Abstract
Ultraviolet B light (UVB) activates nitric oxide synthase(s) (NOS) and nitric oxide (NO•) production, which plays a role in regulation of apoptosis. However the role of NO• in UVB-induced apoptosis remains controversial. In this study, we analyzed expression and activation of constitutive NOSs (cNOSs) and their roles in UV-induced apoptosis of HaCaT keratinocytes. Our data showed that the expression of neuronal NOS (nNOS) was increased while endothelial NOS (eNOS) was uncoupled in the early phase (0–6 h) post-UVB. The expression of both cNOSs peaked at 12 h post-UVB and NO• was transiently elevated with 30 min and then steadily rose from 6–18 h post-UVB. The expression of iNOS was detected at 6 h post-UVB and then sturdily increased. Inhibition of cNOSs with L-NAME reduced the inducibility of NO• in the early and late phases of irradiation. Along with the eNOS uncoupling, an increased level of peroxynitrite (ONOO−) was detected in the early phase, but not in the late phase post-UVB. Inhibition of cNOSs reduced the production of ONOO− in the early time, but led to an increase of ONOO− in the late time after UVB-irradiation. The results indicate that cNOSs regulate NO•/ONOO− imbalance after UVB-irradiation. Our data suggested that the activation of cNOSs in the early phase post-UVB leads to NO•/ONOO− imbalance and promotes apoptosis via a caspase 3-independent pathway. The elevation of NO• in the late phase of UVB-irradiation is mainly produced by inducible NOS (iNOS). However, cNOSs also contribute to the NO• production and to maintain a higher NO•/ONOO− ratio, which reduces caspase 3 activity and protects cells from UVB-induced apoptosis.
Keywords: UVB, cNOS, nitric oxide, caspase 3 and apoptosis
Introduction
UVB-induced NO• production occurs in a dose- and time-dependent manner. In human keratinocytes, the UVB-dose-dependent induction of NO• reaches a maximal NO• release of 3-fold with a treatment of 120 mJ/cm2 UVB. Higher doses of UVB result in similar increases in generation of NO• [1–3]. UVB induces a rapid elevation of NO•, which is recorded at 20 sec and reaches a peak at 40 sec in keratinocytes [4]. A maximal prolonged NO• production occurs between 17 and 48 hours post UVB-irradiation based on cell type [5,6]. Keratinocytes were shown to contain nNOS (NOS1), iNOS (NOS2) and eNOS (NOS3), the three members of NOS family [7–9]. UVB-activated NOSs generate NO• from L-arginine (L-Arg). Since [L-Arg] is low in cells, rapid consumption of L-Arg results in a L-Arg depletion, which leads to cNOS uncoupling and increases production of O2•− [4,10,11]. NO• is able to rapidly react with O2•− to from ONOO−, which is a powerful oxidant [12,13].
An elevated cytosolic [NO•] could have anti- or pro-apoptotic effect on keratinocytes upon UVB-irradiation [14–16]. Superoxide was suggested to play a role in regulation of nitric oxide-mediated anti-or pro-apoptosis after the irradiation [16]. However it is not clear how these NOSs were involved in the regulation of UV-induced apoptosis in a contrasting manner. Our recent reports suggested that the UVB-induced activation of constitutive NOS (cNOS), including nNOS and eNOS, in combination of oxidative stress, promoted apoptosis of keratinocytes [4,17]. In this report, we further elucidate the mechanism for coordinative regulation of apoptosis of keratinocytes by the three NOSs after UVB-irradiation. Our date suggested that cNOSs are the key contributors for a transient elevation of NO• and ONOO− in the early phase post-UVB, while iNOS is a major contributor for NO• production in the late phase post-UVB. However, cNOSs also generated NO• and play a role in maintaining a healthier NO•/ONOO− ratio in the late phase of UVB-irradiation.
Materials and Methods
Cell Culture
All chemicals were obtained from Sigma (St. Louis, MO), unless otherwise mentioned. The HaCat cells (human keratinocyte cell line, kindly provided by Dr. Nihal Ahmad, University of Wisconsin, Madison, WI) were cultured in 100 mm2 cell culture plates in Dulbecco’s modification of eagle’s medium (DMEM, Mediatech, Manassas, VA) with 10% (v/v) fetal bovine serum (FBS, Hyclone, Waltham, MA). Penicillin and streptomycin were added to the culture medium, and the cells were incubated at 37°C and 5% CO2.
Ultraviolet irradiation
Two UVB lamps (UVP, Upland, CA) were measured by a UVP model UVX digital radiometer (UVP, Upland, CA) after the lamps warmed up for 5 min. The cell culture medium was replaced with 1 mL/plate phosphate buffered saline (PBS) and then the cells were irradiated with a total dose of 75 mJ/cm2, which is in the rage of 1 minimal erythema dose (MED) for human [18]. After UVB-irradiation, fresh medium was added to the culture plates and the cells were returned to the incubator for further research.
Cell treatments
L-NG-nitro-arginine methyl ester (L-NAME) [19] was added to HaCat cells to a final concentration of 1 mM at 6 h before UVB-irradiation. After irradiation, the cells were continuously treated with L-NAME (1 mM) for a short or prolonged period of time. For the short treatment, the cells were incubated with the medium containing L-NAME for 0.5 h post-irradiation. Then the medium was replaced with fresh medium without L-NAME and the cells were incubated for another 17.5 h. For prolonged treatment, the cells were incubated with the medium containing L-NAME for a continuous 18 h.
Nitric oxide and peroxynitrite assay
The concentration of NO• or ONOO− was measured by a standard nitric oxide synthase assay [20] kit (Sigma, St. Louis, MO). The cells were placed in a 96-well plate. After treatment, the medium was removed. Then 190 µL reaction buffer, 10 µL L-arginine (1 mM) and 0.1 µL 4,5-diaminofluorescein diacetate (DAF-2DA, for NO•) (5 mM) or diaminorhodamine (DAR, for ONOO−) (5 mM) were added to each well. The cells were incubated for 2 h in dark at room temperature and the fluorescence was read by SpectraMax M2 fluorescence plate reader (Molecular Devices, Sunnyvale, CA) at ex490/em520 nm for DAF-2DA or ex 488/em 515 nm for DAR.
Cell viability was determined by Thiazolyl Blue Tetrazolium Bromide (MTT) assay [21]. 0.01 mL MTT solution (5 mg/mL, Millipore, Billerica, MA) was added into 96-well plate after nitric oxide and peroxynitrite assay. Then the plate was incubated at 37°C for 4 h and 0.1 mL isopropanol with 0.04 M HCl was added to each well. The absorbance of MTT solution was measured by optical reader at 570 nm. The fluorescence intensity of DAF-2DA and DAR was corrected by diving by the cell viability.
Caspase-3 activity assay
Cells were cultured in 100 mm plates. At 18 h post-UVB, cells were harvested by trypsin and rinsed in cold PBS twice. Then cells lysates were prepared in lysis buffer (200 mM Tris, pH 7.5, 2 M NaCl, 20 mM EDTA, 0.2% Triton X-100) by sonication. Same amount of proteins (200 µg) from each sample were diluted to 300 µL with the same lysis buffer in a 96-well plate and the substrate of caspase-3 FAM-DEVD-FMK [22] (10 µL, Cell Technology, Mountain View, CA) was added into each sample. After 1 h incubation at 37°C in the dark, the fluorescence intensity was measured by SpectraMax M2 fluorescence plate reader (Molecular Devices, Sunnyvale, CA) at ex488nm/em515nm.
Apoptosis assay
The apoptotic cell death was analyzed by determination of the loss of membrane phospholipid symmetry and membrane integrity [23] using a fluorescein isothiocyanate (FITC) conjugated-annexin V (ANX5)/propidium iodide (PI) apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ) following the manufacturer’s protocol. Briefly, the cells were harvested by 0.05% trypsin digestion, combined with the cells floating in the medium and washed twice with cold PBS. The cells were then suspended in annexin V binding buffer (10 mM Hepes/NaOH, pH 7.4, 140 mM NaCl and 2.5 mM CaCl2) at 106 cells/mL. 100 µL of the cell suspension was mixed with 5 µL ANX5-FITC and 5 µL PI. The cell mixture was incubated in dark for 15 min and the ANX5/PI double-stained cells were analyzed by using a FACSort Flow Cytometer (BD science, Franklin Lakes, NJ) equipped with CellQuest software (BD science, Franklin Lakes, NJ). A minimum of 1×104 cells was used for each analysis and cell debris is excluded by appropriate forward light scatter threshold setting. The numbers of cells positive for both ANX5 and PI were counted. The relative percentage change of apoptotic cells (relative %AC) was calculated as:
Western blot analysis
The antibodies against nNOS, eNOS, iNOS, caspase-3 and mouse IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-PARP was purchased from Cell Signaling (Danvers, MA). The anti-β-actin was purchased from Sigma (St. Louis, MO) and anti-rabbit IgG was from BioRad (Hercules, CA). The monomers and dimers of cNOSs were analyzed on a low-temperature, SDS-resistant SDS-PAGE [24]. Briefly, the cells were lysed after treatment in NP-40 lysis buffer (2% NP-40, 80 mM NaCl, 100 mM Tris-HCl, 0.1% SDS) containing a Proteinase Inhibitor Cocktail (Complete™, Roche Molecular Biochemicals, Indiana, IN). Then the protein samples were added to five-fold Laemmli buffer (0.32 M Tris-HCl, pH 6.8, 0.5 M glycine, 10% SDS, 50% glycerol, and 0.03% bromophenol blue) without boiling. These proteins were separated on SDS-PAGE with reducing gel (with 2.5% 2-mercaptoethanol) at 4°C before transferred onto a nitrocellulose membrane. Caspase 3 and PARP were analyzed on regular SDS-PAGE. The proteins were boiled in the loading buffer and separated on SDS-PAGE at room temperature before transferred onto a nitrocellulose membrane. The membrane was blocked with 5% (w/v) skim milk in TBST (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20) for 1 h and then incubated with corresponding antibodies at 4°C overnight. After washing with TBST, the membrane was incubated with HRP–conjugated anti-rabbit antibody for 1 h at room temperature. Membranes were washed three times in TBST, two times in TBS, and developed in West Pico Supersignal chemiluminescent substrate (Pierce, Rockford, IL).
Statistics
All values shown in figures were mean±SE. Independent Student’s t-test was used to analyze the statistic significance of data before normalization and applied to compare every two groups. 95% confidence interval (p<0.05) was used to denote the statistical significance.
RESULTS
Time-dependent analysis of cNOSs expression and uncoupling after UVB-irradiation
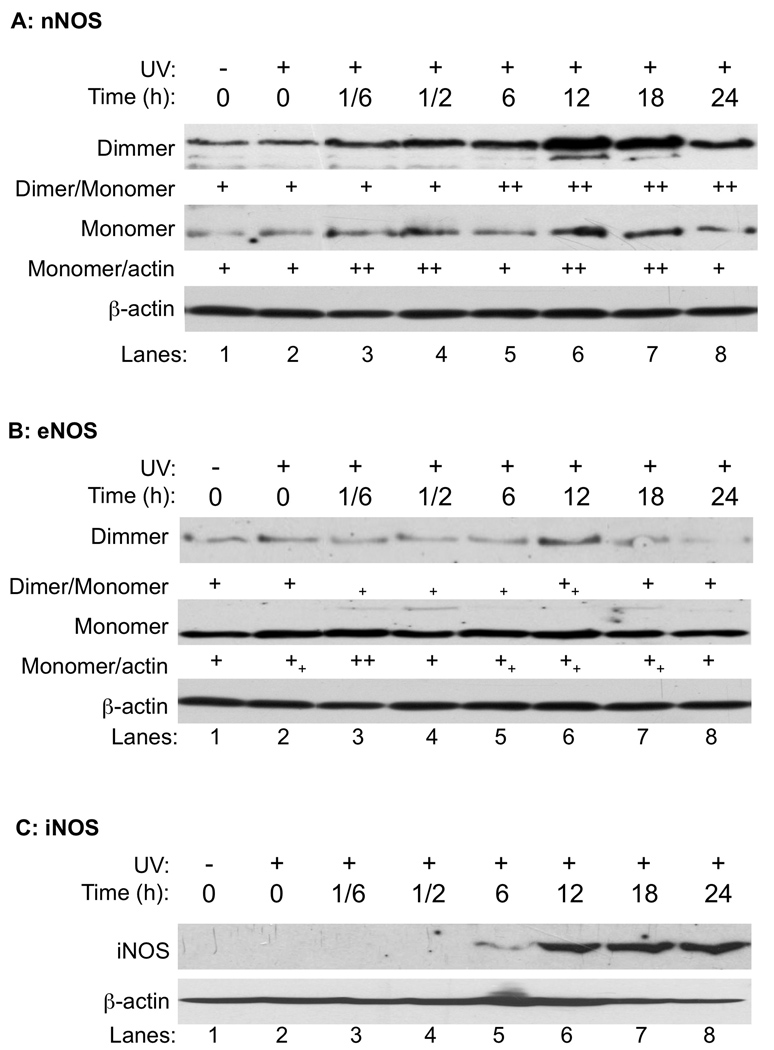
To analyze the roles of cNOSs in UVB-induced apoptosis, we first determined the effects of UVB irradiation on expression and coupling of eNOS and nNOS in human keratinocytes (HaCat). The cNOS uncoupling was detected by low-temperature SDS-resistant SDS-PAGE as previously reported [24]. Both nNOS and eNOS were detected in dimerized and monomerized forms in HaCaT cells (Fig. 1A and 1B). After UVB treatment (75 mJ/cm2), the expression of nNOS was increased shortly after the irradiation and peaked at 12 h post-UVB. The ratio between dimer and monomer of nNOS was not changed in the first half hour after UVB indicating that the newly synthesized nNOS formed both dimers and monomers (Fig. 1A). In contrast to nNOS, the expression of eNOS was not significantly changed until 12 h after UVB treatment (Fig. 1B). However, the ratio between dimer and monomer of eNOS was rapidly reduced with a shift from dimers to monomers (Fig. 1B). The expression of iNOS was detected at 6 h post-UVB and then continuously increased during the whole period of measurement (Fig. 1C).
Fig. 1.
The effect of UVB on cNOSs expression and dimerization. HaCaT cells were UVB-irradiated (75 mJ/cm2) and harvested at different time-points as indicated. The monomers and dimers of cNOSs were separated on a low-temperature SDS-resistant SDS-PAGE and the iNOS expression was determined by regular western blotting. The levels of β-actin were also probed as a loading control. Panel A: nNOS. Panel B: eNOS. Panel C: iNOS. The intensities of the protein bands were semi-quantitated by ImageJ (Version 1.42k, NIH). +: No change; ++: Increased >50%; ++: Increased <50%; +: Decreased <50%.
The contribution of cNOS in UVB-induced elevation of NO• and ONOO−
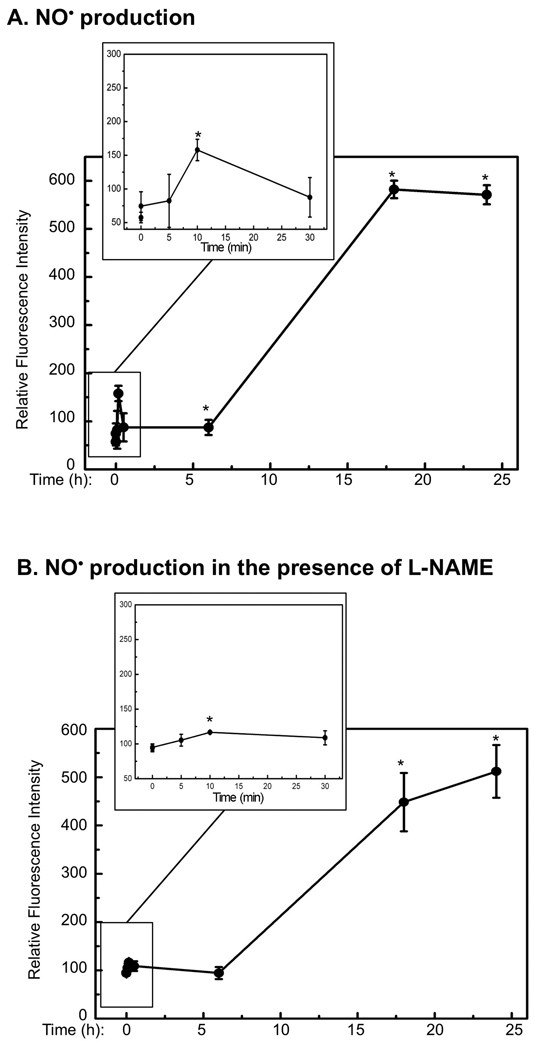
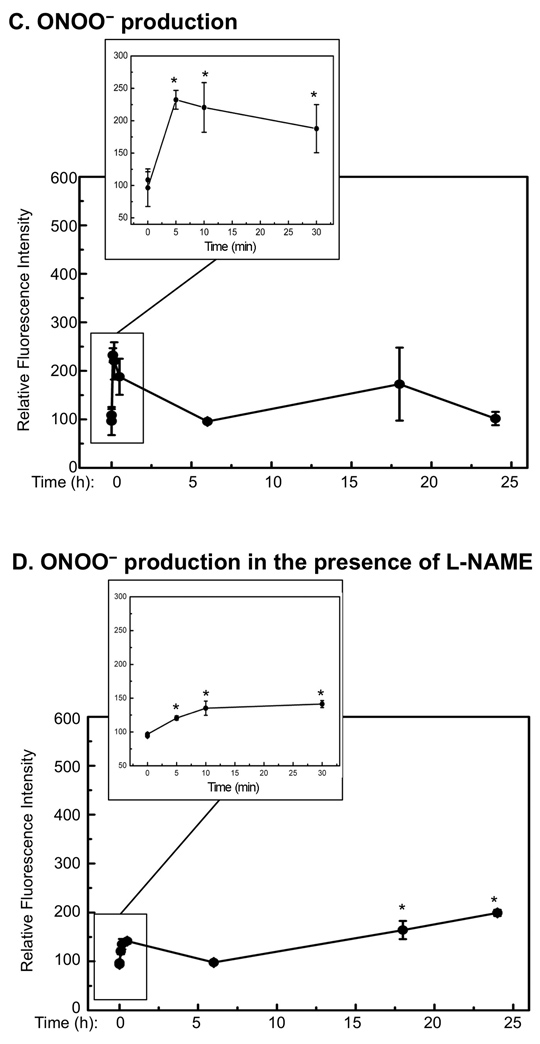
To determine the contribution of cNOS in generating NO• and oxidative stress after UVB-irradiation, we measured the time-dependent production of NO• and ONOO− in the presence and absence of the selective cNOS inhibitor L-NAME [19] using specific fluorescent dyes. Our data showed that the production of NO• increased immediately and generated the first peak, which was approximately 2 times that of the background level, at 10 min after UVB-irradiation (Fig. 2A, Table I). The production of NO• was reduced and remained low for 6 h and then raised to approximately 10 times of the background level at 18 h post-UVB and remained high (Fig. 2A, Table I). The UVB-induced immediate elevation of NO• was significantly inhibited by L-NAME (Fig. 2B, Table I). However, the later elevation of NO• was only reduced slightly by L-NAME (Fig. 2B, Table I). These results suggested that the early elevation of NO• was depended on the activation cNOS upon UVB-irradiation. The UVB-induced late elevation of NO• was largely depended on the increased expression of iNOS, but cNOSs also made a contribution since NO• levels were partially reduced by L-NAME.
Fig. 2.
Quantitative analysis of NO• and ONOO− after UVB-irradiation. HaCaT cells were irradiated with UVB (75 mJ/cm2) in the absence or presence of L-NAME. At the indicated time-points post-UVB-irradiation, the cells were stained with DAF-2DA or DAR. The fluorescence intensity was then measured and corrected with respect to cell viability. The fluorescence intensities of (A) NO• in the absence of L-NAME; (B) NO• in the presence of L-NAME; (C) ONOO− in the absence of L-NAME; and (D) ONOO− in the presence of L-NAME were shown. The error bars present the standard deviations of 3 independent experiments. *: p<0.05 vs. control.
Table I. Relative amount of NO• and ONOO− in the presence or absence of L-NAME after UVB-irradiation.
The data is normalized with NO• or ONOO− respectively produced in HaCaT cells without any treatment. Means±SE of 3 independent experiments were shown.
| Time | 0 | 5 min | 10 min | 30 min | 6 h | 18 h | 24 h | |
|---|---|---|---|---|---|---|---|---|
| NO• | UV alone | 1.3±0.4 | 1.4±0.7 | 2.7±0.3 | 1.5±0.5 | 1.5±0.3 | 10.1±0.3 | 9.9±0.3 |
| UV+L-NAME | 1.7±0.1 | 1.8±0.1 | 2.0±0 | 1.9±0.2 | 1.6±0.2 | 7.8±1.1 | 8.9±0.9 | |
| ONOO− | UV alone | 0.9±0.3 | 2.1±0.1 | 2.0±0.4 | 1.7±0.3 | 0.9±0 | 1.6±0.7 | 0.9±0.1 |
| UV+L-NAME | 0.9±0 | 1.1±0.1 | 1.2±0.1 | 1.3±0 | 0.9±0 | 1.5±0.2 | 1.8±0.1 | |
| NO•/ ONOO− |
UV alone | 1.4 | 0.7 | 1.4 | 0.9 | 1.7 | 6.3 | 11.0 |
| UV+L-NAME | 1.9 | 1.6 | 1.7 | 1.5 | 1.8 | 5.2 | 4.9 |
The UVB-induced production of ONOO− also increased immediately and peaked at approximately 1.5-fold of the background level at 5 min post-irradiation. The production of ONOO− was reduced and remained low without statistically significant change during the whole experiment (Fig. 2C, Table I). The UVB-induced immediate elevation of ONOO− was also significantly inhibited by L-NAME, while ONOO− was only slightly decreased in the late stage of irradiation upon treating with L-NAME (Fig. 2D, Table I). These results suggested that the UVB-induced uncoupling of cNOS could be a source of generating O2•−, and sequentially ONOO− as we previously reported [4,10]. However, cNOS was also contributed to the reduction of oxidative stress and therefore the production of ONOO− at late stage of UVB-irradiation.
The roles of NOSs in regulation of UVB-induced caspase 3 activation and apoptosis
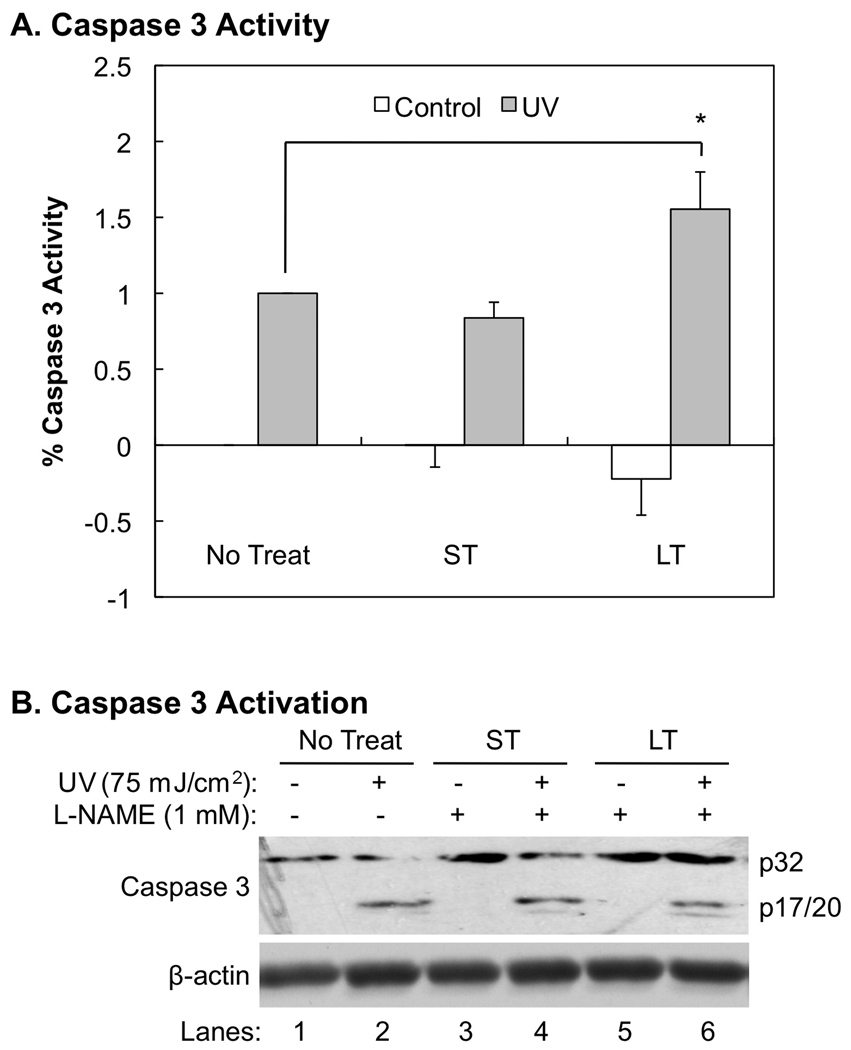
The role of NO• in regulation of apoptosis is controversial [14–16,25–28]. Our recently studies suggested that early activation of NOSs promotes apoptotic death of keratinocytes upon UVB-irradiation [4,17]. To further analyze the roles of NOSs in regulation of UVB-induced apoptosis of keratinocytes, we determined the extent of effects of a short or prolonged inhibition of cNOS on UVB-induced caspase 3 activation and apoptosis. Our data showed that while the short incubation of L-NAME after UVB-irradiation did not statistically significantly affect caspase 3 activity, the prolonged incubation of L-NAME increased caspase 3 activity by 55±3% (Fig. 3A). Either short or prolonged incubation of L-NAME did not statistically significantly affect caspase 3 activity in the absence of UVB-irradiation (Fig. 3A). Our data also showed that the increased caspase 3 activity after prolonged treatment of L-NAME was not a result of an elevation of p20, the active form of caspase 3 (Fig. 3B).
Fig. 3.
The effects of inhibiting cNOSs on UVB-induced caspase-3 activity and cleavage. The cells were treated with L-NAME for a short (ST, 0.5 h post-UVB) or longer period (LT, 18 h post-UVB) of time as described in “Methods”. (A) Caspase 3 activity was determined by using a fluorescent substrate, FAM-DEVD-FMK, of caspase 3. The fluorescence intensity was normalized with the caspase 3 activity in the untreated sample (No Treat). The error bars present the standard deviations of 3 independent experiments. *: p<0.05 vs. control. (B) Western blot analysis of caspase 3 cleavage.
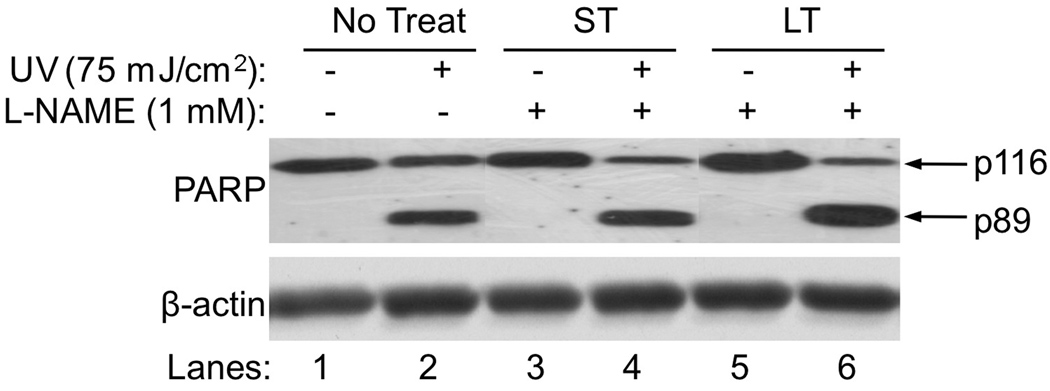
To confirm that the caspase 3 activity was increased without changing the amount of the p20 after prolonged incubation of L-NAME, we determined the cleavage of PARP, a caspase 3 substrate. Our data showed that the cleavage of PARP was increased with the prolonged inhibition of cNOS after UVB-irradiation (Fig. 4, Lane 6 vs. 2 and 4). The cleavage of PARP was not affected by L-NAME alone without the irradiation or a short incubation of L-NAME after UVB-irradiation (Fig. 4, Lane 3,5 vs. 1 and Lane 4 vs. 2). These results suggest that the combination effect of higher [NO•] with lower [ONOO−] in the late phase of UVB-irradiation (Table I) may lower the net amount of the active form of caspase 3.
Fig. 4.
The effects of inhibiting cNOSs on UVB-induced PARP cleavage. UVB-induced PARP cleavage in HaCaT cells that were not treated or treated with L-NAME was determined by western blot analysis. No Treat: without L-NAME treatment. ST: treated with L-NAME for a short of time (0.5 h post-UVB). LT: treated with L-NAME for a longer period of time (18 h post-UVB).
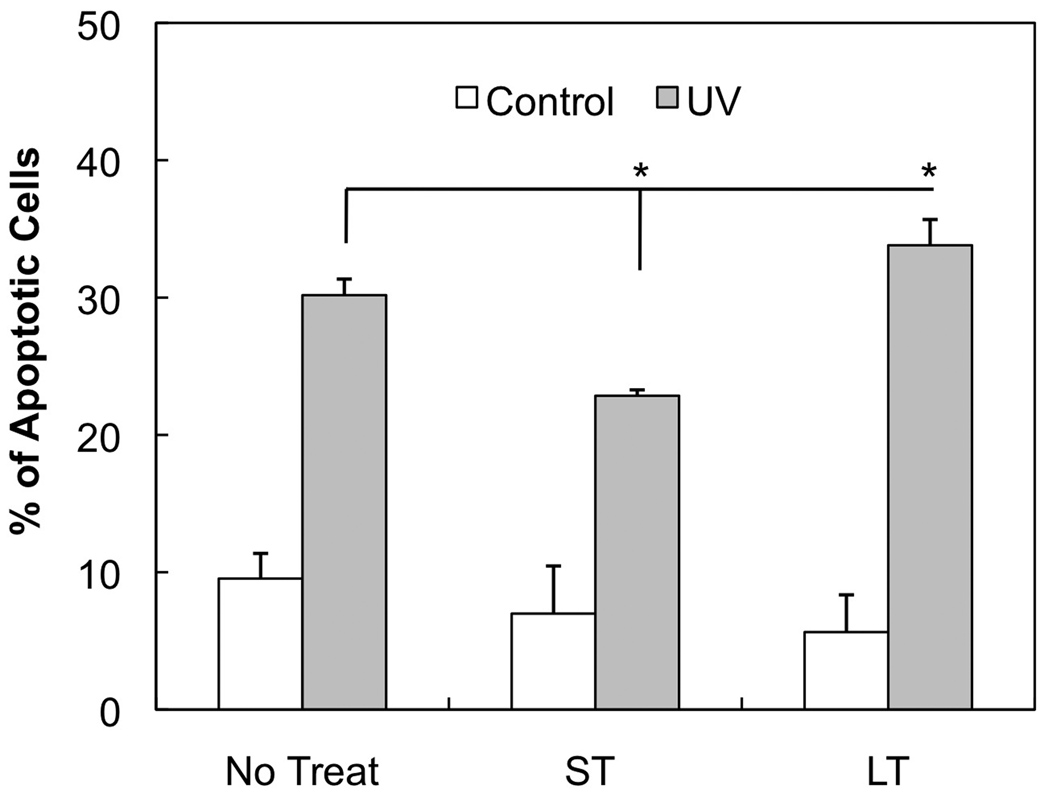
Last we determined the role of cNOS in UVB-induced apoptosis of keratinocytes. After the short period of inhibition of cNOS, the amount of UVB-induced apoptotic cells were reduced by 7.3%, which was a relative 24.2% reduction (Fig. 5). In contrast, the prolonged inhibition of cNOS increased the amount of UVB-induced apoptotic cells by 3.6%, which was a relative 11.9% increase (Fig. 5). These results suggest that the effect of cNOS on UVB-induced apoptosis is via a caspase 3-independed pathway during the early period and a caspase 3-depended pathway in the late period of irradiation.
Fig. 5.
The effects of inhibiting cNOSs on UVB-induced apoptosis. HaCaT cells were UVB irradiated and then were not treated (No Treat) or treated with L-NAME for a short (ST, 0.5 h post-UVB) or longer period (LT, 18 h post-UVB) of time. The cells were then double stained with ANX5-FITC/PI at 18 h post-UVB and apoptosis was analyzed by flow cytometry. The error bars present the standard deviations of 3 independent experiments. *: p<0.05 vs. control.
Discussion
UVB-irradiation activates both extrinsic and intrinsic apoptotic pathways [29]. With the extrinsic pathway, UVB induces the aggregation of Fas receptors, which recruits FADD and activates caspase 8 [30,31]. With the intrinsic pathway, UVB induces reactive oxygen species (ROS) production, which leads to mitochondrial cytochrome c (cyto c) release and caspase 9 activation [32,33]. Both caspase 8 and 9 activate caspase 3, which catalyzes the specific cleavage and activation of many key apoptosis-related proteins [34]. UVB also activates a caspase-independent, p38 mitogen-activated protein kinase (MAPK)-induced cyto c-mediated pathway in promoting apoptosis [35]. The UVB-induced NOSs activation and NO• release play roles in regulation of apoptosis. An elevation of NO• could promote apoptosis through the formation of more reactive oxidative species such as ONOO−, which induces oxidative protein modifications including oxidation or nitration of various amino acid residues, such as methionine and tyrosine [36,37]. However, high levels of NO• could also inhibit apoptosis by s-nitrosylation of caspases [38].
After UVB-irradiation, the activation of NOSs and production of NO• have been shown to be pro- and anti-apoptotic [14–16,25–28]. Our recent reports suggested that UVB-induced early activation of cNOS leads to the uncoupling of cNOS and elevation of O2•−, which reacts with NO• to form ONOO− and promotes apoptosis due to a NO•/ONOO− imbalance [4,10,17]. In this study, we demonstrated that in the early phase of UVB-irradiation, rapid transitional elevations of NO• and ONOO− were mainly dependent on the activation of cNOSs. Inhibition of cNOSs by L-NAME significantly reduced the inducibility of NO• and ONOO− productions after the irradiation (Fig. 2, Panel B vs. A; Panel D vs. C; Table I). The production of ONOO− peaks ahead of NO•, which agreed with our previous report [4]. The UVB-induced up-regulation of nNOS expression was paired in monomers (uncoupled form) and dimers (coupled form) (Fig. 1A, Lanes 1–4). In contrast, the eNOS expression after UVB-irradiation was more in monomer than in dimer. Further more, UVB appears to reduce the dimer and the dimer/monomer ratio of eNOS (Fig. 1B, Lanes 3–5 vs. 1,2). Since the uncoupled eNOS generates O2•− and therefore ONOO− [12], our results suggest that uncoupled eNOS is likely a source for UVB-induced oxidative stress. The inhibition of cNOSs activation at the early phase of the irradiation reduced the apoptotic death of the irradiated keratinocytes (Fig. 5, ST vs. No Treat), which agreed with our previous report [4]. Interestingly, the reduction of apoptosis was not accompanied with a reduced activity of caspase 3 (Figs. 3,4, ST vs. No Treat), which is one of the major players in UVB-induced apoptosis [39,40]. The results indicated that the UVB-induced and cNOS-mediated early signaling for inducing apoptosis is via a caspase 3-independent pathway.
In the late phase of UVB-irradiation, the NO• production is contributed to by both cNOS and iNOS [1,2,41–43]. Our data showed that the expressions of all three NOSs were significantly increased in the late phase of the irradiation (Fig. 1). The increased expressed cNOSs existed mainly as dimers, which increased the ratio of dimer/monomer (Fig. 1A, 1B). The UVB-induced production of NO• was dramatically elevated from 6– 24 h post-UVB, while the ONOO− level was not statistically changed during the same period (Figs. 2A and 2C; Table I). The inhibition of cNOS reduced NO• production by approximately 10–20% at 18 to 24 h post-UVB (Fig. 2A vs. 2B; Table I) suggesting that the iNOS, whose expression was induced during the period after UVB (Fig. 1C) [44], is a key contributor of NO• production during this period. Interestingly, with the inhibition of cNOS, the ONOO− production was steadily increased from 6–24 h post-UVB (Fig. 2D; Table I). These results indicate that cNOSs do not only contribute to NO• production but also to reduce ONOO−, and therefore maintain a healthier balance of NO•/ONOO− ratio, in the late phase of UVB-irradiation. This conclusion agrees with our observation that inhibition of cNOSs promotes apoptosis with an increased activity of caspase 3 and cleavage of PARP after UVB-irradiation (Figs. 3A and 4, LT vs. No Treat). The increased activity of caspase 3 was not correlated to an increased amount of activated caspase 3 (Fig. 3B), indicating that the bioavailability of NO• may direct inhibit activated caspase 3 activity as previously reported [38].
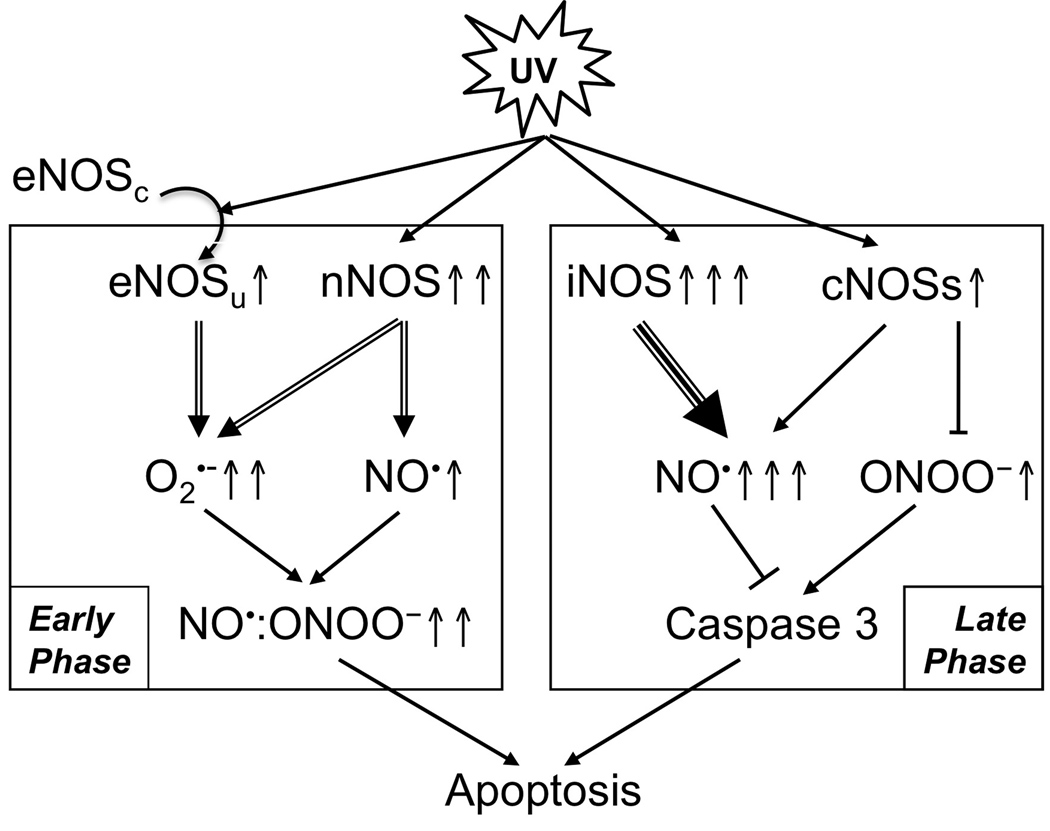
Based on our results, we propose a signaling network for UVB-induced and NOSs-mediated apoptosis (Fig. 6). The UVB-induced expression of nNOS and uncoupling of eNOS are the main sources of NO• and ONOO− productions in the early phase of UVB-irradiation. The elevation of NO•/ONOO− promotes apoptosis via a caspase 3-independent pathway. The elevation of NO• in the late phase of UVB-irradiation is mainly produced by iNOS. However, cNOSs also contribute to the NO• production and to maintain a higher NO•/ONOO− ratio, which reduces caspase 3 activity and protects cells from UVB-induced apoptosis.
Fig. 6.
Proposed NOSs signaling network after UVB-irradiation.
Supplementary Material
Acknowledgments
We thank Ms. Lei Wang for her assistance in analysis of apoptosis using the flow cytometer. We thank Mr. Oliver L. Carpenter for editorial assistance. This work is supported by 2R56CA086928 (to S. W.) and 2RO1CA086928 (to S. W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deliconstantinos G, Villiotou V, Stravrides JC. Release by ultraviolet B (u.v.B) radiation of nitric oxide (NO) from human keratinocytes: a potential role for nitric oxide in erythema production. Br J Pharmacol. 1995;114:1257–1265. doi: 10.1111/j.1476-5381.1995.tb13341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deliconstantinos G, Villiotou V, Stavrides JC. Nitric oxide and peroxynitrite released by ultraviolet B-irradiated human endothelial cells are possibly involved in skin erythema and inflammation. Exp Physiol. 1996;81:1021–1033. doi: 10.1113/expphysiol.1996.sp003986. [DOI] [PubMed] [Google Scholar]
- 3.Deliconstantinos G, Villiotou V, Stavrides JC. Alterations of nitric oxide synthase and xanthine oxidase activities of human keratinocytes by ultraviolet B radiation. Potential role for peroxynitrite in skin inflammation. Biochemical Pharmacology. 1996;51:1727–1738. doi: 10.1016/0006-2952(96)00110-4. [DOI] [PubMed] [Google Scholar]
- 4.Wu S, Wang L, Jacoby AM, Jasinski K, Kubant R, Malinski T. Ultraviolet B Light-induced Nitric Oxide/Peroxynitrite Imbalance in Keratinocytes-Implications for Apoptosis and Necrosis. Photochem Photobiol. doi: 10.1111/j.1751-1097.2009.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez Maglio DH, Paz ML, Ferrari A, Weill FS, Czerniczyniec A, Leoni J, Bustamante J. Skin damage and mitochondrial dysfunction after acute ultraviolet B irradiation: relationship with nitric oxide production. Photodermatol Photoimmunol Photomed. 2005;21:311–317. doi: 10.1111/j.1600-0781.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 6.Seo SJ, Choi HG, Chung HJ, Hong CK. Time course of expression of mRNA of inducible nitric oxide synthase and generation of nitric oxide by ultraviolet B in keratinocyte cell lines. Br J Dermatol. 2002;147:655–662. doi: 10.1046/j.1365-2133.2002.04849.x. [DOI] [PubMed] [Google Scholar]
- 7.Boissel JP, Ohly D, Bros M, Godtel-Armbrust U, Forstermann U, Frank S. The neuronal nitric oxide synthase is upregulated in mouse skin repair and in response to epidermal growth factor in human HaCaT keratinocytes. J Invest Dermatol. 2004;123:132–139. doi: 10.1111/j.0022-202X.2004.22731.x. [DOI] [PubMed] [Google Scholar]
- 8.Nakai K, Kubota Y, Kosaka H. Inhibition of nuclear factor kappa B activation and inducible nitric oxide synthase transcription by prolonged exposure to high glucose in the human keratinocyte cell line HaCaT. Br J Dermatol. 2004;150:640–646. doi: 10.1111/j.0007-0963.2004.05867.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakai K, Fujii S, Yamamoto A, Igarashi J, Kubota Y, Kosaka H. Effects of high glucose on NO synthesis in human keratinocyte cell line (HaCaT) J Dermatol Sci. 2003;31:211–218. doi: 10.1016/s0923-1811(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 10.Lu W, Laszlo CF, Miao Z, Chen H, Wu S. The role of nitric-oxide synthase in the regulation of UVB light-induced phosphorylation of the alpha subunit of eukaryotic initiation factor 2. The Journal Of Biological Chemistry. 2009;284:24281–24288. doi: 10.1074/jbc.M109.008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huk I, Nanobashvili J, Neumayer C, Punz A, Mueller M, Afkhampour K, Mittlboeck M, Losert U, Polterauer P, Roth E, Patton S, Malinski T. L-arginine treatment alters the kinetics of nitric oxide and superoxide release and reduces ischemia/reperfusion injury in skeletal muscle. Circulation. 1997;96:667–675. doi: 10.1161/01.cir.96.2.667. [DOI] [PubMed] [Google Scholar]
- 12.Kalinowski L, Malinski T. Endothelial NADH/NADPH-dependent enzymatic sources of superoxide production: relationship to endothelial dysfunction. Acta Biochim Pol. 2004;51:459–469. [PubMed] [Google Scholar]
- 13.Padmaja S, Huie RE. The reaction of nitric oxide with organic peroxyl radicals. Biochem Biophys Res Commun. 1993;195:539–544. doi: 10.1006/bbrc.1993.2079. [DOI] [PubMed] [Google Scholar]
- 14.Weller R, Schwentker A, Billiar TR, Vodovotz Y. Autologous nitric oxide protects mouse and human keratinocytes from ultraviolet B radiation-induced apoptosis. Am J Physiol Cell Physiol. 2003;284:C1140–C1148. doi: 10.1152/ajpcell.00462.2002. [DOI] [PubMed] [Google Scholar]
- 15.Yamaoka J, Kawana S, Miyachi Y. Nitric oxide inhibits ultraviolet B-induced murine keratinocyte apoptosis by regulating apoptotic signaling cascades. Free Radical Research. 2004;38:943–950. doi: 10.1080/10715760412331284807. [DOI] [PubMed] [Google Scholar]
- 16.Weller R, Billiar T, Vodovotz Y. Pro- and anti-apoptotic effects of nitric oxide in irradiated keratinocytes: the role of superoxide. Skin Pharmacol Appl Skin Physiol. 2002;15:348–352. doi: 10.1159/000064540. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Liu W, Parker SH, Wu S. Nitric oxide synthase activation and oxidative stress, but not intracellular zinc dyshomeostasis, regulate ultraviolet B light-induced apoptosis. Life Sci. 86:448–454. doi: 10.1016/j.lfs.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Placzek M, Gaube S, Kerkmann U, Gilbertz KP, Herzinger T, Haen E, Przybilla B. Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J Invest Dermatol. 2005;124:304–307. doi: 10.1111/j.0022-202X.2004.23560.x. [DOI] [PubMed] [Google Scholar]
- 19.Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000;58:1026–1034. [PubMed] [Google Scholar]
- 20.Marletta MA, Hurshman AR, Rusche KM. Catalysis by nitric oxide synthase. Curr Opin Chem Biol. 1998;2:656–663. doi: 10.1016/s1367-5931(98)80098-7. [DOI] [PubMed] [Google Scholar]
- 21.Boethling RS, Weaver TL. A new assay for diaphorase activity in reagent formulations, based on the reduction of thiazolyl blue. Clin Chem. 1979;25:2040–2042. [PubMed] [Google Scholar]
- 22.Kuzelova K, Grebenova D, Hrkal Z. Labeling of apoptotic JURL-MK1 cells by fluorescent caspase-3 inhibitor FAM-DEVD-fmk occurs mainly at site(s) different from caspase-3 active site. Cytometry A. 2007;71:605–611. doi: 10.1002/cyto.a.20415. [DOI] [PubMed] [Google Scholar]
- 23.Schindl A, Klosner G, Honigsmann H, Jori G, Calzavara-Pinton PC, Trautinger F. Flow cytometric quantification of UV-induced cell death in a human squamous cell carcinoma-derived cell line: dose and kinetic studies. Journal Of Photochemistry And Photobiology. B, Biology. 1998;44:97–106. doi: 10.1016/s1011-1344(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 24.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. The Journal Of Clinical Investigation. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukunaga-Takenaka R, Fukunaga K, Tatemichi M, Ohshima H. Nitric oxide prevents UV-induced phosphorylation of the p53 tumor-suppressor protein at serine 46: a possible role in inhibition of apoptosis. Biochem Biophys Res Commun. 2003;308:966–974. doi: 10.1016/s0006-291x(03)01516-x. [DOI] [PubMed] [Google Scholar]
- 26.Schneiderhan N, Budde A, Zhang Y, Brune B. Nitric oxide induces phosphorylation of p53 and impairs nuclear export. Oncogene. 2003;22:2857–2868. doi: 10.1038/sj.onc.1206431. [DOI] [PubMed] [Google Scholar]
- 27.Suschek CV, Briviba K, Bruch-Gerharz D, Sies H, Kroncke KD, Kolb-Bachofen V. Even after UVA-exposure will nitric oxide protect cells from reactive oxygen intermediate-mediated apoptosis and necrosis. Cell Death Differ. 2001;8:515–527. doi: 10.1038/sj.cdd.4400839. [DOI] [PubMed] [Google Scholar]
- 28.Suschek CV, Krischel V, Bruch-Gerharz D, Berendji D, Krutmann J, Kroncke KD, Kolb-Bachofen V. Nitric oxide fully protects against UVA-induced apoptosis in tight correlation with Bcl-2 up-regulation. J Biol Chem. 1999;274:6130–6137. doi: 10.1074/jbc.274.10.6130. [DOI] [PubMed] [Google Scholar]
- 29.Takasawa R, Nakamura H, Mori T, Tanuma S. Differential apoptotic pathways in human keratinocyte HaCaT cells exposed to UVB and UVC. Apoptosis. 2005;10:1121–1130. doi: 10.1007/s10495-005-0901-8. [DOI] [PubMed] [Google Scholar]
- 30.Bang B, Gniadecki R, Larsen JK, Baadsgaard O, Skov L. In vivo UVB irradiation induces clustering of Fas (CD95) on human epidermal cells. Exp Dermatol. 2003;12:791–798. doi: 10.1111/j.0906-6705.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- 31.Elyassaki W, Wu S. Lipid rafts mediate ultraviolet light-induced Fas aggregation in M624 melanoma cells. Photochem Photobiol. 2006;82:787–792. doi: 10.1562/2005-12-09-RA-748. [DOI] [PubMed] [Google Scholar]
- 32.Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H, Sang S, Ho CT. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol Nutr Food Res. 2008;52:527–537. doi: 10.1002/mnfr.200700157. [DOI] [PubMed] [Google Scholar]
- 33.Paz ML, Gonzalez Maglio DH, Weill FS, Bustamante J, Leoni J. Mitochondrial dysfunction and cellular stress progression after ultraviolet B irradiation in human keratinocytes. Photodermatol Photoimmunol Photomed. 2008;24:115–122. doi: 10.1111/j.1600-0781.2008.00348.x. [DOI] [PubMed] [Google Scholar]
- 34.Claerhout S, Van Laethem A, Agostinis P, Garmyn M. Pathways involved in sunburn cell formation: deregulation in skin cancer. Photochem Photobiol Sci. 2006;5:199–207. doi: 10.1039/b504970a. [DOI] [PubMed] [Google Scholar]
- 35.Assefa Z, Vantieghem A, Garmyn M, Declercq W, Vandenabeele P, Vandenheede JR, Bouillon R, Merlevede W, Agostinis P. p38 mitogen-activated protein kinase regulates a novel, caspase-independent pathway for the mitochondrial cytochrome c release in ultraviolet B radiation-induced apoptosis. J Biol Chem. 2000;275:21416–21421. doi: 10.1074/jbc.M002634200. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn DM, Aretha CW, Geddes TJ. Peroxynitrite inactivation of tyrosine hydroxylase: mediation by sulfhydryl oxidation, not tyrosine nitration. J Neurosci. 1999;19:10289–10294. doi: 10.1523/JNEUROSCI.19-23-10289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Joseph J, Feix J, Hogg N, Kalyanaraman B. Nitration and oxidation of a hydrophobic tyrosine probe by peroxynitrite in membranes: comparison with nitration and oxidation of tyrosine by peroxynitrite in aqueous solution. Biochemistry. 2001;40:7675–7686. doi: 10.1021/bi002958c. [DOI] [PubMed] [Google Scholar]
- 38.Bonavida B, Khineche S, Huerta-Yepez S, Garban H. Therapeutic potential of nitric oxide in cancer. Drug Resist Updat. 2006;9:157–173. doi: 10.1016/j.drup.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Mizu H, Yamauchi H. Molecular response to phototoxic stress of UVB-irradiated ketoprofen through arresting cell cycle in G2/M phase and inducing apoptosis. Biochem Biophys Res Commun. 2007;364:650–655. doi: 10.1016/j.bbrc.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 40.Anand S, Chakrabarti E, Kawamura H, Taylor CR, Maytin EV. Ultraviolet light (UVB and UVA) induces the damage-responsive transcription factor CHOP/gadd153 in murine and human epidermis: evidence for a mechanism specific to intact skin. J Invest Dermatol. 2005;125:323–333. doi: 10.1111/j.0022-202X.2005.23784.x. [DOI] [PubMed] [Google Scholar]
- 41.Deliconstantinos G, Villiotou V, Stavrides JC. Increase of particulate nitric oxide synthase activity and peroxynitrite synthesis in UVB-irradiated keratinocyte membranes. The Biochemical Journal. 1996;320(Pt 3):997–1003. doi: 10.1042/bj3200997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JH, Hong Y, Shim CS. Mechanism of UV light-induced photorelaxation in isolated rat aorta. J Vet Sci. 2000;1:81–86. [PubMed] [Google Scholar]
- 43.Chen YC, Shen SC, Lee WR, Lin HY, Ko CH, Lee TJ. Nitric oxide and prostaglandin E2 participate in lipopolysaccharide/interferon-gamma-induced heme oxygenase 1 and prevent RAW264.7 macrophages from UV-irradiation-induced cell death. J Cell Biochem. 2002;86:331–339. doi: 10.1002/jcb.10230. [DOI] [PubMed] [Google Scholar]
- 44.Chang HR, Tsao DA, Wang SR, Yu HS. Expression of nitric oxide synthases in keratinocytes after UVB irradiation. Arch Dermatol Res. 2003;295:293–296. doi: 10.1007/s00403-003-0433-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.