Abstract
Individuals with body dysmorphic disorder (BDD) are preoccupied with perceived physical defects or flaws, often facial features, which may be due to distorted perception. Previous studies have demonstrated abnormalities in visual processing of faces and figures, and misinterpretations of emotional expressions. The objective of this study was to determine in BDD how viewing faces with emotional expressions affects perception on an identity-matching task. Twelve BDD subjects and eleven healthy controls matched identities of faces with emotional expressions, neutral-expressions, and a control task of ovals and circles. The BDD group made twice as many errors relative to controls for identity-matching of faces with emotional expressions but not for neutral faces or ovals/circles. Mean reaction times were slower for the BDD relative to the control group for emotional faces in general, but there was no effect of specific emotion type. These data suggest that individuals with BDD have abnormalities in facial identification for faces with emotional expressions. This could reflect fundamental abnormalities in visual information processing that are more pronounced for emotional expressions in general, and may relate to their perceptual disturbances.
Keywords: face, facial expression, perceptual distortion
1. Introduction
Body dysmorphic disorder (BDD) is a severe psychiatric condition in which patients are preoccupied with perceived defects in their appearance, resulting in significant suffering and functional impairment (American Psychiatric Association. 2000). BDD affects 1–2% of the population (Mayville et al. 1999; Otto et al. 2001; Rief et al. 2006), yet is vastly under recognized and under studied. Individuals with BDD tend to be self-conscious of what they perceive to be defective, which is often a facial feature (Phillips 2005). They also frequently have ideas of reference, believing others are staring at them and judging them negatively because of their appearance (American Psychiatric Association. 2000). They subsequently tend to engage in compulsive and avoidant behaviors such as mirror-checking, covering up with makeup or clothing, or avoiding social situations. BDD can cause significant impairment in social and occupational functioning and can lead to severe depression, hospitalization, suicide attempts, and high rates of cosmetic surgery (Phillips et al. 1993; Ishigooka et al. 1998; Sarwer et al. 1998; Aouizerate et al. 2003; Phillips et al. 2005; Phillips et al. 2005).
Previous studies have indicated that individuals with BDD have abnormalities in processing of faces with emotional expressions. Buhlmann et al. (2004) found that individuals with BDD had difficulty interpreting facial expressions, more often misidentifying faces as being angry than the obsessive-compulsive disorder (OCD) group and healthy controls (Buhlmann et al. 2004). However, there were no differences in general facial feature recognition accuracy for neutral-expression faces. A more recent study by the same researchers found that BDD subjects had difficulty identifying emotional expressions in self-referent scenarios (i.e. they were told to imagine that the person in the photograph that they were viewing was someone who was looking specifically at them). In these situations, they more often interpreted neutral expressions as angry or contemptuous as compared to controls (Buhlmann et al. 2006). In addition, they more often interpreted neutral emotional expressions as contemptuous in self-referent scenarios as compared to other-referent scenarios (i.e. that the person in the photograph was looking at someone else). These studies suggest an abnormality in emotional face processing in BDD that may be related to recognition biases and/or misinterpretation of faces that are perceived as contemptuous or otherwise negative. This, in turn, may contribute to their poor insight and frequent ideas of reference if they believe that these negative emotional expressions are others’ reactions to them. Whether these recognition biases or misinterpretations are the result of abnormalities in visual processing is not clear.
There is also evidence of abnormal processing of faces with neutral expressions. A recent fMRI study using neutral faces as stimuli found greater left-hemisphere activity in the BDD group relative to healthy controls in an extended visual processing network (Feusner et al. 2007). This suggests greater detail and analytic processing relative to holistic and configural processing, as other studies have shown that the left hemisphere predominates for local (or analytic) processing while the right hemisphere dominates for global (or holistic) processing (Bradshaw et al. 1976; Van Kleeck 1989; Evans et al. 2000). The nature of these abnormalities was similar to what was discovered in a previous neuropsychological test using the Rey-Osterreith Complex Figure Test, in which individuals with BDD overly relied on details to reproduce the figure, as the expense of more global and configural aspects (Deckersbach et al. 2000).
Given these previous findings and the clinical relevance in BDD of face processing, we investigated visual processing of faces with emotional expressions. The objective of this study was to determine how viewing faces with emotional expressions affects perception in BDD (as opposed to interpretation of emotions) on a novel identity-matching task. We designed the experiment with two groups, BDD and healthy controls, and three different stimuli conditions: emotional faces, neutral faces, and ovals/circles. This allowed us to compare performance both between and within groups on an emotional face task vs. a neutral face task vs. a (non-face) control task of ovals and circles. We hypothesized that compared to controls, individuals with BDD would have abnormalities in identity-matching of faces with emotional expressions as reflected in slower reaction times and a higher error rate, but there would be no significant differences for the neutral expression faces or for ovals/circles.
2. Methods
2.1. Subjects
The UCLA Institutional Review Board approved the protocol for the study. We obtained informed consent of the participants after fully explaining the nature of the procedures. We enrolled 12 patients with BDD and 11 healthy controls between the ages of 18 and 64, recruited from the community. The BDD group and controls were matched by gender, age, and level of education. All BDD subjects met the Diagnostic and Statistical Manual (DSM-IV) criteria for Body Dysmorphic Disorder, using the Body Dysmorphic Disorder Module (Phillips et al. 1995), a reliable diagnostic module modeled after the Structured Clinical Interview for DSM. In addition to this module, we performed a clinical psychiatric evaluation on all participants and administered the Mini International Neuropsychiatric Inventory (MINI) (Sheehan et al. 1998) to screen for comorbid diagnoses. All BDD subjects were required to have a Body Dysmorphic Disorder version of the Yale-Brown Obsessive-Compulsive Disorder Scale (BDD-YBOCS) score of ≥20. The BDD-YBOCS is a validated scale that is widely-used to evaluate symptom severity in BDD (Phillips et al. 1997). We allowed subjects with delusional beliefs.
Exclusion criteria for subjects and controls included: active substance abuse, current neurological disorder, pregnancy, and any current medical disorder that might affect cerebral metabolism. We excluded subjects with any concurrent Axis I disorder besides dysthymia, major depressive disorder (MDD), or generalized anxiety disorder (GAD). As depression and anxiety are so frequently comorbid in this population, we believed it would not be a representative sample to exclude these. However, we excluded other frequently-occurring comorbid disorders such as social phobia because we anticipated these would have more overlap in primary symptoms having to do with face processing; self-consciousness in social situations and sensitivity to others’ facial expressions are commonly shared experiences (Wilhelm et al. 1997; Phillips et al. 1998 ; Stein et al. 2002). BDD symptoms had to be the primary concern in every subject, as determined during the initial clinical psychiatric evaluation and from relative severity on the BDD-YBOCS, Hamilton Anxiety Scale (HAM-A) (Hamilton 1969), and the 17-item Hamilton Depression Rating Scale (HAM-D) (Hamilton 1960). We excluded subjects with a HAM-D score >20, to minimize the effect of more severe depressive states on the outcomes, and subjects whom the investigator judged were at current risk of suicide. The MINI, BDD-YBOCS, HAM-D, and HAM-A were administered to all subjects.
All participants were free from psychoactive medications for at least three weeks prior to entering the study, and free of fluoxetine hydrochloride for at least five weeks. Subjects were not receiving any cognitive-behavioral therapy. All participants had normal or corrected vision.
2.2. Stimuli
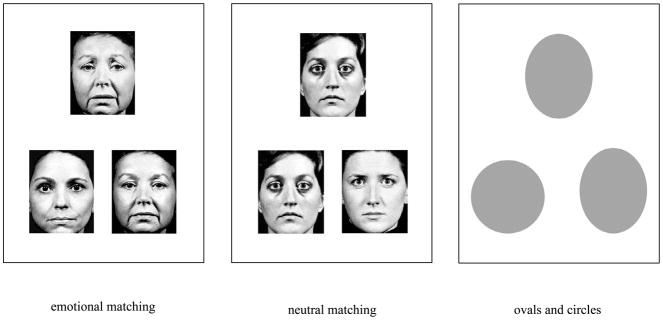
We created a novel emotional face identity-matching task, comprised of 32 sets of digitized photographs: 6 happy, 6 sad, 6 angry, 6 disgusted, 6 fearful, and 2 surprised (Macbrain database (Tottenham et al. 2002), UPenn Facial Emotional Stimuli (Gur et al. 2001)) (see Fig. 1). Each emotional target face was presented with two neutral-expression selection faces: one of the same person and one of a different person (see Fig. 2). The stimuli for the neutral face task were similarly arranged, except all faces had neutral expressions. Stimuli for the non-face control task consisted of ovals and circles, approximately the same size as the faces.
Fig. 1.
Example emotional face stimuli
Fig. 2.
Example matching tasks
2.3. Task
We used MacStim 3.0 software (White Ant Occasional Publishing, West Melbourne, Australia) to present each set of 3 faces for 4 seconds, with a 1 second interstimulus interval. Subjects were told “please select one of the two selection faces or shapes that is the same person or shape as the target, regardless of the emotional expression, by pressing the 1 or 2 button on the keyboard. Please make your selection both as rapidly and as accurately as possible.” MacStim recorded the reaction times (RTs) and responses. Subjects first completed a run of the matching task for emotional faces, in which the different emotional expressions were randomly interspersed. This was followed by a run of the matching task for neutral faces interspersed with the control task of matching ovals and circles. There were 32 trials total for each of the emotional faces, neutral faces, and ovals/circles. For the emotional faces, there were 6 trials each for the angry, sad, happy, fearful, and disgust faces and 2 for the surprise faces. Subjects rated their task-related anxiety on a Likert scale of 0–10 (10 representing the highest anxiety) after the emotional faces task and after the combined neutral faces and ovals/circles tasks.
2.4. Statistical analyses
Due to the skewness of the raw RT data, we log-transformed the values for all the analyses, which yielded a more normal distribution. We analyzed the RT data using mixed-effects ANOVA, modeling subject effects as random effects nested within groups. For pair-wise comparisons, we performed post hoc Bonferroni-corrected t-tests, two-tailed. We also performed a post hoc analysis of specific emotion type (happy, sad, angry, disgusted, fearful, and surprised) by group, again using mixed-effects ANOVA. We analyzed error rates using a log-linear model (rather than Fisher’s exact test or χ2), which is a multivariate extension given that the dependent and independent variables were categorical and there were more than two variables, and that distribution of error rate is non-normal.
3. Results
3.1. Demographics
There were no significant differences between the BDD and control group in age (32±9 and 32±9.3, respectively; t=−0.03, P=0.98), gender (9 females/3 males and 9 females/2 males respectively; Fisher’s exact test, P=0.54), or level of education (15.6±2.7 years and 15.9±1.5 years, respectively; t=−0.35, P=0.73). The BDD group included three individuals with comorbid MDD, one with dysthymic disorder, two with GAD, and one with GAD and MDD. The BDD symptoms were the primary concern in every subject. Typical of this population, 11 subjects had preoccupations with perceived facial defects and one had a preoccupation with a perceived misshapen chest. Of the 11 with facial concerns, six had concerns solely about facial features and five had face and non-face concerns. The average BDD-YBOCS score in the BDD group was 30.7±5.5, the average HAM-A score was 11.50±8.52, and the average HAM-D score was 9.42±6.24.
Anxiety levels during the task were not significantly different between BDD and control groups during the emotional faces task (3.67±2.46 and 2.5±1.72, respectively, P=0.22) and the neutral faces and ovals/circles tasks (3.45±2.38 and 4.0±1.73, respectively, P=0.55).
3.2. Error rates
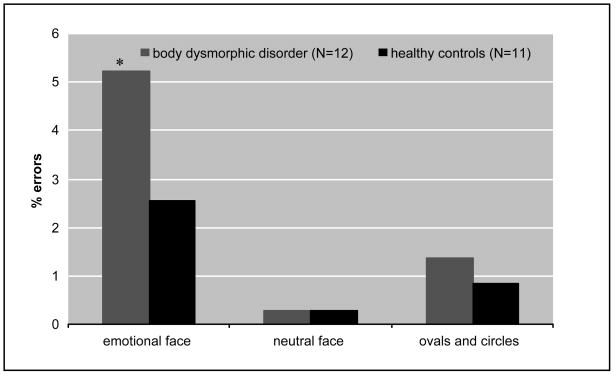
To understand the effects of viewing emotional faces on accuracy of facial identification, we compared error rates between BDD subjects and healthy controls. Error rates for identifying faces with emotional expressions were 5.21% vs. 2.56%; neutral faces 0.27% vs. 0.28%; and for ovals and circles 1.36% vs. 0.85% for the BDD group and the healthy controls, respectively (Fig. 3). There was a significant effect of group (χ2 =4.23, df =1, P=0.04), stimulus type (χ2 =12.98, df=2, P<0.01) and group by stimulus type interaction (χ2 =19.27, df=2, P<0.01) on error rates. Post hoc χ2 tests determined there was a significant difference between groups for emotional faces (χ2=21.5, df=1, P<0.01), but not for neutral faces (χ2=1.05, df=1, P=0.30) or ovals/circles (χ2=1.2, df =1, P=0.27).
Fig. 3. Percent errors on matching tasksa.
aPercent errors for the body dysmorphic disorder and healthy control groups by stimulus type (log-linear model). There was a significant group effect (χ2 =4.23, df=1, P=0.04), stimulus type effect (χ2 =12.98, P<0.01), and group by stimulus type effect (df=2, χ2 =19.27, df=2, P<0.01). Post hoc χ2 tests determined significant difference between groups for:
*emotional faces (χ2=21.5, df=1, P<0.01)
3.3. Reaction times by group and stimulus
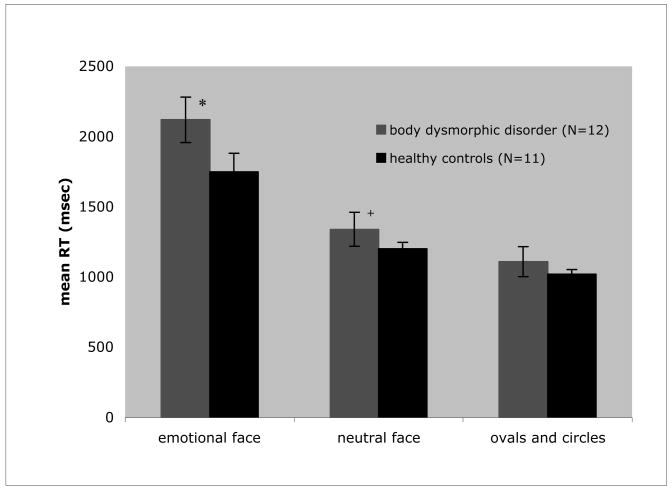
To determine whether individuals with BDD differed from controls in their speed of identifying faces with emotional expressions, neutral faces, and ovals/circles, we performed a mixed-effects ANOVA with diagnosis (BDD or healthy control) as the between-group factor and stimulus type (emotional face, neutral face, or oval/circles) as the within-group factor. There was no significant main effect of group on RT (F=1.68; df=1,21; P=0.21). There was a significant main effect of stimulus type (F=901.05; df=2,2149; P<0.01), with slower RT for emotional faces relative to neutral faces (t=32.5, P<0.01). RT for neutral faces in turn was significantly slower than for oval/circles (t=9.5, P<0.01). Notably, there was a significant interaction between group and stimulus type (F=5.08; df=2,2149; P<0.01) (Figure 4). Post hoc ANOVAs revealed significant differences between groups for emotional faces (F=53.51; df=1,2168; P<0.01) and neutral faces (F=8.261; df=1,2168; P<0.01), with slower RT for the BDD group, but not ovals/circles (F=3.43; df=1,2168; P=0.06). The effect sizes (Cohen’s d) for each stimulus type are as follows: emotional faces 0.54; neutral faces 0.22; ovals/circles 0.14.
Fig. 4. Reaction time performance on matching tasks for group by stimulus type.
RT = reaction time.
There was a significant stimulus type effect (F=901.05; df=2,2149; P<0.01) and group by stimulus type effect (F=5.08; df=2,2149; P<0.01), but the group effect was nonsignificant (F=1.68; df=1,21; P=0.21).
Post hoc ANOVAs revealed significant differences between groups for:
*emotional faces (F=53.51; df=1,2168; P<0.01)
+neutral faces (F=8.261; df=1,2168; P<0.01)
3.4. Reaction time by specific type of emotion
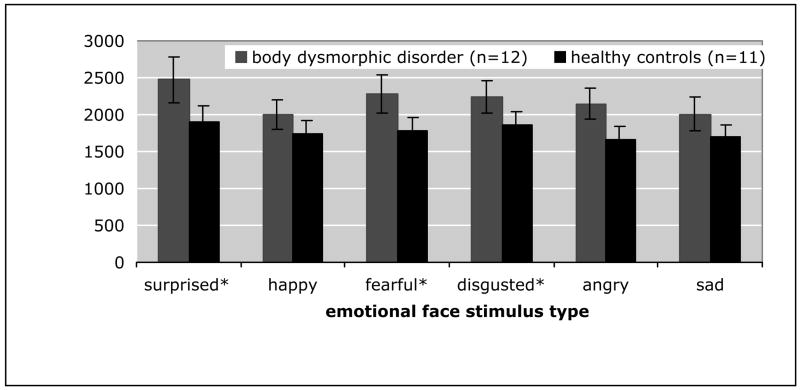
In order to determine the effect of specific emotion type on the speed of face identification, we again performed a mixed effects ANOVA with diagnosis (BDD or healthy control) as the between-group factor and specific emotion type (happy, sad, angry, disgusted, fearful, or surprised) as the within-group factor. There was a significant main effect of specific type of emotion (F=6.3; df=5,702; P<0.01) and group(F=4.3; df=1,20; P=0.05), but no group by emotion interaction (F=0.698; df=5,702; P=0.625) (Fig. 5). We performed all pair-wise post-hoc t-tests for specific emotion type, Bonferroni-corrected. There were no significant differences between groups for individual emotion types. Comparisons between the mean RTs of the different types of emotions showed that RTs across all participants for surprised, fearful, and disgusted faces were not significantly different from each other, but were significantly different (slower) than participants’ RTs for happy, angry, and sad faces (all p values <0.05). In turn, participants’ RTs for happy, angry, and sad faces were not significantly different from each other.
Fig. 5. Reaction time performance on facial matching task by category of emotion.
RT = reaction time. There was a significant effect of specific type of emotion (F=6.3; df=5,702; P<0.01) and group (F=4.3; df=1,20; P=0.05), but no group by emotion interaction (F=0.698; df=5,702; P=0.625). Post hoc Bonferroni-corrected t-tests revealed the following:
*Significantly different than happy, angry, and sad (across all participants) (all p<0.05)
4. Discussion
This study confirmed the hypothesis that individuals with BDD are slower and less accurate than controls at identity-matching of faces with emotional expressions. The BDD group had more than twice the error rate for the matching task with emotional faces compared to the healthy controls. The BDD group showed the greatest difference in reaction time from healthy controls for emotional faces, followed by neutral faces and then ovals/circles. However, there was no differential effect on the BDD group of any specific emotion type. In total, these data suggest that individuals with BDD have abnormalities in the speed and accuracy of processing faces with emotional expressions. This builds on findings from previous studies of abnormal interpretation of emotions (Buhlmann et al. 2004; Buhlmann et al. 2006), to suggest that there may be more fundamental abnormalities for perception of faces with emotional expressions.
These results may be explained in that the matching condition for emotional faces differed from the matching condition for neutral faces on several dimensions that may have affected performance for the BDD subjects. Although the faces had emotional expressions, the explicit task was not to interpret the expression but to match the face with the neutral face of the same person. For the neutral face task, correct choices were identical. The emotional face task, however, required processing of facial features to match the emotional face to the neutral face. This identification process normally involves visual analysis of both details and configural aspects of faces whereas matching neutral faces could be accomplished either by matching configural information (Vuilleumier et al. 2003) or by matching one or more details. Previous neuropsychological testing demonstrated that on a visuospatial task individuals with BDD they tend to focus on isolated details rather than larger, global organizational features (Deckersbach et al. 2000). In addition, a recent functional magnetic resonance imaging (fMRI) study demonstrated greater left-hemisphere activity in BDD compared to a control group while matching identities of others’ neutral-expression faces (Feusner et al. 2007). Although further research is needed to verify this, one possibility is that this finding in BDD may be associated with an imbalance in detailed vs. holistic processing. If BDD subjects overly rely on details for processing emotional faces as well, this slower strategy (Peyrin et al. 2006) may account for delayed reaction times relative to controls. It may also prove to be less accurate, at least within the limited stimulus presentation timeframe in this experiment of 4 seconds. These possible explanations remain to be tested directly. However, the fact that we did not find a significant group by stimulus effect for the different types of emotion supports a face-processing deficit that occurs for faces with emotional expressions in general, rather than an influence of emotion per se, for which we would expect a differential influence on the BDD group depending on the valence of emotion.
Concerning facial processing in general, less is known in BDD compared to social phobia. However, the two disorders share many clinical features including fears of negative evaluation, rejection, ridicule by others (Wilhelm et al. 1997; Phillips et al. 1998), and ideas of reference (American Psychiatric Association. 2000). Several studies of social phobia have demonstrated abnormalities in the processing of emotional faces (Simonian et al. 2001 ; Horley et al. 2004; Juth et al. 2005). In a study of children with social phobia, Simonian et al., found impaired explicit recognition of facial affect that included happy, disgusted, and angry faces (Simonian et al. 2001). Juth, et al. (2005) studied the relationship of facial emotional expression and social anxiety in both healthy and social phobia subjects (Juth et al. 2005). In individuals with or without social anxiety, the processing of faces with fearful or angry expressions was slower and less accurate than for happy faces. In addition, individuals with a diagnosis of social phobia were less accurate than healthy controls in facial identification for fearful and happy averted target faces. Although the explicit task was different, we found similar results of lower accuracy of facial identification in the BDD group for emotional faces. One difference in our study, the significance of which is unclear, is that mean RT for angry faces was not significantly different than for happy faces.
There are other possibilities as to why the BDD group had slower responses to faces with emotional expressions during the matching task. The mean RT for the BDD group relative to the controls progressively diverged from non-face objects (ovals and circles) to neutral faces, to emotional faces (Fig. 4). This could indicate that the degree of general salience of the stimuli corresponded with progressively slower RT. This occurred for both groups, although it was more pronounced for the BDD group. Several studies have shown that the processing of threat-related information is highly prioritized and may occur automatically, even when the threat is not explicitly attended (Morris et al. 1998; Whalen et al. 1998; Vuilleumier and Schwartz 2001). In a study of selective processing of threatening information Buhlmann, et al. (2002) demonstrated that individuals with BDD were more easily distracted than controls by emotional cues in general and most distracted by words related to their disorder, such as “disfigured” or “pretty” (Buhlmann et al. 2002). Thus, feature processing in the BDD group may also be more susceptible to distraction from the presentation of emotional faces, resulting in slower RTs and more errors. Yet if this were the case, we would expect to see proportionally slower RTs in the BDD group for the more threatening faces (angry, disgusted, or fearful), which we did not observe.
An additional factor that may have influenced processing time for emotional faces is failure in inhibition. To respond quickly and accurately to match emotional faces they must attend to the facial identity while implicitly inhibiting attention to the emotional valence. Maxwell, et al., (2005) demonstrated in healthy controls that the presentation of task-irrelevant emotional faces (angry and happy) resulted in more inhibitory errors relative to the presentation of neutral faces on an explicit go/no-go task (Maxwell et al. 2005). Other studies have also shown that the perception of emotional stimuli is able to bias competing information processing schemes (Pessoa et al. 2002; Bishop et al. 2004). Similar inhibitory errors from emotional faces in the current study could account for the higher error rates on the matching task for both groups, relative to the neutral faces and ovals/circles. The fact that the BDD group had approximately twice the error rate for the emotional faces suggests that they may have a more marked failure in inhibition than healthy controls.
Another possible explanation for the differences between groups could be related to the complexity of the visual task, and not specifically to face processing. The observation that error rates were greater in the BDD group for emotional faces than neutral faces and non-face objects (ovals and circles) could be due to the fact that the emotional faces task was a more difficult task. Unlike the other two tasks, it did not just involve matching of identical visual constructs. However, Buhlmann et al. (2004) in a previous study of face processing tested subjects with BDD using the Short Form of the Benton Facial Recognition task (Buhlmann et al. 2004). This tests facial identity matching using neutral-expression target faces that are the same individuals’ face yet presented at a different angle, and was therefore more difficult than the matching task in the current study. In this study individuals with BDD did not perform significantly differently than controls (mean scores of 23.7±2.5 and 23.3±2.7, respectively). This suggests that the differences seen in the current study may not just be a reflection of the task difficulty. Nevertheless, future studies will still be useful to clarify whether these differences are specific for faces as opposed to other types of complex visual stimuli.
Regardless of the cause, aberrant processing of others’ faces may contribute to the symptomatology in BDD. Abnormal perception of faces with emotional expressions could contribute to misinterpretation of emotions and subsequently lead to ideas of reference in which they believe others are regarding them in a contemptuous or threatening manner. As they are usually concerned about others’ judgments of their appearance, this could result in significant distress. If, in fact, the basis of abnormal emotional face processing is aberrant feature processing and this occurs for their own face as well, this could be an important factor in their apparent perceptual distortions of their own appearance.
One of the limitations of the current study is that we do not know the subjects’ interpretation of each emotional expression, as this was not an explicit part of the task. It is therefore unclear if misinterpretation accounted for differences in performance. Similarly, we did not have subjects rate the aversiveness of faces. Although anxiety levels were not significantly different between groups during the task as a whole, we do not know if there were differences in emotional arousal for specific faces. The fact that our face database only included 2 surprised stimuli, as opposed to 6 for the other emotions, was also a limitation.
Another limitation of this study is the small sample size. This may have limited the power of detection of differences between groups, particularly for the individual emotional expressions. It also limited our ability to analyze the effects of comorbid MDD and GAD diagnoses on RTs and accuracy rates. Future, larger studies that address these variables could provide useful information to further understand the basis of delayed reaction times and higher error rates.
This study’s findings of abnormalities in processing of emotional faces in BDD may reflect fundamental differences in the nature of their visual information processing, or may suggest a specific problem in processing of emotional information conveyed by faces. Thus, the perceptual abnormalities seen in BDD may not be specific to misperception of their own faces but may reflect a more fundamental problem in human face perception. Future studies are needed to elucidate the specific causes of aberrant face processing in BDD.
Table 1.
errors and reaction times by stimulus type and group
| ovals | neutral faces | emotional faces | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient # | Gender | Comorbid diagnosis | errors | mean RT (ms) | errors | mean RT (ms) | errors | mean RT (ms) | |
| BDD group | 1 | F | none | 0 | 1200 | 0 | 1780 | 1 | 2136 |
| 2 | M | none | 2 | 1090 | 0 | 2057 | 2 | 2320 | |
| 3 | M | MDD | 1 | 844 | 0 | 905 | 2 | 1715 | |
| 4 | F | GAD | 0 | 1460 | 0 | 948 | 0 | 2240 | |
| 5 | F | none | 0 | 814 | 0 | 1493 | 0 | 1592 | |
| 6 | F | MDD | 1 | 1740 | 0 | 1121 | 3 | 2192 | |
| 7 | F | MDD and GAD | 0 | 949 | 0 | 1016 | 4 | 3024 | |
| 8 | F | GAD | 0 | 929 | 0 | 1963 | 0 | 1782 | |
| 9 | M | none | 0 | 867 | 1 | 1364 | 3 | 2953 | |
| 10 | F | none | 0 | 1369 | 0 | 1344 | 2 | 2480 | |
| 11 | F | MDD | 0 | 1090 | 0 | 1197 | 1 | 1987 | |
| 12 | F | GAD | 1 | 728 | 0 | 846 | 2 | 1022 | |
| mean ± SD | total: 5 (1.3%) | 1170± 368 | total: 1 (0.26%) | 1336±41 3 | total: 20 (5.23%) | 2120 ±562 | |||
| Healthy Control group | 1 | F | N/A | 0 | 1182 | 0 | 1384 | 0 | 1628 |
| 2 | M | N/A | 0 | 1126 | 0 | 1100 | 1 | 1555 | |
| 3 | F | N/A | 0 | 1213 | 0 | 1188 | 1 | 2854 | |
| 4 | F | N/A | 0 | 953 | 0 | 1254 | 1 | 1285 | |
| 5 | F | N/A | 0 | 1165 | 0 | 1146 | 1 | 1715 | |
| 6 | F | N/A | 0 | 1016 | 0 | 1196 | 0 | 1913 | |
| 7 | F | N/A | 0 | 1114 | 0 | 943 | 0 | 1149 | |
| 8 | F | N/A | 0 | 1038 | 0 | 1094 | 0 | 1754 | |
| 9 | F | N/A | 0 | 974 | 0 | 1527 | 2 | 1768 | |
| 10 | M | N/A | 3 | 1071 | 1 | 1289 | 2 | 1979 | |
| 11 | F | N/A | 0 | 864 | 0 | 1059 | 1 | 1691 | |
| mean ± SD | total: 3 (0.85%) | 1065± 108 | total: 1 (0.26%) | 1198±16 2 | total: 9 (2.56%) | 1754± 440 | |||
Abbreviations: RT = reaction time; MDD = major depressive disorder; GAD = generalized anxiety disorder
Acknowledgments
This study was funded by grant T32 MH17140 from the National Institute of Mental Health (Dr. Feusner) and a grant from the Saban Family Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Aouizerate B, Pujol H, Grabot D, Faytout M, Suire K, Braud C, Auriacombe M, Martin D, Baudet J, Tignol J. Body dysmorphic disorder in a sample of cosmetic surgery applicants. Eur Psychiatry. 2003;18:365–8. doi: 10.1016/j.eurpsy.2003.02.001. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Gates A, Patterson K. Hemispheric differences in processing visual patterns. Q J Exp Psychol. 1976;28:667–81. doi: 10.1080/14640747608400593. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Etcoff N, Wilhelm S. Emotional recognition bias for contempt and anger in body dysmorphic disorder. Journal of Psychiatric Research. 2006;40:105–11. doi: 10.1016/j.jpsychires.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, McNally R, Etcoff N, Tuschen-Caffier B, Wilhelm S. Emotion recognition deficits in body dysmorphic disorder. Journal of Psychiatric Research. 2004;38:201–6. doi: 10.1016/s0022-3956(03)00107-9. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, McNally RJ, Wilhelm S, Florin I. Selective processing of emotional information in body dysmorphic disorder. Journal of Anxiety Disorders. 2002;16:289–98. doi: 10.1016/s0887-6185(02)00100-7. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage C, Phillips K, Wilhelm S, Buhlmann U, Rauch S, Baer L, Jenike M. Characteristics of memory dysfunction in body dysmorphic disorder. Journal of the International Neuropsychological Society. 2000;6:673–81. doi: 10.1017/s1355617700666055. [DOI] [PubMed] [Google Scholar]
- Evans MA, Shedden JM, Hevenor SJ, Hahn MC. The effect of variability of unattended information on global and local processing: evidence for lateralization at early stages of processing. Neuropsychologia. 2000;38:225–39. doi: 10.1016/s0028-3932(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Arch Gen Psychiatry. 2007;64:1417–25. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- Gur R, Ragland J, Moberg P, Turner T, Bilker W, Kohler C, Siegel S, Gur R. Computerized Neurocognitive Scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Diagnosis and rating of anxiety. British Journal of Psychiatry. 1969;3:76–79. [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, Gordon E. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Research. 2004;127:43–53. doi: 10.1016/j.psychres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Ishigooka J, Iwao M, Suzuki M, Fukuyama Y, Murasaki M, Miura S. Demographic features of patients seeking cosmetic surgery. Psychiatry Clin Neurosci. 1998;52:283–7. doi: 10.1046/j.1440-1819.1998.00388.x. [DOI] [PubMed] [Google Scholar]
- Juth P, Lundqvist D, Karlsson A, Ohman A. Looking for foes and friends: perceptual and emotional factors when finding a face in the crowd. Emotion. 2005;5:379–95. doi: 10.1037/1528-3542.5.4.379. [DOI] [PubMed] [Google Scholar]
- Maxwell J, Shackman A, Davidson R. Unattented facial expressions asymmetrically bias the concurrent processing of nonemotional information. Journal of Cognitive Neuroscience. 2005;17:1386–1395. doi: 10.1162/0898929054985437. [DOI] [PubMed] [Google Scholar]
- Mayville S, Katz R, Gipson M, Cabral K. Assessing the prevalence of body dysmorphic disorder in an ethnically diverse group of adolescents. Journal of Child and Family Studies. 1999;8:357–362. [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;4:467–70. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. American Journal of Psychiatry. 2001;158:2061–3. doi: 10.1176/appi.ajp.158.12.2061. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Research Cognitive Brain Research. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Peyrin C, Mermillod M, Chokron S, Marendaz C. Effect of temporal constraints on hemispheric asymmetries during spatial frequency processing. Brain Cogn. 2006;62:214–20. doi: 10.1016/j.bandc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Phillips KA. The Broken Mirror. Oxford University Press; New York: 2005. [Google Scholar]
- Phillips KA, Atala KD, Pope HG. Diagnostic instruments for body dysmorphic disorder. New Research Program and Abstracts. American Psychiatric Association 148th Annual Meeting; Miami American Psychiatric Association. 1995. [Google Scholar]
- Phillips KA, Coles ME, Menard W, Yen S, Fay C, Weisberg RB. Suicidal Ideation and Suicide Attempts in Body Dysmorphic Disorder. Journal of Clinical Psychiatry. 2005;66:717–725. doi: 10.4088/jcp.v66n0607. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Gunderson CG, Mallya G, McElroy SL, Carter W. A comparison study of body dysmorphic disorder and obsessive-compulsive disorder. Journal of Clinical Psychiatry. 1998;59:568–75. doi: 10.4088/jcp.v59n1102. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin. 1997;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, McElroy SL, Keck PE, Jr, Pope HG, Jr, Hudson JI. Body dysmorphic disorder: 30 cases of imagined ugliness. American Journal of Psychiatry. 1993;150:302–8. doi: 10.1176/ajp.150.2.302. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Menard W, Fay C, Pagano ME. Psychosocial functioning and quality of life in body dysmorphic disorder. Comprehensive Psychiatry. 2005;46:254–60. doi: 10.1016/j.comppsych.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rief W, Buhlmann U, Wilhelm S, Borkenhagen A, Brahler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychological Medicine. 2006;36:877–85. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- Sarwer DB, Wadden TA, Pertschuk MJ, Whitaker LA. Body image dissatisfaction and body dysmorphic disorder in 100 cosmetic surgery patients. Plast Reconstr Surg. 1998;101:1644–9. doi: 10.1097/00006534-199805000-00035. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar G. The Mini International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Simonian SJ, Beidel DC, Turner SM, Berkes JL, Long JH. Recognition of facial affect by children and adolescents diagnosed with social phobia. Child Psychiatry and Human Development. 2001;32:137–45. doi: 10.1023/a:1012298707253. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus D, Nelson C. Categorization of Facial Expressions in Children and Adults: Establishing a Larger Stimulus Set. Poster presented at the Cognitive Neuroscience Society annual meeting; San Francisco. 2002. [Google Scholar]
- Van Kleeck MH. Hemispheric differences in global versus local processing of hierarchical visual stimuli by normal subjects: new data and a meta-analysis of previous studies. Neuropsychologia. 1989;27:1165–78. doi: 10.1016/0028-3932(89)90099-7. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony J, Driver J, Dolan R. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience. 2003;6:624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S. Beware and be aware: Capture of spatial attention by fear-related stimuli in neglect. Neuroreport. 2001;12:1119–1122. doi: 10.1097/00001756-200105080-00014. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The Journal of Neuroscience. 1998;18:411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Otto MW, Zucker BG, Pollack MH. Prevalence of body dysmorphic disorder in patients with anxiety disorders. Journal of Anxiety Disorders. 1997;11:499–502. doi: 10.1016/s0887-6185(97)00026-1. [DOI] [PubMed] [Google Scholar]