Abstract
Background
Alice in Wonderland syndrome is a perceptual disorder involving brief, transient episodes of visual distortions (metamorphopsia) and can occur in conjunction with certain viral infections. We used functional magnetic resonance imaging to examine visual processing in a 12-year-old boy with viral-onset Alice in Wonderland syndrome during an episode of micropsia (reduction in the perceived size of a form).
Methods
Functional magnetic resonance imaging was conducted in response to a passive viewing task (reversing checkerboard) and an active viewing task (line-length decisions in the context of the Ponzo illusion).
Results
In both tasks, the child with Alice in Wonderland syndrome showed reduced activation in primary and extrastriate visual cortical regions but increased activation in parietal lobe cortical regions as compared to a matched control participant.
Conclusions
The active experience of micropsia in viral-onset Alice in Wonderland syndrome reflects aberrant activity in primary and extrastriate visual cortical regions as well as parietal cortices. The disparate patterns of activity in these regions are discussed in detail.
Introduction
Alice in Wonderland syndrome, named for Lewis Carroll’s titular character, is a disorder characterized by transient episodes of visual hallucinations and perceptual distortions, during which objects or body parts are perceived as altered in various ways (metamorphopsia), including enlargement (macropsia) or reduction (micropsia) in the perceived size of a form.1,2 Such episodes are of short duration (generally less than an hour), variable frequency (up to several times per day), and unpredictable onset.3,4
Several studies have assessed the neurological underpinnings of the visual distortions of acute-stage Alice in Wonderland syndrome (ie, while individuals were experiencing frequent periods of visual distortion) but none during actual episodes of visual hallucinations. No frank structural brain abnormalities have been linked to viral-onset Alice in Wonderland syndrome, based on studies using both computed-tomography (CT) and magnetic resonance imaging (MRI) scans.4-8
Findings from psychophysiological studies of micropsia following viral infection appear consistent with dysfunction in posterior visual cortical regions, although findings are mixed. Across three studies, 6 of 13 people with Alice in Wonderland syndrome were found to have abnormal electroencephalogram (EEG) patterns over parieto-occipital and occipital electrode sites.4-6 Other reports failed to find abnormal EEG in individuals with the disorder (in five of five cases).7-9 Abnormal visual evoked potentials (VEP) recorded in response to a reversing black and white checkerboard have been reported in children with Alice in Wonderland syndrome.4 Blood perfusion of brain tissue using single-photon emission computed tomography (SPECT) has been reported for 9 cases of the syndrome, providing a mixed picture of the disorder.5,7,8 While normal perfusion was reported in one case, the other eight showed a varied pattern of hypoperfusion (abnormally low cerebral blood flow) across distinct neural regions. One case demonstrated right hemisphere occipital hypoperfusion, with two additional cases demonstrating bilateral occipital hypoperfusion.5,7 Hypoperfusion in the right parietal cortex was reported for five cases, whereas left parietal hypoperfusion was not found in any of the nine cases.5,7,8
The extant literature therefore highlights a critical role for the posterior visual processing areas, including particularly occipital and parietal cortical regions, in the visual disturbance of Alice in Wonderland syndrome. Importantly, abnormal findings in occipital and parietal regions observed during the acute stages of the disorder were normal when children were re-tested 4-6 weeks after resolution of their symptoms.4,7 This suggests that the visual distortions in viral-onset Alice in Wonderland syndrome reflect acute, transient functional disruptions in the occipital and parietal regions observed in these studies.
The current study employed blood oxygenation level–dependent (BOLD) functional magnetic resonance imaging (fMRI),10 a technique used to infer the localization of neural activity from the interplay between cerebral blood flow (CBF) and oxygenation levels of blood within the brain.11,12 We report results of a BOLD fMRI study from a 12-year-old child with viral-onset Alice in Wonderland syndrome evaluated during an episode of micropsia. This provided a rare opportunity to directly assess the neurophysiological markers of the visual disturbances that define the syndrome. To the best of our knowledge, ours is the first published report of measurements obtained during an episode of micropsia from a participant with viral-onset Alice in Wonderland syndrome.
Participants and Methods
Child with Alice in Wonderland Syndrome
A 12-year-old, right-handed boy with Alice in Wonderland syndrome was tested during an episode of micropsia. He reported the onset of micropsia shortly before the start of the fMRI scan, and stated that the episode was still ongoing at the completion of the test session, which lasted approximately 90 minutes.
His original visual symptoms began at the age of 10 years, 8 months, within one week of a Streptococcus infection, manifested as scarlet fever, confirmed by throat swab antigen testing. On initial examination, he described these symptoms as “seeing everything as far away,” but on further questioning on the date of the fMRI scan he reported that he perceived objects as reduced in size but at the correct distance and proportion. Two EEG evaluations showed intermittent focal slowing in the left temporal region with no changes during micropsia episodes. Anatomical brain MRI was normal. He had no history of migraine headaches or epilepsy and no indications of drug abuse or other developmental, neuropsychological, or psychiatric disorder.
He experienced multiple episodes of micropsia per day, each lasting 20-30 minutes or longer, over the 16 months prior to his participation in this study. These episodes were not related to a specific activity. He occasionally experienced photophobia and phonophobia with prolonged episodes. His distance visual acuity was tested as 20/20. Visual field perimetry was normal. Both the examining pediatric ophthalmologist and neuro-ophthalmologist ruled out fabrication or psychogenic cause and were convinced symptoms were real. There was no pupillary constriction noted during a reported episode, nor any accommodation noted with dynamic retinoscopy. Although mild hyperopic spectacles did enlarge the near field, making it more comfortable for the patient to continue schoolwork during these episodes, this treatment was not prophylactic. The patient failed trial treatment with gabapentin and riboflavin, which had no impact on his symptoms, for possible partial seizure and migrainous activity.
Typically Developing Child Control
A 13-year-old, right-handed boy was tested with the same fMRI protocol as a control subject. The control participant had no history of developmental, neuropsychological, or psychiatric disorders and had normal vision and hearing.
Both children received $50 for their participation in this study. Each participant and their parent/guardian gave written and oral informed consent. This study was approved by the Institutional Review Board of the University of California–San Diego and conformed to the requirements of the United States Health Insurance Portability and Accountability Act.
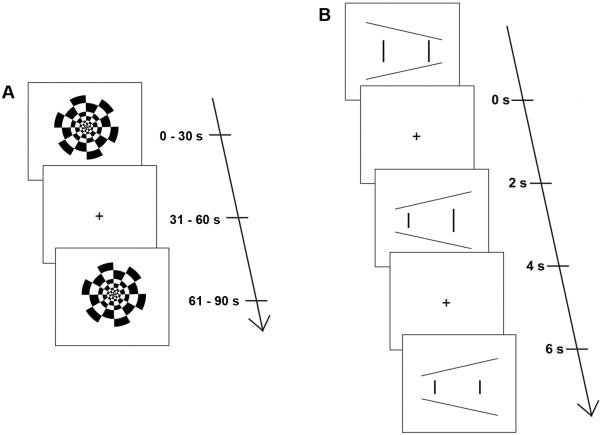
We examined occipital and parietal lobe functional activation using two binocular viewing tasks with demonstrated effectiveness in eliciting occipital and parietal cortical activation. The first task was a passive viewing task—a reversing checkerboard (Figure 1A), a benchmark functional imaging task that reliably elicits activation in visual areas of the occipital lobe, including Brodmann areas 17, 18, and 19.13,14 The second task was an active viewing task—the Ponzo illusion (Figure 1B), a common optical illusion that influences size judgments. In this illusion, viewers are presented with two vertical lines of equal length that are placed inside two converging horizontal lines. The illusion induces viewers to mistakenly judge the vertical lines to be different lengths and is believed to occur because size perception is influenced by external reference frames (ie, the converging horizontal lines) rather than the visual angle that each vertical line subtends on the retina.15 A previous fMRI study of similar illusions has shown these tasks to elicit occipital15 and parietal lobe activation.16,17 In the current fMRI study participants were required to decide whether the two vertical lines (Figure 1B) were equal or different lengths; they reported their response via a binary button press response. Additional details of the methods, including precise definitions of all regions of interest (ROIs), are given in e-Supplement 1 (available at jaapos.org).
FIG 1.
Passive and active viewing tasks. A, The black-and-white radial checkerboard reversed at 8 Hz in 30 second blocks. B, The Ponzo illusion. Each image (n = 64) remained on screen for 2 seconds, with a variable inter-stimulus interval (ISI). Participants were instructed to indicate, by pressing a button, which vertical line appeared longer.
Results
For each task, a nonparametric χ2 analysis was chosen as a conservative statistical approach to contrast the number of active voxels in each ROI between the child with Alice in Wonderland syndrome and the control participant.18-20 Bonferroni-corrected χ2 values are reported. The effective α for the analyses following correction equals 0.05 (given 8 comparisons per task, the significance of each comparison was evaluated with α = 0.00625).
Checkerboard Task
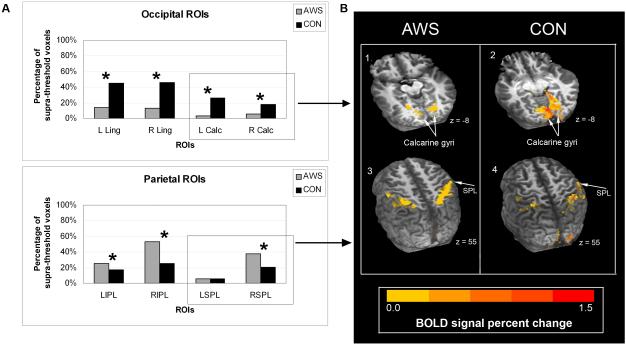
In occipital lobe ROIs, the child with Alice in Wonderland syndrome produced fewer active voxels (hypoactivation) than the control child in the left hemisphere calcarine gyrus ROI (χ2 = 49.8, p < 0.0001), but not the right calcarine ROI (χ2 = 4.0, p = 0.04). No differences were detected in either lingual gyrus ROI (left: χ2 = 1.2, p = 0.26; right: χ2 = 1.7, p = 0.19). In the parietal lobe ROIs, the child with Alice in Wonderland syndrome produced significantly more active voxels (hyperactivation) than the control child bilaterally in the superior parietal lobule ROIs (left: χ2 = 229.8, p < 0.0001; right: χ2 = 212.3, p < 0.0001). No differences were found in the inferior parietal ROIs (left: χ2 = 6.0, p = 0.01; right: χ2 = 4.6, p = 0.03).
Ponzo Illusion Task
Throughout the Ponzo illusion task, participants made a binary decision as to whether the vertical lines were of equal or different lengths; although responses were monitored explicitly during testing (by colored lights keyed to each button on the response box), due to software error these responses were not recorded. By observation of the responses during testing, however, we were able to conclude that participants attended to the task and responded to each trial.
In the occipital lobe ROIs, the child with Alice in Wonderland syndrome produced fewer active voxels (hypoactivation) than the control child bilaterally in the calcarine gyrus ROIs (left: χ2 = 81.2, p < 0.0001; right: χ2 = 26.5, p < 0.0001) and the lingual gyrus ROIs (left: χ2 = 81.6, p < 0.0001; right: χ2 = 95.9, p < 0.0001). The child with Alice in Wonderland syndrome produced significantly more active voxels (hyperactivation) than the control child in the right but not the left hemisphere superior parietal lobule ROI (left: χ2 = 0.02, p = 0.89; right: χ2 = 23.6, p < 0.0001). The child with Alice in Wonderland syndrome also showed hyperactivation relative to the control child in the inferior parietal cortex ROIs bilaterally (left: χ2 = 7.7, p = 0.0054; right: χ2 = 27.2, p < 0.0001).
Discussion
In this study, we had the unique opportunity to directly evaluate brain function in Alice in Wonderland syndrome during an episode of micropsia, the moment that defines the disorder. Previous studies of Alice in Wonderland syndrome have implicated the occipital and parietal lobes specifically in micropsia.4-8 We therefore targeted our investigation to these regions in particular, with two visual processing tasks, one passive (reversing checkerboard) and one active (Ponzo illusion). The current findings demonstrate a consistent pattern across both the passive and active viewing tasks: the patient with Alice in Wonderland syndrome recruited parietal regions more extensively than the matched control participant, who more broadly activated visual regions in the occipital lobe during these same tasks.
In the occipital lobe, the child with Alice in Wonderland syndrome demonstrated reduced activation (hypoactivation) of primary and extrastriate regions of visual cortical areas during his episode of micropsia, relative to the control participant. In the passive viewing task, this hypoactivation was limited to the left hemisphere of the calcarine gyrus, with no differences between participants in other occipital ROIs (Figure 2). For the active viewing task, the child with Alice in Wonderland syndrome demonstrated more extensive hypoactivation, which was seen for both the calcarine and lingual gyrus ROIs bilaterally (Figure 3).
FIG 2.
A, Mean percentage of BOLD signal change for each ROI: supra-threshold positive voxels per ROI for the child with Alice in Wonderland syndrome (AWS) and the control participant (CON). Percentage of positive voxels is shown rather than the raw numbers since each region has a different total number of voxels. Statistically significant differences between participants for each ROI are indicated by an asterisk. B, Representative images of functional activation (vs fixation) for each participant overlaid on their own anatomical MRI, showing occipital lobe (1,2; calcarine gyri shown) and parietal lobe (3,4; superior parietal lobule shown) activation patterns. See e-Supplement 1 for methods and definitions of anatomical boundaries.
ROI, region of interest; L Calc, left calcarine gyrus; R Calc, right calcarine gyrus; L Ling, left lingual gyrus; R Ling, right lingual gyrus; SPL, superior parietal lobule; LIPL, left inferior parietal lobule; RIPL, right inferior parietal lobule; LSPL, left superior parietal lobule; RSPL, right superior parietal lobule.
FIG 3.
A, Mean percentage of BOLD signal change for each ROI: suprathreshold positive voxels per ROI for the child with Alice in Wonderland syndrome (AWS) and the control participant (CON). Percentage of positive voxels is shown rather than the raw numbers since each region has a different total number of voxels. Statistically significant differences between participants for each ROI are indicated by an asterisk. B, Representative images of functional activation (vs fixation) for each participant overlaid on their own anatomical MRI, showing occipital lobe (1,2; calcarine gyri shown) and parietal lobe (3,4; superior parietal lobule shown) activation patterns. See e-Supplement 1 for methods and definitions of anatomical boundaries.
ROI, region of interest; L Calc, left calcarine gyrus; R Calc, right calcarine gyrus; L Ling, left lingual gyrus; R Ling, right lingual gyrus; SPL, superior parietal lobule; LIPL, left inferior parietal lobule; RIPL, right inferior parietal lobule; LSPL, left superior parietal lobule; RSPL, right superior parietal lobule.
Thus it appears that primary and extrastriate visual cortex regions in the occipital lobe do not respond normally to increased demands for visual analysis during micropsia, particularly during an active viewing task. While it has been demonstrated in the literature that these regions are sensitive to low-level perceptual factors, such as proportion of the retina occupied by the stimulus, orientation, and so forth, it is the case here that there is a clear distinction in the patterns of activation found between the control and Alice in Wonderland syndrome participants. The activation of this region of the visual cortex by the control participant allows us to rule out stimulus factors (line length, thickness, etc, in the illusion task) as a possible explanation for the results.
Toward an explanation of this occipital hypoactivation, we note that micropsia has been reported following focal lesion damage in occipital regions,21-24 particularly throughout regions Brodmann areas 18 and 19, which correspond to our calcarine and lingual gyri ROIs. Therefore, a functional lesion (eg, hypoactivation) may contribute to the percept of micropsia. These reports and our results are also consistent with prior neurophysiological studies of viral-onset Alice in Wonderland syndrome, which reported abnormal EEG patterns and hypoperfusion in occipital regions.4-6 First, the hypoactivation we observed in occipital cortical regions appears consistent with findings of abnormal visual evoked potentials, which were elicited by a reversing checkerboard similar to the one used in our study.4-6 Second, as discussed above, prior SPECT studies have reported hypoperfusion of the occipital cortex in Alice in Wonderland syndrome.5,7
However, it is not the case that the child with Alice in Wonderland syndrome shows an overall reduction in BOLD signal. During the episode of micropsia, we see excessive recruitment of parietal regions across both passive (checkerboard) and active (Ponzo illusion) tasks. In the passive viewing task, we observed a large difference between participants in bilateral superior parietal regions, with the child with Alice in Wonderland syndrome exhibiting hyperactivation relative to the control participant but no difference between participants in inferior parietal regions. This suggests over-recruitment of neural regions that may not be typically involved in passive viewing.
More strikingly, in the active viewing task, which requires visual perception, attention, and size comparison between two visual stimuli, we find hyperactivation of the right superior parietal cortex as well as bilateral inferior parietal cortical regions in the child with Alice in Wonderland syndrome relative to the control participant. This right hemisphere parietal bias is perhaps not a surprising result from this active task, as many studies have implicated the right hemisphere in a variety of spatial attention tasks.16,25-29
The notion of aberrant activation of parietal regions is not new to the Alice in Wonderland syndrome literature. Along with the previously published EEG reports demonstrating abnormal wave patterns over parietal and occipital scalp electrodes (see above), neuroimaging data have reported hypoperfusion in the (specifically right) parietal cortex in 5 of 9 Alice in Wonderland syndrome cases.5,7,8 However, it is not clear if these three physiological measures (BOLD, EEG, perfusion) are indicative of the same underlying mechanism.
In sum, across both perceptual tasks, we see a consistent pattern of occipital hypoactivation and parietal hyperactivation in the child with Alice in Wonderland syndrome, relative to the control participant. However, there are some limitations to our results.
First, the use of a single control participant makes it difficult to establish whether the abnormal pattern of activation seen in the child with Alice in Wonderland syndrome actually falls outside the range of the normal BOLD signal variability that would be observed for unaffected individuals. This is particularly the case for the Ponzo illusion task, which, despite being a well-studied effect of visual perception, as reviewed and discussed by Prinzmetal and colleagues,30 does not have the history of use with fMRI that the reversing checkerboard task has. If the child with Alice in Wonderland syndrome had shown a global change in BOLD signal relative to the control participant, it might be argued that the results simply reflect individual differences in BOLD signal responsiveness. However, such individual differences are very unlikely to result in the observed pattern of relative hypoactivation in some regions with concomitant relative hyperactivation in others. Nonetheless, without additional control data that establishes the normal BOLD signal range in all of our ROIs on these tasks, we cannot eliminate the possibility that an unaffected individual would indeed show a pattern similar to that seen in our participant with Alice in Wonderland syndrome.
Second, while our results appear broadly consistent with prior results implicating dysfunctional neurophysiological processing in occipital and parietal regions in Alice in Wonderland syndrome, these prior results were obtained when participants were between episodes of micropsia (ie, not actually experiencing it). Thus in comparison to a healthy neurological system, similar neurological dysfunction may underlie the potential for an episode of micropsia and the abnormal visual experiences that occur during such an episode. The results presented here do not speak to the differences between these two dysfunctional neurological states. The investigation of such differences would require additional control conditions (eg, comparing the same child with Alice in Wonderland syndrome while experiencing micropsia and while not experiencing micropsia), that could not be included in the present study. Nevertheless, together with prior neuroimaging and lesion reports, the fMRI results reported here suggest that micropsia in viral-onset Alice in Wonderland syndrome is linked both to functional impairment of the occipital cortex (under-recruitment of primary and extrastriate visual regions) and to over-recruitment of specific regions in the parietal cortex. While many questions remain, the novel situation explored in this study provides valuable information about the functional underpinnings of micropsia in viral-onset Alice in Wonderland syndrome.
Supplementary Material
Acknowledgments
The authors thank James Clinton, Daniel Sanchez, and Maxwell Moholy for their help in stimulus preparation.
This work was supported by the National Institutes of Health R01DC03861 (TL), T3 DC007361 (KB), and R01HD060595 (FH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was conducted at the University of California, San Diego.
The authors have no conflicts of interest.
This work has not been reported at any national meeting.
Literature Search
A literature search was conducted via PubMed/MEDLINE, Exerpta Medica/EMBASE and Opthalmic Literature, and via Google scholar, using the keywords Alice, Wonderland, AND syndrome; virus, pediatric, micropsia, and metamorphopsia. Also, all references in the articles found by these searches were sought out. For articles reporting neurophysiological measurements (eg, SPECT, EEG), the authors of these articles were contacted by email if there was any ambiguity or doubt as to whether or not the participants in their studies were experiencing micropsia during testing.
References
- 1.Lippman CW. Certain hallucinations peculiar to migraine. J Nerv Ment Dis. 1952;116:346–51. doi: 10.1097/00005053-195210000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Todd J. The syndrome of Alice in Wonderland. CMAJ. 1955;73:701–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Eshel GM, Eyov A, Lahat E, Brauman A. Alice in Wonderland syndrome, a manifestation of acute Epstein-Barr virus infection. Pediatric Infectious Disease Journal. 1986;6:68. doi: 10.1097/00006454-198701000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Lahat E, Berkovitch M, Barr J, Paret G, Barzilai A. Abnormal visual evoked potentials in children with “Alice in Wonderland” syndrome due to infectious mononucleosis. J Child Neurol. 1999;14:732–5. doi: 10.1177/088307389901401109. [DOI] [PubMed] [Google Scholar]
- 5.Kuo Y-T, Chiu N-C, Shen E-Y, Ho C-S, Wu M-C. Cerebral perfusion in children with Alice in Wonderland syndrome. Pediatr Neurol. 1998;19:105–8. doi: 10.1016/s0887-8994(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 6.Liaw S-B, Shen E-Y. Alice in Wonderland syndrome as a presenting symptom of EBV infection. Pediatr Neurol. 1991;7:464–6. doi: 10.1016/0887-8994(91)90032-g. [DOI] [PubMed] [Google Scholar]
- 7.Hung K-L, Liao H-T, Tsai M-L. Epstein-Barr virus encephalitis in children. Acta Paediatrica Taiwanica. 2000;41:140–46. [PubMed] [Google Scholar]
- 8.Gencoglu EA, Alehan F, Erol I, Koyuncu A, Aras M. Brain SPECT findings in a patient with Alice in Wonderland syndrome. Clin Nucl Med. 2005;30:758–9. doi: 10.1097/01.rlu.0000182278.13389.a3. [DOI] [PubMed] [Google Scholar]
- 9.Wang S-M, Liu C-C, Chen Y-J, Chang Y-C, Huang C-C. Alice in Wonderland syndrome caused by coxsackievirus B1. The Pediatric Infectious Disease Journal. 1996;15:470–71. doi: 10.1097/00006454-199605000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huettel SA, Song AW, McCarthy G. Functional magnetic resonance imaging. Sinauer Associates, Inc.; Sunderland, MA: 2004. [Google Scholar]
- 12.Logothetis NK, Wandell BA. Interpreting the BOLD Signal. Annu Rev Physiol. 2004;66:735–69. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 13.Fox PT, Raichle ME. Stimulus rate dependence of regional cerebral blood flow in human striate cortex demonstrated by positron emission tomography. J Neurophysiol. 1984;51:1109–20. doi: 10.1152/jn.1984.51.5.1109. [DOI] [PubMed] [Google Scholar]
- 14.Sereno MI, Dale AM, Reppas JB, et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–93. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 15.Murray SO, Boyaci H, Kersten D. The representation of perceived angular size in human primary visual cortex. Nat Neurosci. 2006;9:429–34. doi: 10.1038/nn1641. [DOI] [PubMed] [Google Scholar]
- 16.Fink GR, Marshall JC, Weiss PH, Zilles K. The neural basis of vertical and horizontal line bisection judgments: An fMRI study of normal volunteers. NeuroImage. 2001;14:S59–67. doi: 10.1006/nimg.2001.0819. [DOI] [PubMed] [Google Scholar]
- 17.Fink GR, Marshall JC, Weiss PH, et al. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: An fMRI study with clinical implications. Neuroimage. 2003;20:1505–17. doi: 10.1016/j.neuroimage.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single-subject analyses of unsmoothed fMRI data. Cereb Cortex. 2009;19:1239–55. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carey JR, Anderson KM, Kimberley TJ, Lewis SM, Auerbach EJ, Ugurbil K. fMRI analysis of ankle movement tracking training in subject with stroke. Exp Brain Res. 2004;154:281–90. doi: 10.1007/s00221-003-1662-7. [DOI] [PubMed] [Google Scholar]
- 20.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2001;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen L, Gray F, Meyrignac C, Dehaene S, Degos J-D. Selective deficit of visual size perception: Two cases of hemimicropsia. J Neurol Neurosurg Psychiatry. 1994;57:73–8. doi: 10.1136/jnnp.57.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frassinetti F, Nichelli P, di Pelligrino G. Selective horizontal dysmetropsia following prestriate lesion. Brain. 1999;122:339–50. doi: 10.1093/brain/122.2.339. [DOI] [PubMed] [Google Scholar]
- 23.Kassubek J, Otte M, Wolter T, Greenlee MW, Mergner T, Lücking CH. Brain imaging in a patient with hemimicropsia. Neuropsychologia. 1999;37:1327–34. doi: 10.1016/s0028-3932(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 24.La Mancusa JC, Cole AR. Visual manifestations of occipital lobe infarction in three patients on a geriatric psychiatry unit. J Geriatr Psychiatry Neurol. 1988;1:231–4. doi: 10.1177/089198878800100409. [DOI] [PubMed] [Google Scholar]
- 25.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, Honda M, Okada T, et al. Attentional modulation of parieto-occipital cortical responses: Implications for hemispatial neglect. J Neurol Sci. 2000;176:136–43. doi: 10.1016/s0022-510x(00)00335-x. [DOI] [PubMed] [Google Scholar]
- 27.Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13:1202–26. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–54. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- 29.Shafritz KM, Gore JC, Marois R. The role of the parietal cortex in visual feature binding. Proc Natl Acad Sci U S A. 2002;99:10917–22. doi: 10.1073/pnas.152694799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prinzmetal W, Shimamura AP, Mikolinski M. The Ponzo illusion and the perception of orientation. Percept Psychophys. 2001;63:99–114. doi: 10.3758/bf03200506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.