Abstract
Sleep duration, sleep continuity, and depression are associated with cardiovascular disease and metabolic disorders. Despite the well-established relationship between sleep and depression, few studies examine these characteristics simultaneously in the development of cardiometabolic disease. Here, we review available studies that include measures of both sleep and depression in relation to cardiometabolic outcomes (cardiovascular disease, diabetes, and the metabolic syndrome). In general, data show that independent of depression, sleep continuity is a risk factor for cardiovascular disease, and short or long sleep duration is a risk factor for diabetes and the metabolic syndrome. Results for associations between sleep duration and cardiovascular disease, and associations between sleep continuity and metabolic disease, are more mixed. Regarding depression, there is preliminary evidence that depression increases risk for cardiovascular disease, independent of sleep continuity. However, there are insufficient data to address whether relationships between depression and cardiovascular and metabolic disease are independent of sleep duration. A number of biobehavioral mechanisms, including inflammation, hypothalamic and sympathetic dysregulation, and obesity and health behaviors, may account for the relationships among sleep, depression, and cardiometabolic disease. After summarizing these mechanisms, we discuss limitations of the extant literature and suggest directions for future research.
Keywords: sleep duration, sleep continuity, depression, cardiovascular disease, diabetes, metabolic syndrome, mechanisms
Introduction
Cardiovascular disease (CVD) remains the leading cause of death in the United States, with an estimated prevalence rate of 37% (1). Type II diabetes, a metabolic disorder and a major risk factor for CVD, affects nearly 8% of the U.S. population and is expected to double in prevalence by the year 2030 (2). Two factors implicated in the development of cardiovascular and metabolic disease include depression and sleep. Individuals who endorse depressive symptoms, as well as other negative emotions, are at increased risk for CVD, diabetes, and related metabolic disorders, such as the metabolic syndrome (3, 4). Analogous associations may exist between sleep and cardiometabolic disease; both short and long sleep, as well as various sleep disturbances, have been related to CVD and diabetes (5, 6). Moreover, both depression and sleep influence physiological pathways believed to play a role in the onset of CVD and diabetes, such as pro-inflammatory activity, dysregulated hypothalamic-pituitary-adrenal functioning, and increased autonomic output (7, 8).
Depressed mood is often accompanied by changes in sleep parameters. As sleep disturbances are part of the diagnostic criteria for mood disorders, it is not surprising that complaints of poor sleep occur in an estimated 50% to 90% of individuals who are diagnosed with depression (as reviewed in 9). Likewise, individuals with insomnia are at increased risk for comborbid mental health disorders, particularly depression (10). Although there is support for the hypothesis that more severe sleep disturbances are concomitant with more severe depressive symptoms, the overlap between sleep and mood is not restricted to clinical sleep and psychiatric disorders. For instance, sleep duration extremes and poor sleep continuity have been associated with symptoms of depression and other negative emotions in non-clinical samples (11).
Despite the connections between sleep and depression, the two bodies of literature examining their roles in cardiovascular and metabolic disease have remained separate. For instance, few of the studies that focus on depression as a predictor of CVD consider the potential role of sleep in this association. Similarly, of the ten studies summarized in a 1999 review on insomnia and coronary heart disease, only two included a measure of depression (5). Since that time, depression has been recognized as a potentially confounding variable in the association between sleep and CVD, and assessment of mood has become more common in such studies. However, the relationship between sleep and depression in the context of subsequent cardiovascular and metabolic disease has not been systematically examined.
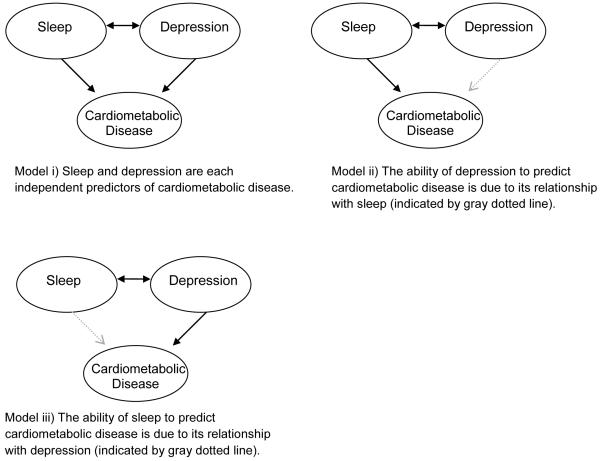
Whether sleep and depression are independent or overlapping risk factors is important for understanding the pathophysiology of cardiometabolic disease, as well as for designing adequate prevention and treatment programs for disease. If sleep and depression are each unique risk factors (as depicted in Figure 1, model i), then both sleep and depression intervention efforts would be needed to reduce risk of cardiometabolic disease. If the sleep disturbances that are common among depressed individuals account for the relationship between depression and disease (model ii), then treatments aimed at first improving sleep may be most efficacious in preventing cardiometabolic disease. Conversely, if depression among individuals with disturbed sleep accounts for the associations between sleep and cardiometabolic disease (model iii), then disease prevention strategies should focus on treating depression as a whole.
Figure 1.
Proposed Models of the Associations among Sleep, Depression, and Cardiometabolic Disease
Our overarching goal is to review the relationships among sleep, depression, and cardiometabolic disease. We begin by defining sleep and depression and briefly summarizing their links. We then review available studies that have Cincluded measures of both sleep and depression in relation to cardiometabolic disease and offer a critique of study methodology. A number of biobehavioral and alternative mechanisms that might account for the observed associations among sleep, depression, and cardiometabolic disease are then described, and we conclude with suggestions and testable hypotheses for future research.
Methodology
Definition and Measurement of Sleep
Sleep is a neurobehavioral process that can be described along several dimensions, such as duration, continuity, architecture, and subjective quality. In turn, a variety of methods are used in sleep assessment, including self-report measures such as questionnaires and interviews, behavioral measures such as actigraphy, and physiological measures such as polysomnography (PSG). By necessity, the current review focuses primarily on self-report measures of sleep duration and continuity, as they have been studied most extensively in conjunction with depression and cardiovascular risk.
Sleep Duration
Sleep duration refers to the total amount of time one spends asleep. In adults, average self-reported sleep durations fall just under 7 hours per night, with actigraphy-assessed sleep slightly lower, although these values may vary by demographic factors such as age, race, and sex (12). The few studies that have examined within-person changes in sleep duration over time suggest that estimates retain moderate to high stability over periods of 1-6 years (13, 14). Many reports linking sleep duration to adverse health outcomes have reported u-shaped associations, with the lowest health risks observed in those reporting seven or eight hours of sleep per night, and the highest risk observed in very short or very long sleepers (e.g., 6).
Sleep Continuity
Sleep continuity refers to the ease or difficulty associated with initiating and maintaining sleep. Self-report assessments of sleep continuity vary across studies; questions might inquire about difficulty falling asleep, difficulty staying asleep, or waking too early, and, less commonly, reports of non-restorative sleep. Other studies ask about “insomnia,” without defining the term, the duration of symptoms, or the degree of daytime impairment, and, therefore, cannot be used to infer an insomnia diagnosis. Thus, although there is some overlap between disturbed sleep continuity and insomnia, the former term is used here to represent more normative variation in difficulty sleeping. Indeed, reports of sleep continuity disturbances are relatively common, with one-year prevalence rates of about 30% in adults (15). One longitudinal study of sleep continuity showed that actigraphy estimates of sleep efficiency remain stable over one year (13); however, a meta-analysis examining separate age cohorts reported that sleep efficiency declines across the lifespan (16).
Definition and Measurement of Depression
Clinical depression, or major depressive disorder (MDD), is a heterogeneous disorder consisting of affective, cognitive, somatic, and behavioral symptoms. To meet diagnosis, one must report a depressed mood or a lack of interest/pleasure in daily activities for a two-week period, along with at least four of the following: changes in appetite and/or weight, insomnia or hypersomnia, psychomotor agitation or retardation, loss of energy, worthlessness or excessive guilt, difficulty concentrating or making decisions, and suicidal ideation or plans. Lifetime prevalence rates for MDD fall between 10% - 25% for women and 5% - 12% for men; point prevalence ranges from 2% - 9% (15). Depression measures most commonly included in studies of sleep, mood, and cardiometabolic disease are self-report questionnaires of depressive symptoms, such as the Center for Epidemiological Studies Depression Scale (CES-D; 17), rather than clinical diagnoses. Such questionnaires may be used to assess symptom levels (i.e., when scored continuously) or to create categorical definitions of depression, although the use of standard cut-off scores may result in low specificity (18). Other common measures are the five-item Mental Health Index of the 36-item Short Form health survey (SF-36 MHI; 19) and the General Health Questionnaire (GHQ; 20). Although these measures were created to assess general mental health or psychological distress rather than depression per se, the face validity of the items is consistent with the affective component of MDD, and there is support for their ability to capture depression adequately (18).
Relationship Between Sleep and Depression
The bi-directional links between sleep and depression have been the subject of much investigation (for reviews, see 9, 10). Below we summarize critical components of this literature in order to better understand how these factors may interact to influence disease risk.
Sleep Duration and Depression
Most studies examining sleep duration and depression reveal a u-shaped association between these two factors, such that elevated depressive symptoms are found in both short and long sleepers. For instance, a large epidemiologic study of Japanese adults showed that those who endorsed more symptoms of depression were more likely to sleep either less than six or more than eight hours a night compared to those with lower depression scores (11). Similar data have recently been reported in U.S. samples (21).
The relationship between acute sleep loss and depression is complex and may vary by clinical status. For instance, partial or total sleep deprivation has rapid but transitory antidepressant effects in individuals with affective disorders (22). In contrast, the impact of sleep deprivation in non-clinical samples appears to be reversed, as healthy individuals undergoing total and partial sleep loss protocols report increased negative emotions (23). At a physiological level, chronic partial sleep restriction in rats leads to changes in both the hypothalamic-pituitary-adrenal and serotonergic systems that are similar to those observed in depressed humans (24). However, the extent to which these findings generalize to chronically short sleep in humans is unknown. Moreover, little experimental work has investigated the links between long sleep and depression.
Sleep Continuity and Depression
Insomnia and MDD are highly co-morbid conditions that overlap in their diagnostic criteria (25), and a vast literature documents the experience of poor sleep continuity in depression. Specifically, individuals with MDD endorse more problems with sleep onset, maintenance, and early morning awakenings than those without MDD (10), and increasing severity of symptoms on depression measures have been associated with greater insomnia frequency and more self-reported awakenings throughout the night (26). The phenomenon of poor sleep continuity in depression is not limited to subjective reports. Common abnormalities in actigraphy and PSG studies include longer sleep latency, increased fragmentation and wake after sleep onset, and disruptions in sleep architecture (9). As overlapping neurobiological mechanisms are involved in the regulation of sleep and mood, it has been suggested that activity in these shared pathways may lead to the common co-occurrence of disrupted sleep and depression (10).
Symptoms of insomnia were often originally considered to occur secondary to depression, and, indeed, depression may precede insomnia (10). However, there is growing support for the theory that insomnia is not just a symptom of depression, but constitutes a separate and distinct condition that commonly occurs in conjunction with, and may even increase risk for, depression and other psychiatric illnesses (25). A 2003 review of eight epidemiological studies reported that insomnia (defined in a variety of ways) was a consistent predictor of subsequent depression (27), and there is evidence that insomnia is associated with poor response to depression treatment (10). However, whether sleep disturbance is causally linked to changes in affect or simply a prodromal symptom of depression remains unclear.
Summary
Sleep and depression are highly related conditions, from the level of shared neurobiology to manifestation in clinical symptoms. At present, it is difficult to distinguish the etiological links between sleep disturbances and depression. For instance, whereas sleep duration and depressive symptoms appear to be related concurrently in a curvilinear fashion, few studies have addressed whether the presence of one of these factors increases risk for the other. With regard to poor sleep continuity, there is growing evidence that insomnia symptoms often precede the onset of subsequent depressive symptoms and may also influence the prognosis of depression. However, a causal relationship between sleep continuity and depression has not been determined. Additional investigation of whether sleep and depression are causally related may not only contribute to our understanding of disorders such as insomnia and depression but may also be relevant to models of cardiometabolic disease. For the purpose of the current review, it is clear that sleep and depression are partly overlapping constructs, and attempts to understand how one of these factors contributes to the development of disease must take the other into consideration.
Cardiometabolic Morbidity and Mortality
Health outcomes in this review are organized in two categories: 1) CVD and cardiovascular mortality and 2) type II diabetes and the metabolic syndrome. The term CVD refers to diseases affecting the heart and/or circulatory system, including coronary heart disease (CHD), myocardial infarction, stroke, angina, hypertension, and atherosclerotic calcification. Type II diabetes is a metabolic disorder characterized by high blood glucose levels in conjunction with insulin deficiency and/or insulin resistance. The metabolic syndrome is a group of anthroprometric and physiological disturbances that tend to cluster together and increase risk for type II diabetes and CVD (28). The core components of the metabolic syndrome typically include excess abdominal or body fat, abnormal insulin or glucose regulation, dyslipidemia, and high blood pressure.
Study Selection Criteria
A literature search was performed in PubMed and PsycInfo that included combinations of the keywords: sleep, duration, quality, insomnia, depression, depressive symptoms, negative affect, cardiovascular disease, coronary heart disease, stroke, myocardial infarction, hypertension, blood pressure, diabetes, metabolic syndrome, mortality. Reference lists of identified articles were reviewed for additional relevant work. To be included in the review, studies had to measure sleep (duration or continuity) and depression, and one of the disease outcomes (in any of the ways described above). The sleep continuity questionnaires included in these studies primarily asked about difficulty initiating or maintaining sleep; however, we also included one study that asked about “restless sleep” and one that asked about general “insomnia.” Studies that included measures which explicitly asked about depressive symptoms or mood were included; however, those that only measured antidepressant use and did not assess depressive symptoms were excluded. Studies that examined sleep and depression as predictors of all-cause mortality were excluded unless they provided findings relevant to CVD-specific mortality. We review here the 18 articles that evaluated sleep and depression in relation to CVD using a longitudinal, prospective design and exclude cross-sectional studies. Because a small number of prospective studies evaluated sleep, depression, and incidence of diabetes (four) or the metabolic syndrome (none), both prospective and cross-sectional studies of these health outcomes were included (eight total).
Literature Review
Sleep Duration, Depression, & CVD
Thirteen studies examined sleep duration, depression, and cardiovascular morbidity or mortality (Table 1). In general, sample populations were very large and consisted of community-dwelling middle-aged or older-aged adults. Follow-up periods ranged from two to 17 years. With one exception (29), duration was assessed by self-report, with a typical reference group of seven or eight hours. Most studies used questionnaires to derive continuous or categorical definitions of depression. All studies excluded participants with baseline CVD, except one which adjusted for baseline disease when examining CHD death (30).
Table 1. Longitudinal Studies of Sleep, Depression, & Cardiovascular Disease.
| Reference | Sample and Follow-Up |
Sleep Measure(s) | Depression Measure |
CVD Outcome | Sleep Findings | Depression Findings |
|---|---|---|---|---|---|---|
| King (29) |
n = 495; 41% M age: 35-47 FU: 5 yrs |
- actigraphy over 6 nights and self-reported habitual sleep duration |
- CES-D - Cook-Medley Hostility Scale |
coronary artery calcification |
actigraphy: decreasing duration = ↑ calcification self-reported duration: NS |
Not Reported |
| Mallon (30) |
n = 1870; 48% M age: 45-65 FU: 12 yrs |
- DIS & DMS severity - avg. duration (< 6, 6-8*, > 8) |
‘Do you feel depressed?’ (Yes/No) |
CVD mortality |
MEN: DIS = ↑ CVD mortality duration = NS WOMEN: ALL NS |
MEN: Depression = ↑ CVD mortality WOMEN: Depression = NS |
| Ayas (31) |
n = 71,617; 100% W age: 45-65 FU: 10 yrs |
avg. duration in 24-hr (≤5, 6, 7, 8*, ≥9) |
≤ 52 MHI (assessed 6 yrs after sleep) |
nonfatal MI or fatal CHD |
≤ 5, ≥ 9 = ↑ CHD | Not Reported |
| Chen (32) |
n = 93,175; 100%W age: 50-79 FU: 7.5 yrs |
avg. duration over past 4 weeks (≤5, 6, 7*, 8, 9, ≥10) |
5-item CES-D scores (sleep item excluded) and history of depression |
fatal/nonfatal ischemic stroke |
adjusted for demographics + depression: ≤ 6, 8, ≥ 9 = ↑ stroke adjusted for CV risk factors: ≤ 6 = NS; 8, ≥ 9 = ↑ stroke excluding baseline CVD: ≤ 6, 8, ≥ 9 = ↑ stroke |
Not Reported |
| Ferrie (33) |
n = 9,781; 68% W age: 35-55 FU1: 17 yrs FU2: 12 yrs |
avg. weeknight duration, assessed at Phase 1 and Phase 3 (~6 yrs later) (≤ 5, 6, 7*, 8, ≥ 9) |
GHQ scores (sleep item excluded) |
CVD mortality |
Phase 1: all NS Phase 3: ≤ 5 = ↑CVD mortality decrease from 6, 7, or 8 hrs. b/w Phase 1-Phase 3 = ↑ CVD mortality |
Not Reported |
| Gangwisch (34) |
n = 4913, 36% M age: 32-86 FU: 8-10 yrs |
- avg. duration (≤ 5, 6, 7-8*, ≥ 9) -frequency of DIS & DMS (combined in 1 scale) |
≥ 16 on CES-D, antidepressant use |
HT (self or hospital report, or cause of death) |
Total Sample: ≤ 5 = ↑ HT - DIS/DMS = ↑ HT - with ≤ 5 & DIS/DMS in same model, both NS ages 32-59: ≤ 5 = ↑ HT - DIS/DMS = ↑ HT ages 60-86: all NS |
Total Sample: Depression = ↑ HT ages 32-59: -Depression = ↑ HT -NS after adjusting for ≤ 5 & DIS/DMS ages 60-86: Depression = NS |
| Ikehara (35) |
n = 98,634; 42% M age: 40-79 FU: 14.3 yrs |
avg. weekday duration (< 4.5, 5, 6, 7*, 8, 9, ≥ 9.5) |
4 study-specific depressive items |
CVD mortality |
MEN: 8, 9, ≥ 9.5 = ↑ CVD mortality WOMEN: 5, 8, 9, ≤ 9.5 = ↑ CVD mortality |
Not Reported |
| Meisinger (36) |
n = 6896; 51% M age: 45-74 FU: 10.1 yrs |
avg. duration (≤5, 6, 7, 8*, ≥9) |
Zerssen affective symptom checklist |
fatal/nonfatal MI, sudden cardiac death |
MEN: All NS WOMEN: ≤ 5 = ↑ MI DIS = ↑ MI |
Not Reported |
| Cappuccio (37) |
n = 5766; 73% M FU: 3 yrs |
avg. weeknight duration (≤ 5, 6, 7*, 8, ≥ 9) |
SF-36 MHI & GHQ scores |
BP ≥ 140/90 or HT medication |
MEN: all NS WOMEN: ≤ 5 = ↑ HT; NS after adjusting for CVD risk factors & depression |
Not Reported |
| Patel (38) |
n = 82,969; 100% W Mean age: 53 FU: 14 yrs |
avg. duration in 24-hr (≤ 5, 6, 7*, 8, ≥ 9) |
≤ 52 MHI (assessed 6 yrs after sleep) |
CVD mortality | ≥ 9 = ↑ CVD mortality | Not Reported |
| Stone (39) |
n = 8101; 100% W age: 69+ FU: 7 yrs |
avg. duration in 24-hr (including naps) (<6, 6-<8, 8-<9*, 9-<10, ≥10) |
≥ 6 Geriatric Depression Scale |
CVD mortality | 9-<10, ≥ 10 = ↑ CVD mortality | Not Reported |
| Lan (40) |
n = 3079; 57% M age: 64+ FU: 10 yrs |
avg. duration (< 7, < 8*, < 9, < 10, ≥ 10) |
≥ 5 on abridged CES-D (possible scores 0-24) |
CVD mortality |
MEN: ≥ 10 = ↑ CVD mortality WOMEN: < 9, < 10, ≥ 10 = ↑ CVD mortality |
Not Reported |
| Lopez- Garcia (41) |
n = 890, 48% M Mean age: 70 FU: 2 yrs |
avg. duration (4-5, 6, 7*, 8, 9, 10-15) |
‘Depression with need for treatment’ (yes/no) |
self-reported hypertension |
All NS | Not Reported |
| Nicholson (42) |
n = 5449; 100% M age: 32-86 FU: 6.8 yrs |
sleep subscale from the GHQ, classified as new, former, or persistent disturbances |
Distress/anxiety & depression GHQ subscales, classified as new, former, or persistent |
CVD events: CHD death, nonfatal MI, angina |
-Persistent & former sleep disturbances = ↑ CVD events -New sleep disturbance = NS |
persistent distress/anxiety subscale = ↑ CVD depression subscale = NS |
| Newman (43) |
n = 5888; 42% M age: 65+ FU: 4.9 yrs |
occurrence of DIS, DMS, daytime sleepiness, & snoring (‘yes/no’) |
10-item CES-D | 1.CVD death and morbidity (MI, angina, CHF, stroke) 2. incident MI 3. CHF |
MEN: all symptoms = NS WOMEN: daytime sleepiness = ↑ CVD death/morbidity -all complaints = NS for MI -daytime sleepiness combined with DMS = ↑ CHF |
CES-D = NS |
| Phillips (44) |
CVD sample n = 11,863; 44% M age: 35-55 FU: 6 yrs |
occurrence of DIS, DMS, & nonrestorative sleep (‘yes/no’) |
Maastricht Questionnaire |
fatal/ nonfatal MI or other cardiac event |
All 3 sleep symptoms = ↑ CV events Individual sleep complaints = NS |
Not Reported |
| HT sample n = 8757; 45% M age: 45-65 FU: 6 yrs |
occurrence of DIS, DMS, & nonrestorative sleep (‘yes/no’) |
Maastricht Questionnaire |
SBP > 160, DBP > 95, or HT medication |
DIS = ↑ HT DMS = ↑ HT |
Not Reported | |
| Phillips (45) |
n = 1419; 41% M age: 64-91 FU: 6 yrs |
occurrence of DIS & DMS (‘yes’/’no’) |
≥ 10 on modified CES-D (0-30 range) |
SBP ≥ 140, DBP ≥ 90, or history of HT + medication |
non-AA Men: DIS = ↓HT AA WOMEN: DIS = ↑ HT (trend) AA men, non-AA WOMEN: all NS |
Not Reported |
| Schwartz (46) |
n = 2960 ; 34% M age: 65+ FU: 3 yrs |
frequency of DIS, DMS, restless sleep, combined in a continuous scale |
modified CES-D | fatal/nonfatal MI |
Adjusted for demographics & education: DIS, restless sleep, & sleep scale = ↑ MI Adjusted for above + self-rated health & depression: DIS, restless sleep, & sleep scale = NS |
CES-D = ↑ MI |
X* = reference group. CES-D = Center for Epidemiological Studies Depression Scale; CHD = coronary heart disease; CHF = coronary heart failure; CV = cardiovascular; CVD = cardiovascular disease; DBP = diastolic blood pressure; DIS = difficulty initiating sleep; DMS = difficulty maintaining sleep; GHQ = General Health Questionnaire; HT = hypertension; FU = follow-up; M = men; MI = myocardial infarction; NS = non-significant; MHI = Mental Health Index of the Short Form-36; SBP = systolic blood pressure; W = women.
Seven of 13 studies reported that sleep durations of less than five or six hours predicted CVD after adjustment for depression and all other covariates (29, 31-36). Of note, most of the studies that observed a link between short sleep and CVD reported that this relationship was either stronger in women (29), present in women only (35-37), or observed in all-female samples (31, 32). Given that women typically sleep longer than men (12), sleep durations of less than five or six hours in women might indicate a more severe deficit with greater cardiometabolic consequences.
In contrast, short sleep and CVD were unrelated in six studies after full multivariate adjustment (30, 37-41). In several cases, authors noted that associations between short sleep and CVD were attenuated after adjustment for all covariates, including depression, but did not evaluate the impact of confounding variables individually (32, 33, 37). Thus, it is unclear if the lack of a relationship between short sleep and CVD in these six studies was due to depression specifically.
With regard to long sleep, six of 13 studies reported that self-reported durations of eight, nine, or ten hours were related to cardiovascular outcomes, after adjusting for a number of covariates, including depressive symptoms (31, 32, 35, 38-40). Seven studies did not observe a similar association between long sleep and CVD after full adjustment for covariates, including depression (29, 30, 33, 34, 36, 37, 41). One of these studies reported that participants with both long sleep and elevated depressive symptoms developed hypertension, supporting a synergistic effect of sleep and depression; however, this effect was observed in middle-aged participants only (34). Thus, the cardiovascular risk associated with short or long sleep duration in studies that measure and adjust for depression remains unclear.
Only two of the 13 above studies reported whether depression was associated with CVD after adjustment for sleep duration and other factors. In the first, depressive symptoms predicted hypertension after adjusting for short sleep (however, this relationship was attenuated in a model that included both short sleep and sleep continuity disruptions; 34). The other study observed that elevated depressive symptoms were associated with CVD mortality in men only (30). Therefore, while about half of the 13 studies assessing sleep, depression, and CVD suggest that extreme sleep duration, either short or long, is a risk factor independent of, or in addition to, depression, less is known about the relationship between depression and CVD, independent of sleep duration.
Sleep Continuity, Depression, & CVD
Eight longitudinal studies included measures of sleep continuity and depression when evaluating cardiovascular morbidity or mortality as an outcome (Table 1). These studies were large, consisted of community-dwelling middle-aged or older-aged adults, and had follow-ups ranging from 3 to 12 years. Depression was measured with questionnaires, with the exception of one study that asked about depressed mood only (30). All of the studies excluded participants with baseline CVD, except one which adjusted for baseline disease when examining CHD death (30).
Six of eight studies reported significant associations among components of poor sleep continuity and CVD, after adjusting for depression (30, 34, 36, 42-44), whereas two did not (45, 46). Of the latter two studies, one showed that poor sleep continuity was no longer associated with CVD after adjustment for scores on the CES-D, consistent with depression being a pathway in the sleep-CVD relationship (46). The other concluded that poor sleep continuity actually led to a reduced risk of hypertension in non-African American men (45). Five of the eight studies evaluated the link between depression and CVD and adjusted for poor sleep continuity. Four of these found that depression (30, 34, 46), or related psychological distress (42), predicted CVD, independent of sleep continuity, while one did not (43). Thus, most of the extant literature suggests that both poor sleep continuity and depression are independent risk factors for CVD, although there is some variation in individual study results (34, 46).
Sleep Duration, Depression, and Metabolic Disease
Five studies evaluated sleep duration, depression, and diabetes or the metabolic syndrome (Table 2). The three prospective studies of diabetes excluded participants with baseline disease and had follow-ups ranging from eight to 12 years (47-49). Sample sizes ranged from 1,170 participants (47) to over 70,000 participants (48), with the latter consisting of women only. All studies used self-report measures of duration.
Table 2. Studies of Sleep, Depression, & Diabetes or the Metabolic Syndrome.
| Reference | Sample and Follow-Up |
Sleep Measure(s) | Depression Measure |
Metabolic Outcome |
Sleep Findings | Depression Findings |
|---|---|---|---|---|---|---|
| Mallon (47) |
n = 1170; ?% M age: 45-65 FU: 12 yrs |
avg. duration (≤5, ≥9) DIS & DMS severity |
‘Do you feel depressed?’ (yes/no) |
self-reported diabetes |
MEN DMS = ↑ diabetes ≤ 5 = ↑ diabetes DIS, ≥9 = NS WOMEN: All NS |
Depression = ↑ diabetes after adjusting for age; risk was eliminated after full adjustment |
| Ayas (48) |
n = 70,026; 100% W Mean age: 52.4 FU: 10 yrs |
avg. duration in 24 hours (≤5, 6, 7, 8*, ≥9) |
≤ 52 MHI | diabetes (asymptomatic & symptomatic) |
All Diabetes ≥ 9 = ↑ diabetes Symptomatic Diabetes ≤ 5, ≥ 9 = ↑ diabetes |
Not Reported |
| Gangwisch (49) |
n = 8992; ?% M age: 32-86 FU: 8-10 yrs |
avg. duration (≤5, 6, 7*, 8, ≥9) |
≤ 16 CES-D | doctor/hospital diagnosed diabetes, or cause of death |
≤ 5, ≥ 9 = ↑ diabetes | CES-D = NS |
| Gottlieb (50) |
n = 1486; 49% M age: 53-93 cross-sectional |
avg. weeknight duration (≤5, 6, 7-8*, ≥9) frequency of DIS, DMS, & sleeping pill use |
2 questions about depressed mood from MHI |
diabetes (oral glucose tolerance test) |
≤5, 6, ≥9 = ↑ diabetes DIS, DMS, sleeping pill use = NS |
Not Reported |
| Hall (51) |
n = 1214; 57% M age: 30-54 cross-sectional |
avg. duration, weighted by weeknight & weekend (<6, <7, <8*, >8) |
CES-D, minus sleep item |
metabolic syndrome |
< 6, 6 - <7, > 8 = ↑ metabolic syndrome | CES-D = NS |
| Eriksson (52) |
n = 5227; 41% M Mean age: 47 FU: 8-10 yrs. |
“frequency of insomnia over the past year” |
frequency of depression, anxiety, apathy over past year |
diabetes (oral glucose tolerance test or medication) |
MEN: insomnia= ↑ diabetes WOMEN: insomnia = NS |
MEN: anxiety, apathy = ↑ diabetes; depression = NS WOMEN: all NS |
| Takeuchi (53) |
n = 1215; 100% M Mean age: 42.5 cross-sectional |
occurrence of DIS & DMS | depression and anxiety items on the POMS |
metabolic syndrome |
Not Reported | Depression = → Metabolic Syndrome Anxiety = NS |
| Vgontzas (54) |
n = 1741; 48% M Mean age: 48.7 cross-sectional |
insomnia = complaint with a duration of at least 1 year; poor sleep = moderate to severe complaint of DIS, DMS, or unrefreshing sleep 1 night of sleep duration measured by PSG (< 5, 5-6, > 6*) |
treatment for depression |
diabetes (fasting blood glucose or diabetes treatment) |
Insomnia = ↑ diabetes, only in those sleeping < 5 hours in PSG study Poor sleep = NS |
Not Reported |
X* = reference group. AA = African American; SBP = systolic blood pressure; CES-D = Center for Epidemiological Studies Depression Scale; CVD = cardiovascular disease; DBP = diastolic blood pressure; DIS = difficulty initiating sleep; DMS = difficulty maintaining sleep; GHQ = General Health Questionnaire; FU = follow-up; M = men; MHI = Mental Health Index of the Short Form-36; NS = non-significant; POMS = Profile of Mood States; PSG = polysomnography; W = women
All four studies that evaluated diabetes found associations with short sleep duration (47-50), and three found associations with long sleep (48-51). All of these associations remained significant after adjusting for cut-offs on depression questionnaires (48, 49) or questions about depressed mood (47, 50). The only study of the metabolic syndrome found higher rates of this syndrome among those reporting either short or long sleep after adjusting for a continuous measure of depression and other relevant covariates (51). When individual components of the metabolic syndrome were evaluated, both short and long sleep were associated with fasting glucose levels, and short sleep was linked to increased waist circumference and trigylceride levels, independent of symptoms of depression (51).
Three studies reported that depression, as measured by a cut-off (49) or total score (51) on the CES-D, or a single item regarding depressed mood (47), was unrelated to diabetes or the metabolic syndrome in multivariate models that included sleep measures. In the other two studies, the relationship between depression and diabetes was not evaluated (48, 50). Although this pattern was found in only a small number of studies and may be limited by inadequate assessment of depressive symptoms, these data are consistent with sleep duration being a pathway that links depression and metabolic disturbances. However, no studies reported whether the lack of an association between depression and metabolic disease was due to sleep duration per se.
Sleep Continuity, Depression, and Metabolic Disease
Five studies evaluated sleep continuity, depression, and diabetes or the metabolic syndrome (Table 2). The two prospective studies excluded participants with baseline diabetes and had follow-up periods of eight to 12 years (47, 52). Sample sizes ranged from 1,170 (47) to 5,227 participants (52), and one study consisted of only men (53). Each used a different depression measure.
With regard to poor sleep continuity, two studies reported significant associations with incident diabetes, after adjusting for depression and other covariates (47, 52). Of the remaining three studies, one reported that persistent sleep continuity difficulties were related to diabetes, but only among those who slept less than five hours as measured by PSG (54), and one reported that frequent sleep continuity difficulties were unrelated to a concurrent diabetes diagnosis (50). The final study evaluated the relationship of depressive symptoms with the metabolic syndrome independent of sleep continuity, but it did not report the metabolic risk associated with sleep continuity (53).
With regard to depression, one study reported that symptoms associated with depression (frequent anxiety, apathy, and fatigue) predicted diabetes and pre-diabetes in men, while overall depression was not a risk factor in men or women (52). A second reported that the bivariate relationship between depression and incident diabetes was eliminated in multivariate models that included poor sleep continuity and other covariates (47). In the third study, symptoms of depression were associated concurrently with the metabolic syndrome in Japanese men after adjusting for sleep disturbances and other covariates (53). The final two studies did not report the relationship between depression and diabetes (50, 54). In sum, data regarding the independent contributions of sleep continuity and depression to diabetes and the metabolic syndrome are mixed at this time, indicating an important area for future research.
Summary of Associations among Sleep, Depression, and Cardiometabolic Disease
We identified 19 articles that evaluated sleep duration, depression, and cardiometabolic disease. These studies offered some evidence of an association between extreme sleep duration and cardiometabolic disease, independent of depression. In particular, extreme sleep duration was consistently related to diabetes or the metabolic syndrome, even after adjustment for depression and other covariates. The literature on sleep duration and incident CVD, independent of depression and other covariates, was more mixed in that about half the studies showed significant relationships and half did not. Short sleep and CVD were more frequently associated in women than in men, and sex differences in the links among sleep, depression, and cardiometabolic disease may deserve further attention. Finally, although one of our aims was to evaluate whether depression was related to cardiometabolic disease, independent of sleep duration, there are insufficient data to address this question at this time.
With regard to sleep continuity, depression, and cardiometabolic disease, we identified 13 relevant articles. Data from these studies offered preliminary support for the hypothesis that sleep continuity and depression each have independent associations with incident CVD. There was more evidence linking poor sleep continuity to increased risk for CVD over and above depression, while fewer studies evaluated depression and CVD, independent of sleep continuity. The links among disturbed sleep continuity, depression, and diabetes/metabolic syndrome were less clear, due to the small number of studies and inconsistent findings.
Limitations of Existing Literature
Study Design & Analysis
Few studies in this review reported whether sleep and depression were related to disease risk in fully adjusted models. While nearly all of the studies reported the cardiometabolic risk associated with extreme sleep duration or poor sleep continuity after including depression as a covariate, very few reported the cardiometabolic risk associated with depression after adjustment for sleep duration or continuity. Thus, the extant literature provides some evidence that sleep may increase risk for cardiometabolic disease independent of depression, but additional work is needed to determine whether the cardiometabolic risk related to depression is independent of sleep.
Additionally, although we had hoped to identify whether sleep and depression were independent risk factors for cardiometabolic disease (consistent with model i in Figure 1), or whether one was more important than the other as in models ii or iii, few studies employed statistical designs that allowed the relationships between sleep and depression to be modeled. Many authors noted that associations between sleep or depression and disease were eliminated after adjustment for covariates but did not report the individual factors that were responsible for the attenuated effect. Moreover, few studies collected repeated measures of sleep and depression over time, and thus could not determine whether changes in sleep preceded other depressive symptoms, changes in depression preceded sleep, or if these two factors fluctuated together. Reporting the change in disease risk related to sleep after adjusting for depression alone, or vice versa, in combination with repeated data points over time, will offer evidence regarding which of these two factors confer a stronger risk for disease. However, experimental designs in which sleep or depression are modified will ultimately be needed in order to support or disconfirm mediational models of sleep, depression, and cardiometabolic disease.
Finally, nearly all of the studies in this review were longitudinal, had moderately long follow-ups, and adjusted for baseline disease, which are important steps in ruling out reverse causation. Moreover, several studies that attempted to evaluate reverse causality did not find evidence for this explanation (32, 36, 38, 42), making it unlikely that CVD and diabetes were responsible for changes in sleep and depression. However, models in which subclinical or other unmeasured disease contributed to changes in sleep or depression, either directly, or through mediating factors such as medication, pain, or other sleep disorders (i.e., restless legs syndrome) cannot be ruled out based on the available literature.
Measurement of Sleep
The vast majority of sleep duration findings were based upon a single, global estimate of habitual sleep time. Advantages of such measures include ease of administration and their suitability for large, epidemiological studies. However, the degree of variability in the use of specifiers (i.e., “on weeknights”, “over the past month”), as well as in how participants interpret such questions, suggests that reports of duration may not be standardized across studies or individuals. Most studies did not inquire about daytime naps, which may influence nocturnal sleep as well as disease risk. Additionally, there is evidence that discrepancies between estimates of typical sleep duration and prospective recordings in nightly diaries increase with depressed mood (55), suggesting that self-reported habitual sleep duration may be a less accurate assessment tool among those with depression.
The manner in which poor sleep continuity was defined also varied across studies. Although the most common method was to inquire about difficulty falling and staying asleep, questionnaires ranged from endorsement of one of these symptoms to more comprehensive measures based upon symptom frequency, severity, or duration. Heterogeneity in assessment resulted in varying rates of disturbed sleep, and, thus, may have influenced observed relationships between sleep and disease. There was no indication that one aspect of sleep continuity was more strongly associated with cardiometabolic disease than the others, as the number of positive findings regarding trouble falling asleep versus staying asleep was similar. However, two studies reported that specific combinations of sleep disturbances predicted outcomes (44, 54), and there was some indication that sleep continuity problems that were defined as persistent (e.g., experienced for at least one year) were more likely to predict disease (42, 54). Thus, comprehensive questionnaires regarding the frequency and persistence of sleep continuity symptoms may be important in future work.
Measurement of Depression
While most studies used well-established depression questionnaires, others used single questions, which are likely less valid. Moreover, many studies relied on a dichotomous definition, with breakdowns based upon pre-existing cut-offs or study-specific criteria. A recent review showed that adopting more stringent criteria for depression increases its ability to predict CVD, perhaps because such definitions reflect more “severe” pathology (3). Therefore, it is possible that studies using less stringent depressive criteria may have been less likely to observe associations between depression and disease, and perhaps more likely to observe links between sleep and disease (in the case that depression was not captured adequately).
The content of depression scales is also important to consider. Whereas some measures inquire about the full range of depressive symptoms (e.g., CES-D), indices of general mental health and well-being were also used in many of the studies included in this review. Such scales focus more exclusively on negative affect, and whether or not sleep predicts disease risk over and above depressed mood is a different question than whether or not sleep predicts disease over and above the entire range of depressive symptoms. Indeed, two studies reported that generalized distress (42), or apathy and anxiety (52), were related to CVD or diabetes, while depression was not, suggesting that more work is needed to disentangle the unique abilities of sleep, depression, and other psychological constructs to predict disease. Finally, it is possible that depression scales containing somatic-vegetative symptoms result in more false positives, particularly among ill or elderly populations. Such measures may also correlate more strongly with sleep than those that focus solely on mood, and, therefore, may be more likely to confound relationships between sleep and other variables.
Covariates
The models in Figure 1 are not exhaustive, and other plausible explanations for the observed links among sleep, depression, and disease, may exist. Nearly all of the findings in the reviewed studies were adjusted for demographics, health behaviors, and a variety of relevant risk factors, such as BMI and cholesterol. Several authors reported that the risk associated with sleep decreased or became non-significant after adjustment for these “traditional” cardiometabolic risk factors or medical co-morbidities (33, 37, 48). Notably, such covariates may lie on the causal pathway between sleep or depression and disease, and thereby may have attenuated effects. For example, sleep or depression may influence obesity, which in turn increases risk for CVD and diabetes.
One key factor missing from the literature on sleep, depression, and cardiometabolic disease is sleep apnea. The repeated awakenings and hypoxemia characteristic of sleep apnea contribute to disease risk through a variety of physiological mechanisms, including surges in autonomic activity, oxidative stress, endothelial dysfunction, and pro-inflammatory activity (56). Although seemingly counterintuitive, reports of poor sleep and insomnia symptoms are increased in those with obstructive sleep apnea (57), a relationship which may be particularly strong in women (58). Sleep apnea is also associated with depression, independent of insomnia and medical conditions, and treatment of apnea has been shown to improve depressive symptoms (59). Given the pathophysiological influence of sleep apnea on cardiometabolic disease and its overlap with depression, sleep duration, and continuity, apnea likely accounts for some of the cardiometabolic risk attributed to sleep and/or depression. Thus, future research should evaluate sleep apnea when possible.
Finally, few studies adjusted for sleep medication or antidepressant use (those that did include 30, 34, 43, 46, 50). Some antidepressants alter aspects of sleep, particularly sleep architecture (60), and there are also data linking the use of antidepressants to sudden cardiac death (61), and frequent hypnotic use to increased mortality (62). Thus, medications may not only be important to consider when defining sleep and depression, but they may also have an impact on cardiometabolic disease, and future studies should aim to assess medications and adjust analyses accordingly.
Mechanisms Linking Sleep, Depression, and Cardiometabolic Disease
Another way to evaluate the role of sleep and depression in cardiometabolic risk is to consider their association with physiological and behavioral mechanisms implicated in disease. In other words, it is important to consider how sleep and depression may lead to cardiometabolic disease and whether or not these pathways are similar or different. In the following section, relationships between sleep and depression and potential biobehavioral pathways to cardiometabolic disease are summarized. When available, key studies that have evaluated both sleep and depression in conjunction with these pathways are highlighted.
Pro-inflammatory Pathways
Chronic, low-grade inflammation, as indexed by levels of the proinflammatory cytokine interleukin (IL)-6 and the acute phase reactant, C-reactive protein, is associated with cardiovascular and diabetes risk, independent of conventional risk factors (63). As short sleep, poor sleep continuity (7), and depression (8) have each been related to increased levels of these markers in some but not all studies, inflammation may represent one pathway linking sleep and depression to cardiometabolic disease. A number of studies simultaneously evaluating sleep continuity and depression or related distress have reported that each is associated with increased inflammation, independent of the other (e.g., 64). However, there is also some evidence that disturbed sleep is responsible for the elevations in inflammation seen in those with depression. For instance, a study of individuals with MDD found that PSG-measured sleep latency and REM density were more strongly associated with IL-6 than depressive status (65). Moreover, depression was no longer associated with inflammatory markers when sleep items were removed from the depression questionnaire, suggesting that this association was at least partially due to disrupted sleep (65). Poor sleep continuity has also been found to drive associations between depression and other aspects of innate immune activity, such as natural killer cell activity (66).
HPA Axis
The hypothalamic-pituitary-adrenal (HPA) axis is a neuroendocrine system involved in the stress response and the regulation of various physiological processes, including immune and inflammatory responses, and sympathetic nervous activity. Alterations in HPA activity are associated with autonomic and metabolic perturbations, and possibly, increased cardiometabolic disease (67). Numerous studies have evaluated the relationships among depression, sleep, and activity of the HPA axis (for a review, see 68). Much of this work has focused on the differential associations between PSG sleep patterns and neuroendocrine abnormalities in depression in an attempt to identify depressive sub-types. Antonijevic summarized findings showing that the melancholic sub-type of depression is associated with HPA over-activation, insomnia, and consistent PSG alterations, whereas the atypical sub-type is associated with HPA under-activation and sleep disturbances that are inconsistent or lacking altogether (68). Thus, it is possible that different presentations of depression, characterized by varying degrees of sleep disturbances, may confer differential risk for disease. Other studies comparing sleep and HPA indices during depressive episodes to those assessed during remission or recovery have found that PSG sleep disturbances are independent of clinical status, while HPA activity tends to normalize during remission or recovery (e.g., 69).
Autonomic Nervous System Activity
Both disrupted sleep and depression are related to increased activity of the autonomic nervous system, which may represent another mechanism by which these factors confer risk for disease. For instance, PSG-measured sleep continuity is related to higher sympatho-adrenal medullary activity, as indexed by nocturnal norepinephrine excretion (70), as well as lower heart rate variability, an indicator of sympathovagal balance (71). Somewhat analogous associations exist between depression and autonomic activity, as hypersecretion of norepinephrine and decreased heart rate variability are observed in individuals with depression (8). However, no studies have evaluated the relationships among sleep, depression, and autonomic functioning directly.
Obesity
As obesity is a well-established risk factor for CVD and diabetes, and is related to short and long sleep durations (72, 73), and depression (74), its role as a potentially common mechanism among these factors should not be overlooked. All of the results reported herein were adjusted for body mass index (BMI); however, several studies noted that the effects of sleep on health outcomes were attenuated after such adjustments, raising the possibility that increases in body weight may lie on the causal pathway linking sleep to CVD or diabetes. There is some evidence that the combination of depression and disturbed sleep may be linked to increased BMI and, by extension, to cardiometabolic disease risk. For instance, Vgontzas et al. (75) recently reported that short sleep duration was related to higher BMI in individuals reporting high emotional distress, but not in those reporting low emotional distress, consistent with a synergistic model of psychological factors and sleep in disease risk.
Health Behaviors
Both sleep and depression have been associated with health behaviors important to cardiometabolic disease risk, including smoking and physical inactivity. For instance, epidemiologic and experimental evidence show that sleep disturbances, including poor quality, PSG indices of continuity, and perhaps decreased duration, are related to smoking and physical inactivity (e.g., 76, 77). Depressive symptoms are likewise related to smoking and physical inactivity, most likely in a bi-directional manner (78). To our knowledge, no studies have examined whether the associations between sleep and these behaviors are independent of depressive symptoms, or vice versa.
Genetic Vulnerability
Some evidence suggests that the covariation among sleep parameters, depressive symptoms, and CVD/diabetes may be partially due to genetic influences. For example, twin studies show that common genetic effects account for significant portions of the phenotypic correlations between depression and sleep problems in children (79), and between depressive symptoms and coronary artery disease (80); however, the possibility that sleep, depression, and CVD may all be influenced by shared genetic vulnerability has not been evaluated. Investigation of common variations in genes implicated in sleep regulation may prove fruitful in this regard. For instance, disruptions of the core clock genes that regulate endogenous circadian rhythmicity are linked to perturbations in glucose metabolism, adipocyte function, and vascular function (81), and others have theorized that clock genes may be implicated in the development of mood disorders (82). It is also possible that genetic variation related to the biobehavioral pathways summarized above (i.e., inflammation, HPA functioning, autonomic imbalance) may contribute to the covariation of sleep, depression, and cardiometabolic disease.
Summary
At present, the extant literature suggests that some components of sleep and depression share associations with common mechanisms implicated in cardiometabolic disease. These data further support the importance of examining sleep and depression as overlapping risk factors and evaluating the nature of their relationship in terms of disease risk. Most of the evidence reflects cross-sectional relationships among sleep, depression, and biobehavioral mechanisms that are likely cyclical in nature and may vary by different depression and sleep characteristics. For instance, the most consistent physiological perturbations are observed in individuals with a clinical diagnosis of depression, rather than elevated symptoms, and in relation to short and fragmented sleep, rather than long sleep. Thus, it is possible that short and long sleep are linked to cardiometabolic risk through different pathways. Improved knowledge of the differential associations between sleep and depression in relation to these mechanisms will contribute to etiological models of CVD and diabetes, and may thus enhance prevention strategies by targeting the most relevant risk factors early in the disease process. More work is also needed to investigate the possibility that common underlying factors, such as shared genetic vulnerability, may be partially responsible for relationships among sleep, depression, and cardiometabolic disorders.
Future Directions
Study Design & Analysis
Additional longitudinal studies of sleep and depression in relation to the development of CVD, diabetes, and the metabolic syndrome are necessary. In particular, future work should aim to collect multiple assessments of sleep, depression, and cardiometabolic disease, and track participants across extended follow-up periods using cross-lagged models. Such data will be essential in supporting a causal role of sleep or depression, or both, in disease and in revealing the impact that within-person fluctuations in these variables have on health over time.
Ideally, experimental work manipulating sleep or depression may be used to delineate causal mechanisms and identify potential points for prevention. In this regard, randomized controlled trials, as well as more acute experimental work in humans and animals, will be beneficial. It may be particularly interesting to intervene in individuals who are experiencing both depressive symptoms and short or fragmented sleep. As an example, future studies might compare short-term health outcomes (such as changes in inflammatory markers or BMI) in individuals who first receive a sleep intervention versus those who first receive a depression intervention, using a cross-over design. Treatments may include cognitive-behavioral or pharmacotherapy options; however, the adverse consequences associated with hypnotics, in particular, should be kept in mind in studies aiming to evaluate pharmacotherapy (62).
While a vast literature supports the role of depression in the development of cardiometabolic disease, there is a paucity of information regarding whether these associations are independent of sleep. Therefore, investigation of whether sleep abnormalities may account for at least some of the links between depression and disease risk is critical. It may be beneficial for future studies to evaluate whether relationships between depression and cardiometabolic disease persist after adjustment for self-reported or objective indices of sleep, or to identify the specific symptoms or cluster of symptoms (i.e., somatic versus cognitive) that drive associations between depression and disease. The potential models of sleep, depression, and cardiometabolic disease offered in Figure 1 may provide a useful framework for such studies.
The available literature offers more information regarding the associations of sleep and cardiometabolic disease, independent of depression. However, conclusions from these studies are limited by the use of dichotomous and often modified measures of depression. Thus, it is critical for future studies of sleep and disease to improve upon the assessment of depression by using well-validated questionnaires that inquire about a range of symptoms, scoring data continuously, and asking about depressive medication, treatment, and history. Additionally, measurement of psychological constructs related to depression and sleep, such as anxiety, hostility, and chronic stress, as well as positive emotions and social support, will further refine models of cardiometabolic disease. Finally, reporting whether sleep and depression are both related to cardiometabolic disease in fully adjusted models will lead to a better understanding of the independent risks associated with each of these factors. If sleep and depression are shown to confer unique health risks, each will need to be considered in future prevention and treatment strategies.
Regarding the measurement of sleep, it is important for future work to be comprehensive in its assessment of sleep parameters. While it may not be feasible for large, population-based studies to include standardized batteries of the major sleep characteristics, researchers should aim to evaluate and report the onset, duration, frequency, and severity of sleep difficulties, as these aspects may be particularly influential in disease risk. Moreover, sleep duration and continuity were evaluated separately in nearly all of the reviewed studies. It may be worthwhile to investigate how these two factors compare in their risk for cardiometabolic disease within one study, as it is possible that one is driving the effects of the other, or that sleep duration and continuity act in a synergistic fashion to influence disease (54). As noted previously, nearly all of the studies evaluating duration used an estimate of habitual sleep, and future work may want to incorporate prospective sleep diaries to improve the reliability of reported data. Relatedly, increased use of other sleep measurement tools, such as actigraphy and PSG, can elucidate which additional aspects of sleep are associated with cardiometabolic disease. As alterations in sleep architecture are common in depression (10), their assessment in relation to either clinical outcomes or biobehavioral disease mechanisms may be a particularly important area of investigation.
Finally, synergistic effects between sleep and depression in the context of disease risk have received little attention. Intriguing questions for future work include determining whether sleep and depression together confer a greater-than-additive risk for disease (34), and evaluating related threshold effects. For instance, does sleep matter at very high levels of depression? Do depressive symptoms matter in those that experience the most extreme sleep durations or continuity problems?
Population
Several of the findings in this review suggest that the associations among sleep, depression, and cardiometabolic disease may be stronger in women. However, this pattern was based on a limited number of studies and was most striking for CVD. In addition, the one study that examined age cohorts separately reported that relationships between sleep and incident hypertension were present in middle-aged but not older adults (34). Thus, future research should investigate the impact of demographic factors (i.e., sex, age) on these relationships more thoroughly, especially in light of sex differences in sleep and depression, and changes in these variables across the lifespan. Moreover, few studies focused on socio-cultural groups that are disproportionately diagnosed with CVD and diabetes (i.e., African Americans and individuals with few socioeconomic resources), highlighting a need for more research in this area. This may be particularly important given the disparities in sleep duration and continuity that have been reported in these groups (12). Finally, the conclusions in this review most likely reflect subclinical elevations in sleep and depressive symptoms. Additional data are needed to determine if effects are similar or stronger in populations with clinical sleep disorders and/or MDD.
Mechanisms
Numerous biobehavioral mechanisms may underlie sleep, depression, and cardiometabolic disease. Additional work testing the unique associations between sleep and depression and these mechanisms, and evaluation of whether these mechanisms mediate the links between sleep and depression and disease, is needed, as this knowledge may be critical in designing prevention strategies that target early disease processes. Findings showing common underlying genetic influences on sleep, depression, and cardiometabolic disease may guide pharmaceutical treatments or the investigation of environmental-gene interactions. Alternatively, studies that support the validity of third-factor (i.e., sleep apnea) and reverse causation models would suggest that the treatment of depression or insomnia would be not be efficacious in reducing CVD, diabetes, or the metabolic syndrome. Ultimately, behavioral, psychological, or pharmacological interventions showing that the successful treatment of sleep and/or depression leads to decreased cardiometabolic disease rates are needed to demonstrate causal pathways.
Summary
Extreme sleep durations, poor sleep continuity, and depression are highly related constructs that have been suggested as risk factors for the development of CVD and diabetes. However, little research has considered whether they contribute to cardiometabolic disease in an independent or overlapping manner. Our purpose in this review was to summarize and evaluate extant data on relationships among sleep, depression, and cardiometabolic disease. Nearly all of the 26 papers included in this review examined the associations of sleep and cardiometabolic outcomes, independent of depression. The most consistent relationships observed in these studies were those between short sleep duration and diabetes or the metabolic syndrome, and poor sleep continuity and CVD, which persisted after adjustment for depressive symptoms. There was also some evidence linking extreme sleep duration to CVD, and poor sleep continuity to diabetes, independent of depression, although the findings regarding these associations were largely mixed. Far fewer studies examined depression and cardiometabolic disease in models that adjusted for sleep duration or continuity, making it difficult to determine whether the contribution of depression to CVD and diabetes is independent of or works through sleep. Thus, it is critical for future research to consider the role of sleep in the depression-cardiometabolic disease relationship. Finally, we summarized data showing common physiological and behavioral mechanisms of both sleep and depression with cardiometabolic disease. Future work should aim to evaluate the links among sleep, depression, and potential biobehavioral mechanisms, and, ideally, test whether these pathways mediate relationships with disease using longitudinal studies and experimental designs.
Over one-third of adults report frequent sleep disturbances or weeknight sleep durations of less than seven hours (83), and about 10% of the population experience a major depressive episode during their lifetime (15). Thus, the question of how sleep and depression interact not only holds important implications for understanding the development of cardiometabolic disease but also for clinical and public health intervention efforts. Studying sleep and depression simultaneously may facilitate the development of more focused and efficacious treatment strategies, and ultimately result in the prevention or delayed onset of CVD and diabetes among individuals with depression and disturbed sleep.
Practice Points:
-
-
It is unclear whether sleep disturbances and depression – two comorbid conditions - are independent or overlapping risk factors for cardiometabolic disease.
-
-
Available literature suggests that some components of sleep may be related to cardiometabolic disease, independent of depression. The most consistent associations are between extreme sleep duration and metabolic disease, and between poor sleep continuity and incident cardiovascular disease.
-
-
There is some evidence that the association between depression and cardiometabolic disease is independent of poor sleep continuity. Few studies of depression and cardiometabolic disease measure and adjust for sleep duration.
Research Agenda:
-
-
Researchers interested in understanding depression as a risk factor for cardiometabolic disease should consider the role of sleep in this relationship.
-
-
Longitudinal data tracking both sleep and depression in relation to cardiometabolic disease over time will help to determine causal associations among these factors.
-
-
Behavioral, psychological, or pharmacological interventions showing that the successful treatment of sleep and/or depression leads to decreased rates of cardiometabolic disease are needed to demonstrate causal pathways.
-
-
Future research should also focus on whether sleep and depression have independent, overlapping, or synergistic effects on physiological and behavioral mechanisms implicated in cardiometabolic disease.
Acknowledgements
This research was supported by grants AG019362, RR024153, HL076379, HL065111, HL065112, R24 HL076852, HL076858, and HL07560 from the National Institutes of Health, Bethesda, MD.
Abbreviations
- BMI
body mass index
- CES-D
Center for Epidemiological Studies Depression Scale
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HPA
hypothalamic-pituitary-adrenal
- IL
interleukin
- MDD
major depressive disorder
- PSG
polysomnography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth J. Mezick, Department of Psychology, University of Pittsburgh, 3811 O’Hara Street, Pittsburgh, PA, 15213; phone: 412-648-7096; fax: 412-648-7160; mezickej@upmc.edu
Martica Hall, Department of Psychiatry, Western Psychiatric Institute and Clinic, 3811 O’Hara Street, E-1101, Pittsburgh, PA,15213; phone: 412-246-6431, fax: 412-246-5300; hallmh@upmc.edu
Karen A. Matthews, Department of Psychiatry, University of Pittsburgh School of Medicine, 3811 O’Hara Street, Pittsburgh, PA 15213; phone: 412-648-7158; fax: 412-648-7160
References
- 1.American Heart Association . Heart disease and stroke statistics — 2008 update. American Heart Association; Dallas (TX): 2008. [Google Scholar]
- 2.Centers for Disease Control and Prevention . National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. U. S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta (GA): 2008. [Google Scholar]
- 3.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 4.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz S, McDowell Anderson W, Cole SR, Cornoni-Huntley J, Hays JC, Blazer D. Insomnia and heart disease: a review of epidemiologic studies. J Psychosom Res. 1999;47:313–333. doi: 10.1016/s0022-3999(99)00029-x. [DOI] [PubMed] [Google Scholar]
- 6.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 7.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewart HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003;54:248–261. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- 9.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- *10.Benca RM, Peterson MJ. Insomnia and depression. Sleep Med. 2008;9:S3–S9. doi: 10.1016/S1389-9457(08)70010-8. [DOI] [PubMed] [Google Scholar]
- 11.Kaneita Y, Ohida T, Uchiyama M, Takemura S, Kawahara K, Yokoyama E, et al. The relationship between depression and sleep disturbances: a Japanese nationwide general population survey. J Clin Psychiatry. 2006;67:196–203. doi: 10.4088/jcp.v67n0204. [DOI] [PubMed] [Google Scholar]
- 12.Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, et al. Objectively measured sleep characteristics among early-middle-aged adults. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 13.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 2007;30:793–796. doi: 10.1093/sleep/30.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Med. 2002;3:305–314. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., text revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 16.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 18.McDowell I. Measuring health: a guide to rating scales and questionnaires. 3rd ed. Oxford University Press; New York: 2006. [Google Scholar]
- 19.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 20.Goldberg DP. The detection of psychiatric illness by questionnaire. Oxford University Press; London: 1972. [Google Scholar]
- 21.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–1063. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361–377. [PubMed] [Google Scholar]
- 23.Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, Killgore WD. The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 2007;8:215–221. doi: 10.1016/j.sleep.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Novati A, Roman V, Cetin T, Hagewoud R, den Boer JA, Luiten PGM, et al. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31:1579–1585. doi: 10.1093/sleep/31.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institutes of Health National Institutes of Health State of the Science conference statement on manifestations and management of chronic insomnia in adults. Sleep. 2005;28:1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 26.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–1464. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 27.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–259. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 28.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome - a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 29.King CR, Knuston KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 31.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Brunner RL, Ren H, Wassertheil-Smoller S, Larson JC, Levine DW, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008;39:3185–3192. doi: 10.1161/STROKEAHA.108.521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II Cohort. Sleep. 2007;30:1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Gangwisch JE, Malaspina D, Posner K, Babiss LA, Heymsfield DB, Turner JB, et al. Insomnia and sleep duration as mediators of the relationship between depression and hypertension incidence. Am J Hypertens. 2010;23:62–69. doi: 10.1038/ajh.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikehara S, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32:295–301. doi: 10.1093/sleep/32.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg Cohort Study. Sleep. 2007;30:1121–1127. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cappuccio FP, Stranges S, Kandala N, Miller MA, Taggart FM, Kumari M, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, et al. Sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 39.Stone KL, Ewing SK, Ancoli-Israel S, Ensrud KE, Redline S, Bauer DC, et al. Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. J Am Geriatr Soc. 2009;57:604–611. doi: 10.1111/j.1532-5415.2008.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lan T, Lan T, Wen C, Lin Y, Chuang Y. Nighttime sleep, Chinese afternoon nap, and mortality in the elderly. Sleep. 2007;30:1105–1110. doi: 10.1093/sleep/30.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Garcia E, Faubel R, Guallar-Castillon P, Leon-Munoz L, Banegas JR, Rodriguez-Artalejo F. Self-reported sleep duration and hypertension in older Spanish adults. J Am Geriatr Soc. 2009;57:663–668. doi: 10.1111/j.1532-5415.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- *42.Nicholson A, Fuhrer R, Marmot M. Psychological distress as a predictor of CHD events in men: the effect of persistence and components of risk. Psychosom Med. 2005;67:522–530. doi: 10.1097/01.psy.0000171159.86446.9e. [DOI] [PubMed] [Google Scholar]
- 43.Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, et al. The Cardiovascular Health Study Research Group Daytime sleepiness predicts mortality and cardiovascular disease in older adults. J Am Geriatr Soc. 2000;48:115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 44.Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3:489–494. [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips B, Buzkova P, Enright P. Insomnia did not predict hypertension in older adults in the Cardiovascular Health Study. Sleep. 2009;32:65–72. [PMC free article] [PubMed] [Google Scholar]
- *46.Schwartz SW, Cornoni-Huntley J, Cole SR, Hays JC, Blazer DG, Schocken DD. Are sleep complaints an independent risk factor for myocardial infarction? Ann Epidemiol. 1998;8:384–392. doi: 10.1016/s1047-2797(97)00238-x. [DOI] [PubMed] [Google Scholar]
- *47.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 48.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- *49.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Sleep duration as a risk factor for diabetes incidence in a large US sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–868. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- *51.Hall MH, Muldoon MF, Jennings R, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–643. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *52.Eriksson AK, Ekbom A, Granath F, Hilding A, Efendic S, Ostenson CG. Psychological distress and risk of pre-diabetes and type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabetes Med. 2008;25:834–842. doi: 10.1111/j.1464-5491.2008.02463.x. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi T, Nakao M, Nomura K, Yano E. Association of metabolic syndrome with depression and anxiety in Japanese men. Diabetes Metab. 2009;35:32–36. doi: 10.1016/j.diabet.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009 July 29; doi: 10.2337/dc09-0284. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bliwise DL, Friedman L, Yesavage JA. Depression as a confounding variable in the estimation of habitual sleep time. J Clin Psychol. 1993;49:471–477. doi: 10.1002/1097-4679(199307)49:4<471::aid-jclp2270490403>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 56.Malhotra A, Loscalz J. Sleep and cardiovascular disease: an overview. Prog Cardiovasc Dis. 2009;51:279–284. doi: 10.1016/j.pcad.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benetó A, Gomez-Siurana E, Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009;13:287–293. doi: 10.1016/j.smrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, Lavie P. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaire. Sleep. 2007;30:312–319. doi: 10.1093/sleep/30.3.312. [DOI] [PubMed] [Google Scholar]
- 59.Baran AS, Richert AC. Obstructive sleep apnea and depression. CNS Spectr. 2003;8:128–134. doi: 10.1017/s1092852900018356. [DOI] [PubMed] [Google Scholar]
- 60.Holshoe JM. Antidepressants and sleep: a review. Perspect Psychiatr Care. 2009;45:191–197. doi: 10.1111/j.1744-6163.2009.00221.x. [DOI] [PubMed] [Google Scholar]
- 61.Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol. 2009;53:950–958. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kripke DF, Klauber MR, Wingard DL, Fell RL, Assmus JD, Garfinkel L. Mortality hazard associated with prescription hypnotics. Biol Psychiatry. 1998;43:687–693. doi: 10.1016/s0006-3223(97)00292-8. [DOI] [PubMed] [Google Scholar]
- 63.Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. J Periodontol. 2008;79:1544–1551. doi: 10.1902/jop.2008.080249. [DOI] [PubMed] [Google Scholar]
- 64.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: The Chicago Health, Aging, and Social Relations Study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- *65.Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- 66.Cover H, Irwin M. Immunity and depression: insomnia, retardation, and reduction of natural killer cell activity. J Behav Med. 1994;17:217–223. doi: 10.1007/BF01858106. [DOI] [PubMed] [Google Scholar]
- 67.Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- 68.Antonijevic IA. HPA axis and sleep: identifying subtypes of major depression. Stress. 2008;11:15–27. doi: 10.1080/10253890701378967. [DOI] [PubMed] [Google Scholar]
- 69.Rao U, Poland RE. Electroencephalographic sleep and hypothalamic-pituitary-adrenal changes from episode to recovery in depressed adolescents. J Child Adolesc Psychopharmacol. 2008;18:607–613. doi: 10.1089/cap.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mausbach BT, Ancoli-Israel S, von Känel R, Patterson TL, Aschbacher K, Mills PJ, et al. Sleep disturbance, norepinephrine, and D-dimer are all related in elderly caregivers of people with Alzheimer disease. Sleep. 2006;29:1347–1352. doi: 10.1093/sleep/29.10.1347. [DOI] [PubMed] [Google Scholar]
- 71.Hall MH, Vasko R, Buysse D, Ombao H, Chen Q, Cashmere JD, et al. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 72.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–298. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;170:805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blaine B. Does depression cause obesity?: A meta-analysis of longitudinal studies of depression and weight control. J Health Psychol. 2008;13:1190–1197. doi: 10.1177/1359105308095977. [DOI] [PubMed] [Google Scholar]
- 75.Vgontzas AN, Lin HM, Papaliaga M, Calhoun S, Vela-Bueno A, Chrousos GP, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes. 2008;32:801–809. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 76.Htoo A, Talwar A, Feinsilver SH, Greenberg H. Smoking and sleep disorders. Med Clin North Am. 2004;88:1575–1591. doi: 10.1016/j.mcna.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24:355–365. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gregory AM, Rijsdijk FV, Dahl RE, McGuffin P, Eley TC. Associations between sleep problems, anxiety, and depression in twins at 8 years of age. Pediatrics. 2006;118:1124–1132. doi: 10.1542/peds.2005-3118. [DOI] [PubMed] [Google Scholar]
- 80.Scherrer JF, Xian H, Bucholz KK, Eisen SA, Lyons MJ, Goldberg J, et al. A twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosom Med. 2006;65:548–557. doi: 10.1097/01.psy.0000077507.29863.cb. [DOI] [PubMed] [Google Scholar]
- 81.Prasai MJ, George JT, Scott EM. Molecular clocks, type 2 diabetes and cardiovascular disease. Diab Vasc Dis Res. 2008;5:89–95. doi: 10.3132/dvdr.2008.015. [DOI] [PubMed] [Google Scholar]
- 82.Turek FW. From circadian rhythms to clock genes in depression. Int Clin Psychopharmacol. 2007;22:S1–S8. doi: 10.1097/01.yic.0000277956.93777.6a. [DOI] [PubMed] [Google Scholar]
- 83.National Sleep Foundation . Sleep in America poll: summary of findings. National Sleep Foundation; Washington DC: 2005. 2005. [Google Scholar]