Abstract
Introduction
Esophageal adenocarcinomas commonly express the Epidermal Growth Factor Receptor (EGFR). This trial assessed the six month overall survival probability in metastatic esophageal cancer patients treated with cetuximab as second line therapy.
Methods
This was a multicenter, open-label phase II study of single agent cetuximab for metastatic esophageal adenocarcinoma patients who failed one prior chemotherapy regimen. Adequate organ function and Zubrod performance status of 0-2 were required. Patients received cetuximab 400mg/m2 IV on week one, and 250 mg/m2 IV weekly thereafter. The primary objective was to determine 6 month overall survival. Secondary endpoints included progression-free survival, response rate, and toxicity. Tumor tissue was collected for correlative studies.
Results
Sixty-three patients were registered, with 8 ineligible or never treated. Fifty-five eligible patients (male=49, female=6; median age=61.2 years [range 30.7-88.5]) were enrolled. Twenty patients survived > 6 months for a 6-month overall survival rate of 36% (95% CI: 24%, 50%). The median overall survival was 4.0 months (95% CI: 3.2, 5.9). Median progression-free survival was 1.8 months (95% CI: 1.7, 1.9). One partial response and 2 unconfirmed partial responses were observed. Two patients experienced grade 4 fatigue. There was one treatment-related death due to pneumonitis. Germline polymorphisms of EGFR, EGF, IL-8, COX-2, VEGF, CCND1, NRP1 and Kras mutational status were not associated with response or survival.
Conclusions
The 6-month overall survival rate of 36% observed on this study failed to meet the primary survival objective. Thus, cetuximab alone cannot be recommended in the second-line treatment of metastatic esophageal cancer.
Keywords: Cetuximab, esophageal cancer, second-line therapy
INTRODUCTION
It is estimated that in 2009, 16,470 patients will be diagnosed with esophageal cancer, and that 14,530 will die of the disease.1 Because a significant proportion of these patients present with or will develop metastatic disease, the prognosis is poor. Chemotherapy has had only a minimal impact on the natural history of metastatic esophageal cancer. First-line chemotherapy results in median survival of up to 11.2 months.2 Little data exists on the benefit of second-line therapy, with one study demonstrating a median time to progression of 7 weeks and a median survival of 5 months), 3 and another with a median survival of 5.6 months in 423 patients.4 A recent second-line randomized study of irinotecan vs. BSC in patients with metastatic gastric and gastroesophageal adenocarcinomas showed an improvement in overall survival for the irinotecan arm (123 vs. 72.5 days).5 To improve the outcome of patients with metastatic esophageal cancer it is imperative that more effective agents be developed.
The Epidermal Growth Factor Receptor (EGFR) is a commonly expressed trans-membrane glycoprotein of the tyrosine kinase growth factor receptor family. EGFR is expressed in many normal human tissues, and activation of this proto-oncogene results in over-expression in many types of human tumors cells in culture.6 In order to inhibit proliferation of EGFR-rich cells, EGFR antagonists, which block the ligand-binding site have been developed. Specifically, monoclonal antibodies to EGFR have been shown to inhibit the proliferation of cells that produce both Transforming Growth Factor (TGF) and Epidermal Growth Factor (EGF).7 Approximately 65% of esophageal adenocarcinomas have been shown to over-express EGFR, and amplification of the EGFR gene has been found in approximately 11%. Patients with esophageal adenocarcinomas overexpressing EGFR appear to have a poorer prognosis than those whose tumors do not overexpress EGFR.8
Cetuximab, a chimerized antibody of the IgG1 subclass, was originally derived from a mouse myeloma cell line.9 Cetuximab blocks binding of EGF and TGFα to EGFR and inhibits ligand-induced activation of this tyrosine kinase receptor. Cetuximab also stimulates EGFR internalization, effectively removing the receptor from the cell surface for interaction with ligand.10 Studies in advanced colorectal cancer have shown cetuximab to have clinical antitumor activity, with an 11% response rate in monotherapy and a 23% response rate in combination with irinotecan.11 Cetuximab has also been approved for use in Head and Neck cancer with radiotherapy for locally advanced disease or with platinum-based chemotherapy for recurrent or metastatic disease.
In a phase I multiple dose clinical trial conducted to examine the tolerability of anti-EGFR in patients with advanced cancer, one out of three esophageal cancer patients demonstrated stable disease for seven months.12
Given the poor prognosis of patients with advanced esophageal cancer, the preclinical rationale for EGFR antagonists and the early clinical data, this phase II study examined cetuximab in metastatic esophageal cancer patients who had failed first line chemotherapy.
MATERIALS AND METHODS
Patients
Eligibility included 1) a histologic diagnosis of adenocarcinoma of the thoracic esophagus or gastroesophageal junction; 2) measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST); 3) one prior regimen of chemotherapy for metastatic or recurrent disease. Patients may have received one prior regimen of adjuvant or neoadjuvant chemotherapy if administered at the time of initial diagnosis with localized disease. 4) No prior cetuximab or other therapy targeting the EGF pathway. 5) Patients were required to have a Zubrod performance status of 0, 1, or 2, and adequate bone marrow, hepatic and renal function. Prior radiation and thoraco-abdominal surgery were allowed.
All patients or their guardians provided informed consent in accordance with institutional and federal guidelines that included permission for the submission of tissue for correlative science. This study (ClinicalTrials.gov Identifier: NCT00096031) was approved by a local Human Investigation Committees and was conducted in accord with an assurance filed with and approved by the Cancer Therapy and Evaluation Program Central Institutional Review Board (CIRB), National Cancer Institute, Department of Health and Human Services.
Study Design
This phase II, open-label, multicenter trial was administered and monitored by SWOG. Patients received a loading dose of cetuximab at 400 mg/m2 IV over two hours on day one and on subsequent weeks cetuximab at 250 mg /m2 IV over one hour. Patients were pre-medicated with diphenhydramine 50 mg IV or p.o. 30 to 60 minutes prior to cetuximab. Treatment continued until disease progression or unacceptable toxicity.
Baseline assessments included medical history and physical examination, performance status, CBC with differential and platelet count, serum chemistries, diagnostic tumor imaging and tumor markers as clinically indicated. During the study, CBC with differential and platelet count was performed weekly, history and physical exam were performed every other week, and serum chemistries were performed at the start of every 4-week cycle.
Patients were monitored for toxicity weekly, with adverse events reported to the SWOG Statistical Center after every 28 day treatment cycle. Toxicity was graded according to the NCI-CTCAE version 3 criteria. In addition, serious adverse events were reported to the NCI via the AdEERS reporting system. Tumor response using RECIST criteria was assessed every 8 weeks.
Correlative Science
Genotyping
Formalin-fixed paraffin–embedded tumor samples were submitted and examined for Kras mutational status, polymorphisms for EGFR, EGF, IL-8, VEGF, COX-2, NRP1 and CCND1. Genomic DNA was extracted using the QIAamp kit (Qiagen). Genotype analysis was performed for most polymorphisms using PCR-RFLP technique.
Forward and reverse primers were used for PCR amplification, and PCR products were digested by restriction enzymes (New England Biolab). Digested fragments were separated on a 4% NuSieve ethidium bromide stained agarose gel. In case no matching restriction enzyme was found, direct sequencing was used.
K-ras Mutational Analysis
Genomic DNA from microdisected tumor samples was extracted, and forward and reverse Primers for Exon 2, codon 12 and 13 k-ras mutation were used for PCR amplification. PCR fragments were sequenced on an ABI 3100A Capillary Genetic Analyzer (Applied Biosystems) and analyzed in antisense direction for the presence of heterozygous mutations. DNA sequence analyses were performed by using the ABI Sequencing Scanner v1.0 (Applied Biosystems).
Statistical Design
This study was monitored by the Data and Safety Monitoring Committee of the Southwest Oncology Group. The primary endpoint of this trial was overall survival. Based on historical survival rates in this population, it was judged that this therapy would be of considerable interest if the overall 6-month survival probability was 50% or greater, but would be of no further interest if it were 30% or less. Secondary objectives included (1) assessment of overall response rate, (2) progression-free survival and time to treatment failure, (3) evaluation of toxicity in these patients, and (4) exploratory analyses of germline polymorphisms of genes involved in the EGFR, DNA repair and angiogenesis pathways.
A two-stage design was used for patient accrual. Thirty patients were to be accrued to the first stage. If at least nine of these survived past 6 months, an additional 25 would be accrued. Of these 55 patients, if a 6-month survival rate of at least 42% was observed, the null hypothesis would be rejected, and this regimen would be considered for further study. This design has a power of 90% at a significance level of 0.04. Overall survival curve was plotted using the Kaplan Meier method.13
RESULTS
Patients
The first patient cohort was accrued from February to November 2005, and the study was temporarily closed to assess survival in these patients. The observed survival rate was sufficiently high to re-open the study, and the second cohort of patients was enrolled from April 2006 to January 2007. In total, sixty-three patients were registered to this study. Eight of these patients are excluded from the analysis. Six of these patients were ineligible: two had a gastric primary; three did not have metastatic disease, and another patient had insufficient baseline documentation. Two additional eligible patients did not receive any treatment and are not evaluable for any endpoint. The demographic information and patient characteristics for the 55 eligible and evaluable patients are listed in Table 1.
Table 1. Baseline Patient Characteristics.
| Median age (range) | 61 (31-89) |
|---|---|
|
Gender Male Female |
49 (89%) 6 (11%) |
|
Performance status 0 1 2 |
14 (25%) 36 (65%) 5 (10%) |
|
Disease status Initial diagnosis Recurrence |
16 (29%) 39 (71%) |
| Prior surgery | 20 (36%) |
| Prior radiation | 34 (62%) |
|
Number of metastatic sites 1 2 3+ |
19 (35%) 20 (36%) 16 (29%) |
Treatment Efficacy
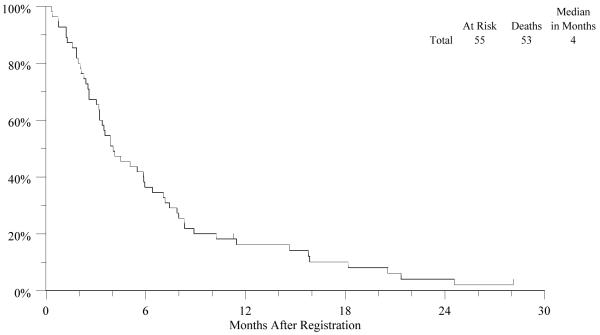
The median number of cetuximab doses was 7 (range 1-40). A major treatment deviation was recorded for one patient who received non-protocol irinotecan and cisplatin while on study. Out of 55 eligible and evaluable patients, 20 survived at least 6 months, for a 6-month overall survival rate of 36% (95% CI: 24%, 50%). The Kaplan-Meier estimate of median overall survival is 4.0 months (95% CI: 3.2, 5.9) [Fig. 1]. Both median progression-free survival and time to treatment failure are 1.8 months (95% CI: 1.7, 1.9).
Figure-1.
The Kaplan-Meier curve for overall survival in patients with metastatic esophageal cancer treated with Cetuximab as second-line therapy
The objective responses to cetuximab are summarized in Table 2. There were 2 partial responses and 1 unconfirmed partial response, for an overall response rate of 5% (95% CI: 1%, 15%). For the 9 patients with either a partial response or stable disease, the median overall survival is 8.3 months (range 4.1-11.3), and the median progression-free survival is 4.0 months (range 3.0-11.3)
Table 2. Treatment -Related Adverse Events.
| Adverse Event | Grade 2 N (%) |
Grade 3+ N (%) |
|---|---|---|
| Dermatologic | 20 (36%) | 4 (7%) |
| Flu-like Symptoms | 13 (24%) | 8 (15%) * |
| Gastrointestinal | 16 (29%) | 4 (7%) |
| Hematologic | 3 (5%) | 1 (2%) |
| Immunological | 3 (5%) | 1 (2%) |
| Infection | 2 (4%) | 1 (2%) |
| Pulmonary | 2 (4%) | 2 (4%) ** |
| Metabolic | 4 (7%) | 6 (11%) |
| Neurologic | 2 (4%) | 1 (2%) |
| Pain | 9 (16%) | 5 (9%) |
Includes 2 patients with Grade 4 fatigue
Includes 1 patient with Grade 5 pneumonitis
Toxicity
The frequency and severity of cetuximab-related toxicities is shown in Table 2. Two patients experienced grade 4 fatigue. Twenty patients experienced grade 3 toxicities, including four patients with grade 3 rash. There was one treatment-related death due to pneumonitis. Dose reductions or treatment delays were reported for twenty-nine of 55 patients (53%).
Correlative Science
Sufficient tissue was available for analysis in 42 of 55 patients. The germline polymorphisms studied included EGFR, EGF, IL-8, COX-2, VEGF, CCND1 and NRP1. Polymorphisms, as performed in this trial, were not found to be associated with response, overall survival, progression-free survival, time to treatment failure or toxicity for any of the genes tested.
Kras mutation was present in 1 out of 42 pts (2%). Table 3 summarizes these findings.
Table 3. Results of Biomarker Analyses.
| Marker | N (%) | Median OS (95% CI), months | p* |
|---|---|---|---|
| CCND1 +870A>G(N=39) | |||
| (rs 17852153) | |||
| AA | 8 (21%) | 2.1 (1.2, 5.9) | 0.18 |
| AG | 23 (58%) | 5.5 (3.4, 7.2) | |
| GG | 8 (21%) | 3.7 (1.8, 15.9) | |
| EGFR +497 G>A (N=38) | |||
| (rs 11543848) | |||
| AA/AG | 31 (81%) | 3.9 (3.0, 7.1) | 0.27 |
| GG | 7 (19%) | 3.5 (1.8, 6.4) | |
| IL-8-251 T>A (N=39) | |||
| (rs 4073) | |||
| AA | 8 (21%) | 5.7 (3.5, 6.4) | 0.60 |
| AT | 17 (44%) | 3.9 (2.4, 8.3) | |
| TT | 14 (35%) | 3.2 (1.3, 5.8) | |
| COX-2 +8473 T>C(N=33) | |||
| (rs 5275) | |||
| CC | 7 (21%) | 3.2 (1.6, 8.3) | 0.69 |
| CT | 15 (46%) | 3.9 (2.6, 6.4) | |
| TT | 11 (33%) | 4.1 (2.1, 8.3) | |
| EGF +61A>G (N=37) | |||
| (rs 4444903) | |||
| AA | 10 (27%) | 6.1 (3.2, 8.0) | 0.94 |
| AG | 10 (27%) | 3.2 (1.6, 8.3) | |
| GG | 17 (46%) | 3.5 (2.6, 7.1) | |
| VEGF +936C>T(N=40) | |||
| (rs 3025039) | |||
| CC | 34 (85%) | 3.7 (2.6, 5.9) | 0.26 |
| CT | 6 (15%) | 7.7 (5.5, 11.5) | |
| NRP-1 C>T(N=34) | |||
| (rs 3750733) | |||
| CC | 10 (29%) | 6.2 (3.9, 11.5) | 0.27 |
| CT | 16 (47%) | 3.7 (1.8, 5.8) | |
| TT | 8 (24%) | 7.7 (2.4, 15.9) |
p values from Cox regression test for heterogeneity across subgroups.
DISCUSSION
This phase II trial examined the efficacy and safety of single agent cetuximab in patients with previously treated metastatic esophageal adenocarcinoma. Patients enrolled on this trial achieved an overall six-month survival probability of 36%. This represented a failure to meet the primary objective of the study. However, the median survival of 4 months is similar to that reported in the other second line trials of chemotherapy in metastatic esophageal adenocarcinoma.3-5 The overall response rate of 5% suggests a similar level of single agent activity as that seen with cetuximab in patients with refractory metastatic colon cancer.14 In metastatic colorectal cancer, the addition of cetuximab to cytotoxic chemotherapy increases anti-tumor response rates,15,16 Phase 2 trials in untreated, metastatic gastric or gastroesophageal junction adenocarcinoma demonstrated that the combination of cetuximab with chemotherapy has a high level of activity, with response rates greater than 40%.17,18
Treatment with single agent cetuximab in this setting was shown to be well-tolerated with the frequency of adverse events comparable to that seen in other trials.14 The frequency and severity of cetuximab-induced rash has been shown to be associated with both response rate and survival.19 In this study, 4 patients developed Grade 3 rash, 3 of whom survived for more than 6 months. The patient who died from pneumonitis is concerning because current trials are combining cetuximab with radiation and chemotherapy for esophageal cancer patients. This is in contrast to large studies of cetuximab in both colorectal and head and neck cancers in which no cases of pneumonitis were reported.20,21 Nonetheless, we recommend that close attention be paid to pulmonary toxicity for patients on these trials.
Evaluation of tissue for a variety of germ line polymorphisms failed to show a relationship between genotype and outcome or toxicity for any of the genes tested, although our ability to detect any differences was limited by the lack of objective responses and the small sample size.
Almost all of the tumors tested were Kras wild-type, with Kras mutation present in only one out of 42 patients (2%). Although the 3 responses in this group of patients were found in patients with Kras wild-type tumors, we see no reason to test patients for Kras mutational status prior to therapy on current clinical trials for patients with adenocarcinoma of the esophagus. Low frequency of Kras mutations have also been observed in squamous cell carcinoma of the esophagus (0%) and gastric adenocarcinoma (11%).22,23 This is in contrast to recent data in colorectal cancer, which clearly demonstrates a correlation between Kras status and the efficacy of anti-EGFR targeted therapy.24,25
This trial failed to meet the primary endpoint. Therefore, cetuximab monotherapy is not recommended as second-line therapy in the treatment of metastatic esophageal adenocarcinoma.
Acknowledgments
Support: This work was supported in part by the following Public Health Service Cooperative Agreement grant numbers awarded by the National Cancer Institute, Department of Health and Human Services: CA32102, CA38926, CA20319, CA45450, CA12644, CA58882, CA35090, CA35176, CA46113, CA76448, CA67575, CA11083, CA45807, CA58416, CA46368, CA35178, CA45377, CA68183, CA67663, CA74647, CA76429, CA27057, CA16385, CA35431, CA45808, CA37981, CA42777 and supported in part by Bristol Myers Squibb, ImClone Systems, Inc., and Response Genetics
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Philip J. Gold, Swedish Cancer Institute, Seattle, WA
Bryan Goldman, Southwest Oncology Group Statistical Center, Seattle, WA
Syma Iqbal, University of Southern California, Los Angeles, CA
Lawrence P. Leichman, Comprehensive Cancer Center at Desert Regional Medical Center, Palm Springs, CA
Wu Zhang, University of Southern California, Los Angeles, CA
Heinz-Josef Lenz, University of Southern California, Los Angeles, CA
Charles D. Blanke, University of British Columbia, and British Columbia Cancer Agency, Vancouver, BC, Canada
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2009. American Cancer Society; Atlanta: 2009. p. 4. [Google Scholar]
- 2.Cunningham D, Starling N, Rao S, et al. Capecitabine and Oxaliplatin for Advanced Esophagogastric Cancer. N Engl J Med. 2008;358:36–46. 208. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Baker J, Pisters PW, et al. Irinotecan/cisplatin in advanced, treated gastric or gastroesophageal junction carcinoma. Oncol. 2002;16:16–18. [PubMed] [Google Scholar]
- 4.Chau I, Norman AR, Ross J, et al. Multivariate prognostic factor analysis and second line treatment in locally advanced and metastatic oesophago-gastric cancer: pooled analysis of 1,080 patients from three multicentre randomized controlled trials using individual patient data; Proc Am Soc Clin Oncol Gastrointest Cancer Sympos; 2004; Abstract #5. [DOI] [PubMed] [Google Scholar]
- 5.Thuss-Patience PC, Kretzschmar A, Deist T, et al. Irinotecan versus best supportive care (BSC) as second-line therapy in gastric cancer: A randomized phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) J Clin Oncol. 2009;27(suppl):15s. doi: 10.1016/j.ejca.2011.06.002. abstr 4540. [DOI] [PubMed] [Google Scholar]
- 6.Yarden Y, Ullrich A. Growth factor receptor tyrosine kinases. Ann Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Norton L, Masui H, et al. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993;85:1327–1333. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- 8.Yacoub I, Goldman H, Odze RD. Transforming growth factor-alpha, epidermal growth factor receptor and MiB-1 expression in Barrett’s associated neoplasia: correlation with prognosis. Mod Pathol. 1997;10:105–112. [PubMed] [Google Scholar]
- 9.Goldstein NI, Prewett M, Zuklys K, et al. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 10.Waksal HW. Role of an anti-epidermal growth factor receptor in treating cancer. Cancer Metastasis Rev. 1999;18:427–436. doi: 10.1023/a:1006302101468. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham D, Humblet Y, Siena S, et al. Cetuximab (C225) alone or in combination with irinotecan (CPT-11) in patients with epidermal growth factor receptor (EGFR)-positive, irinotecan-refractory metastatic colorectal cancer (MCRC) Proc Am Soc Clin Oncol. 2003;22:1012a. [Google Scholar]
- 12.Figlin R, Belldegrun, et al. ABX-EGF, a fully human anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) in patients with advanced cancer: phase I clinical results. Proc Am Soc Clin Oncol. 2002;21:35. [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;52:457–481. [Google Scholar]
- 14.Saltz LB, Meropol NJ, Loehrer PJ, Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 15.Saltz L, Rubin MS, Hochster HS, et al. Cetuximab (IMC-C225) plus irinotecan (CPT-11) is active in CPT-11 refractory colorectal cancer that expresses epidermal growth factor receptor. Proc Am Soc Clin Oncol. 2001;20:3a. [Google Scholar]
- 16.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 17.Pinto C, Di Fabio F, Siena S, et al. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study) Ann of Oncol. 2007;18(3):510–517. doi: 10.1093/annonc/mdl459. [DOI] [PubMed] [Google Scholar]
- 18.Pinto C, Di Fabio F, Barone C, et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesphageal junction adenocarcinoma (DOCETUX study) Br J Cancer. 2009;101:1261–1268. doi: 10.1038/sj.bjc.6605319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Mayer RJ, Gold P, et al. Correlation of acne skin rash and tumor response with cetuximab monotherapy in patients with colorectal cancer refractory to both irinotecan and oxaliplatin. Eur J Cancer. 2004;85(supp 2) Abstract 279. [Google Scholar]
- 20.Lenz HJ, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, et al. Multicenter Phase II and Translational Study of Cetuximab in Metastatic Colorectal Carcinoma Refractory to Irinotecan, Oxaliplatin, and Fluoropyrimidines. J Clin Oncol. 2006;24:4914–4921. doi: 10.1200/JCO.2006.06.7595. [DOI] [PubMed] [Google Scholar]
- 21.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus Cetuximab for Squamous Cell Carcinoma of the Head and Neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzen S, Schuster T, Porschen R, et al. Cetuximab plus cisplatin-5-Fluorouracil versus cisplatin-5-fuorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2009;20(10):1667–1673. doi: 10.1093/annonc/mdp069. [DOI] [PubMed] [Google Scholar]
- 23.Stella G, Rojas Llimpe FL, Barone C, et al. KRAS and BRAF mutational status and response to cetuximab combination therapy in advanced gastric cancer (GC) patients; Proc Am Soc Clin Oncol Gastrointest Cancers Sympos; 2009; Abstract #34. [Google Scholar]
- 24.Hecht JR, Mitchell EP, Baranda J, et al. Panitumumab (pmab) efficacy in patients (pts) with metastatic colorectal cancer (mCRC) with low or undetectable levels of epidermal growth factor receptor (EGFr): Final efficacy and KRAS analyses; Am Soc Clin Oncol Gastrointest Cancers Sympos; 2008; abstract #343. [Google Scholar]
- 25.Tol J, Koopman M, Cats A, et al. Chemotherapy, Bevacizumab, and Cetuximab in Metastatic Colorectal Cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]