Abstract
With the relative global lack of immunity to the pandemic influenza A/H1N1/2009 virus that emerged in April 2009 as well as the sustained susceptibility to infection, rapid and accurate diagnostic assays are essential to detect this novel influenza A variant. Among the molecular diagnostic methods that have been developed to date, most are in tandem monoplex assays targeting either different regions of a single viral gene segment or different viral gene segments. We describe a dual-gene (duplex) quantitative real-time RT-PCR method selectively targeting pandemic influenza A/H1N1/2009. The assay design includes a primer-probe set specific to only the hemagglutinin (HA) gene of this novel influenza A variant and a second set capable of detecting the nucleoprotein (NP) gene of all swine-origin influenza A virus. In silico analysis of the specific HA oligonucleotide sequence used in the assay showed that it targeted only the swine-origin pandemic strain; there was also no cross-reactivity against a wide spectrum of noninfluenza respiratory viruses. The assay has a diagnostic sensitivity and specificity of 97.7% and 100%, respectively, a lower detection limit of 50 viral gene copies/PCR, and can be adapted to either a qualitative or quantitative mode. It was first applied to 3512 patients with influenza-like illnesses at a tertiary hospital in Singapore, during the containment phase of the pandemic (May to July 2009).
Since the first case of pandemic influenza A/H1N1/2009 virus of swine-origin appeared in Mexico in April 2009,1,2,3 various diagnostic assays such as real-time and conventional reverse transcription-polymerase chain reaction assays (RT-PCRs), antigen tests, Luminex xTAG Respiratory Viral Panel, direct immunofluorescence tests, and R-Mix viral culture,4,5,6,7,8 including the Emergency Use Authorization assay [Centers for Disease Control and Prevention (CDC): CDC protocol of real-time RT-PCR (rRT-PCR) for swine influenza A(H1N1), http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf, last accessed April 29, 2009], have been developed to provide accurate and rapid detection of this novel virus. This has allowed timely treatment and infection control measures to be taken to limit the spread of this virus. To date, most of the real-time RT-PCR diagnostic methods described elsewhere are either monoplex assays,9,10 in tandem monoplex assays targeting different regions at a single gene segment only (eg, hemagglutinin or matrix protein),4,11 or in tandem monoplex assays targeting different viral gene segments.12,13 To provide for more cost- and labor-effective diagnostic services for a possible second wave of the A/H1N1/2009 virus, including the ability to closely monitor the viral loads of patients with influenza A gene mutants leading to drug resistance,14,15 a dual-gene quantitative real-time RT-PCR (qRT-PCR) assay targeting the relatively more variable hemagglutinin (HA) envelope protein gene and the conserved internal nucleoprotein (NP) gene that would selectively detect the pandemic influenza A/H1N1/2009 virus was developed in a single-tube format.
When first developed, it was optimized as a qualitative assay (rRT-PCR) for rapid detection of infectivity; at a later date, it was adapted into a quantitative test (qRT-PCR) to enable viral load measurements. Both will be discussed here. The clinical performance of this assay was validated during an enhanced surveillance (containment) period (May to July 2009) at a large teaching hospital in Singapore, the National University Hospital (NUH), and its related primary care clinics. Use of the assay protocol on patient samples was approved by the Institutional Review Board in mid-May 2009 (National Healthcare Group DSRB 205-001).
Materials and Methods
Clinical Specimen Preparation
Nasopharyngeal, nasal, or throat swabs, cerebrospinal fluid, and sputum were collected from symptomatic patients or their contacts who presented to NUH or its affiliated primary care clinics. These included patients who attended the outpatient clinics or emergency medicine department at NUH, public and private primary care clinics, inpatients with influenza-like symptoms, as well as patients or staff with compatible contact or travel histories (surveillance program).
For swabs that were kept in 3 ml of universal transport medium (Copan Diagnostics Inc., Corona, CA) at 4°C, viral RNA was extracted from 200 μl of universal transport medium with either the Qiagen EZ1 Virus mini kit v2.0 or the QIAsymphony Virus/Bacteria mini kit, using their respective proprietary Bio Robot EZ1 and QIAsymphony automated platforms (Qiagen, Valencia, CA), according to the manufacturer's instructions. For dry/gel swabs, the swabs were preprocessed in 500 μl of 1×-PBS, 200 μl of which was used for extraction as described above. For cerebrospinal fluid specimens, 200 μl of cerebrospinal fluid was directly used for extraction. For sputum specimens, 250 μl of sputum was resuspended with 500 μl of 1% N-acetylcysteine in 1×-PBS, before RNA extraction from 200 μl of the suspension. For all sample types, the extracted viral RNA was eluted into a final volume of 60 μl of elution buffer.
Primer and Probe Design
The published NP and HA gene sequences of the novel influenza A/H1N1/2009 virus, and those of other human and swine origin influenza viruses, were downloaded from the Global Initiative on Sharing Avian Influenza Database (GISAID, http://platform.gisaid.org, last accessed May 2, 2009) and aligned using the online alignment tool (NCBI Influenza Virus Resource, http://www.ncbi.nlm.nih.gov/genomes/Flu last accessed June 26, 2009). Visual inspection of the aligned sequences revealed several conserved regions of the NP gene, unique to the swine-origin influenza viruses (suitable for universal swine-origin influenza detection), and highly discriminative regions of the HA gene segment that were specific for the A/H1N1/2009 virus.
Using published sequences of A/California/04/2009 (HA segment; FJ966082; NP segment; FJ966083) as references, we designed the primer and TaqMan probe combinations for the assay (Figure 1, A–D). To confirm their targeted specificity, the HA primer-probe combinations were further checked against the HA gene sequences of 489 non-pandemic, swine-origin influenza A strains (obtained from the NCBI Influenza Virus Resource) in silico (see Supplemental Figure 1 at http://jmd.amjpathol.org).
Figure 1.
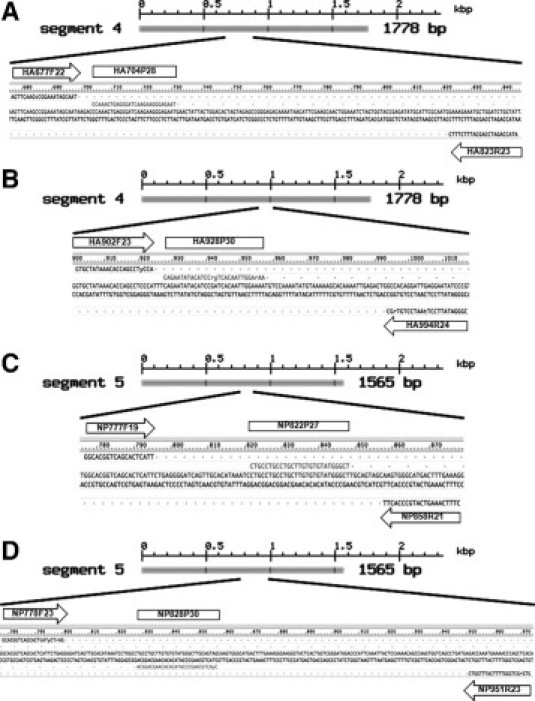
A and C show the locations of the hemagglutinin (HA) and nucleoprotein (NP) primer and probe sequences for the dual-gene duplex pandemic influenza A/H1N1/2009-specific assay. B and D show the locations of the corresponding HA and NP primer and probe sequences for the CDC/World Health Organization website method (http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf, last accessed April 29, 2009).
RNA Transcript Control Standards
A positive influenza A/H1N1/2009 RNA control (A/Auckland/H1N1/2009), provided by Dr. Ian Barr, World Health Organization Collaborating Laboratory, Melbourne, Australia, was used to generate cloned positive plasmid controls and in vitro transcribed RNA standards, for routine diagnostic work. Both NP and HA amplicons containing the selected primer and probe binding sites, with amplicon size of 102 bp and 169 bp respectively, were individually cloned into the pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA). The plasmid DNA from each construct was extracted with the QIAprep Spin Miniprep Kit (Qiagen, Valencia, CA), and in vitro transcribed into RNA transcripts with the T7 RiboMAX Express Large Scale RNA Production System (Promega, Madison, WI). Both transcripts were quantified using the NanoDrop ND-1000 UV-Vis Spectrophotometer (Nanodrop Technologies, Wilmington, DE), at an absorption wavelength of 260 nm, then serially diluted with RNase-free water from 107–101 copies per μl, for lower detection limit determination and quantitative standard curve analysis.
rRT-PCR Assay (Qualitative)
The one-step rRT-PCR amplification was performed using the SuperScript III Platinum One-step qRT-PCR reagents (Invitrogen, Carlsbad, CA), on the LightCycler v2.0 system (Roche Molecular Diagnostics, Pleasanton, CA). Each LightCycler used was color-compensated for both FAM and HEX fluorescence signals generated by the duplex assay, according to the manufacturer's protocol, to minimize cross talk between the different channels of the real time PCR instrument. The 20-μl reaction volume contained 5 μl extracted RNA template, 0.5 μl SuperScript III RT/Platinum Taq Mix, 10 μl 2× Reaction Mix, 0.2 μmol/L NP forward primer (NP777F19, 5′-GGCACGGTCAGCACTCATT-3′), 0.3 μmol/L NP reverse primer (NP858R21 5′-CTTTCAAAGTCATGCCCACTT-3′), 0.15 μmol/L NP probe (NP822P27 5′FAM-CTGCCTGCCTGCTTGTGTGTATGGGCT-3′BHQ1), and 0.3 μmol/L HA forward primer (HA677F22 5′AGTTCAAGTCGGAAATAGCAAT-3′), 0.3 μmol/L HA reverse primer (HA823R23 5′ATACCAGATCCAGCATTTCTTTC-3′), and 0.15 μmol/L HA probe (HA704P28 5′HEX-CCAAAGTGAGGGATCAAGAAGGGAGAAT-3′BHQ1). All primers and probes were obtained from Eurogentec AIT (Seraing, Belgium). Cycling conditions included initial reverse transcription (55°C, 8 minutes) and denaturation (95°C, 2.5 minutes) steps, followed by 45 cycles of amplification and data acquisition stages at 94°C for 8 s, 60°C for 30 s, and 68°C for 15 s. Fluorescence signals were detected at 68°C of each cycle. The NP and HA amplification curves were analyzed at absorption wavelengths of 530 nm and 560 nm, respectively. Positivity was defined as Ct (crossover/threshold cycle) of <40 cycles, for both gene targets. Appropriate positive controls and negative nontemplate controls were included in every test run.
Clinical Validation of Analytical and Diagnostic Specificity
The analytical specificity of the dual-gene assay was first tested against a panel of archived respiratory viruses isolated from patient samples, which included seasonal influenza A (H3N2 and H1N1), influenza B, respiratory syncytial virus, parainfluenza virus 3, rhinovirus, coronaviruses 229E and OC43, and human metapneumovirus, to verify noncross reactivity with these viruses. It was then used as part of our influenza A/H1N1/2009 diagnostic strategy, alongside universal influenza A16 and influenza B17 screening assays, and seasonal influenza A H3 and H1 subtyping assays [Centre for Health Protection (Hong Kong): CHP molecular diagnostic protocols for the detection of human swine influenza virus type A (subtype H1), http://www.01.chp.gov.hk/files/pdf/CHP_Protocols_for_the_Detection_of_Human_Swine_Influenza.pdf, last accessed May 26, 2009]. After RNA extraction, all samples were screened for influenza A viruses of human, avian, and swine origin, influenza B virus, and the pandemic influenza A/H1N1/2009 simultaneously. For samples with detected influenza A, but tested negative for the pandemic strain, further seasonal influenza A subtyping rRT-PCR assays (to human H1 or H3 viruses) ensued. All four screening and subtyping assays used in our diagnostic suite were from previously published literature (as above) and were optimized to conform to our laboratory setting (eg, instrument/platform availability). A small but important modification was made to Spackman's universal influenza A assay16 with the inclusion of an additional reverse primer, M124R′ 5′-TGCAAAGACACTTTCCAGTCTCTG-3′, to enhance the detection of swine-origin influenza virus (Table 1).16,17 The CDC's Emergency Use Authorization assay was run in parallel for the first 100 influenza A/H1N1/2009 positive samples during the initial clinical validation of our in-house assay.
Table 1.
Primer and Probe Sequences Used in this Study
| Primers | Sequence | Gene target | Forward or reverse orientation | 5′ Position |
|---|---|---|---|---|
| Universal influenza A* | 5′-AGATGAGTCTTCTAACCGAGGTCG-3′ | Matrix | Forward | 25 |
| 5′-TGCAAAAACATCTTCAAGTCTCTG-3′ | Reverse | 124 | ||
| 5′-FAM-TCAGGCCCCCTCAAAGCCGA-TAMRA-3′ | Forward | 64 | ||
| Influenza A (H1 subtype)† | 5′-TGCAAAGACACTTTCCAGTCTCTG-3′§ | Hemagglutinin (H1) | Reverse | 124 |
| 5′-AACATGTTACCCAGGGCATTTCGC-3′ | Forward | 347 | ||
| 5′-GTGGTTGGGCCATGAGCTTTCTTT-3′ | Reverse | 461 | ||
| 5′-HEX-GAGGAACTGAGGGAGCAATTGAGTTCAG-TAMRA-3′ | Forward | 378 | ||
| Influenza A (H3 subtype)† | 5′-ACCCTCAGTGTGATGGCTTCCAAA-3′ | Hemagglutinin (H3) | Forward | 266 |
| 5′-TAAGGGAGGCATAATCCGGCACAT-3′ | Reverse | 373 | ||
| 5′-FAM-ACGCAGCAAAGCCTACAGCAACTGTT-TAMRA-3′ | Forward | 315 | ||
| Influenza B‡ | 5′-AAATACGGTGGATTAAACAAAAGCAA-3′ | Hemagglutinin (Influenza B) | Forward | 970 |
| 5′-CTCCGAAGAAACCCCTTTCC−3′ | Reverse | 1110 | ||
| 5′-FAM-CACCCATATTGGGCAATTTCCTATGGC-TAMRA-3′ | Forward | 1004 | ||
| Influenza A (H1N1/2009) | 5′-GGCACGGTCAGCACTCATT-3′ | Nucleoprotein (Influenza A) | Forward | 777 |
| 5′-CTTTCAAAGTCATGCCCACTT-3′ | Reverse | 858 | ||
| 5′-FAM-CTGCCTGCCTGCTTGTGTGTATGGGCT-BHQ1–3′ | Forward | 822 | ||
| 5′-AGTTCAAGTCGGAAATAGCAAT-3′ | Hemagglutinin (Influenza A) | Forward | 677 | |
| 5′-ATACCAGATCCAGCATTTCTTTC-3′ | Reverse | 823 | ||
| 5′-HEX-CCAAAGTGAGGGATCAAGAAGGGAGAAT-BHQ1–3′ | Forward | 704 |
The underlined nucleotides represent the modifications made.
From Spackman et al.16
From Krafft et al.17
From Centre for Health Protection (Hong Kong) [CHP molecular diagnostic protocols for the detection of human swine influenza virus type A (subtype H1), http://www01.chp.gov.hk/files/pdf/CHP_Protocols_for_the_Detection_of_Human_Swine_Influenza.pdf, last accessed May 26, 2009].
Modified from Spackman et al.16
Quantitative Measurement of A/H1N1/2009 Viral Loads
The rRT-PCR described above was further adapted for quantification of the viral loads in infected patients. The PCR amplification efficiency and lower detection limit of the qRT-PCR assay were determined from the seven-point standard curves constructed from amplification of duplicates of serially diluted RNA transcript controls containing 5 × 101 to 5 × 107 viral gene copies per PCR. The PCR efficiency values were calculated with the formula E = 10−1/slope. The slope of the standard curve, which describes the kinetics of the PCR amplification and indicates how quickly the amount of target nucleic acid can be expected to increase with the amplification cycles, is related to as the efficiency of the amplification reaction. To determine the interassay reproducibility, 15 replicates of the six-point, 10-fold serially diluted NP and HA RNA transcripts (5 × 102 to 5 × 107 copies per PCR) were prepared in equivalent copy numbers for the NP and HA gene segments and analyzed in 15 consecutive batch runs by two different operators.
After adequate validation, the qRT-PCR was used to measure the viral loads in 286 clinical specimens on two separate occasions, by two different operators so as to determine the reproducibility of the qRT-PCR assay in clinical specimens. The 286 patient specimens analyzed represented the full range of possible viral load concentrations obtained in our patient cohort during the containment period, from May to July 2009. All repeat testing was done on thawed out aliquots that had been kept at −80°C immediately after diagnostic testing, with a single freeze-thaw cycle. All statistical analysis were performed using SPSS Statistics 17.0 (IBM Company, Chicago, IL) and Microsoft Office Professional Plus Excel 2007 (Microsoft Corporation, Redmond, WA).
Results
None of the archived noninfluenza A/H1N1/2009 respiratory viruses tested showed any cross-reactivity with the highly specific HA primer-probe set of the assay, which also did not cross-react with any of the HA genes of the 489 nonpandemic swine influenza subtypes, when analyzed in-silico. There was also no cross-reactivity observed with the NP primer-probe set against the panel of noninfluenza viruses tested.
During the period of heightened surveillance between May 1 and July 22, 2009, 3512 patients with influenza-like symptoms were tested using this dual-gene assay, alongside universal influenza A16 and universal influenza B17 screening assays, and seasonal influenza A H3 and H1 subtyping assays. Of these, there were 20 influenza B, 17 seasonal H1 influenza A, 143 seasonal H3 influenza, and 943 cases of A/H1N1/2009 virus infection (see Table 2). The first 100 positive cases of A/H1N1/2009 detected by the dual-gene duplex assay gave a 100% concordance with the CDC method. The assay appears to be highly specific for the pandemic influenza A variant, in the presence of seasonal influenza virus A(H1N1), A(H3N2) and B subtypes—none of the samples showing positivity for either seasonal influenza A or influenza B gave a positive result with the dual-gene assay. There were, however, six HA-only and 16 NP-only positive results (of the total 943 cases of A/H1N1/2009-positives), which were found in patients who were shown to be infected by the pandemic influenza strain by a sister laboratory that used another laboratory-developed method of their own (unpublished data). All 22 cases were found to be patients on post-antiviral treatment follow-up, with low viral titers (detection threshold >35 cycles), where virus detection probably became stochastic at the later stages of viral shedding. When such a scenario arose, we would repeat the assay and in the event that the repeats were consistently discrepant, we used our backup assay (the CDC EUA assay) to provide the correct result. In our hands, the dual-gene duplex assay had clinically validated sensitivity of 97.7% and specificity of 100%, based on 943 cases, positively infected with influenza A/H1N1/2009, and a rapid turnaround time of about 2.5 hours from sample receipt to result output.
Table 2.
Summary of the Clinical Performance of the Quantitative Dual-Gene Assay on 3497 Patients during the Containment (Mitigation) Phase of the Pandemic (May 1 to July 22, 2009) at a Tertiary Hospital in Singapore
| Sample types | NP gene (+) | NP gene (−) | HA gene (+) | HA gene (−) |
|---|---|---|---|---|
| Influenza A/H1N1/2009 | 921 | — | 921 | — |
| — | 6 | 6 | — | |
| 16 | — | — | 16 | |
| Non-influenza virus | — | 2389 | — | 2389 |
| Influenza A (Seasonal H1) | — | 17 | — | 17 |
| Influenza A (Seasonal H3) | — | 143 | — | 143 |
| Influenza B | — | 20 | — | 20 |
All samples were tested with universal influenza A16 and influenza B17 screening assay. Influenza A/H1N1/2009 specimens were detected using the quantitative dual-gene assay, including 100 cases that were confirmed with CDC/WHO method (http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf, last accessed April 29, 2009). Seasonal H1 and H3 specimens were detected using seasonal influenza A H3 and H1 subtyping assay (http://www01.chp.gov.hk/files/pdf/CHP_Protocols_for_the_Detection_of_Human_Swine_Influenza.pdf, last accessed May 26, 2009).
Figure 2, A and B shows the standard amplification curves of both the NP and HA RNA transcripts in duplicates. The lower limit of detection for both NP and HA targets, defined as the lowest dilution with reproducible amplification, was 50 copies per PCR. The PCR efficiencies were 1.818 for the NP gene target and 1.772 for the HA gene. Boxplots, drawn using the amplification curve threshold cycle (Ct) values of each NP- and HA-serially diluted RNA transcript controls (six points for each gene) performed in 15 separate runs are shown in Figure 3, A and B. Percentage coefficient of variation (CV%) and SD of each control level were derived to assess the interassay imprecision and gave an overall CV% of <3.5% and SD of <1.5 for all six points. The best fit line and 95% confidence interval against the calculated mean were drawn using the Ct values of the 15 consecutive separate runs, showing an R2 value of 0.988 for both NP and HA (Figure 3, C and D).
Figure 2.
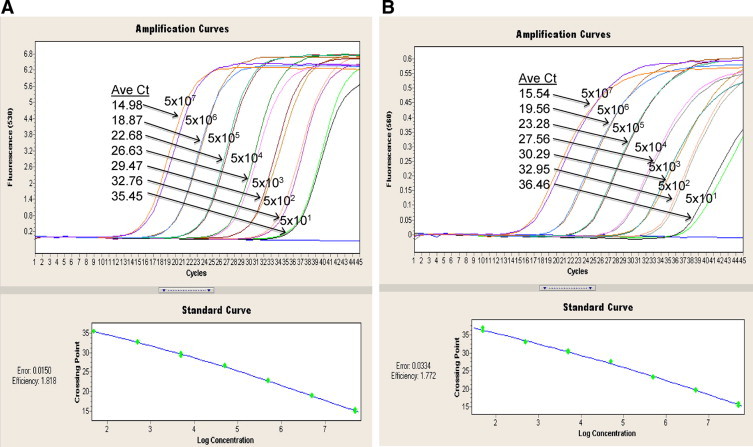
Amplification curves of duplicates of serially diluted RNA transcripts (in RNase-free water). The gene copy number for each of the six dilutions is indicated. Standard curves were plotted with threshold cycle (Ct) values against log10 concentrations of RNA transcripts. A: Representative amplification profile of the 10-fold serially diluted NP RNA transcript. The PCR efficiency of 1.818 was calculated by the LC v2.0 software. B: Corresponding amplification profile of the 10-fold serially diluted HA RNA transcript, with calculated PCR efficiency of 1.772.
Figure 3.
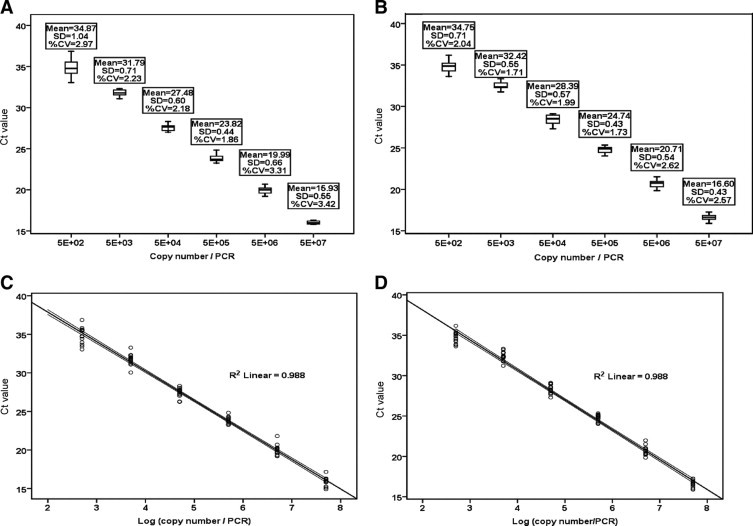
A: Boxplot for Ct values of 15 consecutive runs for the six-point standard curve of the 10-fold serially diluted NP RNA transcript (5 × 102–5 × 107 copies per PCR). Percentage coefficients of variation (%CV) for all six standards were less than 3.5%, and SD, less than 1.5. B: Boxplot for Ct values of 15 consecutive runs for the corresponding six-point standard curve of the 10-fold serially diluted HA RNA transcript (5 × 102–5 × 107 copies per PCR), with %CV for all six standards of less than 3%, and SD, less than 0.8. Boxes in both charts contain the middle 50% of the Ct values, with the upper hinge of the box indicating the 75th percentile of the Ct values, the lower hinge indicating the 25th percentile, and the line in the box denoting the median value of the data. The ‘whiskers’ indicate the minimum and maximum Ct values for each set of 15 replicates. X-Y (scatter) plots of Ct values in 15 consecutive runs against the log10 viral gene copies/PCR were drawn. The best fit lines for the amplification of NP (C) and HA (D) transcripts gave R2 = 0.988 for both.
The difference between the first and second Ct value readings was obtained from the duplicate testing of 286 clinical specimens. The best fit lines for NP (Figure 3A) and HA (Figure 3B) gene target scatter plots for Ct values of the initial diagnostic testing against the repeat testing of these 286 archived specimens were derived (y = 0.9806x + 0.2976 for the NP plot and y = 0.9341x + 1.1087 for the HA plot, with R2 = 0.94685 and R2 = 0.89384, respectively) (Figure 4, A and B).
Figure 4.
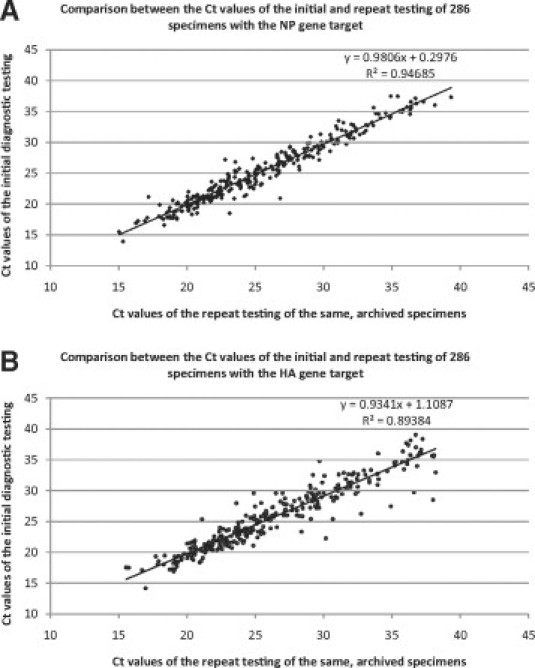
X-Y (scatter) plots of the Ct values of the initial diagnostic testing against the Ct values of the repeat testing of the same 286 archived patient specimens. The two runs were performed on different days, by different operators, using different reaction mixes. The best fit lines for NP (A) and HA (B) gene target scatter plots were derived, with slopes/gradients of 0.9806 and 0.9341, respectively.
We did not find any significant differences, in a subset of 10 samples, when we performed the duplicate testing for patient samples with RNA stored from the previous extraction or with RNA, which was reextracted from the VTM for the duplicate test. Thereafter, we only used the stored RNA for the remaining data collection. We stored excess RNA extracts in the eluent buffer in a −80°C freezer, in suitable aliquots for further usage. In our hands, such stored RNA remained intact for up to 6 months.
Discussion
Issued World Health Organization guidelines recommended that any influenza A/H1N1/2009-positive case should be confirmed by the detection of two different influenza A/H1N1/2009 gene targets [World Health Organization: World Health Organization Information for Laboratory Diagnosis of New Influenza A (H1N1) Virus in Humans, http://www.who.int/csr/resources/publications/swineflu/WHO_Diagnostic_RecommendationsH1N1_20090521.pdf, last accessed May 25, 2009]. This in-house dual-gene rRT-PCR assay targeted a region in the HA gene segment that could reliably discriminate influenza A/H1N1/2009 virus from other influenza A(H1) strains (both human and swine) and influenza B. As the HA gene is known to have a relatively high mutation rate, which may cause primer/probe mismatches and a subsequent loss of assay sensitivity, a second region on the more conserved internal NP gene was also targeted in the design of the dual-gene assay. The region targeted in the NP gene is common to only swine-origin influenza, increasing the specificity and confirmation of this assay against influenza A/H1N1/2009 from other human seasonal influenza.
The assay has a demonstrated optimum PCR efficiency of 1.818 and 1.772, respectively, for the NP and HA genes (obtained from in vitro transcribed RNA, not viral RNA from clinical samples) of the influenza A/H1N1/2009 virus, and an acceptable interbatch variability, with %CV for all six standards of both NP and HA Ct values falling below 3.5%, and SDs of <1.04 and <0.71, respectively. The R2 obtained for 15 separate amplifications of both the NP and HA genes (obtained from plasmids, not clinical samples) were 0.988, confirming the suitability of this assay for viral load quantification of the influenza A/H1N1/2009 variant. Repeat analysis of 286 clinical specimens performed in two separate runs several days apart showed that the majority of Ct values of the initial versus the repeat testing were well correlated (R2 of 0.94685 for NP and 0.89384 for HA), substantiating the high reproducibility of this RNA-based assay in clinical specimens over the wide range of viral loads expected in infected patients. The differences between duplicates in patients were, as expected, not as good as those for duplicate analyses from plasmid transfects. We attribute this to the likely presence of inherent “contaminants” in the former, which could act as potential inhibitors of PCR amplification, often referred as the “matrix effect.”
As the northern hemisphere enters the annual seasonal influenza period during the winter months, the presence of this pandemic strain may increase the morbidity and mortality above the usual levels in these regions [World Health Organization: Preparing for the second wave: lessons from current outbreaks, http://www.who.int/csr/disease/swineflu/notes/h1n1-second-wave-20090828/en/index.html, last accessed August 30, 2009]. Unlike with previous pandemic influenza viruses, which displaced the previous seasonal influenza strains to become the current predominant circulating strain18, there appears to be a recent sporadic resurgence of seasonal influenza H3 and B viruses in certain parts of the world [World Health Organization: Pandemic (H1N1) 2009. update 98, http://www.who.int/csr/don/2010_04_30a/en/index.html, last accessed 2 July, 2010]. However, given its continued global circulation, a reliable diagnostic assay will still be required to screen for this novel influenza A/H1N1/2009 subtype, rapidly and accurately. In addition, the quantitative capability of this assay for viral load measurements enables better monitoring for drug-resistant influenza A/H1N1/2009 mutations, which are beginning to evolve in some infected patients post-treatment (eg, oseltamivir resistant influenza A/H1N1/2009, with probable human-to-human transmission, was first reported on November 20, 200919). A recent study describing the natural viral load profile of the pandemic virus reported a reduction rate of 0.638 log10 copies/ml/day (post symptom onset) in patients who received oseltamivir within two days of symptom onset versus −0.409 log10 copies/ml/day in those who did not receive treatment, and viral load was undetectable by the day 7 of oseltamivir treatment.20 Observation of persistently high viral load in a clinically suspicious or oseltamivir-treated patient would suggest the presence of the drug-resistant A/H1N1/2009 mutant and may warrant further identification study.20 In this context, this dual-gene duplex rRT-PCR assay in both its qualitative and quantitative modes, offers a robust, sensitive, and specific option for detection and quantitative viral load determination of the influenza A/H1N1/2009 variant.
Acknowledgements
We thank Dr. Ian Barr (Director, World Health Organization Collaborating Laboratory, Melbourne, Australia) for kindly providing the positive influenza A/H1N1/2009 RNA controls. We also thank the other staff members of the Molecular Diagnosis Centre where the diagnostic testing was performed for their contribution to the testing of the 3512 patients who were included in our study cohort, between May and July 2009.
Footnotes
Supported by the H1N1 Advance Urgent Funds, #R-179-000-045-720, from the Yong Loo Lin School of Medicine, National University of Singapore.
P.A.T. has research support from ADAMAS and Baxter, Board membership of the Asia Pacific Advisory Committee on Influenza and honoraria from Novartis, in relation to H1N1 work. None of the other authors disclosed any relevant financial relationships.
Supplemental material for this article can be found on http://jmd.amjpathol.org.
Web Extra Material
List of Genbank accession numbers for 489 non-pandemic swine subtype HA sequences, for the in-silico test of the specificity of the HA primers of the dual-gene duplex pandemic influenza A/H1N1/2009-specific assay.
References
- 1.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team, Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Peiris JSM, Poon LLM, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009;45:169–173. doi: 10.1016/j.jcv.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris M, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 4.Carr MJ, Gunson R, Maclean A, Coughlan S, Fitzgerald M, Scully M, O'Herlihy B, Ryan J, O'Flanagan D, Connell J, Carman WF, Hall WW. Development of a real-time RT-PCR for the detection of swine-lineage influenza A (H1N1) virus infections. J Clin Virol. 2009;45:196–199. doi: 10.1016/j.jcv.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginocchio CC, Zhang F, Manji R, Arora S, Bornfreund M, Falk L, Lotlikar M, Kowerska M, Becker G, Korologos D, Geronimo M, Crawford JM. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009;45:191–195. doi: 10.1016/j.jcv.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeBlanc JJ, Li Y, Bastien N, Forward KR, Davidson RJ, Hatchette TF. Switching gears for an influenza pandemic: validation of a duplex reverse transcriptase PCR assay for simultaneous detection and confirmatory identification of pandemic (H1N1) 2009 influenza virus. J Clin Microbiol. 2009;47:3805–3813. doi: 10.1128/JCM.01344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahony JB, Hatchette T, Ojkic D, Drews SJ, Gubbay J, Low DE, Petric M, Tang P, Chong S, Luinstra K, Petrich A, Smieja M. Multiplex PCR tests sentinel the appearance of pandemic influenza viruses including H1N1 swine influenza. J Clin Virol. 2009;45:200–202. doi: 10.1016/j.jcv.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasoo S, Stevens J, Singh K. Rapid antigen tests for diagnosis of pandemic (swine) influenza A/H1N1. Clin Infect Dis. 2009;49:1090–1093. doi: 10.1086/644743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang T, Kang XP, Deng Y, Zhao H, Li X, Yu X, Yu M, Qin E, Zhu Q, Yang Y, Qin C. Development of a real-time RT-PCR assay for a novel Influenza A (H1N1) virus. J Virol Methods. 2010;163:470–473. doi: 10.1016/j.jviromet.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Poon LL, Chan KH, Smith GJ, Leung CSW, Guan Y, Yuen KY, Peiris JSM. Molecular detection of a novel human influenza (H1N1) of pandemic potential by conventional and real-time quantitative RT-PCR assays. Clin Chem. 2009;55:1555–1558. doi: 10.1373/clinchem.2009.130229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R, Sheng ZM, Taubenberger JK. Detection of novel (swine origin) H1N1 influenza A virus by quantitative real-time reverse transcription-PCR. J Clin Microbiol. 2009;47:2675–2677. doi: 10.1128/JCM.01087-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bose ME, Beck ET, Ledeboer N, Kehl SC, Jurgens LA, Patitucci T, Witt L, LaGue E, Darga P, He J, Fan J, Kumar S, Henrickson KJ. Rapid semiautomated subtyping of influenza virus species during the 2009 swine origin influenza A H1N1 virus epidemic in Milwaukee. Wisconsin J Clin Microbiol. 2009;47:2779–2786. doi: 10.1128/JCM.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiley DM, Bialasiewicz S, Bletchly C, Faux CE, Harrower B, Gould AR, Lambert SB, Nimmo GR, Nissen MD, Sloots TP. Detection of novel influenza A(H1N1) virus by real-time RT-PCR. J Clin Virol. 2009;45:203–204. doi: 10.1016/j.jcv.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Update: drug susceptibility of swine-origin influenza A(H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:433–435. [PubMed] [Google Scholar]
- 15.Chen H, Cheung CL, Tai H, Zhao P, Chan JFW, Cheng VCC, Chan KH, Yuen KY. Oseltamivir-resistant influenza A pandemic (H1N1) 2009 virus. Hong Kong, China. Emerg Infect Dis. 2009;15:1970–1972. doi: 10.3201/eid1512.091057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krafft AE, Russell KL, Hawksworth AW, McCall S, Irvine M, Daum LT, Connoly JL, Reid AH, Gaydos JC, Taubenberger JK. Evaluation of PCR testing of ethanol-fixed nasal swab specimens as an augmented surveillance strategy for influenza virus and adenovirus identification. J Clin Microbiol. 2005;43:1768–1775. doi: 10.1128/JCM.43.4.1768-1775.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson NM, Galvani AP, Bush RM. Ecological and immunological determinants of 375 influenza evolution. Nature. 2003;422:428–433. doi: 10.1038/nature01509. [DOI] [PubMed] [Google Scholar]
- 19.Gulland A. First cases of spread of oseltamivir resistant swine flu between patients are reported in Wales. BMJ. 2009;339:b4975. doi: 10.1136/bmj.b4975. [DOI] [PubMed] [Google Scholar]
- 20.Li IW, Hung IF, To KK, Chan KH, Wong SS, Chan JF, Cheng VC, Tsang OT, Lai ST, Lau YL, Yuen KY. The natural viral load profile of patients with pandemic swine-origin influenza A H1N1 2009 (pH1N1) and the effect of oseltamivir treatment. Chest. 2010;137:759–768. doi: 10.1378/chest.09-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of Genbank accession numbers for 489 non-pandemic swine subtype HA sequences, for the in-silico test of the specificity of the HA primers of the dual-gene duplex pandemic influenza A/H1N1/2009-specific assay.


