Abstract
Inter-alpha Inhibitor proteins (IaIp) are serine proteases inhibitors which modulate endogenous protease activity and have been shown to improve survival in adult models of sepsis. We evaluated the effect of IaIp on survival and systemic responses to sepsis in neonatal mice. Sepsis was induced in 2-day-old mice with LPS, E. coli and Group B Streptococci. Sepsis was associated with 75% mortality. IaIp, given by intraperitoneal administration at doses between 15–45 mg/kg from 1–6 hours following the onset of sepsis improved survival to nearly 90% (p = 0.0159) in both LPS induced sepsis and with live bacterial infections. The greatest effect was on reversal of hemorrhagic pneumonitis. The effects were dose and time dependent. Systemic cytokine profile and tissue histology were examined. Survival was compared in interleukin 10 knock out animals. Systemic cytokine levels, including TNF-α and IL-10 were increased following induction of sepsis and modulated significantly following IaIp administration. Because the effect of IaIp was still demonstrable in IL-10 deficient mice, we conclude the beneficial effect(s) of IaIp is due to suppression of pro-inflammatory cytokines like TNF-α rather than augmentation of IL-10. IaIp may offer significant benefits as a therapeutic adjunct to treatment of sepsis in neonates and adults.
INTRODUCTION
Sepsis is a leading cause of morbidity and mortality in newborn and preterm infants (1–3). In a large, multicenter, prospective, cross-sectional study from the NICHD Neonatal Research Network, the incidence of late onset nosocomial infection was 16% of 2,416 infants enrolled at 12 sites (3). The high continuing morbidity and mortality due to sepsis, septic shock, and multiple organ failure may be attributable to the fact that the mediators/factors responsible for the pathophysiologic alterations of sepsis are not fully understood (4). Antibiotic therapy may not be sufficient to reverse systemic inflammation and consequent organ injury accompanying sepsis.
Mediators involved in the progression of sepsis can induce the activation of phagocytes that release neutrophil proteases (5). Experimental and clinical data have demonstrated that increased activity of neutrophil-derived serine proteases, such as leukocyte elastase and cathepsin G, play a prominent role in sepsis-related tissue damage (6). Administration of protease inhibitors has been proposed as a therapeutic strategy to restore the balance between proteases and protease inhibitors in sepsis (7). One such inhibitor is the Inter-alpha Inhibitor protein (IaIp) family (8–10). This is a group of structurally related serine protease inhibitors found at relatively high concentrations in human plasma. IaIp proteins are composed of heavy and light polypeptide subunits that are covalently linked by a glycosaminoglycan chain (10–12). The light chain, also called bikunin, contains the serine protease inhibitory activity of the molecules (13). Bikunin is inactive when cross-linked in these complexes until it is released by partial proteolytic degradation. After cleavage from the complex, bikunin is cleared rapidly from plasma by glomerular filtration and receptor-mediated uptake(14).
IaIp proteins are involved in many physiological and pathological activities. This includes tumor invasion and metastasis (15), stabilization of the extracellular matrix (8), inflammation and wound healing (16). Recently, the involvement of IaIp in inflammatory diseases has become an area of intensive investigation. The release of neutrophil proteases, especially human leukocyte elastase, has been implicated in the progression of complications in both adult and neonatal patients with sepsis(9,17,18). Plasma IaIp is particularly sensitive to cleavage by neutrophil elastase. The light chain released from the IaIp complex then exerts its inhibitory activity on serine proteases (19). In vitro, IaIp inhibits several serine proteases that are involved in inflammation, including elastase, plasmin, and cathepsin G (20). Clinical studies of patients with severe sepsis have revealed significant reduction in circulating IaIp due to consumption and accompanied by an extended secretion of elastase (9,17,18).
We have demonstrated markedly decreased concentrations of IaIp in plasma of septic adults (9,21,22) and neonates (23,24). Further, the degree of reduction of IaIp in adults correlates with increase mortality (9,21,22). In experimental models of sepsis, we have demonstrated improved survival, improved hemodynamic and physiological stability, reduced hepatic injury and reduced inflammatory cytokine production following IaIp (25,26). Jourdain et al. have shown administration of human IaIp markedly improved hemodynamics, tissue oxygenation and pulmonary hypertension in a porcine model of endotoxic shock (27). There are no experimental studies on the effects of IaIp administration in neonates. The goal of our studies was to examine the effects of IaIp administration on survival and inflammatory cytokine production in microbiologically relevant models of neonatal sepsis (28,29).
MATERIALS AND METHODS
IaIp Preparation
Human IaIp was purified as previously described by a combination of cryo-precipitation, solid phase extraction and a series of chromatographic separations involving ion-exchange, heparin affinity and hydroxyl apatite columns (25,26). The purity is greater than 95%. This highly purified IaIp has shown no side effects including toxicity, thrombogenicity, or hypotension in studies in adult model systems (25,26,30).
Neonatal Sepsis Models
All animal experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee, which conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and in accordance with the Declaration of Helsinki principles. All mice used in this study were housed in a specific pathogen-free facility under the care of the Central Research Department of Rhode Island Hospital. All strains of mice, C57BL/6 Interleukin 10 deficient animals (IL-10−/−), C57BL/6 wild type and Balb/C wild type, were obtained from the Jackson Laboratory (Bar Harbor, ME).
The dose dependent effects of LPS were compared by injection of 5, 10 or 25 micrograms subcutaneously in 2-day-old, mixed-gender BALB/c wild type mice. The LPS preparation used was serotype O26:B6 E. coli LPS (Sigma Chemical, St. Louis, Missouri). Injection of 25 micrograms of LPS subcutaneously resulted in survival of 25% after 80 hours. This dose was used in subsequent experiments. Two day old mice (n=22) were injected with LPS and randomized into two groups. The IaIp animals (n=12) received 30 mg/kg body weight of highly purified human IaIp intraperitoneally one hour after LPS. Controls (n=10) received equal amount of human serum albumin (HSA). Survival was observed every 2 hours for 80 hours. Results were compared in the two groups. T the effect of delayed administration of IaIp was tested by administration of IaIp up to 6 hours after the LPS dose. The dependence of survival on the dose of IaIp was compared by administration of three different doses, 15 mg/kg (n=5), 30 mg/kg (n=10) and 45 mg/kg (n=6) body weight 3 hours after LPS. This dosing range was based on prior studies in adult animal models (25,26)
Survival was also determined following injection of live E. coli or Type 1 GBS. The virulent strain of K1 subtype of E coli we used was a clinical obtained from Dr. Stephen Opal Memorial Hospital of Rhode Island. The GBS strain was a clinical isolate obtained at Women & Infants Hospital of Rhode Island. Bacteria from a fresh overnight culture was inoculated into LB broth and grown to log phase in a shaking culture flask at 37°C and monitored spectrophotometrically until achieving an OD560 of 0.38. Previous experiments using serial dilutions and direct plating of that innoculum were used to generate a standard curve for colony forming units (CFU)/ml of culture. Fresh culture was serially diluted into sterile phosphate buffered saline. The final injection volume for all doses of bacteria was 25 microliters. Survival was examined following subcutaneous administration of 104 to 107 E. coli CFU/pup. The effects of exogenous IaIp were examined following administration of 30 mg/kg IaIp or HSA intraperitoneally one hour following the E. coli. Similarly, the dose dependent effects of GBS were determined by subcutaneous administration of 104 to 107 CFU/pup and the effects on survival were examined following a dose of 30 mg/kg IaIp one hour after the GBS. All experiments with animals were approved by the Institutional Animal Care & Use Committee. The effect of IaIp was also compared in wild type animals and in animals in which the anti-inflammatory cytokine IL-10 had been disrupted (IL-10−/−). Because of their heightened mortality in response to LPS, the dose response for lethality was first established in IL-10−/− neonatal mice. The effect of IaIp on survival was then examined after LPS injection as in the experiments with wild type animals by intraperitoneal injection of 30mg/kg IaIp or human albumin 1 hour after subcutaneous LPS.
Inflammatory Cytokine Responses
In order to examine the effects of IaIp on inflammatory responses, plasma cytokine levels were measured in IaIp and albumin treated groups. LPS administered subcutaneously as above was followed 1 hr later by 30 mg/kg body weight of intraperitoneal IaIp or Albumin. Animals were sacrificed at intervals and pooled trunk blood was collected from three pups at 2, 6, 12, 24, 48 and 72 hours after the LPS injection, from both IaIp and albumin treated groups. Plasma was separated immediately by centrifugation and stored at −70°C for measurement of MCP-1, TNF-a, IL-10, IL-12p70, IFN-g, and IL-6 by radioimmunoassay (9).
Statistics
The log rank test was used to calculate statistical differences in survival between the IaIp and the control group. Unpaired t-testing was used to compare group means were appropriate. In both cases, p <0.05 was considered as statistically significant.
RESULTS
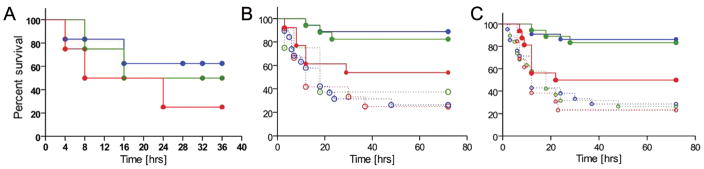
We administered graded doses of LPS subcutaneously to 2–3 day old BALB/c wild type mice (Figure 1, panel A). As can been seen, there was a dose dependent mortality which reached 60–70% by 48 hours following the 25 microgram per pup dose. In Figure 1B, it can be seen that administration of 30 mg/kg intraperitoneally of IaIp one hour after LPS challenge reversed mortality from 60–70% by 40 hours to prolonged survival in nearly 90% of the pups. Increasing the dose to 45 mg/kg had no additional salutary effect. However, reducing the dose to 15 mg/kg decreased survival to 60% which was still statistically significant, p<0.05. We examined the timing of IaIp administration. Administration of IaIp as long as up to 3 hours after the LPS was still highly protective. If IaIp was administered one to three hours following LPS survival remained between 80 and 90%, Figure 1C. Delaying administration for six hours reduced survival, which was nonetheless still significantly better than vehicle alone.
Figure 1.
The effect of IaIp on mortality in 2 day old mice. (A) Survival following 5 (blue, n=5), 10 (green, n=4) or 25 (red, n=4) micrograms of LPS per pup. (B). Dose dependent effects of 15 (red, n=13), 30 (blue, n=18) or 45 (green, n=17) mg/kg IaIp intraperitoneally one hour later following LPS as compared to HSA controls at the same concentrations:15 (red-dotted; n=12), 30 (blue-dotted, n=19) and 45 (green-dotted; n=9) mg/kg. Both 30 and 45 mg/kg were significantly different from the 15 mg/kg group, p<0.004. (C) Effect of delayed administration. Animals received 30 mg/kg IaIp at one (blue, n=23), three (green, n=18) or six (red, n=16) hours after LPS. IaIp resulted in significantly better survival than each respective control, p<0.001. HSA controls at the same time points: 1 hour (blue-dotted, n=19), 3 hours (green-dotted, n=19), and 6 hours (red-dotted, n=13)
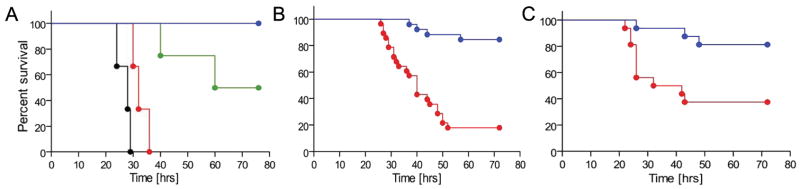
We examined the effects of IaIp on E. coli and Group B β hemolytic Streptococcus (GBS) sepsis, the two most relevant pathogens in neonatal sepsis in human infants, to assess veracity of protection (2,3,28,29,31). The virulent subtype of E coli was grown to logarithmic phase in culture and then administered subcutaneously at doses from 4×105 up to 2×107 CFU/animal, Figure 2A. There was a high degree of lethality associated with the two highest doses, whereas 4×105 CFU per pup resulted in reproducible lethality between 60 and 80% (Figure 2A, 2B). Administration of 30 mg/kg of IaIp one hour following the E coli improved survival to nearly 90%, Figure 2B. Similar lethality and response to administration of IAIP was seen with a clinical isolate of Type I GBS, Figure 2C. Subcutaneous administration of GBS resulted in 60% overall mortality in the albumin-treated group. Survival was increased to 80% following administration of IaIp in the animals injected with GBS.
Figure 2.
The effect of IaIp administration on survival following subcutaneous administration of live bacteria in 2-day-old BALB/c mice. (A) Survival following subcutaneous injection of 104 (blue), 105 (green),106 (red), 107 (black) CFU E. coli per pup. (B). Dose dependent effect of IaIp on survival following E. coli administration. Control animals (red, n=28) received intraperitoneal HSA, and IaIp treated animals (blue, n=26) received 30 mg/kg IaIp intraperitoneally one hour later. IaIp treated animals had significantly better survival than either control group, p<0.05. (C) The effect of IaIp administration on survival following subcutaneous administration of group B streptococci. IaIp treated animals (blue, n=17) received 30 mg/kg IaIp intraperitoneally one hour later. IaIp treated animals had significantly better survival than the HSA control group (red, n=16).
To examine the mechanism(s) of protection of IaIp, tissue samples were taken from randomly selected pups twenty four hours following administration of 25 micrograms of LPS. Histological sections were reviewed by a pathologist not aware of the treatment groups. Sections of the lung parenchyma following LPS from an animal that received IaIp and a sections following LPS in a control animal that received albumin are shown in Figure 3. The control lung showed marked thickening of the alveolar septae, a pronounced polymorphonuclear leukocyte infiltration and some pulmonary hemorrhage, Figure 3A. In contrast, IaIp administration was associated with preservation of largely normal lung parenchymal architecture, Figure 3B. Changes in other organs (liver, kidney, intestine) were similar but less pronounced (not shown). Whole blood and plasma were collected and examined by Western blot and ELISA to determine the time course for decrements in endogenous, mouse IaIp and the Pre-alpha Inhibitor. The plasma concentration of both IaIp and PaI decreased significantly the following LPS induced sepsis, Figure 4A. The differences between each time point are all significant, 4B p<0.05.
Figure 3.
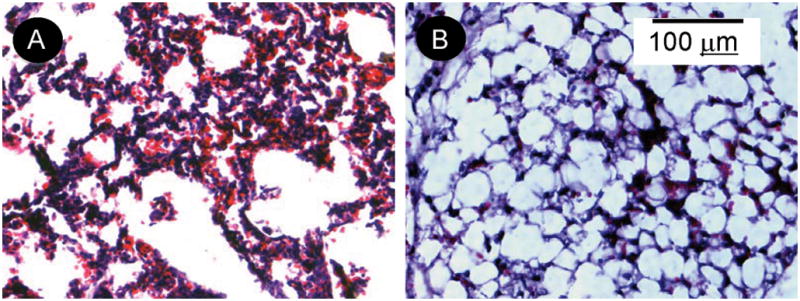
The effect of IaIp on lung histology following LPS. Animals were given LPS followed by HSA (A) or 30 mg/kg IaIp (B). Hematoxylin and eosin stain. Magnification 40X.
Figure 4.
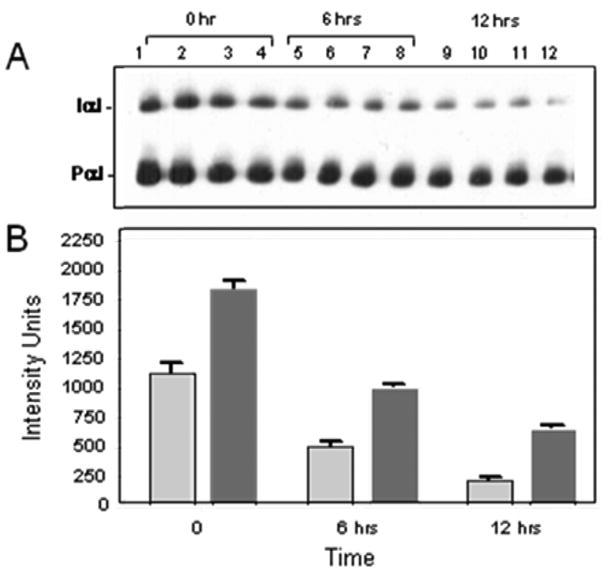
Western blot (A) showing changes in endogenous, mouse IaIp (gray) and the Pre-alpha Inhibitor (dark gray) following administration of LPS. (B) The differences between each time point are all significant, p<0.05.
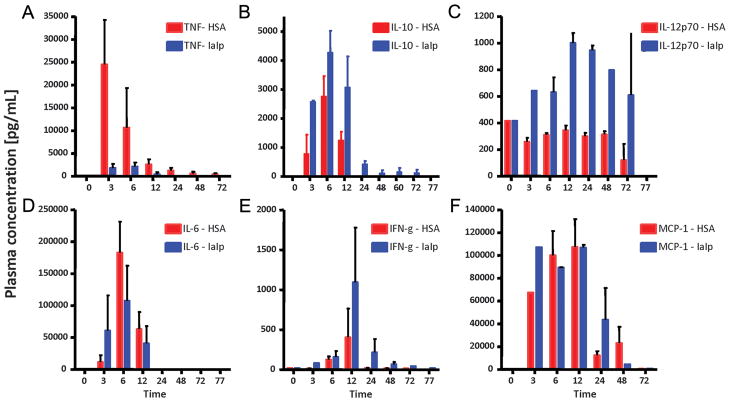
In order to examine the effect of IaIp on systemic immune responses, LPS was administered to animals as described above followed by timed sacrifice and collection of trunk blood for cytokine measurements. The results are shown in Figure 5. By 3 hours after LPS, the plasma concentration of the pro-inflammatory cytokine TNF-α was increased to almost 25,000 pg/ml and remained elevated for up to 12 hours. Similarly, the immunomodulatory cytokine IL-10 was increased following LPS injection, reaching a peak concentration of over 2,500 pg/ml at 6 hours. Most remarkably, administration of IaIp (30 mg/kg) one hour following LPS injection nearly completely attenuated the increase in the plasma concentration of TNF-α. TNF-α was reduced to almost baseline levels at 3 hours after IaIp (p<0.03). In contrast, the increase in IL-10 was augmented and prolonged by IaIp (p<0.04). IaIp similarly augmented the increase in plasma IL-12p70 (p< 0.002). The increase in IL-6 at 6 hours, followed the maximum increase in TNF-α and was modestly attenuated by IaIp administration. There was much less of an effect of IaIp on the changes in MCP-1 following LPS.
Figure 5.
The effect of LPS on systemic cytokine levels. Animals received LPS, HSA and IaIp as described in Methods. Cytokines were measured by radioimmunoassay 3, 6, 12, 24, 48 and 72 hours later. Results were compared by Two-way ANOVA. A) TNF-alpha, p=0.0313; B) IL-10, p=0.0377; C) IL-12p70, p=0.0024; D) IL-6, p=0.649; E) IFN-gamma, p=0.141; F) MCP-1, p=0.4948.
In order to determine the contribution(s) to the beneficial effect of IaIp on survival from suppression of pro-inflammatory cytokine production or augmentation of IL-10 secretion, we carried out similar experiments in animals in which the IL-10 gene had been disrupted (IL-10−/−). The IL-10−/− animals showed a markedly heightened susceptibility to mortality following LPS. We systematically compared 10, 2.5 and 0.25 micrograms in IL-10−/− animals (Figure 5B). LPS was highly lethal but by reducing the dose to 0.25 micrograms/mouse, we observed a survival of up to 40% at 60 hours. Administration of IaIp to IL-10−/− animals one hour following 0.25 micrograms LPS was associated with over 90% survival (Figure 6B). IaIp prolonged survival in the IL-10−/− animals receiving the higher LPS doses, albeit death was observed in all by 50 hours (p<0.05).
Figure 6.
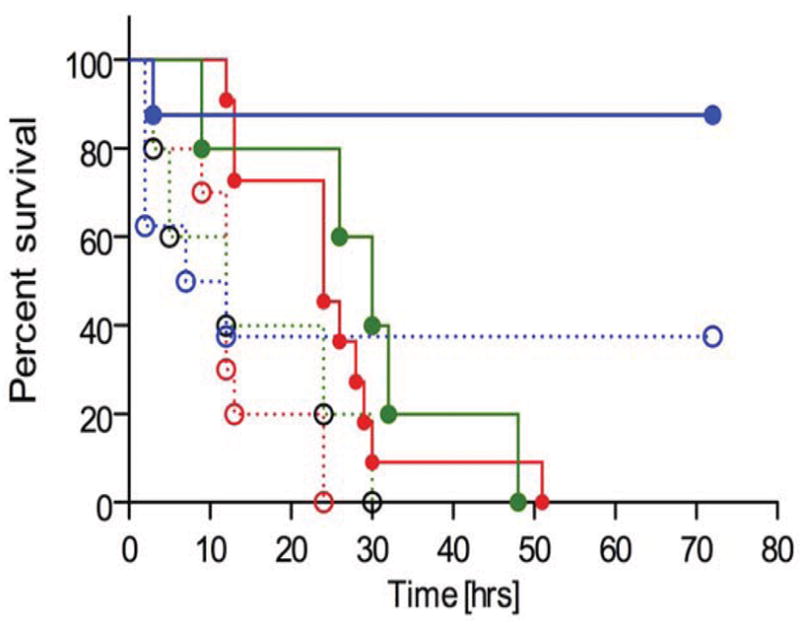
Survival following LPS administration in IL-10−/− mice. Animals were treated with LPS, either 0.25, 2.5 or 10 μg per animal, as described in Methods. Animals were treated with IaIp (IaIp + LPS 0.25 (blue, n=8); IaIp + LPS 2.5 (green, n=5); IaIp + LPS 10 (red, n=10)) or HSA (HSA + LPS 0.25 (blue-dotted, n=8); HSA + LPS 2.5 (green-dotted, n=5); HSA + LPS 10 (red-dotted, n=10)) as described in Methods 1 hour following LPS. Animals were examined every 4 hours for survival. All IaIp treated animals had significantly better survival than each of their respective control groups, p = 0.006.
DISCUSSION
In these studies, we show improved survival following the administration of IaIp in neonatal sepsis due to LPS and live E. coli and GBS, the most important organisms in the epidemiology of neonatal sepsis (3,20,31). Taken together with our other findings, these results suggest that administration of IaIp elicits potent immunomodulatory actions that lead to survival in neonatal sepsis even when administered at delayed time points. These observations suggest that deficiency of these regulatory protease inhibitors may play a pathophysiologic role in systemic inflammation and sepsis (32,33).
The host immune response leads to a proinflammatory cascade, which continues even after the responsible organisms are killed despite administration of antibiotics. Recently, studies in adult humans with severe sepsis and organ dysfunction have demonstrated an improvement in survival following administration of antibiotics and recombinant activated protein C (34). The reduction in the risk of death was modest (6%) however there was an apparent increase in associated risk of bleeding. Activated protein C promotes fibrinolysis and suppresses immune system activation. The increase in bleeding with activated protein C administration is a relatively strong contraindication in newborn infants, especially preterm infants. Indeed, a randomized, controlled trial in children ages 38 weeks gestation to 17 years of age failed to identify any benefits of activated protein C but noted an increase in the risk of bleeding in infants less than 60 days of age (35).
Clinical studies in patients with severe sepsis show significant reduction in the circulating concentration of IaIp which correlate with increased mortality in septic adult patients (9,21–24). We and others have shown improvement in survival of adult animals in experimental models of sepsis associated with a decrease in organ dysfunction and a marked reduction in the production/secretion of the pro-inflammatory cytokine TNF-α. (25–27). The experiments reported here demonstrate that intraperitoneal administration of IaIp to 2-day-old mice following induction of sepsis syndrome was associated with a dose and time dependent improvement in survival, suppression of secretion of pro-inflammatory cytokines like TNF-α and augmentation of IL-10 and IL-12p70 production. The improvement in survival was seen in animals with genetic deficiency in IL-10, suggesting that the beneficial effect of IaIp on survival resulted from alteration in TNF-α levels and other cytokines rather than augmentation of IL-10 or IL-12p70 secretion.
Neutrophils, monocytes, macrophages and Kupfer cells all produce significant amounts of TNF-α when exposed to high levels of endotoxin. Coeshutt and coworkers have further shown that neutrophils contribute to augmented TNF-α and IL-1β secretion by release of proteases such as proteas-3, elastase and cathepsin G in a TACE (TNF-α converting enzyme) and ICE (IL-1β converting enzyme) independent manner (38). Il-6 is likewise produced in response to stimulation by endotoxin IL-1β and TNF-α (36). We observed marked suppression in the levels of TNF-α in the animals treated with IaIp. Taken together, these findings stress different pathways of TNF-α biosynthesis/processing and provide insight into mechanisms for the immunomodulatory effect of the protease inhibitor IaIp. Hepatic Kupfer cells are the main source of IL-10, an immunomodulatory cytokine that protects mice against endotoxic shock by preventing excessive production of pro-inflammatory cytokines (37). Because the effect of IaIp on survival was still demonstrable in IL-10 deficient mice, we conclude the beneficial effect(s) of IaIp is due more to suppression of pro-inflammatory cytokines like TNF-α and others rather than augmentation of IL-10 production.
Models of sepsis in experimental animals have been used widely to develop new and effective therapies. Models that employ live organisms better recapitulate the characteristics of disease progression and organ dysfunction that accompanies sepsis than models using endotoxin alone (8,29). IaIp has been demonstrated to be effective now in a wide-range of conditions (25,26,30). IaIp is not only therapeutic but may have both diagnostic and prognostic value. As already noted, low levels of IaIp are associated with sepsis in adult humans and human neonates (9,21–24). Most recently, we have demonstrated that in longitudinal studies of IaIp levels in severely septic patients that failure of IAIP levels to recover over the first five days of sepsis is associated with a poor outcome (22). Additionally, our most recent data looked cross-sectionally at large numbers of human newborns with sepsis and gestational age and postnatally aged matched controls. We studied the receiver/operating characteristics needed to identify optimal predictive values of plasma IaIp levels in neonatal sepsis (24). Using a computer derived cutoff value of 177 mcg/ml, we demonstrated a sensitivity of 89.5%, a specificity of 99%, a positive predictive value of 95% and negative predictive value of 98%. The area under the ROC curve was 0.94, P < 0.001.
In the present studies, we observed comparable survival following induction of a systemic immune response syndrome (SIRS) mimicking sepsis due to LPS in animals that received either 30 or 45 mg/kg body weight IaIp, which suggests that larger doses are unlikely to offer additional benefits. We also compared administration of IaIp after one hour with delayed administration of IaIp until 3 or 6 hours. The beneficial effect(s) on survival were seen even when IaIp administration was delayed 3 to 6 hours after induction of sepsis. The results further demonstrate the effects of IaIp administration on immune system activation and organ dysfunction. Our results suggest that administration of IaIp exerts potent immunomodulatory actions that lead to a beneficial effect in septic newborn mice. IaIp may be an effective adjunct to treatment of severe sepsis in neonates or adults and warrants clinical investigation.
Acknowledgments
SOURCE OF SUPPORT: This project was supported by Grants from the NIH NICHD R21 HD047600 and NCRR P20 RR018728.
We appreciate intellectual contributions from Michael P. Sherman, MD, Professor, Southern Illinois University School of Medicine and Rashmin C. Savani, Professor and Chief, Division of Neonatology, University of Texas Southwestern Medical Center.
Abbreviations
- CFU
Colony forming units
- GBS
Group B β hemolytic Streptococcus
- HSA
Human serum albumin
- IaIp
Inter alpha Inhibitor Proteins
- LPS
Lipopolysaccharide
- IL-10−/−
10 deficient animals
Footnotes
DISCLOSURE:Y-P.L. has equity in the company ProThera Biologics where the IaIp protein preparations used in these experiments were produced and where the Western blot assays were performed.
References
- 1.Hyde TB, Hilger TM, Reingold A, Farley MM, O’Brien KL, Schuchat A. Active Bacterial Core surveillance (ABCs) of the Emerging Infections Program Network. Trends in incidence and antimicrobial resistance of early-onset sepsis; Population-based surveillance in San Francisco and Atlanta. Pediatrics. 2002;110:690–695. doi: 10.1542/peds.110.4.690. [DOI] [PubMed] [Google Scholar]
- 2.Puopolo KM, Madoff LC, Eichenwald EC. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics. 2005;115:1240–1246. doi: 10.1542/peds.2004-2275. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, Laptook AR, Walsh M, Oh W, Hale E. Very low birth weight preterm infants with early onset neonatal sepsis: The predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J. 2005;24:635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 5.Jochum M, Gippner-Steppert C, Machleidt W, Fritz H. The role of phagocyte proteases and proteinase inhibitors in multiple organ failure. Am J Respir Crit Care Med. 1994;150:S123–S130. doi: 10.1164/ajrccm/150.6_Pt_2.S123. [DOI] [PubMed] [Google Scholar]
- 6.Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- 7.Siebeck M, Fink E, Weipert J, Jochum M, Fritz H, Spannagl M, Kroworsch P, Shimamoto K, Schweiberer L. Inhibition of plasma kallikrein with aprotinin in porcine endotoxin shock. J Trauma. 1993;34:193–198. doi: 10.1097/00005373-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bost F, Diarra-Mehrpour M, Martin JP. Inter-alpha-trypsin inhibitor proteoglycan family--a group of proteins binding and stabilizing the extracellular matrix. Eur J Biochem. 1998;252:339–346. doi: 10.1046/j.1432-1327.1998.2520339.x. [DOI] [PubMed] [Google Scholar]
- 9.Lim Y-P, Bendelja K, Opal SM, Siryaporn E, Hixson DC, Palardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis. 2003;188:919–926. doi: 10.1086/377642. [DOI] [PubMed] [Google Scholar]
- 10.Zhuo L, Hascall VC, Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem. 2004;279:38079–38082. doi: 10.1074/jbc.R300039200. [DOI] [PubMed] [Google Scholar]
- 11.Blom AM, Morgelin M, Oyen M, Jarvet J, Fries E. Structural characterization of inter-alpha-inhibitor. Evidence for an extended shape. J Biol Chem. 1999;274:298–304. doi: 10.1074/jbc.274.1.298. [DOI] [PubMed] [Google Scholar]
- 12.Fries E, Blom AM. Bikunin--not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000;32:125–137. doi: 10.1016/s1357-2725(99)00125-9. [DOI] [PubMed] [Google Scholar]
- 13.Wachter E, Hochstrasser K. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor: IV. The amino acid sequence of the human urinary trypsin inhibitor isolated by affinity chromatography. Hoppe Seylers Z Physiol Chem. 1981;362:1351–1355. doi: 10.1515/bchm2.1981.362.2.1351. [DOI] [PubMed] [Google Scholar]
- 14.Sjoberg EM, Blom A, Larsson BS, Alston-Smith J, Sjöquist M, Fries E. Plasma clearance of rat bikunin: evidence for receptor mediated uptake. Biochem J. 1995;308:881–887. doi: 10.1042/bj3080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi H, Suzuki M, Hirashima Y, Terao T. The protease inhibitor bikunin, a novel anti-metastatic agent. Biol Chem. 2003;384:749–754. doi: 10.1515/BC.2003.083. [DOI] [PubMed] [Google Scholar]
- 16.Fries E, Kaczmarczyk A. Inter-alpha-inhibitor, hyaluronan and inflammation. Acta Biochim Pol. 2003;50:735–742. [PubMed] [Google Scholar]
- 17.Kingsmore SF, Kennedy N, Halliday HL, Van Velkinburgh JC, Zhong S, Gabriel V, Grant J, Beavis WD, Tchernev VT, Perlee L, Lejnine S, Grimwade B, Sorette M, Edgar JD. Identification of diagnostic biomarkers for infection in premature neonates. Mol Cell Proteomics. 2008;7:1863–1875. doi: 10.1074/mcp.M800175-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizon C, Piva F, Queyrel V, Balduyck M, Hachulla E, Mizon J. Urinary bikunin determination provides insight into proteinase/proteinase inhibitor imbalance in patients with inflammatory diseases. Clin Chem Lab Med. 2002;40:579–586. doi: 10.1515/CCLM.2002.100. [DOI] [PubMed] [Google Scholar]
- 19.Hirose J, Ozawa T, Miura T, Isaji M, Nagao Y, Yamashiro K, Nii A, Kato K, Uemura A. Human neutrophil elastase degrades inter-alpha-trypsin inhibitor to liberate urinary trypsin inhibitor related proteins. Biol Pharm Bull. 1998;21:651–656. doi: 10.1248/bpb.21.651. [DOI] [PubMed] [Google Scholar]
- 20.Potempa J, Kwon K, Chawla R, Travis J. Inter-alpha-trypsin inhibitor. Inhibition spectrum of native and derived forms. J Biol Chem. 1989;64:15109–15114. [PubMed] [Google Scholar]
- 21.Rucevic M, Fast LD, Jay GD, Trespalcios FM, Sucov A, Siryaporn E, Lim YP. Altered levels and molecular forms of granzyme k in plasma from septic patients. Shock. 2007;27:488–493. doi: 10.1097/01.shk.0000246905.24895.e5. [DOI] [PubMed] [Google Scholar]
- 22.Opal SM, Lim Y-P, Siryaporn E, Moldawer LL, Pribble JP, Palardy JE, Souza S. Longitudinal studies of inter-alpha inhibitor proteins in severely septic patients: a potential clinical marker and mediator of severe sepsis. Crit Care Med. 2007;35:387–392. doi: 10.1097/01.CCM.0000253810.08230.83. [DOI] [PubMed] [Google Scholar]
- 23.Baek YW, Brokat S, Padbury JF, Pinar H, Hixson DC, Lim Y-P. Inter-alpha inhibitor proteins in infants and decreased levels in neonatal sepsis. J Pediatr. 2003;143:11–15. doi: 10.1016/S0022-3476(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 24.Chaaban H, Singh K, Lam J, Siryaporn E, Lim Y-P, Padbury JF. The Role of Inter-alpha Inhibitor Protein in the Diagnosis of Neonatal Sepsis. J Pediatr. 2009;154:620–622. doi: 10.1016/j.jpeds.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Wu R, Cui X, Lim Y-P, Bendelja K, Zhou M, Simms HH, Wang P. Delayed administration of human inter-alpha inhibitor proteins reduces mortality in sepsis. Crit Care Med. 2004;32:1747–1752. doi: 10.1097/01.ccm.0000132903.14121.0e. [DOI] [PubMed] [Google Scholar]
- 26.Yang S, Lim YP, Zhou M, Salvemini P, Schwinn H, Josic D, Koo DJ, Chaudry IH, Wang P. Administration of human inter-alpha-inhibitors maintains hemodynamic stability and improves survival during sepsis. Crit Care Med. 2002;30:617–622. doi: 10.1097/00003246-200203000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Jourdain M, Carrette O, Tournoys A, Fourrier F, Mizon C, Mangalaboyi J, Goudemand J, Mizon J, Chopin C. Effects of inter-[alpha]-inhibitor in experimental endotoxic shock and disseminated intravascular coagulation. Am J Respir Crit Care Med. 1997;156:1825–1833. doi: 10.1164/ajrccm.156.6.9611100. [DOI] [PubMed] [Google Scholar]
- 28.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 29.Esmon CT. Why do animal models (sometimes) fail to mimic human sepsis? Crit Care Med. 2004;32:S219–S222. doi: 10.1097/01.ccm.0000127036.27343.48. [DOI] [PubMed] [Google Scholar]
- 30.Opal SM, Artenstein AW, Cristofaro PA, Jhung JW, Palardy JE, Parejo NA, Lim Y-P. Inter-alpha-inhibitor proteins are endogenous furin inhibitors and provide protection against experimental anthrax intoxication. Infect Immun. 2005;73:5101–5105. doi: 10.1128/IAI.73.8.5101-5105.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker CJ. Group B streptococcal infections. Clin Perinatol. 1997;24:59–70. [PubMed] [Google Scholar]
- 32.Garantziotis S, Hollingsworth JW, Ghanayem RB, Timberlake S, Zhuo L, Kimata K, Schwartz DA. Inter-alpha-trypsin inhibitor attenuates complement activation and complement-induced lung injury. J Immunol. 2007;179:4187–4192. doi: 10.4049/jimmunol.179.6.4187. [DOI] [PubMed] [Google Scholar]
- 33.Wakahara K, Kobayashi H, Yagyu T, Matsuzaki H, Kondo T, Kurita N, Sekino H, Inagaki K, Suzuki M, Kanayama N, Terao T. Bikunin suppresses lipopolysaccharide-induced lethality through down-regulation of tumor necrosis factor- alpha and interleukin-1 beta in macrophages. J Infect Dis. 2005;191:930–938. doi: 10.1086/428134. [DOI] [PubMed] [Google Scholar]
- 34.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficiency & safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 35.Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, Sundin DP, Giroir B. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Researching severe Sepsis and Organ dysfunction in children: a gLobal perspective (RESOLVE) study group. Lancet. 2007;369:836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 36.Robache-Gallea S, Morand V, Bruneau JM, Schoot B, Tagat E, Realo E, Chouaib S, Roman-Roman S. In vitro processing of human tumor necrosis factor-alpha. J Biol Chem. 1995;270:23688–23692. doi: 10.1074/jbc.270.40.23688. [DOI] [PubMed] [Google Scholar]
- 37.van der Poll T, Marchant A, Buurman WA, Berman L, Keogh CV, Lazarus DD, Nguyen L, Goldman M, Moldawer LL, Lowry SF. Endogenous IL-10 protects mice from death during septic peritonitis. J Immunol. 1995;155:5397–5401. [PubMed] [Google Scholar]
- 38.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci USA. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]