential interactions) except for age on the relationship between DI and conversion to diabetes (P = 0.038). The strength of the relation of DI and SG to conversion to diabetes differed little between categories of age, sex, race/ethnicity, glucose tolerance status, BMI, and family history of diabetes (Fig. 2A–F). Additionally, the interaction term DI × SG was not statistically significant (P ≥ 0.71). DI and SG were independent risk factors across different degrees of insulin sensitivity and insulin secretion (Fig. 2G and H).
Figure 2.
Risk of developing diabetes associated with DI and SG by ethnicity, sex, glucose tolerance status, BMI and age categories, family history of diabetes, and tertiles of SI and AIR. Estimates expressed for a 1 SD unit change. Age, sex, race/ethnicity, research center, BMI, IGT, family history of diabetes, DI, and SG were all included as independent variables in all eight models. Log-transformed values of DI were used to improve discrimination and calibration of the models and to minimize the influence of extreme observations.
CONCLUSIONS
In cross-sectional analyses, SG is directly related to insulin sensitivity and first-phase insulin secretion. Further in prospective analyses, SG and DI were found to be independent predictors of conversion to diabetes similarly across race/ethnic groups, varying states of glucose tolerance, family history of diabetes, and obesity. The predictive power of DI is comparable to that of the combination of SI and AIR.
Expanding a previous analysis by Martin et al. (4), Goldfine et al. (5) examined the risk of conversion to diabetes associated with directly measured DI in 155 offspring of parents who both had type 2 diabetes and 181 subjects with normal glucose tolerance and without family history of diabetes. DI predicted the development of type 2 diabetes only in the former group. Goldfine et al. concluded that DI and particularly insulin resistance was not sufficient for the development of diabetes in individuals without family history of the disease. These individuals, however, had a very low incidence of diabetes (6 cases during a mean follow-up period of 25 years). In another study of Pima Indians, incident diabetes was predicted by DI independent of the effect of demographic variables and percentage of body fat (7). Our results validate previous studies and extend the findings to men and women, all three race/ethnic groups, and varying states of glucose tolerance, family history of diabetes, and adiposity.
Ferrannini and Mari (10) have questioned the hyperbolic paradigm of the relationship between insulin sensitivity and first-phase insulin secretion. In a recent cross-sectional study (22), however, these authors confirmed the paradigm while arguing against the compensation for insulin resistance as the sole mechanism responsible for hyperglycemia. They also indicated that an intrinsic β-cell function defect is the most important mechanism for hyperglycemia. In agreement with the hyperbolic paradigm, our results suggest that the predictive power of DI is comparable with that of its components. DI, however, is not the only independent risk factor for the development of diabetes. Since fasting and 2-h plasma glucose values remain strong predictors of diabetes, we cannot disregard other determinants of glucose homeostasis, such as additional aspects of secretory response to glucose stimulation (e.g., β-cell glucose sensitivity and potentiation), from the disease process.
Lopez et al. (23) recently reported that SG is related to SI in individuals with family history of diabetes but not in those without such a history. Our results, however, indicate that family history of diabetes has little influence on the relationship between SG and SI. This is not surprising. SG is independent of the dynamic insulin response but is influenced by the basal insulin level (13). In other words, SG is influenced by the effect of insulin on glucose uptake by insulin-dependent tissues (basal insulin–dependent component) (12). Similarly, in longitudinal studies by Martin et al. (4) and Goldfine et al. (5), SG predicted conversion to diabetes independent of DI but only among offspring of type 2 diabetic parents. Individuals without family history of diabetes had a very low risk of future diabetes and neither SG nor DI predicted diabetes. Osei et al. (18) examined the risk of progression to IGT or diabetes associated with SG in 81 first-degree relatives of African Americans with type 2 diabetes. SG as well as directly measured insulin resistance and secretion were independent predictors of worsening glucose tolerance status. Nevertheless, none of these studies adjusted their results for glucose tolerance status and adiposity. In the IRAS, SG is an independent risk factor for future diabetes in individuals with family history of diabetes and similar results are demonstrated in all other categories regardless of age, sex, race/ethnicity, glucose tolerance, and adiposity.
SG is directly related to first-phase insulin secretion. Experiments in canines have suggested that this relationship is an artifact of the minimal model method (24). In mice, however, the effect of first-phase insulin secretion (in physiological conditions) on SG is minimal (20). Furthermore, FSIGTT with the minimal model analysis may overestimate SG. In human subjects, the difference between minimal model- and clamp-derived SG appears to be related to the assumption of mono-compartmental distribution of glucose by the minimal model analysis; however, both measures are highly correlated (25). Therefore, minimal model-derived SG is considered an adequate measure of nonsteady-state glucose kinetics and a dependable index.
In conclusion, the predictive discrimination of DI for future diabetes is comparable with that of the combination of SI and AIR; therefore, our results support the validity of the hyperbolic paradigm. DI and SG are important, independent determinants of diabetes in different ethnic groups and varying states of glucose tolerance, family history of diabetes, and obesity. SG has a direct relationship with SI and AIR. Prospective studies are needed to examine the natural course of SG relative to that of insulin resistance and β-cell function.
Supplementary Material
Acknowledgments
This study was supported by the National Heart, Lung, and Blood Institute grants HL-47887, HL-47889, HL-47890, HL-47892, HL-47902 and the General Clinical Research Centers Program (National Center for Research Resources, M01 RR431, M01 RR01346).
No potential conflicts of interest relevant to this article were reported.
C.L. wrote the manuscript, contributed to the discussion, and reviewed/edited the manuscript. L.E.W. researched the data, contributed to the discussion, and reviewed/edited the manuscript. M.J.R. reviewed/edited the manuscript. A.J.K. researched the data, contributed to the discussion, and reviewed/edited the manuscript. R.N.B. contributed to the discussion and reviewed/edited the manuscript. A.J.G.H. contributed to the discussion and reviewed/edited the manuscript. S.M.H. researched the data, wrote the manuscript, contributed to the discussion, and reviewed/edited the manuscript.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–27 June 2010.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Lorenzo C, Wagenknecht LE, D'Agostino RB, Jr, Rewers MJ, Karter AJ, Haffner SM: Insulin resistance, β-cell dysfunction, and conversion to type 2 diabetes in a multiethnic population: the Insulin Resistance Atherosclerosis Study. Diabetes Care 2010;33:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP: Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects: evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 3. Festa A, Williams K, D'Agostino R, Jr, Wagenknecht LE, Haffner SM: The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes 2006;55:1114–1120 [DOI] [PubMed] [Google Scholar]
- 4. Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR: Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 1992;340:925–929 [DOI] [PubMed] [Google Scholar]
- 5. Goldfine AB, Bouche C, Parker RA, Kim C, Kerivan A, Soeldner JS, Martin BC, Warram JH, Kahn CR: Insulin resistance is a poor predictor of type 2 diabetes in individuals with no family history of disease. Proc Natl Acad Sci U S A 2003;100:2724–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stumvoll M, Tataranni PA, Stefan N, Vozarova B, Bogardus C: Glucose allostasis. Diabetes 2003;52:903–909 [DOI] [PubMed] [Google Scholar]
- 7. Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE: Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Retnakaran R, Qi Y, Goran MI, Hamilton JK: Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med 2009;26:1198–1203 [DOI] [PubMed] [Google Scholar]
- 9. Hücking K, Watanabe RM, Stefanovski D, Bergman RN: OGTT-derived measures of insulin sensitivity are confounded by factors other than insulin sensitivity itself. Obesity 2008;16:1938–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrannini E, Mari A: Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia 2004;47:943–956 [DOI] [PubMed] [Google Scholar]
- 11. Pacini G, Thomaseth K, Ahrén B: Contribution to glucose tolerance of insulin-independent vs. insulin-dependent mechanisms in mice. Am J Physiol Endocrinol Metab 2001;281:E693–E703 [DOI] [PubMed] [Google Scholar]
- 12. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP: The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes 1994;43:587–592 [DOI] [PubMed] [Google Scholar]
- 13. Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN: Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 1996;19:1018–1030 [DOI] [PubMed] [Google Scholar]
- 14. Tonelli J, Kishore P, Lee DE, Hawkins M: The regulation of glucose effectiveness: how glucose modulates its own production. Curr Opin Clin Nutr Metab Care 2005;8:450–456 [DOI] [PubMed] [Google Scholar]
- 15. Burattini R, Di Nardo F, Boemi M, Fumelli P: Deterioration of insulin sensitivity and glucose effectiveness with age and hypertension. Am J Hypertens 2006;19:98–102 [DOI] [PubMed] [Google Scholar]
- 16. Kahn SE, Larson VG, Beard JC, Cain KC, Fellingham GW, Schwartz RS, Veith RC, Stratton JR, Cerqueira MD, Abrass IB: Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol Endocrinol Metab 1990;258:E937–E943 [DOI] [PubMed] [Google Scholar]
- 17. Escalante-Pulido M, Escalante-Herrera A, Milke-Najar ME, Alpizar-Salazar M: Effects of weight loss on insulin secretion and in vivo insulin sensitivity in obese diabetic and non-diabetic subjects. Diabetes Nutr Metab 2003;16:277–283 [PubMed] [Google Scholar]
- 18. Osei K, Rhinesmith S, Gaillard T, Schuster D: Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans: implications for primary diabetes prevention. Diabetes Care 2004;27:1439–1446 [DOI] [PubMed] [Google Scholar]
- 19. Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, Henkin L, Howard G, Savage PJ, Saad MF: The Insulin Resistance Atherosclerosis Study objectives, design, and recruitment results. Ann Epidemiol 1995;5:464–472 [DOI] [PubMed] [Google Scholar]
- 20. Bergman RN, Finegood DT, Ader M: Assessment of insulin sensitivity in vivo. Endocr Rev 1985;6:45–86 [DOI] [PubMed] [Google Scholar]
- 21. Welch S, Gebhart SS, Bergman RN, Phillips LS: Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 1990;71:1508–1518 [DOI] [PubMed] [Google Scholar]
- 22. Mari A, Tura A, Natali A, Laville M, Laakso M, Gabriel R, Beck-Nielsen H, Ferrannini E: RISC Investigators. Impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia 2010;53:749–756 [DOI] [PubMed] [Google Scholar]
- 23. Lopez X, Bouché C, Tatro E, Goldfine AB: Family history of diabetes impacts on interactions between minimal model estimates of insulin sensitivity and glucose effectiveness. Diabetes Obes Metab 2009;11:123–130 [DOI] [PubMed] [Google Scholar]
- 24. Finegood DT, Tzur D: Reduced glucose effectiveness associated with reduced insulin release: an artifact of the minimal model method. Am J Physiol Endocrinol Metab 1996;271:E485–E495 [DOI] [PubMed] [Google Scholar]
- 25. Ni TC, Ader M, Bergman RN: Reassessment of glucose effectiveness and insulin sensitivity from minimal model analysis: a theoretical evaluation of the single-compartment glucose distribution assumption. Diabetes 1997;46:1813–1821 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.