Abstract
OBJECTIVE
A1C is an optional method for diagnosing diabetes and also for detecting individuals at increased risk of the disease. However, how A1C compares with fasting (FPG) and 2-h plasma glucose for detecting at-risk individuals is not well known.
RESEARCH DESIGN AND METHODS
A 2-h glucose tolerance test, frequently sampled intravenous glucose tolerance test, and A1C were obtained at the follow-up examination in 855 participants in the Insulin Resistance Atherosclerosis Study (IRAS). For this report, 385 individuals were at increased risk of diabetes as defined by A1C between 5.7 and 6.4%, impaired glucose tolerance (IGT), and/or impaired fasting glucose (IFG).
RESULTS
IFG and IGT identified 69.1 and 59.5% of all individuals at increased risk of diabetes, respectively. A1C 5.7–6.4% detected 23.6% of all at-risk individuals, although more African Americans (31.4%) and Hispanics (35.2%) than non-Hispanic whites (9.9%). Relative to A1C, FPG was more strongly related to fasting insulin (r = 0.38 vs. 0.26; P < 0.01), acute insulin response (r = – 0.20 vs. – 0.09; P < 0.01), and waist circumference (r = 0.43 vs. 0.25; P < 0.001) by the Spearman correlation test. Similarly, 2-h plasma glucose was more strongly related to Si (r = – 0.40 vs. – 0.27; P < 0.01) and triglycerides (r = 0.30 vs. 0.08; P < 0.001).
CONCLUSIONS
A1C 5.7–6.4% is less sensitive for detecting at-risk individuals than IFG and IGT, particularly among non-Hispanic whites. Single determinations of FPG and 2-h plasma glucose seem to be more precise correlates of insulin resistance and secretion than A1C and, in general, better for other metabolic disorders.
A1C has been proposed by the American Diabetes Association (ADA) as an optional assay for diagnosing diabetes and also for detecting individuals at increased risk of the disease (1). A1C has been shown to predict future onset of diabetes (2–4) and is better than fasting plasma glucose (FPG) for predicting microvascular complications (1). A1C may be superior to FPG in predicting mortality and cardiovascular risk in nondiabetic individuals (5) but inferior to 2-h glucose concentration (2-h plasma glucose) in most studies (6–8), albeit not all (9). The A1C assay has advantages over the measurement of plasma glucose including convenience (not requiring fasting samples) and superior technical attributes (1). Conversely, the number of individuals diagnosed with diabetes by the 6.5% A1C threshold is significantly smaller than the number of those diagnosed by the 2003 American Diabetes Association (ADA) criteria (10–13). A1C, FPG, and 2-h plasma glucose assess different aspects of glucose metabolism (1), but differences in the relation of these three glycemic measures to insulin resistance, insulin secretion, and other metabolic abnormalities have not been described.
A1C between 5.7 and 6.4% (A1C 5.7–6.4%) is now considered a category of increased risk for diabetes in addition to impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) (1). However, studies that compare A1C 5.7–6.4% with IFG and IGT are lacking. Therefore, our aim was twofold: 1) to analyze A1C, FPG, and 2-h plasma glucose for their ability to identify individuals at increased risk of diabetes; and 2) to examine the relation of these glycemic measures to other metabolic abnormalities, particularly measured insulin resistance and secretion in nondiabetic subjects.
RESEARCH DESIGN AND METHODS
The Insulin Resistance Atherosclerosis Study (IRAS) is an epidemiologic study of the relationships among insulin resistance, cardiovascular disease, and its known risk factors (sociodemographic variables, family history, blood pressure, dyslipidemia, obesity, and chronic inflammation) in three ethnic groups (non-Hispanic whites, African Americans, and Hispanics) and different states of glucose tolerance (normal glucose tolerance, IGT, and type 2 diabetes). The design and methods of this study have been described previously (14). In brief, the study was conducted at four clinical centers: Oakland and Los Angeles, California, San Antonio, Texas, and San Luis Valley, Colorado. A total of 1,625 individuals were enrolled between October 1992 and April 1994 (mean age 54.6 years, range 40–69 years; 56% women). After an average of 5.2 years (range 4.5–6.6 years), follow-up examinations were conducted using the same baseline protocol. The response rate was 81%, and those who attended the follow-up examination were similar to those who did not in terms of ethnicity, sex, baseline glucose tolerance status, and BMI (all comparisons, P > 0.32). The IRAS protocol was approved by local institutional review committees, and all participants provided written informed consent.
Subjects described in this study have been part of many previous reports. Among 1,065 participants who were free of diabetes (2003 ADA criteria), 22 died during the follow-up period and 890 (85.3%) returned to the follow-up examination. We excluded 13 participants because of treatment with glucose-lowering agents and 22 others because of missing information. This report is limited to 855 of 1,030 eligible participants (83.0%) who attended the follow-up examination, because A1C was only measured at this time point (mean age 59.8 years; range 44–75 years).
Clinical measurements and procedures
Baseline and follow-up examinations required two visits. During the first visit, a 75-g OGTT was administered to assess glucose tolerance status. During the second visit a week later, insulin sensitivity and first-phase insulin secretion were directly measured by the frequently sampled intravenous glucose tolerance test with two modifications to the original protocol. First, an injection of regular insulin was used to ensure adequate plasma insulin levels for the accurate computation of insulin sensitivity across a broad range of glucose tolerance (14). Second, the reduced sampling protocol (12 samples) was used because of the large number of subjects. Insulin sensitivity, expressed as the insulin sensitivity index (Si), was calculated using mathematical modeling methods (MINMOD version 3.0, 1994, Los Angeles, CA; courtesy of Richard Bergman, PhD). First-phase insulin secretion, expressed as acute insulin response (AIR), was computed as the mean of 2- and 4-min insulin concentrations after glucose administration.
Anthropometric variables were measured by trained personnel. Plasma glucose and serum lipid, lipoprotein, and insulin concentrations were determined as described previously (14). A1C was measured by an automated microparticle immunoassay using whole blood (Medlantic Research Institute, Washington, DC) (15).
Diabetes was defined as having diabetes according to the 2003 ADA criteria (FPG ≥126 mg/dl and/or 2-h plasma glucose ≥200 mg/dl) or A1C ≥6.5% (1). Individuals taking glucose-lowering medications were excluded. In the absence of diabetes, IFG was defined as FPG 100–125 mg/dl and IGT as 2-h plasma glucose 140–199 mg/dl. In the absence of diabetes, participants with A1C 5.7–6.4% and/or IFG and/or IGT were considered at increased risk (1). Metabolic syndrome and metabolic disorders were defined according to the “harmonizing the metabolic syndrome” criteria (16).
Statistical analyses
Analyses were performed using SAS statistical software (version 9.1; SAS Institute, Cary, NC). Differences in continuous and dichotomous variables between groups were analyzed by ANCOVA and logistic regression analysis, respectively. Spearman correlation coefficients were used to examine the relationship between glycemic measures as well as with other metabolic variables. Linear regression analysis was also used to assess the independent relation of glycemic measures to Si and AIR. The strength of these associations was determined by calculating the R2 statistics. In these analyses, log-transformed values of all continuous variables were used to minimize the influence of extreme observations. We also used the log transformation of (Si + 1) given that some participants had Si = 0 and the logit transformation of Framingham risk score. We used the area under the receiver operating characteristic curve (AUC) to assess the ability to detect subjects with Si (or AIR) in the lower quartile (or ≥2 metabolic abnormalities with hyperglycemia excluded).
RESULTS
Characteristics of participants by A1C categories are presented in Table 1. Most metabolic variables including Si and AIR worsened with increasing A1C.
Table 1.
Characteristics by categories of A1C adjusted for age, sex, race/ethnicity, and research center
| 1: A1C <5.7% | 2: A1C 5.7–6.4% | 3: A1C ≥6.5% |
P value |
|||
|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||
| n | 673 | 138 | 44 | — | — | — |
| Age (years)* | 59.6 ± 0.3 | 60.7 ± 1.3 | 59.6 ± 1.3 | 0.177 | 0.984 | 0.418 |
| Female (%)* | 57.2 (53.4–60.9) | 55.8 (47.4–63.9) | 61.4 (46.4–74.4) | 0.760 | 0.589 | 0.516 |
| Ethnicity (%)* | <0.001 | 0.006 | 0.544 | |||
| African Americans | 23.6 (20.6–27.0) | 30.4 (23.3–38.6) | 38.7 (25.6–53.6) | |||
| Hispanics | 31.5 (28.1–35.1) | 45.7 (37.5–54.0) | 36.4 (23.6–51.4) | |||
| Non-Hispanic whites | 44.9 (41.2–48.7) | 23.9 (17.5–31.7) | 25.0 (14.4–39.7) | |||
| BMI (kg/m2) | 28.1 ± 0.2 | 32.5 ± 0.5 | 32.2 ± 0.8 | <0.001 | <0.001 | 0.600 |
| Waist circumference (cm) | 90.4 ± 0.4 | 100.3 ± 1.5 | 100.5 ± 1.7 | <0.001 | <0.001 | 0.879 |
| Systolic blood pressure (mmHg) | 125.8 ± 0.7 | 127.9 ± 1.5 | 130.9 ± 2.6 | 0.207 | 0.059 | 0.459 |
| Diastolic blood pressure (mmHg) | 77.5 ± 0.3 | 78.4 ± 0.8 | 79.7 ± 1.4 | 0.295 | 0.139 | 0.661 |
| Antihypertensive medications (%) | 18.9 (15.7–22.6) | 31.2 (23.6–39.9) | 17.6 (9.2–30.9) | 0.002 | 0.793 | 0.085 |
| Triglycerides (mg/dl)† | 109.1 ± 2.2 | 125.3 ± 5.5 | 142.2 ± 11.2 | 0.018 | 0.001 | 0.128 |
| HDL cholesterol (mg/dl) | 50.7 ± 0.5 | 45.6 ± 1.2 | 43.4 ± 2.1 | <0.001 | 0.001 | 0.402 |
| Total cholesterol (mg/dl) | 209.0 ± 1.4 | 208.1 ± 3.1 | 202.2 ± 5.4 | 0.786 | 0.214 | 0.316 |
| Treatment for high cholesterol (%) | 6.1 (4.0–9.3) | 14.2 (8.3–23.3) | 8.5 (2.6–24.5) | 0.763 | 0.608 | 0.534 |
| Fasting glucose (mg/dl) | 95.8 ± 0.6 | 110.8 ± 1.4 | 160.5 ± 2.5 | <0.001 | <0.001 | <0.001 |
| 2-h glucose (mg/dl) | 128.9 ± 1.8 | 171.8 ± 4.0 | 272.2 ± 7.1 | <0.001 | <0.001 | <0.001 |
| Metabolic syndrome (%) | 38.9 (34.7–43.2) | 73.7 (65.2–80.7) | 82.2 (67.8–91.0) | <0.001 | <0.001 | 0.247 |
| Fasting insulin (μU/ml)† | 14.3 ± 0.3 | 22.6 ± 1.2 | 21.8 ± 1.8 | <0.001 | <0.001 | 0.655 |
| Si (× 10−4 min−1 · μU−1 · ml−1)† | 1.27 ± 0.05 | 0.67 ± 0.07 | 0.46 ± 0.11 | <0.001 | <0.001 | 0.156 |
| AIR (μU/ml)† | 68.8 ± 2.0 | 49.6 ± 3.4 | 27.1 ± 3.3 | 0.041 | <0.001 | <0.001 |
Data are mean ± SEM or rates with 95% CI.
*Unadjusted results.
†Log-transformed variables. These variables were then back-transformed to their units for presentation in the table.
Ability of A1C ≥6.5% to detect of individuals with diabetes
A total of 136 of 855 (15.9%) individuals had diabetes. A1C ≥6.5% identified 32.3% of all individuals with diabetes. FPG ≥126 mg/dl and 2-h plasma glucose ≥200 mg/dl detected a larger percentage (44.8 and 86.8%, respectively). The combination of A1C ≥6.5% and/or FPG ≥126 mg/dl detected 52.2% of diabetic subjects and the combination of IFG and/or IGT detected 97.1%.
Relation of glycemic measures to metabolic variables in nondiabetic individuals
FPG was more strongly correlated with A1C than was 2-h plasma glucose (r = 0.39 vs. 0.25; P < 0.001) in nondiabetic subjects (Table 2). The correlations of 2-h plasma glucose with Si, systolic blood pressure, and triglycerides were stronger than the corresponding correlations of A1C. FGP had also more robust correlations with fasting insulin, AIR, obesity, and systolic blood pressure than did A1C.
Table 2.
Spearman correlation coefficients for the relationship between glycemic measures as well as with other metabolic variables in nondiabetic participants
| A1C | Fasting glucose | 2-h glucose | |
|---|---|---|---|
| Fasting glucose | 0.39 | — | — |
| 2-h glucose | 0.25 | 0.34 | — |
| Fasting insulin | 0.26 | 0.38* | 0.28 |
| S i | −0.27 | −0.34 | −0.40* |
| AIR | −0.09 | −0.20* | −0.18 |
| BMI | 0.20 | 0.28† | 0.24 |
| Waist circumference | 0.25 | 0.43‡ | 0.22 |
| Systolic blood pressure | 0.10 | 0.21* | 0.29‡ |
| Diastolic blood pressure | 0.08 | 0.15 | 0.10 |
| Total cholesterol | 0.07 | 0.03 | 0.07 |
| Triglycerides | 0.08 | 0.14 | 0.30‡ |
| HDL cholesterol | −0.13 | −0.21 | −0.16 |
P value for test of difference in the correlation of each plasma glucose measure with individual metabolic variables relative to the correlation of A1C with the same metabolic variable.
*P < 0.01;
†P < 0.05;
‡P < 0.001.
Relationship among glycemic measures, Si, and AIR in nondiabetic individuals
In linear regression analysis, A1C explained 7.4% of the Si variance and FPG and 2-h plasma glucose accounted for 10.3 and 13.8%, respectively. Si accounted for 11.3% of the AIR variance, but addition of A1C to the model increased the variance explained by 4.2%, addition of FPG by 10.2%, and addition of 2-h plasma glucose by 8.7%.
A multivariate linear regression model was fitted with Si as the dependent variable and age, sex, race/ethnicity, research center, and all three glycemic measures as independent variables. Expressed per 1 SD, regression coefficients demonstrated that A1C (β = −0.04 ± 0.02, P = 0.038), FPG (β = −0.08 ± 0.02, P < 0.001), and 2-h plasma glucose (β = −0.14 ± 0.02, P < 0.001) were independently related to Si. Similarly, we fitted a second model with AIR as the dependent variable and demographic variables, Si, and all three glycemic measures as independent variables. A1C (β = −0.11 ± 0.03; P < 0.001), FPG (β = −0.16 ± 0.03, P < 0.001), and 2-h plasma glucose (β = −0.15 ± 0.03, P < 0.001) were also independently associated with AIR.
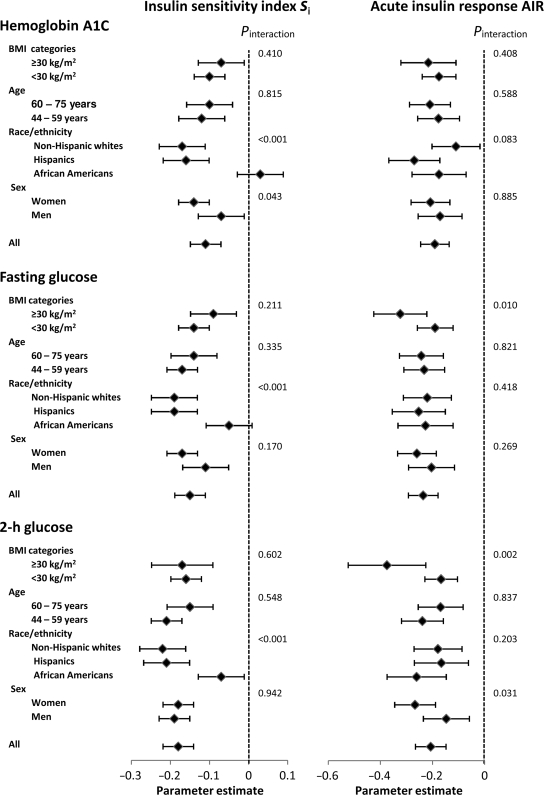
In separate models, there was strong effect modification of race/ethnicity on the relation of each glycemic measure to Si (Fig. 1). In African Americans, 2-h plasma glucose was weakly related and A1C and FPG were not related to Si. Sex had an interaction effect on the relationship between A1C and Si and between 2-h plasma glucose and AIR (stronger in women for both). Obesity had a similar effect on the relation of each plasma glucose measure to AIR (stronger in obese individuals).
Figure 1.
Effect of age, sex, race/ethnicity, and obesity on the relation of glycemic measures to Si and AIR. In linear regression models with Si as the dependent variable, age, sex, race/ethnicity, and center were included as covariates. In models with AIR as the dependent variable, age, sex, race/ethnicity, research center, and Si were included as covariates. Continuous variables were log transformed to meet the specifications of the test. Estimates are expressed per 1 SD unit change.
Identification of at-risk individuals
A total of 385 of 719 (53.5%) nondiabetic individuals were at increased risk of diabetes (Table 3). The proportion of these individuals identified by A1C 5.7–6.4%, IFG, and IGT was 23.6, 69.1, and 59.5%, respectively. The combination of IFG and/or IGT detected 95.8% and the combination of IFG and/or A1C 5.7–6.4% detected 75.6%. At-risk individuals were more frequently identified by IFG if they were men, African Americans, or non-Hispanic whites, and if they were young, overweight, or obese. At-risk individuals were more commonly detected by IGT if they were women or Mexican Americans. A1C 5.7–6.4% detected few non-Hispanic whites at increased risk of diabetes and was more effective with worsening BMI.
Table 3.
Sensitivity of A1C 5.7– 6.4%, IFG, and IGT for detecting individuals at increased risk of diabetes by age, sex, race/ethnicity, and BMI categories
| Nondiabetic individuals | At-risk individuals | A1C 5.7– 6.4% sensitivity | IFG sensitivity | IGT sensitivity | |
|---|---|---|---|---|---|
| All | 719 | 385 (53.5) | 23.6 | 69.1 | 59.5 |
| Age categories | |||||
| 44–54 years | 237 | 108 (45.6) | 20.4 | 72.2 | 47.2 |
| 55–64 years | 253 | 146 (57.7) | 27.4 | 67.8 | 65.1 |
| 65–75 years | 229 | 131 (57.2) | 22.1 | 67.9 | 63.4 |
| Sex | |||||
| Men | 314 | 182 (58.0) | 24.7 | 81.9 | 48.3 |
| Women | 405 | 203 (50.1) | 22.7 | 57.6 | 69.5 |
| Race/ethnicity | |||||
| African Americans | 184 | 102 (55.4) | 31.4 | 73.5 | 45.1 |
| Hispanics | 240 | 122 (50.8) | 35.2 | 59.0 | 71.3 |
| Non-Hispanic whites | 295 | 161 (54.6) | 9.9 | 73.9 | 59.6 |
| BMI categories | |||||
| <25 kg/m2 | 183 | 64 (35.0) | 10.9 | 57.8 | 62.5 |
| 25–29.9 kg/m2 | 337 | 186 (55.2) | 19.9 | 72.0 | 58.1 |
| ≥30 kg/m2 | 199 | 135 (67.8) | 34.8 | 70.4 | 60.0 |
Data are n, n (%), or %. Nondiabetic individuals with A1C 5.7–6.4%, IFG, or IGT were considered at increased risk of diabetes.
We used AUCs to assess the ability to detect individuals in the lower quartile of Si (supplementary Figure, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0679/DC1). The AUC of A1C was smaller than that of 2-h plasma glucose (0.620 vs. 0.682; P = 0.048) but was not statistically different from that of FPG (0.654; P = 0.239). The three glycemic measures performed poorly in detecting individuals in the lower quartile of AIR, although FPG (0.583; P = 0.029) and 2-h plasma glucose (0.590; P = 0.027) displayed larger AUCs than did A1C (0.520). Finally, the AUC of A1C for detecting individuals with ≥2 metabolic abnormalities (excluded hyperglycemia) was smaller than that of 2-h plasma glucose (0.598 vs. 0.702; P < 0.001) but was not significantly different from the AUC of FPG (0.643; P = 0.064).
CONCLUSIONS
A1C 5.7– 6.4% identifies a much smaller proportion of individuals at increased risk of diabetes than do IFG and IGT, particularly among non-Hispanic whites and lean individuals. A1C is a less precise correlate of insulin resistance and insulin secretion in studies of metabolism than single determinations of FPG and 2-h plasma glucose and, in general, is worse for other metabolic disorders.
In participants in the 2003–2006 National Health and Nutrition Examination Survey (NHANES), A1C between 6.0 and 6.4% missed 90% of the individuals at increased risk of diabetes (11). Our results suggest that the newly proposed category, A1C 5.7–6.4%, still misses three-quarters of them. Similarly, in a recent study from Qingdao, China, A1C was unable to distinguish individuals with IFG and/or IGT from those with normal glucose tolerance (17). Conversely, in a study from Chennai, India, A1C ≥5.7% detected two-thirds of individuals with IFG and/or IGT (18). The performance of A1C 5.7–6.4% may differ among studies owing to differences in the target population. Ginde et al. (19) have reported that risk stratification improves the ability of A1C to screen for undiagnosed diabetes. In our study, A1C 5.7–6.4% performs poorly among non-Hispanic whites and lean individuals. The 2010 ADA report indicated that the risk of diabetes associated with A1C 5.7–6.4% is comparable to that in participants in the Diabetes Prevention Program (DPP) (1). Because DPP participants in the control arm had an 11% per year incidence of diabetes (20), it would be beneficial to detect individuals at an earlier stage of the disease process so that they may benefit from lifestyle changes. In clinical and epidemiological settings, a significant proportion of individuals even with A1C <5.5% have either IFG or IGT (21). Thus, A1C 5.7–6.4% may be inadequate as the only criterion for detecting individuals at increased risk of the disease.
Inoue et al. (4) have reported than both IFG and A1C between 5.5 and 6.4% are independent predictors of conversion to diabetes (4). In nondiabetic individuals, little of the Si (and AIR) variance is explained by A1C; in this regard, FPG and 2-h plasma glucose seem to be somewhat better. FPG correlates better with fasting insulin and first-phase insulin secretion compared with A1C. Similarly, 2-h plasma glucose correlates better with directly measured insulin resistance than does A1C. Nevertheless, each of the three glycemic measures is related to both Si and AIR, independently of the effect of the other two measures. This is probably so because each glycemic measure reflects unique domains of glucose metabolism. Furthermore, FPG and 2-h plasma glucose may be superior to A1C in their relationship with metabolic variables other than insulin resistance and insulin secretion. The preeminence of A1C as a better indicator of future complications, at least relative to fasting glucose (1,5), is in agreement with the concept of “metabolic memory,” which postulates that hyperglycemia leaves a very early imprint on the progression to complications (22). However, A1C is inferior for metabolic abnormalities (insulin resistance and secretion, obesity, and triglycerides), which may be more determined by acute levels of glycemia.
A1C ≥6.5% identifies one-third of patients with diabetes. A larger percentage is detected by FPG ≥126 mg/dl and 2-h plasma glucose ≥200 mg/dl (45 and 87%, respectively). These results are almost identical to those derived from 2003–2006 NHANES data (30% of diabetic individuals detected by A1C ≥6.5%, 46% by FPG ≥126 mg/dl, and 90% by 2-h plasma glucose ≥200 mg/dl) (11). In Qingdao, China, the 6.5% A1C cut point also detects 30% of individuals with diabetes (2003 ADA criteria) (17). In Chennai, India, however, A1C ≥6.5% detects 78% of individuals with newly diagnosed diabetes (18). Furthermore, the 6.5% A1C threshold seems to be specific for detecting undiagnosed diabetes defined by a single measure of FPG (12,23) and identifies retinopathy better than do FPG ≥126 mg/dl (1) and 2-h plasma glucose ≥200 mg/dl (13). It seems logical to use A1C ≥6.5% as a criterion for diagnosing diabetes provided that the plasma glucose criteria stay in use. The 6.5% A1C threshold misses a large percentage of undiagnosed diabetes, and the clinical consequences of A1C screening remain unknown. Less sensitivity could lead to undertreatment for cardiovascular disease if future recommendations integrate the risk of diabetes as part of the algorithm.
There are practical considerations as well. Although A1C is convenient, given that it does not require the fasting state, it is substantially more expensive to analyze than plasma glucose tests. Patients are commonly asked to come in fasting for other types of tests (e.g., LDL cholesterol and triglycerides), and there is little added effort required if the alternative FPG is paired with other fasting tests. There is also the concern of potential racial differences in the interpretability of A1C. The DPP and others have reported ethnic differences in the way A1C correlates to glucose levels among individuals with IGT, suggesting the potential for further detection disparities (24). In this regard, our results suggest that A1C does not reflect the same domain of glucose metabolism across ethnic groups. Furthermore, our study indicates that A1C 5.7–6.4% detects at-risk individuals better as BMI worsens. Others have reported that A1C in diabetic patients with chronic kidney disease (stages 3–4) has been shown to underestimate actual glucose values (25), nor is A1C accurate in individuals with anemia and hemoglobinopathies (1). Whether this is a concern in detection of at-risk individuals is unclear.
A significant limitation of our study is the use of single determinations of plasma glucose values. Nevertheless, single determinations of FPG and 2-h plasma glucose seem to be more precise correlates of insulin resistance and secretion than A1C and, in general, better for other metabolic disorders.
In summary, the 6.5 and 5.7% A1C thresholds have a low sensitivity for detecting individuals with diabetes and at increased risk for the disease, respectively. A1C 5.7–6.4% performs poorly for identifying at-risk individuals among non-Hispanic whites but is more effective as BMI increases. A1C is a less precise correlate of insulin resistance and insulin secretion in studies of metabolism than FPG and 2-h plasma glucose and, in general, is worse for other metabolic disorders. Further studies are needed for assessing the relation of glycemic measures to metabolic abnormalities.
Supplementary Material
Acknowledgments
This study was supported by the National Heart, Lung, and Blood Institute (grants HL-47887, HL-47889, HL-47890, HL-47892, and HL-47902) and the General Clinical Research Centers Program (National Center for Research Resources M01-RR431 and M01-RR-01346).
No potential conflicts of interest relevant to this article were reported.
C.L. contributed to discussion, wrote the manuscript, and reviewed/edited the manuscript. L.E.W. and S.M.H. researched data, contributed to discussion, and reviewed/edited the manuscript. A.J.G.H. and M.J.R. contributed to discussion and reviewed/edited manuscript. A.J.K. researched data and reviewed/edited the manuscript.
Parts of this study were presented in poster form at the 70th annual meeting of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33:S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Droumaguet C, Balkau B, Simon D, Caces E, Tichet J, Charles MA, Eschwege E: DESIR Study Group. Use of HbA1c in predicting progression to diabetes in French men and women: data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2006;29:1619–1625 [DOI] [PubMed] [Google Scholar]
- 3. Pradhan AD, Rifai N, Buring JE, Ridker PM: Hemoglobin A1c predicts diabetes but not cardiovascular disease in non-diabetic women. Am J Med 2007;120:720–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inoue K, Matsumoto M, Akimoto K: Fasting plasma glucose and HbA1c as risk factors for type 2 diabetes. Diabet Med 2008;25:1157–1163 [DOI] [PubMed] [Google Scholar]
- 5. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL: Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barr EL, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE: Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia 2009;52:415–424 [DOI] [PubMed] [Google Scholar]
- 7. de Vegt F, Dekker JM, Ruhé HG, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ: Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 1999;42:926–931 [DOI] [PubMed] [Google Scholar]
- 8. Qiao Q, Dekker JM, de Vegt F, Nijpels G, Nissinen A, Stehouwer CD, Bouter LM, Heine RJ, Tuomilehto J: Two prospective studies found that elevated 2-hr glucose predicted male mortality independent of fasting glucose and HbA1c. J Clin Epidemiol 2004;57:590–596 [DOI] [PubMed] [Google Scholar]
- 9. Park S, Barrett-Connor E, Wingard DL, Shan J, Edelstein S: GHb is a better predictor of cardiovascular disease than fasting or postchallenge plasma glucose in women without diabetes. The Rancho Bernardo Study. Diabetes Care 1996;19:450–456 [DOI] [PubMed] [Google Scholar]
- 10. Lorenzo C, Haffner SM: Performance characteristics of the new definition of diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes Care 2010;33:335–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, Bainbridge KE, Fradkin JE: Prevalence of diabetes and high risk for diabetes using hemoglobin A1C criteria in the U.S. population in 1988–2006. Diabetes Care 2010;33:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carson AP, Reynolds K, Fonseca VA, Muntner P: Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults. Diabetes Care 2010;33:95–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kramer CK, Araneta MR, Barrett-Connor E: A1C and diabetes diagnosis: The Rancho Bernardo Study. Diabetes Care 2010;33:101–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, Henkin L, Howard G, Savage PJ, Saad MF: The Insulin Resistance Atherosclerosis Study (IRAS): objectives, design, and recruitment results. Ann Epidemiol 1995;5:464–472 [DOI] [PubMed] [Google Scholar]
- 15. Mayer-Davis EJ, Dhawan A, Liese AD, Teff K, Schulz M: Towards understanding of glycaemic index and glycaemic load in habitual diet: associations with measures of glycaemia in the Insulin Resistance Atherosclerosis Study. Br J Nutr 2006;95:397–405 [DOI] [PubMed] [Google Scholar]
- 16. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr: Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 17. Zhou X, Pang Z, Gao W, Wang S, Zhang L, Ning F, Qiao Q: Performance of an A1C and fasting capillary blood glucose test for screening newly diagnosed diabetes and pre-diabetes defined by an oral glucose tolerance test in Qingdao, China. Diabetes Care 2010;33:545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohan V, Vijayachandrika V, Gokulakrishnan K, Anjana RM, Ganesan A, Weber MB, Narayan KM: A1C cut points to define various glucose intolerance groups in Asian Indians. Diabetes Care 2010;33:515–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ginde AA, Cagliero E, Nathan DM, Camargo CA, Jr: Value of risk stratification to increase the predictive validity of HbA1c in screening for undiagnosed diabetes in the US population. J Gen Intern Med 2008;23:1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu ZX, Walker KZ, O'Dea K, Sikaris KA, Shaw JE: A1C for screening and diagnosis of type 2 diabetes in routine clinical practice. Diabetes Care 2010;33:817–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ceriello A: Hypothesis: the “metabolic memory,” the new challenge of diabetes. Diabetes Res Clin Pract 2009;86(Suppl. 1):S2–S6 [DOI] [PubMed] [Google Scholar]
- 23. Selvin E, Zhu H, Brancati FL: Elevated A1C in adults without a history of diabetes in the U.S. Diabetes Care 2009;32:828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E: Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen HS, Wu TE, Lin HD, Jap TS, Hsiao LC, Lee SH, Lin SH: Hemoglobin A1c and fructosamine for assessing glycemic control in diabetic patients with CKD stages 3 and 4. Am J Kidney Dis 2010;55:867–874 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.