Abstract
Memantine is a partial NMDA receptor antagonist that has been shown to improve learning and memory in several animal models, and is approved for the treatment of Alzheimer’s disease. Chronic treatments using memantine in animal models of Alzheimer’s disease show disease-modifying effects and suggest a potential neuroprotective function. The present study assessed the effects of both short- and long-term memantine treatment in a mouse model of Down syndrome, the Ts65Dn mouse. The Ts65Dn mouse contains a partial trisomy of murine chromosome 16, and exhibits hippocampal-dependent memory deficits, as well as progressive degeneration of basal forebrain cholinergic neurons. Ts65Dn mice were treated with memantine for a period of six months, beginning at four months of age. At the end of treatment the mice underwent memory testing using novel object recognition and water radial arm maze tasks, and then histologically analyzed for markers of neurodegeneration. Memantine treatment improved spatial and recognition memory performance in the Ts65Dn mice, though not to the level of normosomic littermate controls. Despite these memory improvements, histological analysis found no morphological signs of neuroprotection of basal forebrain cholinergic or locus coeruleus neurons in memantine-treated Ts65Dn mice. However, memantine treatment of Ts65Dn mice gave rise to elevated brain-derived neurotrophic factor expression in the hippocampus and frontal cortex, suggesting a mechanism of behavioral modification. Thus, our findings provide further evidence for memory facilitation of memantine, but suggest pharmacological rather than neuroprotective effects of memantine both after acute and chronic treatment in this mouse model.
Keywords: Alzheimer’s disease, radial arm maze, novel object recognition, brain derived growth factor, BDNF, hippocampus, cholinergic neuron, inflammation
Introduction
Down syndrome (DS) is the most common genetic cause of mental retardation, resulting from a trisomy of chromosome 21. In addition to developmental disabilities and learning impairments, DS individuals acquire the symptoms of dementia in the 4th and 5th decades of life at high rates and show neuropathology consistent with Alzheimer’s disease (AD) with near uniformity [17]. While the early-onset dementia and neuropathology that develops in DS has much in common with that seen in AD, it is not clear whether cognitive decline in DS individuals arises through similar biological mechanisms.
Currently the most complete animal model for DS is the Ts65Dn mouse, which contains a partial trisomy of murine chromosome 16 confined to the region homologous to human chromosome 21. Ts65Dn mice exhibit characteristic age-related pathology associated with both AD and DS: degeneration of basal forebrain cholinergic neurons (BCFNs) [40], impaired hippocampal long-term potentiation [71], as well as increases in inflammation and oxidative stress [42, 52]. In addition, these mice exhibit memory deficits on both spatial and non-spatial cognitive tasks [33, 44, 64]. Triplication of the amyloid precursor protein (APP) gene is required for a number of these pathologies [66], suggesting that elevated levels of APP or its toxic cleavage products, such as β-amyloid, may contribute to these deficits. In addition to its inflammatory potential, β-amyloid, has also been implicated in contributing to neuronal death by triggering excessive calcium signaling through NMDA receptors [22].
Other candidate genes for DS phenotype that may modulate NMDA receptor function, Dyrk1A and DSCR1, are also triplicated in Ts65Dn mice. Dyrk1A, a dual specificity kinase, prolongs calcium uptake after NMDA receptor stimulation when over-expressed [4], while DSCR1, which encodes calcipressin, can similarly facilitate Ca2+ influx by increasing NMDA receptor activity through the inhibition of calcineurin [49]. Ts65Dn mice exhibit an age-dependent reduction in Calbindin D-28k, a Ca2+ binding protein and important regulator of Ca2+ intracellularly, in the CA1 of the hippocampus [42]. The loss of Calbindin D-28k may further sensitize these neurons to excitotoxicity, since they would be less able to handle the elevated calcium load. Taken together, these studies provide strong evidence for altered NMDA receptor function, and consequent disrupted calcium signaling, increasing the potential for excitotoxic damage in DS, but chronic studies using neuroprotective therapies targeting calcium regulation or NMDA receptors have not been performed in Ts65Dn mice to date.
Conventional therapies for AD for many years have focused on cholinesterase inhibitors, which function by blocking acetyl-cholinesterase enzymatic activity resulting in increased levels of acetylcholine at the synapse, and have been used as the conventional therapy for AD for many years [for review: [37]]. More recently NMDA receptor antagonists, such as memantine, have shown promise in their ability to not only facilitate cognitive function, but also to potentially halt or reduce the progression of the disease [For review: [50]]. Glutamatergic NMDA receptors are required for several forms of synaptic plasticity and memory formation [6, 20], but over-activation of these channels can disrupt these processes and lead to intracellular calcium toxicity. NMDA receptors, due to their high permeability to Ca2+ and their slow-gating kinetics, are also implicated in excitotoxic neuronal damage that has been implicated in several neurodegenerative disorders, including AD [50]. Cerebrospinal fluid levels of glutamate are elevated in AD patients [63], and impaired glutamate transporter function in these individuals may result in excessive glutamate at the synaptic cleft [54]. The potential efficacy of memantine lies in its low affinity, noncompetitive antagonism for the NMDA receptor, which paradoxically acts as a stronger inhibitor under increasing glutamate levels [60], thus allowing memantine to preferentially reduce pathological NMDA receptor activity without impairing physiologic functioning necessary for learning and memory [59, 67]. Importantly, several preclinical animal studies have found that memantine improves hippocampal-dependent memory function [57, 84] and human clinical trials have found that memantine improves cognition and global mental status significantly in individuals with mild or moderate dementia after 28 weeks of treatment [29]. In addition, preclinical studies show that memantine attenuates neuropathological hallmarks, such as reducing amyloid plaque burden and neuroinflammation in AD mouse models [56, 68], but the neuroprotective effects of this compound in animal models for DS have not been investigated.
In the present study, we first examined whether long-term oral memantine treatment in Ts65Dn mice would improve performance in measures of hippocampal- as well as frontal cortex-dependent learning and memory tasks. An oral dosage of 20 mg/kg memantine was implemented, as this route is consistent with clinical administration. We hypothesized that since Ts65Dn mice share similar cognitive and neuropathological hallmarks to those observed in murine models of AD-like pathology, memantine treatment could have significant behavioral and morphological benefits in the Ts65Dn mouse such as facilitation of learning and memory function, neuroprotection of BFCNs and calbindin D-28k-positive cells in the hippocampus, and reduction of microglial activation observed in Ts65Dn mice [38, 42, 52]. Therefore in addition to evaluating Ts65Dn mice via water radial arm maze (WRAM) and novel object recognition (NOR) testing, we also assessed markers of cholinergic and noradrenergic degeneration, neuroinflammation, and hippocampal morphology after a six-month chronic memantine administration. In a second study, we also evaluated the cognitive effects of acute administration of memantine in a separate set of Ts65Dn mice. We found that memantine facilitated performance in the WRAM and NOR tasks in Ts65Dn but not in normosomic mice in both chronic and acute settings, but did not alter markers of neurodegeneration in Ts65Dn mice.
Methods
Study 1: Long-term memantine treatment
Subjects
Mice partially trisomic for a segment of murine chromosome 16 just proximal to the gene for App and extending to the gene for myxovirus resistance (Mx) were developed by M. Davisson at Jackson Laboratories [21]. Controls for this experiment were normosomic littermates (NS) to the Ts65Dn mice with the same genetic background (B6C3HF1). As the C3H mouse strain carries the retinal degeneration allele (rd), the Ts65Dn and NS were screened and found free of retinal degeneration at Jackson Laboratories before shipment to MUSC. The trisomy is maintained by mating female carriers (the males are sterile) to C57Bl/6Jeicher × C3H/HeSnJ F1 males on a segregated genetic background [21].
Subjects consisted of male Ts65Dn mice and their NS littermates which were acquired from Jackson Laboratories (Bar Harbor, ME) and housed in our animal facility for 1 to 2 months before testing. All mice were singly housed, received food and water ad libitum, and were maintained on a 12-hour light/dark cycle. Due to the subtle nature of the Ts65Dn phenotype, behavioral testing was conducted blind to the genotype of the animals. All experimental procedures were approved by the institutional animal care and use committee (IACUC) of the Medical University of South Carolina.
Treatment
Long-term memantine treatment in study 1 was initiated at 4 months-of-age (Fig. 1 schematically depicts the experimental timeline). Memantine hydrochloride (Tocris, Bristol, UK) was administered orally (via drinking water) at doses of 20 mg/kg/day on average for a period of six months, and was maintained during behavioral testing (see Fig. 1). This method of administration has proven successful previously in prolonged studies, producing steady state plasma levels within the therapeutic range [57]. Concentrations of 20 mg/kg have previously shown neuroprotective effects as well as memory facilitation in models of AD and stroke [12, 19, 23, 82]. Water consumption was measured weekly throughout treatment to confirm average daily memantine intake over the course of each week. Controls received drinking water, and were also measured for water consumption. There were no differences in liquid consumption between groups (NS Control = 4.8±0.7 ml/day; NS Mem = 5.1±0.06 ml/day; Ts65Dn Control = 5.5±0.3 ml/day; Ts65Dn Mem = 5.2±0.5 ml/day; p>0.2). During novel object recognition testing, a subset of mice were taken off oral memantine prior to the third session and switched to normal drinking water for one week, in order to determine whether memantine treatment had sustained effects (see Fig. 1, Oral Memantine). Following this session of recognition testing, mice were returned to oral memantine treatment of 20 mg/kg/day for a final session of recognition testing and until sacrifice.
Figure 1.
Study timelines. Study 1 consisted of a long-term oral memantine treatment in which Ts65Dn mice and normosomic controls (NS) received memantine (20 mg/kg) for 6 months, beginning at 4 months of age. Mice from Study 1 were first tested on a 3-day win-stay RA maze at 8.5 months of age. Following spatial memory testing, the mice underwent a series of four weekly NOR testing sessions. Prior to the 3rd NOR testing session, mice that had previously received memantine were switched to control fluids, such that the 3rd session occurred with mice “off” treatment. Following this session, mice were returned to their treatments for session 4. Study 2 evaluated the effects of acute memantine administration on recognition memory in Ts65Dn mice, as naïve Ts65Dn mice received acute memantine injections (10 mg/kg, i.p.) immediately prior to NOR training and testing periods.
Spontaneous Activity
Ts65Dn and NS mice were tested to evaluate the effects of memantine-treatment on motor activity. Spontaneous activity (total distance traveled) was assessed in a Digiscan Animal Activity Monitor system for 1 h (Omnitech Electronics Columbus, OH), as described previously (Boger et al. 2006). Each activity unit contains 16-photo beams positioned 5 cm apart, 8 on the x-axis, and 8 on the y-axis. The Digiscan analyzer is interfaced with an IBM XT computer using ILAM software (Coulbourn Instruments, Lehigh Valley, PA). Total activity is recorded as total centimeters traveled over the testing intervals described for each experiment. Center time measures the proportion of time spent in the center of the arena (a percentage of total time). On the day of testing, the mice were transferred from the animal colony into the laboratory in groups of six and tested in a darkened environment.
Win-stay water radial arm maze (WRAM) testing procedure: assessment of spatial reference memory
Testing of mice from study 1 began 4.5 months following initiation of treatment, at 8.5 months of age (12 NS Control; 6 NS Mem; 9 Ts65Dn Control; 8 Ts65Dn Mem). This is a win-stay spatial reference memory task that minimizes thigmotactic behavior, which has been documented in Ts65Dn mice [16, 28]. The 3 d win-stay WRAM has been modified from a procedure described previously [79], on an eight-arm maze apparatus. Briefly, on day 1, 12 trials were run on two blocks of six trials, with mice run in cohorts of six, permitting a short rest between each trial and a longer rest while the other cohort of mice were run. This testing procedure was established in order to facilitate rapid testing with less fatigue than other water maze paradigms. The goal arm containing the platform was kept consistent through all trials of testing, while the start arm was varied for each trial (win-stay). Day 1 of the WRAM is a training day, in which the visible and hidden platforms were alternated to shape mice into identifying platform location, a technique shown to be effective by Alamed et al. as a way to reduce acquisition time [2]. In the final three trials of Day 1 the platform remained hidden. On days 2 and 3, the two days of testing, all trials were run using the hidden platform. The maximum trial time for all trials was 60 s. Incorrect arm entries were counted as errors. Consistent with previous studies [2], inactive mice were assigned one error for every 20 s a mouse failed to make an arm selection. Mouse inactivity was only seen during the first few trials of testing, and no errors caused by inactivity were therefore assigned in days 2 or 3 for either of the groups. For statistical measurements, errors were averaged into three trial blocks, with four blocks per day. These trial blocks were analyzed statistically by ANOVA using Statview (SAS Institute, Cary, NC).
Novel Object Recognition
One week following WRAM testing, mice from Study 1 began habituation for the novel object recognition (NOR) task. This cognitive task is non-aversive and can be repeatedly used to measure memory across treatment regimens, and has been described previously [33]. Briefly, Ts65Dn and NS mice were submitted to daily handling sessions and allowed to habituate to a black, acrylic, open field arena (40 cm × 40 cm wide × 30 cm high) for 5 min the day before testing. During a 15 min training phase, the mice were exposed to either two identical objects (simple protocol) or two different objects (complex protocol) positioned in two opposite corners of the apparatus. These objects varied in material composition (plastic, metal, and glass), color scheme, and shape, though they all maintained a similar size and volume and were attached to a flat washer for stability. Twenty-four h after training, a 15 min testing phase occurred, in which the mice were presented with one object explored previously during the training phase (familiar), and a new object. Both phases were recorded, and the time spent exploring each object was measured by two blinded observers. Exploration was defined as any investigative behavior, such as head orientation or sniffing within 1.0 cm, or deliberate contact with the object. Mice that did not explore the objects for at least 30 s during the training phase were excluded from analysis. This resulted in the exclusion of one normosomic mouse throughout testing. The proportion of time spent investigating the novel object relative to the familiar one, i.e. the discrimination index, was used to quantify recognition memory [Discrimination Index = (novel object exploration time / total exploration time) – (familiar object exploration time / total object exploration time) × 100] according to previous studies in this mouse model [33]. Between each mouse, the objects and apparatus were cleaned with 70% ethanol and allowed to dry before the next mouse was tested. Mice were subjected to multiple sessions of recognition testing, with each session separated by one-week intervals, and each separate session using new object sets. This paradigm treats each mouse as a naïve subject, and each session is considered an independent observation [32]. The long-term memantine study was conducted with the complex protocol (two different objects during the training session), while the acute memantine study utilized the simple protocol (two identical objects).
Tissue Preparation
All Study 1 mice were used for histochemical analysis and were anesthetized with isoflurane and sacrificed by decapitation. After removal from the skull, the brain was blocked sagittally, with the entire frontal lobe and the remaining right hemisphere of the brain post-fixed in 4% paraformaldehyde, while the hippocampus was immediately dissected from the left hemisphere, frozen in dry ice and kept at −80°C. Post-fixed brain regions were kept in paraformaldehyde for 48 h, after which they were transferred to 30% sucrose in 0.1 M phosphate buffer for at least 48 h before sectioning. All sections were generated at a thickness of 45 µm on a Microm cryostat.
Quantitative real-time PCR
Total RNA was extracted from hippocampal tissue using 1 ml Trizol (Invitrogen, Carlsbad, CA). After vigorous mixing in chloroform (0.2 ml), the samples were centrifuged for 15 minutes at 14,000 × g. The aqueous phase was transferred to a fresh tube and mixed with an equal volume of isopropyl alcohol. RNA was then isolated using RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized by reverse transcription of 1 µg RNA using high-capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA). Realtime quantitative RT-PCR was performed on an ABI 7000 (Applied Biosystems) by monitoring the increase in FAM reporter dye fluorescence resulting from Taqman probe binding to DNA. For a 20 µl PCR reaction, 100 ng DNA was added to Gene Expression Master Mix and BDNF Taqman Gene Expression Assay (Applied Biosystems, Assay ID: Mm01334042_m1). The reaction was run using the default setting profile (95°C, 15 s; 60°C, 1 m; 40 cycles). All PCR reactions were performed in duplicate (n=3 per group). Gene expression changes were quantified by the ΔΔCt method, which calculates relative fold changes normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Assay ID: Mm99999915_g1).
BDNF ELISA
BDNF was assessed on frontal cortex tissue (the most medial 1.5–2 mm anterior pole) using a commercially available assay kit from R&D (R&D Systems, Minneapolis, MN) according to our standard protocol [8]. In brief, flat-bottom plates were coated with the BDNF capture antibody, which binds the soluble, captured neurotrophin. Tissue homogenate was then incubated for 2 hours. The captured BDNF was bound by a second specific antibody, which was detected using a species-specific antibody conjugated to horseradish peroxidase as a tertiary reactant. All unbound conjugates were removed by subsequent wash steps according to the R&D protocol. After an incubation period with chromagenic substrate, color change was measured in an ELISA plate reader at 450 nm. Using these kits, BDNF can be quantified in the range of 7.8–500 pg/ml. For each assay kit, cross-reactivity with other trophic proteins is <2–3%. Quantification was normalized via weight (mg tissue), and group differences for BDNF levels were analyzed by ANOVA.
Immunohistochemistry
Immunohistochemistry for the high-affinity NGF receptor TrkA, a reliable marker for cholinergic neurons [72], tyrosine hydroxylase (TH), and the pan-microglial marker CD45 in the basal forebrain, locus coeruleus, and hippocampus were conducted as previously reported utilizing the avidin-biotin method followed by nickel-enhanced diaminobenzidine (DAB) development [38]. Briefly, free-floating sections were rinsed with 0.01 M tris buffered saline (TBS), incubated in a solution containing 0.3% hydrogen peroxide and 20% methanol in TBS to inhibit residual endogenous peroxidase, and blocked for 1 h in 10% normal goat serum (NGS) and 0.3% Triton X-100. Sections were incubated for 48 h at 4 °C in a primary antibody solution (rabbit anti-TrkA, 1:10,000, generously provided by Dr. L. Reichardt; rabbit anti-TH, 1:2000, Pel-Freeze; rat anti-CD45, 1:1000, Serotec) containing 3% NGS and 0.3% Triton X-100. Next, sections were incubated for 1 h secondary antibody (TrkA and TH: biotinylated anti-rabbit secondary antibody, 1:200; CD45: biotinylated anti-rat secondary antibody, 1:200; Vector Laboratories, Burlingame, CA) followed by incubation in an avidin–biotin complex (ABC Elite; Vector) before development in (DAB). Immunohistochemistry for Calbindin D-28k (Cal 28k) in the hippocampus used the peroxidase antiperoxidase method described previously [42], using primary rabbit anti- Cal 28k (1:1000, Chemicon), biotinylated goat anti-rabbit secondary (1:200, Covance), and incubation with rabbit peroxidase-antiperoxidase (1;200, Covance) before DAB development. Sections were mounted on subbed slides, dehydrated with increasing gradients of ethanol, cleared with two incubations of xylene, and coverslipped with Permount. To control for antibody specificity, we incubated one section without primary antibody and one section without secondary antibody for each different type of staining. No immunostaining was observed in any of these sections. All sections for each different antibody used were processed together under the same conditions to avoid batch-to-batch differences. Antibody penetration throughout the full section depth was determined by stepwise focusing through the z-plane of several sections.
Stereology and image analysis
Stereology of LC
Quantitative estimates of the total number of TH-positive neurons in the locus coeruleus (LC) were performed using the optical fractionator method (StereoInvestigator software; MicroBrightfield, Colchester, VT). This is an unbiased, stereological cell counting method that is not affected by the volume of reference (LC) or size of the counted elements (TH-positive neurons) that has been used frequently in our laboratory [9, 42]. The software performed a system of systemic random sampling of the region of interest. As a result, all objects within the defined region had the same chance of being counted. The landmarks outlining the area of interest were determined by using a Mouse Brain stereotaxic atlas [35]. After randomly selecting the initial section, every 3rd subsequent section was stained for TH (resulting in 7–8 sections for counting), providing for a systematic random design. The tissue was measured at 24 µm thick, and a 3 µm guard zone was employed to prevent the counting of artifacts in the tissue section. Disector counting frames of 75 × 75 µm were used (with a sampling grid of 125 × 125 µm, resulting in a sampling frame area of 0.36), and neurons were counted using a 60X objective lens (1.4 numerical aperture).
BFCN cell size
Cell size measurements for TrkA-positive neurons were performed on every 3rd serial section stained for TrkA (yielding 8–9 sections), using the nucleator probe within StereoInvestigator [39]. The nucleator uses a series of six rays that extend out from a point marked at the nucleus. Each intersection of the rays with the cell boundary is located and marked, and together they provide an estimation of cell size. Neurons were randomly selected via the optical fractionator program, with at least 10 neurons sampled per section and at least 60 neurons sampled per brain by an independent and blinded investigator, with neurons measured using a 60× objective lens (1.4 numerical aperture). Cal 28k and CD45 density: Staining intensity of Cal 28k and CD45 immunoreactivity in the cornu ammonis 1 and 2 regions of the hippocampus (CA1/CA2) was performed as described previously [42]. In brief, staining intensity was determined using NIH Image software which measures a gray scale value within the range of 0 to 256, where 0 represents white and 256 black. Images were captured with a Nikon Eclipse E-600 microscope. Staining density was obtained when background staining was subtracted from mean staining intensities on every 6th section through the hippocampus using the same systemic random design described above for cell counts.
STUDY 2: Acute Memantine Treatment
Treatment
Acute memantine was administered to a separate, naïve group of Ts65Dn mice at 8–10 mo of age, an age at which Ts65Dn mice have been shown to display behavioral impairments. Memantine (10 mg/kg) was dissolved in sterile 0.9% sodium chloride, filtered, and delivered via intraperitoneal (i.p.) injections (in a volume of 5.0 ml/kg) on two occasions 24 hours apart (see Fig. 1). The dose was reduced from 20 to 10 mg/kg in Study 2, as i.p. administration raises plasma levels more sharply than oral administration, and acute injections of 10 mg/kg are sufficient to produce plasma levels at or above the therapeutic dose [84]. These injections were given 30 minutes prior to both the training and testing phases of the NOR task (see methods above). Control animals received i.p. injections of saline at equivalent volumes, generating the following groups: NS Sal, n=9; Ts65Dn Sal, n=5; Ts65Dn Mem, n=7.
Statistics
All data were analyzed by one-way analysis of variance (ANOVA) unless otherwise noted. When the main effects of one-way ANOVA showed overall group differences, Fisher's post hoc analysis was used to assess differences between individual groups. Two-way ANOVAs (treatment × genotype) were also implemented to establish treatment and genotype effects and assess possible treatment × genotype interactions. The WRAM was analyzed using a 1-between (group) × 1-within (days) repeated-measures ANOVA (Statview Version 4.0). A paired t-test was used to determine error reduction within each group between Day 1 (the training day) and Day 2 (the first testing day) on the WRAM. A one-way t-test was used to determine discrimination in the novel object recognition task, with the hypothesized mean set to “0”. Significance was set at a p value less than 0.05 for all statistical analyses.
Results
Study 1: Long-term memantine administration
In order to investigate whether modulating glutamatergic signaling attenuates declarative memory deficits and neuropathology in Ts65Dn mice, we orally administered the partial NDMA receptor antagonist, memantine hydrochloride, (20 mg/kg/day) for a period of 6 months beginning when the mice were 4 months of age (Fig. 1). Age-matched, untreated Ts65Dn mice and normosomic (NS) littermates were maintained on regular drinking water. Oral administration of memantine did not alter fluid consumption, body weight, or general well-being of the animals studied (data not presented).
Memantine did not alter spontaneous activity in Ts65Dn mice
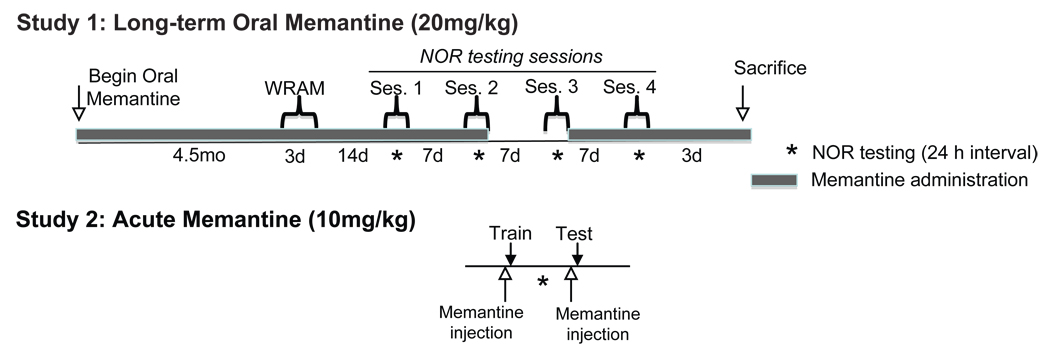
Ts65Dn and NS mice were tested for general activity to measure genotypic and drug effects on locomotor activity and exploration prior to the WRAM testing. A two-way ANOVA showed a genotype effect on total distance [Fig. 2A, Genotype Effect: F(1,32)=8.685, p<0.01], where Ts65Dn mice had greater activity during one hour of testing as measured by total distance travelled regardless of treatment. Long-term memantine treatment did not significantly elevate activity in either NS or Ts65Dn mice [Treatment Effect: F(1,32)=0.107]. Similarly, an analysis of the amount of time spent in the center of the apparatus during the first 20 minutes of testing revealed a genotypic effect [two-way ANOVA: F(1,32)=7.905; p=0.008], as Ts65Dn mice, regardless of genotype, spent less time in the center than NS mice. When confronted with a new location, mice typically develop a “home base” in one of the corners. Thus the time spent traveling through the center of the apparatus is often indicative of “exploratory activity”. Thus, the heightened exploratory activity combined with reduced center time in Ts65Dn mice suggests thigmotactic behavior (remaining close to the walls as a protective measure) and a heightened state of anxiety. Memantine had no significant effects on this behavior.
Figure 2.
Spatial memory deficits were attenuated following memantine treatment in Ts65Dn mice. A, Activity was measured over a one-hour session (distance traveled, cm). Ts65Dn Control and Ts65Dn Mem mice show greater spontaneous activity than NS Control and Mem mice. Memantine treatment did not significantly affect either genotype (Genotype effect: **p<0.01). B, Performance in a win-stay RA maze task is indicated by errors per trial across days. NS Control mice show reduced errors across days. While Ts65Dn Control mice performed significantly more errors on days 2 and 3 than NS Controls, Ts65Dn Mem mice show a reduction in errors relative to Ts65Dn Controls. Memantine treatment had no effect on NS mice (Days 2–3: *p<0.05 compared with NS mice, ^p<0.05 between Ts65Dn Mem and Ts65Dn control groups; mean ± SEM).
Improved spatial reference memory in memantine-treated Ts65Dn mice
At 8.5 months of age, mice were tested on the win-stay WRAM, which assessed hippocampal-dependent spatial learning and memory function over three days of testing. Under this testing paradigm, revealed that NS Control (n=12) and NS Mem (n=6) mice exhibited a significant decrease in errors between Day 1, the training day, and Day 2, the first day of testing (Fig. 2B), indicating that these groups were able to learn the task rapidly (Paired t-test between Day 1 and 2: NS Control t11=4.343, p<0.001; NS Mem t5=2.841, p<0.05). While Ts65Dn Controls (n=9) did not improve during the second day of testing (t8=0.898, p=0.4), Ts65Dn Mem mice (n=8) did show a significant error reduction (t7=3.974, p<0.01). A one-way ANOVA of errors collapsed across the final two days of testing [Days 2–3: F(3,31)=8.456, p<0.001] revealed a significant group-wise effect. Further analysis using Fisher’s post-hoc tests revealed that NS Controls made significantly fewer errors than Ts65Dn Control (p<0.0001) and Ts65Dn Mem (p<0.05) mice. While memantine treatment showed no effect in NS (NS Mem) mice at any time point, Ts65Dn mice receiving memantine treatment made fewer errors relative to their untreated counterparts on days 2 and 3 of behavioral testing (p<0.05 collapsed across days). This improved performance over the final two days of testing suggests that memantine treatment partially reduces spatial learning and memory deficits in Ts65Dn mice, though not to the level of performance observed with NS mice. The inability of memantine-treated Ts65Dn mice to reduce their error rate to NS levels in this spatial task suggests that memantine exerted only moderate effects on performance in the WRAM in Ts65Dn mice, and no beneficial effects for spatial reference memory in NS mice. In order to assess the effects of memantine on a non-spatial task, we therefore tested these mice using a novel object recognition task.
Memantine recovered object discrimination ability In Ts65Dn mice
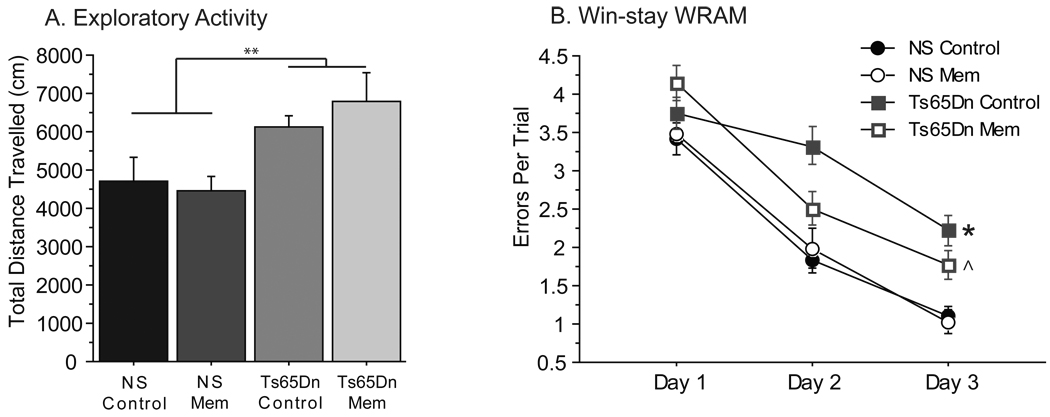
The novel object recognition (NOR) task utilizes the innate tendency of mice to differentially explore novel objects relative to favorable ones [26], and has been used previously to demonstrate deficits in recognition memory in the Ts65Dn mouse at longer time intervals [33]. Following habituation to the apparatus, Ts65Dn and NS mice began two weekly sessions of NOR testing using the “complex” protocol (yielding NS Control, n=14; NS Mem, n=12; Ts65Dn Control, n=12; Ts65Dn Mem, n=10 independent observations). At a delay between training and testing phases of 24 hours, commonly used to evaluate long-term memory in rodents [10], a one-group t-test revealed that NS Control and NS Mem mice were able to effectively discriminating the novel object, as measured by the Discrimination Index (DI) (Fig. 3A, DI>0, NS Control: t13=6.314, p<0.001; NS Mem: t11=3.095, p<0.05). On the other hand, Ts65Dn control mice showed no preference for the novel object during the testing phase (t11=0.913, p>0.3), and had a DI significantly lower [one-way ANOVA: F(3,44)=4.267, p=0.01] than both NS control and memantine groups (post-hoc analyses: p=0.001, p=0.04, respectively). Memantine treatment of Ts65Dn mice enabled this group to detect object novelty (t9=2.664, p<0.05), indicating a significant improvement with treatment in performance on this task. Improvement in object discrimination was not based on altered exploratory behavior, as there were no group differences between the groups for total exploration time during the testing phase (Fig. 3B, p>0.8). In order to determine whether the improved NOR memory function in Ts65Dn Mem mice could be maintained following a drug washout period, mice formerly receiving oral memantine solution were switched to water for one week, during which they underwent an additional exploration session. Removal of the memantine treatment during the 3rd session of testing resulted in deficits in ability to detect the novel object in the Ts65Dn Mem group (t4=1.277) – this group no longer performed statistically different from the null hypothesis (DI = 0) – and a significant difference in DI between NS and Ts65Dn mice [Fig. 3C, washout, F(1,8)=18.459, p<0.01]. A 4th exploration session, carried out one week after the reinstatement of memantine-treatment at the same dose, recovered the discriminatory function of these Ts65Dn mice, such that they detected the novel object as well as NS Mem mice (p>0.6). Thus, when receiving memantine treatment, these Ts65Dn mice discriminated the objects (trials 1 and 3: t13 = 0.01), while when memantine was removed Ts65Dn mice did not distinguish between objects. In addition, a genotype by treatment interaction [F(1,36)=7.759, p<0.01] during this partial cross-over study indicated that memantine selectively improved performance in this task in Ts65Dn mice, but not in NS mice, and that memantine treatment did not result in long-lasting memory improvements in Ts65Dn mice.
Figure 3.
Memantine-treatment recovered novel object recognition in Ts65Dn mice following both chronic and acute delivery. A, Novel object recognition performance, as shown by discrimination indices (DI), indicate Ts65Dn did not discriminate object novelty, showing no preference for novel objects and a reduced DI compared to NS control mice. Ts65Dn Mem mice, in contrast, were able to discriminate object novelty. B, Total exploration time was not altered by memantine-treatment (n = 10–14). C, A cross-over trial was carried out in the memantine-treated mice to determine whether memantine-treatment would sustain performance following removal of the drug. Following memantine removal of one week, these Ts65Dn mice were no longer able to discriminate object novelty, while reinstatement of memantine recovered their preference for novel objects (*p<0.05 compared with NS mice; mean ± SEM).
Effects of memantine treatment on basal forebrain cholinergic neurons
In order to determine whether memantine exerted neuroprotective effects on the progressive cholinergic degeneration observed in Ts65Dn mice with aging, we performed immunohistochemistry for the high-affinity nerve growth factor receptor TrkA in the medial septal nucleus following long-term memantine treatment. No statistical differences between treatments in NS mice were observed in any morphologic analysis, thus NS Control and NS Mem groups were combined for statistical measurements. As illustrated in Figure 4, TrkA-positive neurons in the medial septal nucleus of NS mice (Fig. 4A) appeared larger and more darkly stained with increased processes than BFCNs of both untreated (Fig. 4B) and memantine-treated Ts65Dn (Fig. 4C) mice. A reliable indicator of cholinergic neuron function, cell size correlates strongly with memory loss [47], and progressive cell atrophy has been documented in both DS [3] and Ts65Dn mice [38] with aging. Cell measurements using the Nucleator method confirmed reductions in TrkA-positive cell sizes of nearly 15% in Ts65Dn mice regardless of treatment [Fig. 4D, ANOVA: F(2,20)=15.858, p<0.001], indicating that memantine treatment was not sufficient to attenuate BFCN atrophy in Ts65Dn mice.
Figure 4.
Memantine-treatment did not prevent cholinergic neuron atrophy in basal forebrain. A–C show TrkA-positive cholinergic neurons in the medial septal nucleus in NS control (A), Ts65Dn control (B), and Ts65Dn Mem (C). Arrows show normal BFCN phenotype, while arrowheads indicate smaller neurons exhibiting less robust TrkA-immunoreactivity (40x magnification, scale bar = 40 µm). D, Cell size measurements depict significant reductions in cell area in control and memantine-treated Ts65Dn mice relative to NS (*p<0.05).
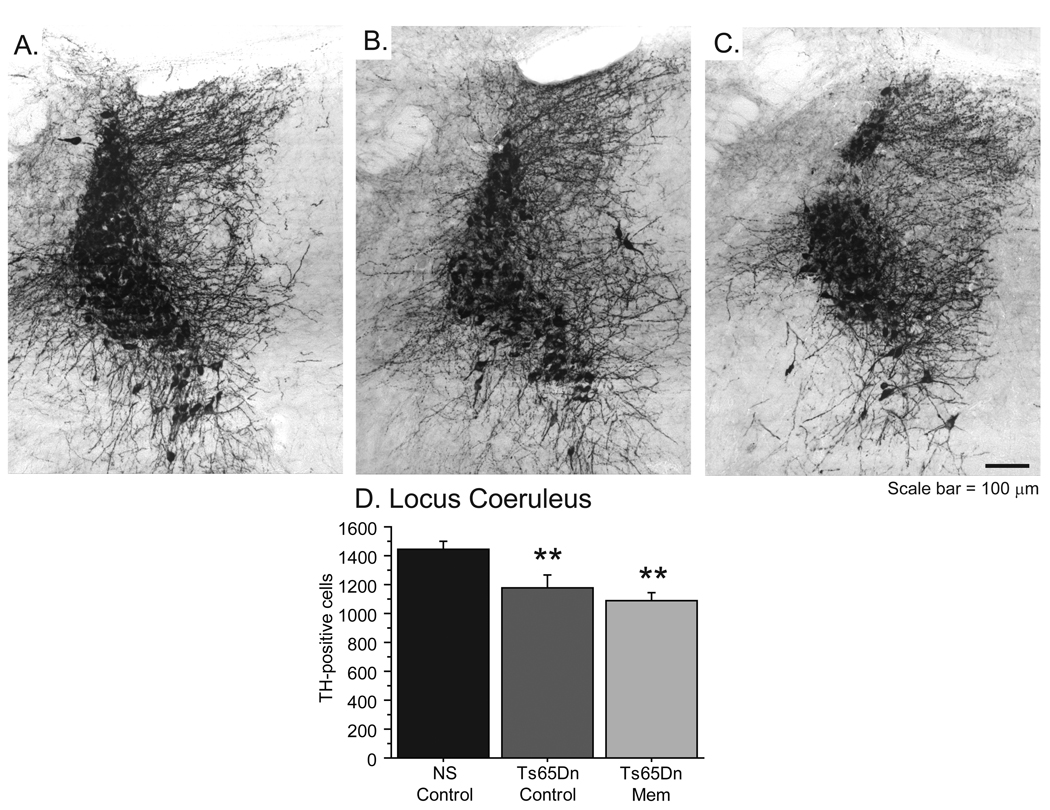
Neuronal loss in the LC of Ts65Dn mice not alleviated following memantine
Though less studied than cholinergic pathology, Locus Coeruleus (LC) degenerates in both AD and DS, and cell loss appears to correlate with a decline in memory function [83]. Our laboratory has recently documented a reduction in TH-positive neurons in the LC of Ts65Dn mice with age [51]. To determine if memantine may have neuroprotective effects on LC neuron degeneration, we performed stereological cell counts and overall fiber density measurements of TH-positive neurons in all groups after chronic memantine treatment. Reductions in TH-immunoreactive (TH-IR) fiber density and neuron number were apparent in Ts65Dn mice, regardless of treatment (Ts65Dn Control, Fig. 5B; Ts65Dn Mem, Fig. 5C) compared with the robust TH-IR visible in NS mice (Fig. 5A). Stereologic cell counts verified a reduction in TH-positive neurons in Ts65Dn Control and Ts65Dn Mem groups [ANOVA: Fig. 5D; F(2,24)=7.832; p<0.01]. Thus, chronic memantine treatment was not able to preserve TH expression in LC neurons in Ts65Dn mice.
Figure 5.
Locus Coeruleus degeneration in Ts65Dn mice was not altered following memantine administration. A–C, TH immunostaining of the rostral pons illustrates reduced fiber density and neuron number in Ts65Dn control (B) and Ts65Dn memantine (C; scale bar = 100 µm) compared to NS mice (A). D, Stereologic measurements of TH-positive cells in the Locus Coeruleus confirm a reduction in LC neurons in both Ts65Dn control and memantine mice (**p<0.01).
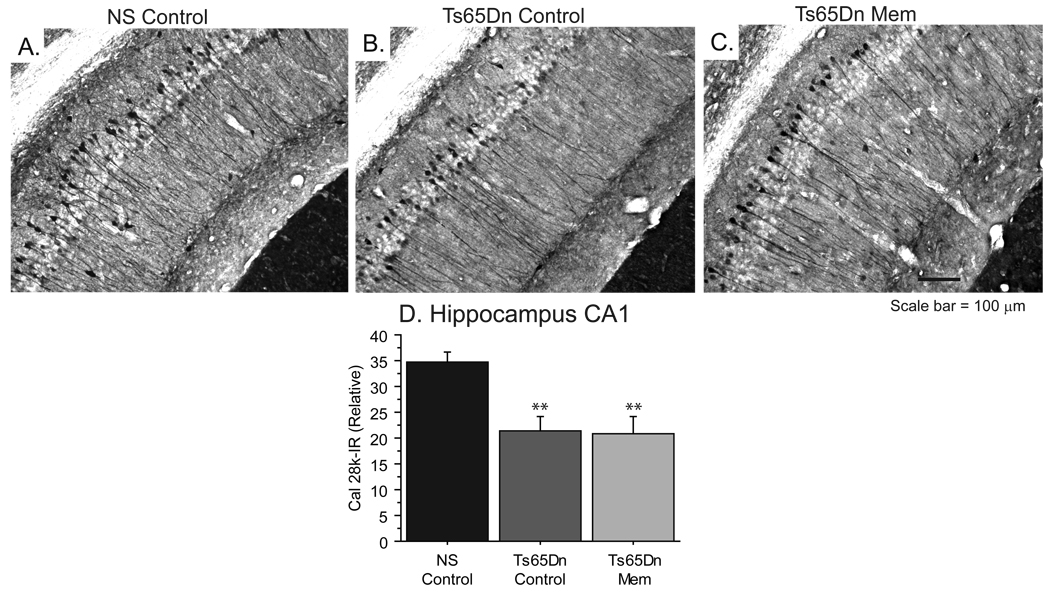
Memantine does not alter hippocampal Cal 28k morphology
Since memantine had moderate effects on performance in WRAM, a hippocampal-dependent memory task in Ts65Dn mice, we also assessed markers of hippocampal morphology in this model. Calbindin D-28k (Cal 28k) is a calcium binding protein that plays an important role in modulating Ca2+ levels in several neuron populations affected in AD [76], and previous studies in our laboratory have shown that reduced Cal 28k-immunoreactivity occurs with aging in the Ts65Dn hippocampus [42]. As seen in Figure 6, Ts65Dn mice (Fig. 6B) exhibit weaker immunoreactivity in Cal 28k-positive neurons in the pyramidal and oriens layers of CA1, as well as in apical dendrites of pyramidal neurons compared with NS mice (Fig. 6A). Reduced immunostaining of Cal 28k in these areas of the hippocampus were also observed in Ts65Dn Mem mice (Fig. 6C). Densitometric analysis of the CA1 confirmed these reductions in Cal 28k-immunoreactivity [ANOVA: Fig 6D, F(2,23)=9.870, p<0.001], and suggested that memantine did not exert neuroprotective effects on Cal 28k expression in the hippocampus of Ts65Dn mice.
Figure 6.
Cal 28k, a calcium binding protein lost early in Ts65Dn mice, was not altered following memantine-treatment. A–C, Immunohistochemical analysis of hippocampal neurons in the CA1 reveals Cal 28k immunoreacivity in the cell bodies and apical dendrites of pyramidal cells in NS mice (A). Hippocampal neurons of Ts65Dn control (B) and memantine (C) mice show fewer immunoreactive pyramidal neurons and dendrites (scale bar = 100 µm). D, Densitometry revealed that NS mice demonstrate significantly higher Cal 28k-immunoreactivity than Ts65Dn groups (**p<0.01; Immunoreactivity normalized to background; mean ± SEM).
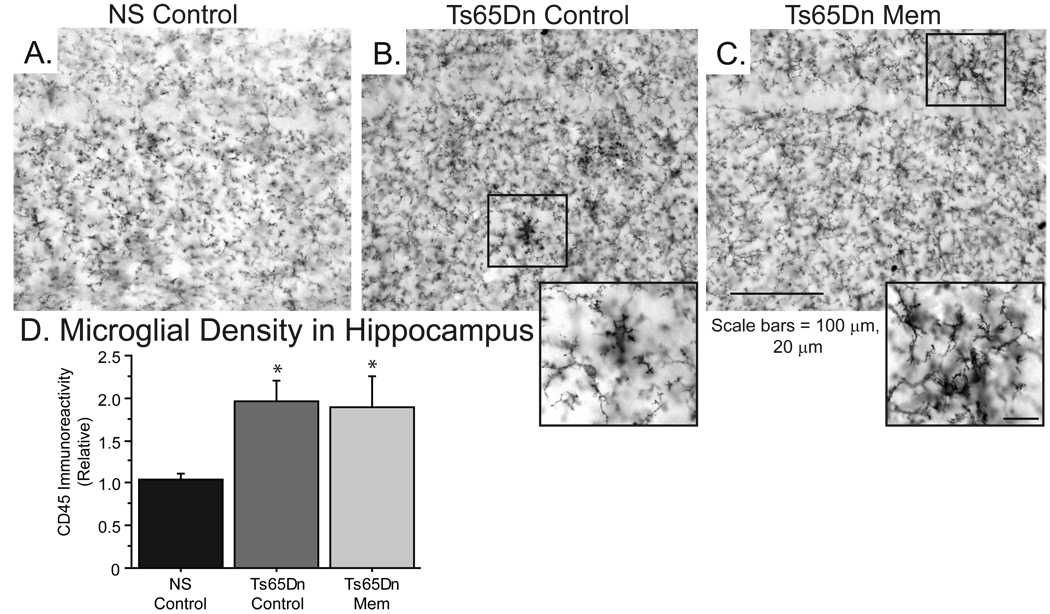
Microglial activation in Ts65Dn mice
Previous studies have indicated that memantine can reduce inflammatory pathology in the brain of Alzheimer mouse models [56, 68]. In order to determine whether memantine might affect microglial activation in Ts65Dn mice, we analyzed microglial activation in the hippocampus. Using the pan-microglial marker CD45, we found an overall increase in CD45 immunostaining in Ts65Dn mice relative to the NS mice, suggesting increased microglial activation (Fig. 7A) in both the Ts65Dn Control (Fig. 7B) and Ts65Dn Mem mice (Fig. 7C), as well as an increased number of enlarged, reactive microglia (Fig. 7C insert). Densitometric analysis of CD45-immunoreactivity in the CA1 region revealed increased microglial density in this region in Ts65Dn Control and Ts65Dn Mem groups when compared to NS mice [ANOVA: Fig. 7D; F(2,19)=7.893; p<0.01], suggesting that neuroinflammation was not relieved in response to chronic memantine treatment.
Figure 7.
Microglial activity was not reduced in the hippocampus of Ts65Dn mice following memantine treatment. A–C, CD45-positive microglia in the CA1 of the hippocampus exhibit lower immunoreactivity indicative of a “resting” state in NS Control mice (A). Ts65Dn control (B) and memantine-treated (C) mice show larger microglia with greater staining density, consistent with an increased microglial activity (scale bars = 100 µm, 20 µm for inset). D, Microglial immunoreactivity in the hippocampus is elevated in Ts65Dn mice, and is not affected by memantine-treatment (*p<0.05; Immunoreactivity normalized to background; mean ± SEM).
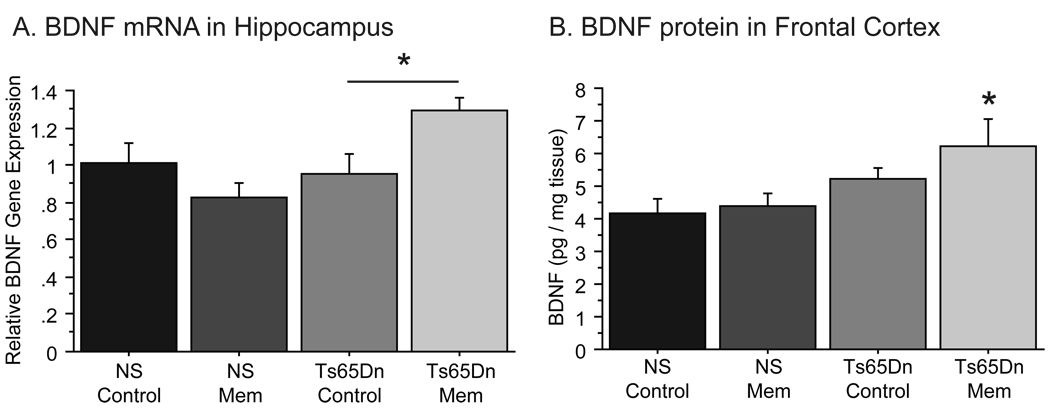
Memantine upregulates BDNF levels in forebrain
Previous studies have indicated that memantine may facilitate memory function by upregulating BDNF expression [53]. As growth factor delivery is altered in Ts65Dn mice, we investigated whether memantine treatment increased BDNF expression in Ts65Dn mice. Bdnf expression in the hippocampus of Ts65Dn and NS mice was detected using quantitative RT-PCR (Fig. 8A). While no significant differences existed between untreated Ts65Dn and NS mice, there was a significant treatment by genotype effect on BDNF expression [F(1,8)=8.405, p<0.05]. Further analysis by one-way ANOVA revealed that Ts65Dn mice treated with chronic memantine (20 mg/kg) demonstrated 30% higher Bdnf mRNA levels in the hippocampus when compared with Ts65Dn Control mice [ANOVA: F(3,8)=4.698, p<0.05; Ts65Dn Control vs. Ts65Dn Mem, p<0.05]. Importantly, this increase in Bdnf mRNA levels was not observed in NS mice, as NS Mem mice showed no relative increase in Bdnf mRNA, thus indicating that memantine treatment affected BDNF expression in the genotypes differently.
Figure 8.
Memantine increased BDNF gene expression in the hippocampus of Ts65Dn mice. A, Ts65Dn Mem mice exhibited higher BDNF mRNA levels in the hippocampus compared to normosomic controls (p<0.05), while NS Mem mice did not differ from NS control mice. B, Protein measurements in frontal cortex tissue showed a significant increase in BDNF levels in Ts65Dn Mem mice relative to NS controls. This increase in BDNF was not observed in NS Mem mice (*p<0.05; Mean ± SEM).
Analysis of BDNF protein levels in the frontal cortex by ELISA (Fig. 8B) also showed that memantine influences BDNF production in Ts65Dn mice [F(3,19)=3.444; p<0.05]. While Ts65Dn Control mice were not statistically different from NS Control mice, Ts65Dn Mem mice exhibited an increase in BDNF levels (p<0.01) relative to Ts65Dn controls. This effect of memantine was only seen in Ts65Dn mice, as memantine-treated NS mice showed no difference from NS Controls. Thus, we here provide evidence that memantine increased both Bdnf mRNA and BDNF protein levels in the hippocampus and frontal cortex, respectively, in Ts65Dn mice. Increased BDNF expression following memantine treatment suggests a possible mechanism for the behavioral improvements seen in Ts65Dn mice.
Study 2: Acute memantine
Performance on the NOR is enhanced in Ts65Dn mice following acute memantine
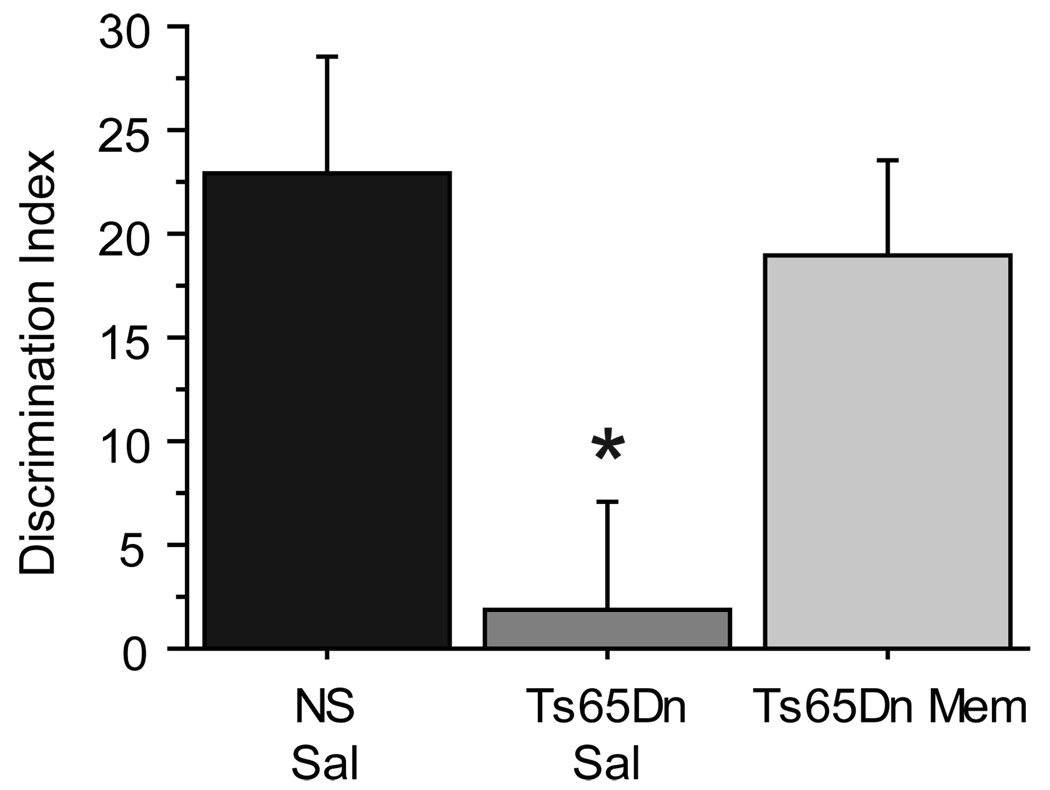
To determine if the improvements in performance on the WRAM and NOR tasks observed following chronic memantine treatment in Ts65Dn mice could be replicated in the acute treatment setting, a separate group of naïve, 8–10 mo Ts65Dn (Ts65Dn Sal n=5; Ts65Dn Mem n=7) and NS mice (NS Sal n=9) were subjected to the simple version (two identical objects during the training session) of the NOR task immediately following memantine (10 mg/kg, i.p., 30 minutes prior to training and testing sessions; see Fig. 1) or saline administration. Acute memantine treatment improved performance significantly on the NOR 24-h task in Ts65Dn mice (Fig. 3D, t6=3.741, p<0.01), demonstrating that the performance in this task in Ts65Dn Mem mice was significantly greater than that seen in the saline-treated Ts65Dn mice [F(2,18)=3.976, p=0.037; post-hoc: p=0.05]. The recovery of object discrimination following acute memantine treatment suggests that altered NMDA receptor function in Ts65Dn mice contributes to observed frontal- and hippocampal-dependent memory deficits, and that even in the acute setting memantine is able to restore normal memory function.
Discussion
There are several lines of evidence that suggest that altered NMDA receptor activity contributes to the memory impairment and neurodegeneration that occurs in DS. Therefore, we sought to determine whether a clinically relevant dose of memantine, a partial NMDA receptor antagonist currently used as pharmacotherapy of AD patients, would alleviate memory loss and prevent changes in neuronal phenotype in the Ts65Dn mouse, a mouse model of DS. Ts65Dn mice treated with 20 mg/kg memantine daily for six months exhibited enhanced cognitive performance in both spatial and non-spatial tasks: the WRAM and NOR, respectively. While these studies showed that memantine facilitated cognitive performance in Ts65Dn mice, we found that cholinergic and LC neuronal markers that decline with age in Ts65Dn mice [40, 51] were not altered following memantine treatment. On the other hand, memantine treatment elevated BDNF levels in the frontal cortex and hippocampus, indicating one potential mechanism for memory facilitation. In fact, acute injections of memantine prior to NOR testing also resulted in improved memory performance in Ts65Dn mice. This supports our conclusion that memantine may provide therapeutic benefits for the cognitive symptoms seen in AD and DS, but does not function via neuroprotection of cholinergic or noradrenergic neuronal integrity.
We assessed hippocampal-dependent memory in a 3-day “win-stay” version of the WRAM. We found that memantine showed moderate benefits in spatial learning in Ts65Dn mice but not in NS controls, as Ts65Dn Mem mice made fewer errors than Ts65Dn Controls on days 2 and 3, the latter portion of testing (Fig. 2B). Despite this improvement, they did not reach the level of performance seen in NS mice on the third day, suggesting that memantine only partially improved hippocampal-dependent function in Ts65Dn mice. This improvement was consistent with several studies in aging and AD rodent models that have found that memantine improves reference memory function on similar spatial tasks [5, 57]. Other studies have shown memantine to have detrimental effects on spatial learning [18]. These studies often detail hyperactivity or disrupted motor function following acute memantine treatment [15, 18]. While increased activity was shown in naive Ts65Dn mice (Fig. 2A), memantine did not have observable effects on this behavior in either genotype in the current study. Ts65Dn mice, despite their elevated activity, did not exhibit increased exploration time in the NOR task (Fig. 3B). The lack of negative side effects of memantine on spontaneous locomotion in our study may be due in part to using an oral dosing regimen which leads to more stable plasma levels, and may explain why we did not observe detrimental performances in spatial memory function in either Ts65Dn or NS mice. This is contrary to previous studies, which have utilized subcutaneous injections of memantine, leading to greater "spikes" in dosing during short intervals [84].
While memantine treatment led to moderate improvements in spatial memory function in Ts65Dn mice, more profound treatment effects were seen in a task evaluating non-spatial visual recognition memory, the NOR task. Specifically, we found that memantine administration allowed recovery of long-term recognition memory (Fig. 3A). These findings are consistent with data showing that memantine facilitates NOR performance in healthy and impaired rodents [62, 68]. But while memantine has been shown to elicit beneficial effects in disease models [57, 68], its effects on healthy young rodents have been more equivocal, with other studies finding detrimental effects in young rats [65]. Previous work has shown that comparable doses of memantine can maintain its effects on NOR in aged rats days after removal of the drug, indicating a level of sustained neuroprotection [61]. When we tested neuroprotective potential on behavioral performance in the Ts65Dn mouse by performing a washout period prior to testing (Fig. 3C), we found a substantial decrease in Ts65Dn performance on the NOR. This deficit was recovered by reinstating the memantine treatment in Ts65Dn mice. This NOR washout study indicated a lack of sustained neuroprotection, and suggested that the behavioral improvements in cognitive performance observed in Ts65Dn mice in the present study did not require long-term memantine treatment before effects are seen. Memantine had no observable effects on behavioral components in the NS mice.
The failure of Ts65Dn mice to maintain performance following memantine removal led us to test whether acute memantine delivery was sufficient to recover normal discrimination on the NOR in Ts65Dn mice. We found that memantine treatment (10 mg/kg, i.p.) delivered immediately prior to both training and testing sessions fully recovered recognition memory in the NOR task in Ts65Dn mice to the level of performance seen in the normosomic mice (Study 2, Fig. 9). Acute memantine delivery has been shown to improve performance in the object recognition task in normal adult rats [62], but most studies in memory impaired mice have been focused on chronic or sub-chronic treatment paradigms [68]. It is not clear why we found no beneficial effects of acute memantine administration in NS mice in the current study, though it lends support to previous studies which suggest that even moderate doses of memantine may impair normal functioning of NMDA receptors and alter learning in healthy individuals [18]. Variations in dosing as well as administration routes may be responsible for the differing results seen in normal rodents across studies. Species-specific effects may also be involved, since some of the previous work was performed in rats in contrast to the mouse studies performed here. A recent study in the Ts65Dn mouse detailed improved fear conditioning following acute memantine [15], suggesting that memantine may also be effective with non-declarative forms of memory. The current study provides even stronger support for memantine as a facilitator of memory in the Ts65Dn mouse, but based on the studies contained herein this effect is most likely a pharmacological rather than a neuroprotective mechanism.
Figure 9.
Acute memantine restored object discrimination in Ts65Dn mice. In study 2, a separate group of mice underwent acute memantine injections immediately prior to training and testing phases. Acute memantine treatment effectively recovered novel object preference in Ts65Dn mice. *p<0.05 compared to NS control (Mean ± SEM).
Interestingly, memantine appeared to have more robust effects on recognition memory than on spatial memory in Ts65Dn mice. Spatial reference memory has consistently been shown to be hippocampal-dependent, and to rely on cholinergic projections from the basal forebrain [48, 70]. The literature on object recognition memory is more conflicted, as several studies have found that rodents exhibit no performance deficits after fornix or hippocampal ablation [25, 27], while others do show impairment [10, 13]. These discrepancies may in part be due to the delay interval, as longer delay intervals (such as 24 h) appear to result in greater hippocampal dependency [74]. Importantly, Ts65Dn mice appear to exhibit normal object recognition function at short intervals (less than 1 hour) [31], suggesting that deficits in hippocampal function may account for the more pronounced impairments observed in over-night object recognition in the present study. The more robust improvement of Ts65Dn mice on the NOR task compared to the WRAM task may be due to the fact that object recognition memory, which also is dependent on the frontal cortex, appears to be maintained even with higher levels of hippocampal damage [10]. An alternate explanation as to why memantine elicited more mild improvements in the WRAM task would be the effects of stress on the Ts65Dn mice. Water serves as an aversive stimulus to mice, and therefore functions as a negative motivator and stressor. Ts65Dn mice appear more sensitive to aversive stimuli, leading to performance deficits that also involve amygdala and are dependent on multiple factors including attention, motivation, and stress-response abilities [75]. As NOR takes advantage of rodents’ innate behaviors, there is no positive or negative stimulus, and as a result does not introduce any potential effects of stress handling.
A possible biological mechanism by which memantine may be exerting its effects on memory function in Ts65Dn mice is by modulating calcium ion signaling. Hippocampal DS neurons exhibit prolonged calcium influx following NMDA receptor activation in vitro [69]. Synaptic increases to calcium has been theorized to disrupt normal neuronal signaling, and contribute to learning and memory decline. This is supported by work in mice transgenic for DYRK1A. This gene is triplicated in DS, and not only is this prolonged decay rate of NMDA receptor activation recapitulated in TgDYRK1A mice [4], these mice also exhibited deficits in spatial learning and memory [1]. Therefore memantine, which is thought to prevent calcium influx during overactive neuronal firing, may be functioning to correct this aberrant calcium flow in the NMDA receptors of DS models. Since regulation of calcium influx is an acute phenomenon, this would be concurrent with our observed acute effects of memantine in the present model. Yet an effective regulation of aberrant calcium signaling would, potentially, also lead to reduced neurodegeneration [see e.g. [80]] - a phenomenon that was not observed in the present study. Memantine exerts effects on transmitter systems other than NMDA-related signaling as well. A recent study demonstrated that administration of memantine to the hippocampal slice gave rise to enhanced cholinergic signaling via muscarinic receptors [24]. The investigators concluded that cholinergic effects of memantine are likely to be involved in it's memory-enhancing effects, since the cholinergic system plays a major role in both learning and memory processes. Dopaminergic and serotonergic neurotransmission are also affected by memantine [58], and we cannot discount the role of these systems in the observed memory effects in the present study. Even though we did not see morphological evidence of memantine effects on cholinergic or noradrenergic neurons in the current study, it is possible that receptor-mediated neurotransmission of these nuclei was enhanced by memantine treatment. This will be closely examined in continued studies.
In addition to calcium irregularities, Ts65Dn mice also exhibit alterations in calcineurin [36]. This is consistent with the elevated gene load of DSCR1, which encodes calcipressin, a calcineurin inhibitor. Alterations in synaptic protein levels may contribute to the deficits in long-term potentiation (LTP) [71], alterations in synaptic spines [7], and neurotrophic signaling [14] in Ts65Dn mice. BDNF is an important neurotrophic factor for several neuronal subtypes in the forebrain, including cholinergic neurons [55], and its expression is regulated in an activity dependent manner [81]. Our laboratory has previously shown that BDNF protein levels in the frontal cortex of Ts65Dn mice correlate with performance of cognitive tasks [8]. In the present study, we found that hippocampal levels of Bdnf mRNA and frontal cortex BDNF protein levels were elevated in Ts65Dn mice following memantine treatment (Fig. 9). While enhanced BDNF expression has not been documented under chronic treatment conditions of memantine, it is consistent with acute studies, which show that memantine administration leads to an up-regulation of Bdnf mRNA after 4 hours in cortical and hippocampal regions in the rat [53]. The increase in hippocampal and frontal cortex BDNF levels following memantine treatment seen here is contrasted with studies showing that BDNF is reduced following administration of the high affinity NMDA receptor blocker MK-801 in normal rodents [11, 41], and suggests that memantine is acting in Ts65Dn mice by an alternate mechanism: preventing prolonged NMDA receptor activity without disrupting normal signaling. Exacerbated glutamate release and/or NMDA receptor signaling in Ts65Dn mice may explain why memantine had a far more exaggerated effect on both behavior and BDNF levels in this mouse model compared to effects seen in normosomic littermate controls. Improved memory function along with enhanced BDNF production suggest improved signaling in Ts65Dn mice, and future studies should evaluate whether memantine strengthens long-term potentiation in Ts65Dn mice, as it has in other models of neurodegeneration [34]. While increases in BDNF may contribute to the reported cognitive improvement in Ts65Dn mice treated with memantine, it is not known at the present time how rapidly BDNF levels are elevated after acute administration of memantine in Ts65Dn mice, nor is it known whether the mechanisms by which memantine affects memory in the chronic and acute paradigms studied here are similar. We also examined BDNF protein levels in the frontal cortex, and found that memantine-treatment increased BDNF in Ts65Dn mice in both hippocampus and frontal cortex, demonstrating memantine-mediated effects on biological substrates in both of these brain regions, and therefore providing a possible mechanism for its effects on both of the tasks employed here. Future studies will investigate whether memantine alleviates synaptic alterations and LTP deficits in the hippocampus and frontal cortex of Ts65Dn mice, and also further explore whether BDNF is involved in observed effects on these two behavioral components.
In addition to BDNF levels, we also assessed a number of morphological markers that decline with age in Ts65Dn mice. Memantine has been shown to protect cholinergic neurons following excitotoxic insults [78], but no studies, to our knowledge, have evaluated the efficacy of memantine in vivo in models of progressive neuronal degeneration. We measured cholinergic atrophy in the medial septal nucleus (Fig. 4F) and loss of noradrenergic phenotype in the LC (Fig. 5D), and found no beneficial effects of memantine treatment in either of these two neuronal populations. Cal 28k histology (Fig. 6D), which shows reductions in the CA1 in Ts65Dn mice, did not improve following treatment, either. In fact, we were unable to uncover any structural correlates of memantine neuroprotection in our model, contrary to findings in amyloid transgenic models described below. Since previous studies have shown that BFCNs, noradrenergic LC neurons, and Cal 28k neurons all respond to neurotrophic support from BDNF [30, 46, 73], it is surprising that increases in BDNF levels in the frontal cortex and hippocampus in this study provided no neuroprotection to these brain regions, at least not in the markers examined here. Impairments in retrograde transport of NGF have been well documented in Ts65Dn mice [14, 66]. The lack of neuroprotection seen here may indicate that enhanced BDNF does not compensate for NGF transport deficits in Ts65Dn mice, or alternatively, that these transport deficits may also involve BDNF signaling. Both the BFCNs and noradrenergic neurons of the LC rely on retrograde transport of BDNF [73], and these elevations in the cortex and hippocampus may not reach the target regions, and would thus be unable to exert the needed trophic effects in specific neuronal populations. Future studies will include sub-cellular signaling effects of memantine treatment, to fully understand the biological substrates involved in its effects reported here for NOR and WRAM performance. Since more substantial effects were observed for NOR, rather than WRAM, in our model, it is possible that future studies should focus on frontal cortex-related mechanisms rather than hippocampal effects.
Despite the great interest in memantine as a clinical treatment paradigm for AD patients, chronic studies assessing the neuroprotective effects of memantine in animal models have thus far been inconclusive. Researchers have primarily focused on murine models of amyloid accumulation, showing modest amyloid plaque reductions and behavioral improvements [23, 57, 68]. These reductions in plaque load may be due to altered APP expression, as memantine reduces APP levels in Tg2576 mice [77]. Reducing amyloid may lead to a dampening of the inflammatory cascade and therefore lower excitotoxic damage, at least in the Alzheimer models examined to date. Triplication of APP appears necessary for many of the pathologies seen in Ts65Dn mice [66], though these mice do not develop amyloid plaques. As cholinergic and inflammatory changes have been linked to APP gene alterations [45, 66], and are often more robust than protein levels of amyloid, our experiments did not include β-amyloid measurements. Our laboratory has previously shown that an anti-inflammatory drug, minocycline, reduced microglial activation and attenuated neuronal loss in Ts65Dn mice [42]. Contrary to our earlier findings with minocycline, inflammatory morphology, as measured by CD45-immunoreactivity, was not reduced in memantine-treated Ts65Dn mice (Fig. 7D), suggesting that this compound does not exert its effects via microglial de-activation or signaling in our hands. It is possible that memantine may have effects on neuronal signaling via altered translation or cleavage of APP into different peptides, even though this notion is not supported by earlier studies. Rather, the rapid improvements and lack of neuroprotective effects following memantine treatment in this study suggest that memantine more likely provides its beneficial mechanism through a partial antagonism of the NMDA receptors, which are hyper-active in DS due to elevated glutamate levels, is involved in behavior-modifying effects of the drug.
In conclusion, we showed that memantine treatment significantly improved cognitive performance on the NOR and moderate effects on the WRAM task in Ts65Dn mice, but not normosomic mice, thereby suggesting that memantine provides some cognitive benefit to DS individuals. Notably, the dosage and regimen of memantine used in this study did not alter the progression of neuropathology found in this mouse model; thus, the behavioral effects noted here are likely related to other mechanisms, possibly including, but not limited to, neurotrophic factors such as BDNF, as well as direct glutamatergic signaling effects mediated via a partial NMDA antagonism or via other transmitter systems, as discussed above. Combined with previous evidence that chronic memantine treatment (10 and 20 mg/kg for six-months) can lead to increased axonal degeneration in normal adult mice [23], our findings suggest that further study is required to better understand the nature of memantine’s memory-enhancing benefits, and whether behavioral efficacy can be achieved with minimal neuronal toxicity.
Acknowledgments
This work was supported by the Anna and John J. Sie Foundation for Down Syndrome and the National Institutes on Aging (AG12122). We thank Dr. Narayan Bhat for valuable scientific discussions and Selena Sumner, Alfred Moore, and Claudia Umphlet for outstanding technical assistance. In addition, we would like to thank Dr. Louis Reichardt for the gift of TrkA antibodies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahn KJ, Jeong HK, Choi HS, Ryoo SR, Kim YJ, Goo JS, Choi SY, Han JS, Ha I, Song WJ. DYRK1A BAC transgenic mice show altered synaptic plasticity with learning and memory defects. Neurobiology of disease. 2006;22:463–472. doi: 10.1016/j.nbd.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–1679. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- 3.Allen SJ, Dawbarn D, Wilcock GK. Morphometric immunochemical analysis of neurons in the nucleus basalis of Meynert in Alzheimer's disease. Brain research. 1988;454:275–281. doi: 10.1016/0006-8993(88)90827-x. [DOI] [PubMed] [Google Scholar]
- 4.Altafaj X, Ortiz-Abalia J, Fernandez M, Potier MC, Laffaire J, Andreu N, Dierssen M, Gonzalez-Garcia C, Cena V, Marti E, Fillat C. Increased NR2A expression and prolonged decay of NMDA-induced calcium transient in cerebellum of TgDyrk1A mice, a mouse model of Down syndrome. Neurobiol Dis. 2008;32:377–384. doi: 10.1016/j.nbd.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Barnes CA, Danysz W, Parsons CG. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. The European journal of neuroscience. 1996;8:565–571. doi: 10.1111/j.1460-9568.1996.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 6.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 7.Belichenko PV, Masliah E, Kleschevnikov AM, Villar AJ, Epstein CJ, Salehi A, Mobley WC. Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. J Comp Neurol. 2004;480:281–298. doi: 10.1002/cne.20337. [DOI] [PubMed] [Google Scholar]
- 8.Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003;139:47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- 9.Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm AC, McGinty JF. Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. J Neurosci. 2007;27:8816–8825. doi: 10.1523/JNEUROSCI.1067-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castren E, da Penha Berzaghi M, Lindholm D, Thoenen H. Differential effects of MK-801 on brain-derived neurotrophic factor mRNA levels in different regions of the rat brain. Experimental neurology. 1993;122:244–252. doi: 10.1006/exnr.1993.1124. [DOI] [PubMed] [Google Scholar]
- 12.Chen HS, Wang YF, Rayudu PV, Edgecomb P, Neill JC, Segal MM, Lipton SA, Jensen FE. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience. 1998;86:1121–1132. doi: 10.1016/s0306-4522(98)00163-8. [DOI] [PubMed] [Google Scholar]
- 13.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper JD, Salehi A, Delcroix JD, Howe CL, Belichenko PV, Chua-Couzens J, Kilbridge JF, Carlson EJ, Epstein CJ, Mobley WC. Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc Natl Acad Sci U S A. 2001;98:10439–10444. doi: 10.1073/pnas.181219298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa AC, Scott-McKean JJ, Stasko MR. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test. Neuropsychopharmacology. 2008;33:1624–1632. doi: 10.1038/sj.npp.1301535. [DOI] [PubMed] [Google Scholar]
- 16.Costa AC, Walsh K, Davisson MT. Motor dysfunction in a mouse model for Down syndrome. Physiol Behav. 1999;68:211–220. doi: 10.1016/s0031-9384(99)00178-x. [DOI] [PubMed] [Google Scholar]
- 17.Coyle JT, Oster-Granite ML, Gearhart JD. The neurobiologic consequences of Down syndrome. Brain research bulletin. 1986;16:773–787. doi: 10.1016/0361-9230(86)90074-2. [DOI] [PubMed] [Google Scholar]
- 18.Creeley C, Wozniak DF, Labruyere J, Taylor GT, Olney JW. Low doses of memantine disrupt memory in adult rats. J Neurosci. 2006;26:3923–3932. doi: 10.1523/JNEUROSCI.4883-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer's disease: preclinical evidence. International journal of geriatric psychiatry. 2003;18:S23–S32. doi: 10.1002/gps.938. [DOI] [PubMed] [Google Scholar]
- 20.Danysz W, Zajaczkowski W, Parsons CG. Modulation of learning processes by ionotropic glutamate receptor ligands. Behav Pharmacol. 1995;6:455–474. [PubMed] [Google Scholar]
- 21.Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res. 1990;360:263–280. [PubMed] [Google Scholar]
- 22.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 23.Dong H, Yuede CM, Coughlan C, Lewis B, Csernansky JG. Effects of memantine on neuronal structure and conditioned fear in the Tg2576 mouse model of Alzheimer's disease. Neuropsychopharmacology. 2008;33:3226–3236. doi: 10.1038/npp.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drever BD, Anderson WG, Johnson H, O'Callaghan M, Seo S, Choi DY, Riedel G, Platt B. Memantine acts as a cholinergic stimulant in the mouse hippocampus. J Alzheimers Dis. 2007;12:319–333. doi: 10.3233/jad-2007-12405. [DOI] [PubMed] [Google Scholar]
- 25.Ennaceur A, Aggleton JP. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res. 1997;88:181–193. doi: 10.1016/s0166-4328(97)02297-3. [DOI] [PubMed] [Google Scholar]
- 26.Ennaceur A, Aggleton JP. Spontaneous recognition of object configurations in rats: effects of fornix lesions. Exp Brain Res. 1994;100:85–92. doi: 10.1007/BF00227281. [DOI] [PubMed] [Google Scholar]
- 27.Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- 28.Escorihuela RM, Fernandez-Teruel A, Vallina IF, Baamonde C, Lumbreras MA, Dierssen M, Tobena A, Florez J. A behavioral assessment of Ts65Dn mice: a putative Down syndrome model. Neurosci Lett. 1995;199:143–146. doi: 10.1016/0304-3940(95)12052-6. [DOI] [PubMed] [Google Scholar]
- 29.Farlow MR, Graham SM, Alva G. Memantine for the treatment of Alzheimer's disease: tolerability and safety data from clinical trials. Drug Saf. 2008;31:577–585. doi: 10.2165/00002018-200831070-00003. [DOI] [PubMed] [Google Scholar]
- 30.Fawcett JP, Alonso-Vanegas MA, Morris SJ, Miller FD, Sadikot AF, Murphy RA. Evidence that brain-derived neurotrophic factor from presynaptic nerve terminals regulates the phenotype of calbindin-containing neurons in the lateral septum. J Neurosci. 2000;20:274–282. doi: 10.1523/JNEUROSCI.20-01-00274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez F, Garner CC. Episodic-like memory in Ts65Dn, a mouse model of Down syndrome. Behav Brain Res. 2008;188:233–237. doi: 10.1016/j.bbr.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez F, Garner CC. Object recognition memory is conserved in Ts1Cje, a mouse model of Down syndrome. Neurosci Lett. 2007;421:137–141. doi: 10.1016/j.neulet.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- 34.Frankiewicz T, Parsons CG. Memantine restores long term potentiation impaired by tonic N-methyl-D-aspartate (NMDA) receptor activation following reduction of Mg2+ in hippocampal slices. Neuropharmacology. 1999;38:1253–1259. doi: 10.1016/s0028-3908(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 35.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. Vol. [Google Scholar]
- 36.Gardiner K. Predicting pathway perturbations in Down syndrome. Journal of neural transmission. 2003:21–37. doi: 10.1007/978-3-7091-6721-2_2. [DOI] [PubMed] [Google Scholar]
- 37.Giacobini E. Cholinesterase inhibitors stabilize Alzheimer's disease. Annals of the New York Academy of Sciences. 2000;920:321–327. doi: 10.1111/j.1749-6632.2000.tb06942.x. [DOI] [PubMed] [Google Scholar]
- 38.Granholm AC, Sanders LA, Crnic LS. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down's syndrome. Experimental neurology. 2000;161:647–663. doi: 10.1006/exnr.1999.7289. [DOI] [PubMed] [Google Scholar]
- 39.Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Apmis. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 40.Holtzman DM, Santucci D, Kilbridge J, Chua-Couzens J, Fontana DJ, Daniels SE, Johnson RM, Chen K, Sun Y, Carlson E, Alleva E, Epstein CJ, Mobley WC. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc Natl Acad Sci U S A. 1996;93:13333–13338. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes PE, Young D, Preston KM, Yan Q, Dragunow M. Differential regulation by MK801 of immediate-early genes, brain-derived neurotrophic factor and trk receptor mRNA induced by a kindling after-discharge. Brain research. 1998;53:138–151. doi: 10.1016/s0169-328x(97)00288-x. [DOI] [PubMed] [Google Scholar]
- 42.Hunter CL, Bachman D, Granholm AC. Minocycline prevents cholinergic loss in a mouse model of Down's syndrome. Ann Neurol. 2004;56:675–688. doi: 10.1002/ana.20250. [DOI] [PubMed] [Google Scholar]
- 43.Hunter CL, Bimonte-Nelson HA, Nelson M, Eckman CB, Granholm AC. Behavioral and neurobiological markers of Alzheimer's disease in Ts65Dn mice: effects of estrogen. Neurobiol Aging. 2004;25:873–884. doi: 10.1016/j.neurobiolaging.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Hunter CL, Bimonte HA, Granholm AC. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav Brain Res. 2003;138:121–131. doi: 10.1016/s0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 45.Hunter CL, Isacson O, Nelson M, Bimonte-Nelson H, Seo H, Lin L, Ford K, Kindy MS, Granholm AC. Regional alterations in amyloid precursor protein and nerve growth factor across age in a mouse model of Down's syndrome. Neurosci Res. 2003;45:437–445. doi: 10.1016/s0168-0102(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 46.Knusel B, Beck KD, Winslow JW, Rosenthal A, Burton LE, Widmer HR, Nikolics K, Hefti F. Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J Neurosci. 1992;12:4391–4402. doi: 10.1523/JNEUROSCI.12-11-04391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh S, Chang P, Collier TJ, Loy R. Loss of NGF receptor immunoreactivity in basal forebrain neurons of aged rats: correlation with spatial memory impairment. Brain Res. 1989;498:397–404. doi: 10.1016/0006-8993(89)91125-6. [DOI] [PubMed] [Google Scholar]
- 48.Koller G, Satzger W, Adam M, Wagner M, Kathmann N, Soyka M, Engel R. Effects of scopolamine on matching to sample paradigm and related tests in human subjects. Neuropsychobiology. 2003;48:87–94. doi: 10.1159/000072883. [DOI] [PubMed] [Google Scholar]
- 49.Lieberman DN, Mody I. Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- 50.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nature reviews. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 51.Lockrow J, Boger HA, Gerhardt GA, Granholm AC. Locus Coeruleus Degeneration Modulates Neuroinflammation and Alzheimer's-like pathology in Ts65Dn mice. Eur J Neurosci (Submitted) 2009 [Google Scholar]
- 52.Lockrow J, Prakasam A, Huang P, Bimonte-Nelson H, Sambamurti K, Granholm AC. Cholinergic degeneration and memory loss delayed by vitamin E in a Down syndrome mouse model. Experimental neurology. 2008 doi: 10.1016/j.expneurol.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marvanova M, Lakso M, Pirhonen J, Nawa H, Wong G, Castren E. The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Molecular and cellular neurosciences. 2001;18:247–258. doi: 10.1006/mcne.2001.1027. [DOI] [PubMed] [Google Scholar]
- 54.Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 55.Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miguel-Hidalgo JJ, Alvarez XA, Cacabelos R, Quack G. Neuroprotection by memantine against neurodegeneration induced by beta-amyloid(1–40) Brain research. 2002;958:210–221. doi: 10.1016/s0006-8993(02)03731-9. [DOI] [PubMed] [Google Scholar]
- 57.Minkeviciene R, Banerjee P, Tanila H. Memantine improves spatial learning in a transgenic mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2004;311:677–682. doi: 10.1124/jpet.104.071027. [DOI] [PubMed] [Google Scholar]
- 58.Onogi H, Ishigaki S, Nakagawasai O, Arai-Kato Y, Arai Y, Watanabe H, Miyamoto A, Tan-no K, Tadano T. Influence of memantine on brain monoaminergic neurotransmission parameters in mice: neurochemical and behavioral study. Biological & pharmaceutical bulletin. 2009;32:850–855. doi: 10.1248/bpb.32.850. [DOI] [PubMed] [Google Scholar]
- 59.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 60.Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, Krzascik P, Hartmann S, Danysz W. Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology. 1995;34:1239–1258. doi: 10.1016/0028-3908(95)00092-k. [DOI] [PubMed] [Google Scholar]
- 61.Pieta Dias C, Martins de Lima MN, Presti-Torres J, Dornelles A, Garcia VA, Siciliani Scalco F, Rewsaat Guimaraes M, Constantino L, Budni P, Dal-Pizzol F, Schroder N. Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience. 2007;146:1719–1725. doi: 10.1016/j.neuroscience.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 62.Pitsikas N, Sakellaridis N. Memantine and recognition memory: possible facilitation of its behavioral effects by the nitric oxide (NO) donor molsidomine. European journal of pharmacology. 2007;571:174–179. doi: 10.1016/j.ejphar.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 63.Pomara N, Singh R, Deptula D, Chou JC, Schwartz MB, LeWitt PA. Glutamate and other CSF amino acids in Alzheimer's disease. Am J Psychiatry. 1992;149:251–254. doi: 10.1176/ajp.149.2.251. [DOI] [PubMed] [Google Scholar]
- 64.Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 65.Reus GZ, Valvassori SS, Machado RA, Martins MR, Gavioli EC, Quevedo J. Acute treatment with low doses of memantine does not impair aversive, non-associative and recognition memory in rats. Naunyn-Schmiedeberg's archives of pharmacology. 2008;376:295–300. doi: 10.1007/s00210-007-0235-x. [DOI] [PubMed] [Google Scholar]
- 66.Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 67.Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer's disease: current status and new perspectives. Lancet Neurol. 2003;2:539–547. doi: 10.1016/s1474-4422(03)00502-7. [DOI] [PubMed] [Google Scholar]
- 68.Scholtzova H, Wadghiri YZ, Douadi M, Sigurdsson EM, Li YS, Quartermain D, Banerjee P, Wisniewski T. Memantine leads to behavioral improvement and amyloid reduction in Alzheimer's-disease-model transgenic mice shown as by micromagnetic resonance imaging. J Neurosci Res. 2008;86:2784–2791. doi: 10.1002/jnr.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schuchmann S, Muller W, Heinemann U. Altered Ca2+ signaling and mitochondrial deficiencies in hippocampal neurons of trisomy 16 mice: a model of Down's syndrome. J Neurosci. 1998;18:7216–7231. doi: 10.1523/JNEUROSCI.18-18-07216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sethy VH, Kuhar MJ, Roth RH, Van Woert MH, Aghajanian GK. Cholinergic neurons: effect of acute septal lesion on acetylcholine and choline content of rat hippocampus. Brain Res. 1973;55:481–484. doi: 10.1016/0006-8993(73)90318-1. [DOI] [PubMed] [Google Scholar]
- 71.Siarey RJ, Stoll J, Rapoport SI, Galdzicki Z. Altered long-term potentiation in the young and old Ts65Dn mouse, a model for Down Syndrome. Neuropharmacology. 1997;36:1549–1554. doi: 10.1016/s0028-3908(97)00157-3. [DOI] [PubMed] [Google Scholar]
- 72.Sobreviela T, Clary DO, Reichardt LF, Brandabur MM, Kordower JH, Mufson EJ. TrkA-immunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J Comp Neurol. 1994;350:587–611. doi: 10.1002/cne.903500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sobreviela T, Pagcatipunan M, Kroin JS, Mufson EJ. Retrograde transport of brain-derived neurotrophic factor (BDNF) following infusion in neo- and limbic cortex in rat: relationship to BDNF mRNA expressing neurons. J Comp Neurol. 1996;375:417–444. doi: 10.1002/(SICI)1096-9861(19961118)375:3<417::AID-CNE6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 74.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 75.Stasko MR, Costa AC. Experimental parameters affecting the Morris water maze performance of a mouse model of Down syndrome. Behav Brain Res. 2004;154:1–17. doi: 10.1016/j.bbr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Sutherland MK, Wong L, Somerville MJ, Yoong LK, Bergeron C, Parmentier M, McLachlan DR. Reduction of calbindin-28k mRNA levels in Alzheimer as compared to Huntington hippocampus. Brain research. 1993;18:32–42. doi: 10.1016/0169-328x(93)90171-k. [DOI] [PubMed] [Google Scholar]
- 77.Unger C, Svedberg MM, Yu WF, Hedberg MM, Nordberg A. Effect of subchronic treatment of memantine, galantamine, and nicotine in the brain of Tg2576 (APPswe) transgenic mice. The Journal of pharmacology and experimental therapeutics. 2006;317:30–36. doi: 10.1124/jpet.105.098566. [DOI] [PubMed] [Google Scholar]
- 78.Wenk GL, Zajaczkowski W, Danysz W. Neuroprotection of acetylcholinergic basal forebrain neurons by memantine and neurokinin B. Behav Brain Res. 1997;83:129–133. doi: 10.1016/s0166-4328(97)86056-1. [DOI] [PubMed] [Google Scholar]
- 79.Wilcock DM, Alamed J, Gottschall PE, Grimm J, Rosenthal A, Pons J, Ronan V, Symmonds K, Gordon MN, Morgan D. Deglycosylated anti-amyloid-beta antibodies eliminate cognitive deficits and reduce parenchymal amyloid with minimal vascular consequences in aged amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5340–5346. doi: 10.1523/JNEUROSCI.0695-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu JT, Chang RC, Tan L. Calcium dysregulation in Alzheimer's disease: from mechanisms to therapeutic opportunities. Progress in neurobiology. 2009;89:240–255. doi: 10.1016/j.pneurobio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 81.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. Embo J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zajaczkowski W, Quack G, Danysz W. Infusion of (+) -MK−801 and memantine --contrasting effects on radial maze learning in rats with entorhinal cortex lesion. Eur J Pharmacol. 1996;296:239–246. doi: 10.1016/0014-2999(95)00716-4. [DOI] [PubMed] [Google Scholar]
- 83.Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Archives of neurology. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 84.Zoladz PR, Campbell AM, Park CR, Schaefer D, Danysz W, Diamond DM. Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane. Pharmacol Biochem Behav. 2006;85:298–306. doi: 10.1016/j.pbb.2006.08.011. [DOI] [PubMed] [Google Scholar]