Abstract
A secondary mutation (T790M) in epidermal growth factor receptor (EGFR) is a hallmark of acquired resistance to EGFR inhibitors used to treat non-small-cell lung cancer (NSCLC). Therefore, identifying the T790M mutation is crucial to guide treatment decisions. Given that DNA sequencing methods are time-consuming and insensitive, we developed and investigated the feasibility of using molecular beacons for the detection of the T790M mutation in EGFR. A molecular beacon complementary to the region of the secondary EGFR mutation (T790M) was designed and used in NSCLC samples bearing drug-sensitive and -resistant EGFR mutations. For a rapid and simple assay, we attempted to use the molecular beacon with real-time PCR and in situ fluorescence imaging. The ability of the designed molecular beacon to specifically detect the T790M mutation of EGFR was tested for samples from two patients with drug resistance and compared with conventional DNA sequencing methods. The molecular beacon successfully detected the T790M mutation in patient samples with drug resistance. The sensitivity of the molecular beacon, which detected as little as 2% of genomic DNA from the drug-resistant cells (H1975), was much higher than direct sequencing. Furthermore, in situ fluorescence imaging with the molecular beacon gave rise to a distinguishable signal for the T790M mutation in drug-resistant cells. The molecular beacon-based approach enabled rapid and sensitive detection of the EGFR mutation (T790M) in NSCLC with in situ fluorescence imaging, which can be directed to determine various treatment options in patients with cancer.
Lung cancer is the leading cause of cancer-related death and is responsible for 1.3 million deaths worldwide annually. Despite marked progress in cancer treatment, the 5-year survival rate of patients with lung cancer has remained at around 15% for over the past 30 years.1 In an attempt to provide improved treatment, a variety of targeted therapeutics have been investigated based on the progress in our understanding of the molecular mechanisms underlying lung tumorigenesis. The epidermal growth factor receptor (EGFR) is one such therapeutic target in non-small-cell lung cancer (NSCLC), which has led to the development of effective anticancer agents, such as EGFR tyrosine kinase inhibitors (EGFR-TKIs). EGFR-TKIs, including gefitinib and erlotinib, have been approved for clinical treatment and are currently prescribed in patients with advanced NSCLC. Extensive clinical trials of EGFR-TKIs have shown a survival benefit associated with EGFR-TKIs in patients with lung cancer.2 Clinical and molecular analyses have revealed that EGFR mutations in the kinase domain, most commonly small in frame deletions in exon 19 or a substitution mutation of leucine 858 to arginine (L858R) in exon 21, are associated with a high clinical response rate of tumors to gefitinib or erlotinib in patients with NSCLC.3,4
Most patients carrying EGFR mutations initially show an impressive response to EGFR-TKIs. However, they eventually develop resistance after prolonged treatment. Previous studies have revealed that a secondary EGFR mutation, as a result of a threonin-to-methionine amino acid change at the 790th codon (T790M), is often observed in the tumors of patients with NSCLC that develop acquired resistance.4,5 From the clinical standpoint, detection of the secondary mutation (T790M) in patients who have disease progression after prolonged treatment with gefitinib or erlotinib is crucial to planning alternative treatment options.
Various methods for the detection of EGFR mutations in patient samples have been developed.6,7,8 Currently, the most common method for the detection of EGFR mutations in tumor tissue samples is direct sequencing of EGFR fragments amplified from genomic DNA. Since the amount of sample available for clinical analysis from patients with advanced NSCLC is limited, and often mixed with an excess amount of normal tissue, the detection of a mutation by direct sequencing may be inaccurate if the number of cancer cells is below a minimum of 20% of the total specimen.8 Therefore, an issue of great importance is the development of methods that allow for unambiguous detection of EFGR mutation in patients with cancer in a rapid and sensitive way.
A molecular beacon (MB) is a nucleotide strand in a distinct stem-loop structure with a fluorescence dye attached on one end and a quencher dye on the other.9,10 In the hairpin conformation, the proximity between the fluorescence-quencher pair allows quenching due to fluorescence resonance energy transfer.11 Only when hybridized to the specific target, the probe separates and expands its stem region, pushing away the dye-attached-ends of the complex. This results in the restoration of the fluorescence unaffected by the distant quencher. As the fluorescence intensity varies according to the changes in the probe conformation to the target DNA, a much more specific and selective signal can be detected. Applying a molecular beacon probe to real-time PCR allows for rapid, simple, and cost-effective detection of the target without post-PCR manipulation.12,13,14 Even though the molecular beacon has been used for a variety of purposes, there has been no prior report on its application to the analysis of mutations in the EGFR gene.
In an effort to develop a rapid and sensitive assay for the identification of acquired resistance to EGFR-TKIs in patients with NSCLC, we demonstrate the use of a molecular beacon for the detection of the secondary mutation, T790M, in EGFR by real-time PCR and in situ fluorescence imaging approaches. Under optimized molecular beacon and real-time PCR conditions, we determined that the detection sensitivity was comparable to or better than other recently developed EGFR mutation detection systems. Furthermore, fluorescence imaging of cells with the T790M mutation using the molecular beacon also provided a simple and rapid detection method for acquired resistance in situ. Therefore, the molecular beacon-based EGFR mutation detection system offers a potentially useful technical platform for the clinical diagnosis of lung cancer samples and ultimately might help to guide treatment decisions for lung cancer patients.
Materials and Methods
Lung Cancer Cell Lines
NSCLC cell lines H1975 and PC9 were obtained from Samsung Medical Center (Seoul, Korea). H1975 contained both mutations L858R in exon 21 and T790M in exon 20 of the EGFR gene. PC9 cell line has a deletion mutation in exon 19. H1975 and PC9 cell lines were cultured in RPMI-1640 medium. Culture media were purchased from Gibco and supplemented with 10% fetal bovine serum (Gibco, Invitrogen, New York, NY), 2 mmol/L l-glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin.
Direct Sequencing for the Detection of the EGFR Mutation
The T790M EGFR mutation in exon 20 was detected by PCR-based direct sequencing. Genomic DNA samples from lung cancer cell lines were isolated by using the Wizard genomic DNA Purification Kit (Promega, Madison, WI). PCR amplification was performed with 10 ng of genomic DNA. The following EGFR primers were used: exon 20–300 forward, 5′-CTCCAGGAAGCCTACGTGAT-3′ and exon 20–300 reverse, 5′-GTCTTTGTGTTCCCGGACAT-3′. DNA was amplified for 35 cycles at 95°C for 30 seconds, 59°C for 1 minute, and 72°C for 30 seconds followed by 7 minutes of extension at 72°C. The sequences were compared with the GenBank human sequence for EGFR (accession number AF288738). Mutant-enriched PCR was performed to determine the presence and type of mutation in the EGFR gene in the lung cancer cell line.
Design and Synthesis of Molecular Beacons for Detection of the T790M Mutation
The molecular beacon probes were designed following the guidelines published by Marras et al.12 The prediction of the secondary structure was determined by using Mfold (version 3.2). DNA sequences reported for EGFR were used for the design of the EGFR molecular beacon and primers: GenBank accession number AF288738. The designs of the molecular beacons targeting T790M mutation in the EGFR gene are shown in Table 1. For each molecular beacon design, the sequence of one arm of the stem and the loop region of the molecular beacons was complementary to the segment of its target gene. The molecular beacon for the T790M mutation was synthesized by Bioneer Inc. (Korea). An FAM fluorophore was conjugated to the 5′-end of each oligonucleotide, and a quencher, benzoic acid (Dabcyl), was linked to its 3′-end.
Table 1.
Design of Molecular Beacons and Synthesized Template
| Synthetic nucleotides* | Sequences |
|---|---|
| T790M-MB | 5′-FAM-TCGGAGCTGCATGATGAGTCCGA-Dabcyl-3′ |
| Control-MB | 5′-Cy5-CCGTGAAAAAAAAAAAAAACACGG-BHQ2–3′ |
| Target DNA | 5′-ACCTCCACCGTGCAGCTCATCATGCAGCTCATGCCCTTGGCTGCCTCCT-3′ |
| Mismatched DNA | 5′-ACCTCCACCGTGCAGCTCATCACGCAGCTCATGCCCTTGGCTGCCTCCT-3′ |
T790M mutation indicates the substitution of ACG nucleotides with ATG at codon 790 in the EGFRgene as marked in bold. Underlined bases are added to create the stem domain. T790M-MB T790M mutation specific molecular beacon; control-MB, scrambled molecular beacon; target DNA, complementary templates to T790M; mismatched DNA, single mismatched to T790M.
Determination of the Specificity of the Molecular Beacons in Solution
The oligonucleotide targets for the respective molecular beacons were synthesized by Bioneer Inc. (Korea) and are listed in Table 2. Binding of the target DNAs to the respective molecular beacons was analyzed by using a fluorescence microplate reader (Infinite TM M200, TECAN, Austria). Next, 200 nmol/L of the molecular beacons were mixed with 1 μmol/L of the oligonucleotide targets in 100 μl of binding buffer in 96-well plates. The binding buffer contained 50 mmol/L NaCl and 5 mmol/L MgCl2 in 20 mmol/L Tris buffer (pH 8.0). After incubation at 37°C for 1 hour, the fluorescence intensity in each well was measured by using a fluorescence microplate reader.
Table 2.
Patient Characteristics and Results of Sequencing Analysis
| Patient no. | Histology | Gefitinib response | EGFR activating mutations | T790M mutation |
|---|---|---|---|---|
| Sample 1 | Contralateral lung cancer | Resistant | L858R | + |
| Sample 2 | Primary lung cancer | Resistant | L858R | + |
Thermal Denaturing Profiles of the Molecular Beacons
Changes in the fluorescence intensity of the molecular beacon were traced in the presence of the target and mismatched oligonucleotides as a function of temperature by using a thermal cycler (BioRad Laboratories, Hercules, CA). Briefly, 200 nmol/L of the molecular beacon were mixed with 1 μmol/L of the target or mismatched oligonucleotides in binding buffer, and the fluorescence intensity of molecular beacon was measured as a function of temperature. The temperature was decreased from 95°C to 10°C by 1°C steps, and the fluorescence was measured during a 30-second hold at each temperature. The same experiment was performed with the molecular beacon alone. Changes in the fluorescence of the MB during thermal denaturing were expressed as the relative fluorescence intensity.
Real-Time PCR with the Molecular Beacon
Based on the thermal denaturing profiles of the molecular beacon in the presence of the target and mismatched oligonucleotides, real-time PCR conditions were optimized. The reaction mixture contained the template genomic DNA, 0.2 μmol/L of each primer set, 0.2 μmol/L of each dNTP, 500 nmol/L of the molecular beacons, 0.5 U ExTaq DNA polymerase, and 5 mmol/L MgCl2 in 50 μl of 1× PCR buffer. Sequences of the designed molecular beacons and primers for different sizes of the amplicon are listed in Table 1. The thermal cycling program included 5 minutes at 95°C for activation of the DNA polymerase, followed by 50 cycles of denaturing at 95°C for 30 seconds, 59°C for 30 seconds to allow annealing, and extension at 72°C for 30 seconds. Fluorescence was measured at the 59°C annealing step in a 96-well spectrofluorometric thermal cycler (Bio-Rad iCycler IQ).
Fluorescence Imaging of the Cells Using the Molecular Beacon
The PC9 and H1975 cell lines from human non-small-cell lung cancer were plated on chamber slides and fixed with ice-cold acetone for 7 minutes. After air drying, the slides were stained immediately or stored in 4°C in PBS buffer until use. A solution of 200 nmol/L of the T790M specific molecular beacon was diluted in PBS and incubated with the fixed cells at 50°C for 40 minutes. The slides were washed briefly with PBS and examined under a confocal microscope (LSM 510, Carl Zeiss Microimaging, Inc, Thornwood, NY). The fluorescent images were obtained by using the same instrument for each color.
Results
Design and Construction of the Molecular Beacon for the Detection of the T790M Secondary Mutation
We first designed molecular beacons that were complementary to a region of EGFR exon 20 that contains the T790M mutation. As depicted in Figure 1A, the molecular beacon specific for the secondary mutation had 23 stem and loop nucleotide sequences complementary to the T790M mutant (red region) of target EGFR DNA. The loop region of the molecular beacon was designed to accommodate this single mutation and to discriminate between the wild-type and the T790M mutation sequences. It also has a hairpin-induced oligonucleotide, which is tethered to fluorophores (5′ FAM) and quencher (3′ Dabcyl). Fluorescence quenching is achieved by a fluorescence resonance energy transfer in a quiescent state, but the loop can be open by hybridization to complementary regions on the target DNA, leading to the strong fluorescence emission during real-time PCR. This designed molecular beacon is also capable of detecting the secondary mutation in drug resistance-acquired cells by using in situ fluorescence imaging (Figure 1B). The sequences of the molecular beacons and the synthetic template are listed (Table 1). We used the scramble molecular beacon as a negative control to ensure the nonspecific binding or cleavage in real-time PCR as well as in situ fluorescence hybridization.
Figure 1.
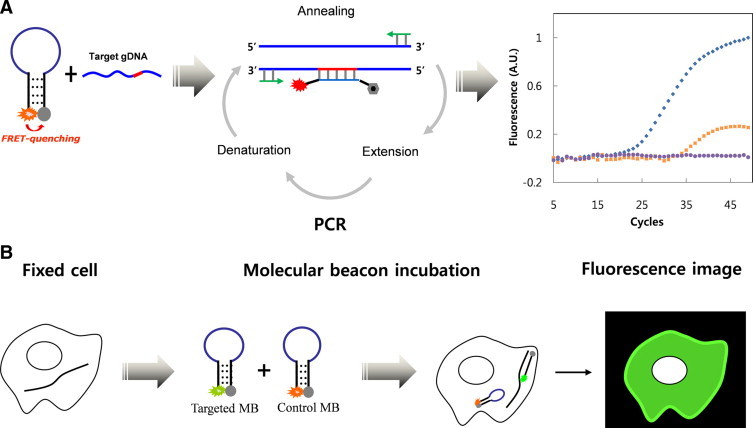
Schematic representation of the detection principle using a molecular beacon. A: Molecular beacon-based real-time PCR. Molecular beacons complementary to a target DNA bind at the annealing temperature, dissociating the stem, and generate a fluorescence signal during real-time PCR. With an increasing number of PCR cycles, fluorescence intensity increases, enabling the detection of the mutation in real-time. B:In situ fluorescence imaging of individual cells carrying a specific mutation. The molecular beacons bind to a target mRNA in the fixed cells, dissociating the stem, and restore a fluorescent signal.
To determine the optimal hybridization temperatures for the molecular beacon targeting the secondary mutation T790M (T790M-MB), we examined the effects of annealing temperature on the binding specificity of the T790M-MB to the synthesized DNA templates. Using a thermal cycler, we monitored changes in the fluorescence signal at temperatures ranging from 10°C to 90°C after mixing the T790M-MB with two types of synthetic templates. Figure 2A shows the status of the T790M specific molecular beacon in conjunction with the complementary or mismatched synthetic DNA over the thermal denaturing profiles. Higher fluorescence emission at the dissociation temperatures perfectly differentiated the complementary targets from the mismatched oligonucleotides, known as the “window of discrimination.” Based on the thermal denaturing profiles, it was inferred that the T790M-MB had an optimal annealing temperature at 60°C to ensure an intense fluorescence signal in the presence of the perfect complementary synthetic DNA and a negligible fluorescence signal in the presence of a single mismatched oligonucleotide.
Figure 2.
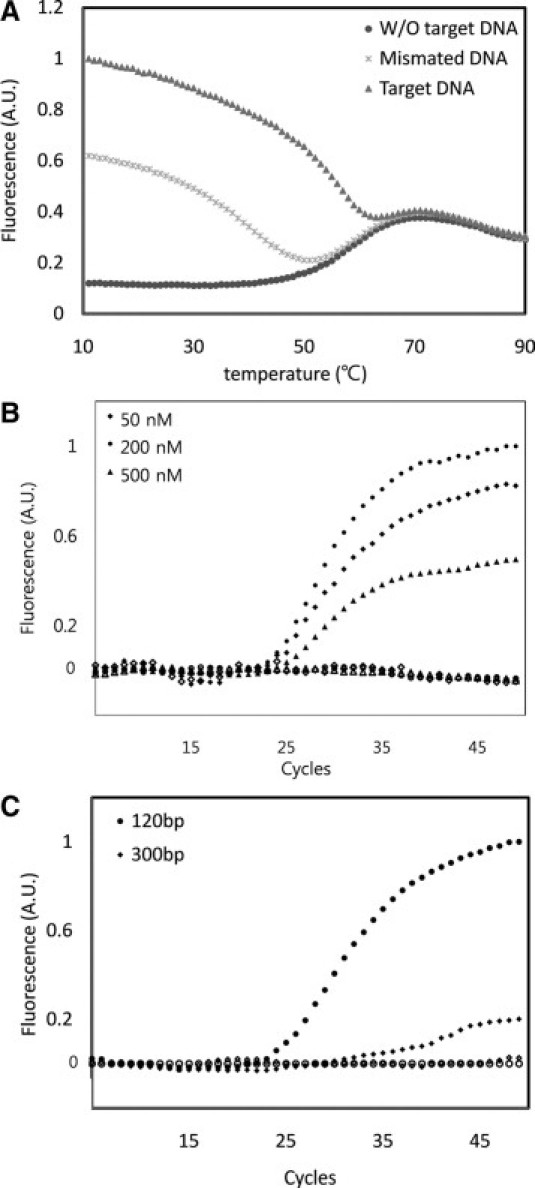
A: Thermal denaturing profiles of molecular beacons in the presence of mismatched DNA, target DNA, or no target. B: Effect of molecular beacon concentration on the fluorescence signal in MB-based real-time PCR. Concentrations of the MB were 50 nmol/L (diamond), 200 nmol/L (circle), and 500 nmol/L (triangle). C: Effect of amplicon size on the sensitivity of the real-time PCR assay. Genomic DNA was amplified with two different primer sets: (circle) 120-bp amplicon and (diamond) 300-bp amplicon. Closed and open symbols represent genomic DNA from the cell lines H1975 and PC9, respectively.
Detection of the T790M Mutation by Real-Time PCR with the Molecular Beacon
The ability of the designed molecular beacon to specifically detect the T790M mutation of EGFR by real-time PCR was investigated. To optimize the PCR conditions and monitor the amplification of nonspecific products, various factors including template concentrations and amplicon sizes were tested. We used genomic DNA from H1975 cell lines as a positive template for carrying the secondary T790M mutation of EGFR, and the PC9 cell lines as a negative template. The PCR primers used in the amplification of EGFR exon 20, encompassing the T790M mutation, were performed robustly in a wide range of annealing temperatures between 40°C and 65°C. Other conditions, such as the number of thermal cycles and salt concentration, were optimized to accomplish a maximum signal-to-background ratio without drastically compromising the yield and specificity. Testing of the molecular beacon concentration, ranging from 50 nmol/L to 500 nmol/L, revealed that 200 nmol/L of the molecular beacon showed the strongest fluorescent intensity (Figure 2B). The shorter length (120 bp) of amplicon allowed for a better result on the real-time PCR assay than 300 bp (Figure 2C).
Under conditions optimized for the detection of the T790M mutation in real-time, we examined whether the T790M-MB could distinguish an amplicon with a single mutation from others during real-time PCR. Figure 3A shows that, as real-time PCR proceeded, a significant fluorescence signal was observed only from the genomic DNA of the H1975 cells with the secondary mutation, demonstrating that T790M-MB specifically bound its exact complementary target DNA. No significant fluorescence was detected from the control PC9 genomic DNA among cells without the T790M mutation. To confirm that this marked difference in fluorescence was not due to variations in the PCR efficiency, the PCR products obtained from H1975 and PC9 were run on an agarose gel. As shown in Figure 3B, no significant difference in the intensities was observed among the PCR bands by densitometry from the PC9 and H1975 genomic DNA. The signal from the molecular beacon did not increase even in the presence of single mismatched amplicons. These results show that the T790M specific molecular beacon was bound specifically only to its intended target.
Figure 3.
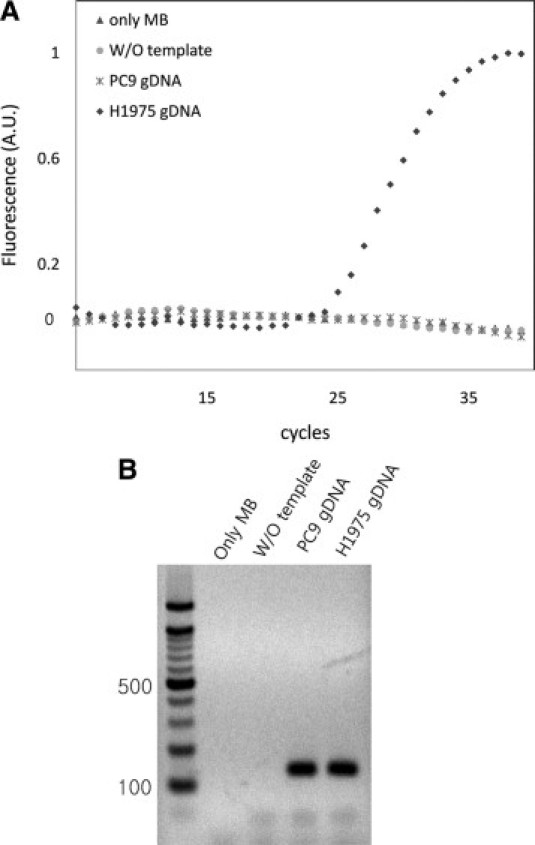
Molecular beacon-based real-time PCR. A: Real-time PCR in the presence of different templates or absence of template as a control. B: Electrophoresis gel image after real-time PCR. Lane 1: PCR with only MB. Lane 2: PCR without template. Lane 3: PCR with genomic DNA from PC9 cells (PC9 gDNA). Lane 4: PCR with H1975 genomic DNA (H1975 gDNA).
Detection Sensitivity of the Molecular Beacon-Based Real-Time PCR Assay
We next set out to test whether the MB based real-time PCR detection of the EGFR point mutation offered higher sensitivity than conventional direct sequencing. Ten-fold serial dilutions of H1975 genomic DNA were used to determine the detection limit of the MB based detection assay (Figure 4A). A fluorescence signal that was 10-fold higher than the SD of the mean baseline emission was used as the quantitative criterion for determining whether the amplicon contained a perfect complementary match. In Figure 4B, the threshold cycle number (Ct), which represents the number of PCR cycles required to cross the threshold value for positive detection, displayed a linear relationship with the concentrations of the genomic DNA template over a wide range of initial target concentrations.
Figure 4.
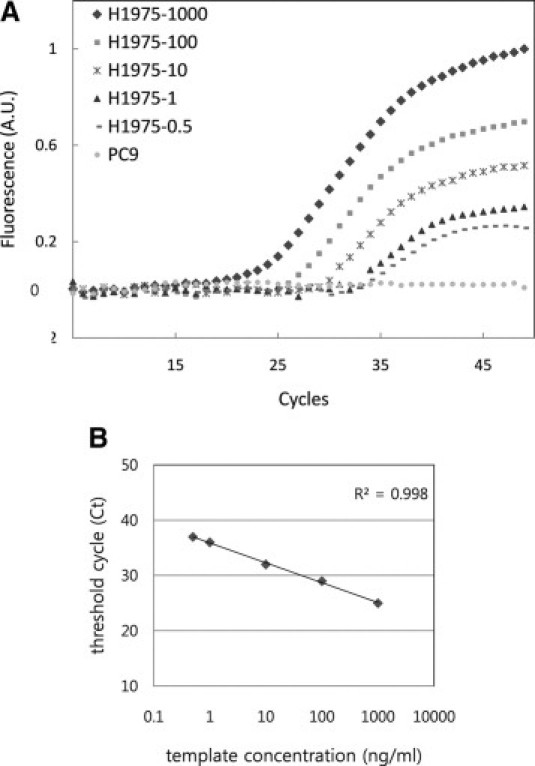
A: Detection sensitivity to template concentration. B: standard curve of the real-time PCR assay. Serial diluted H1975 genomic DNA was used as template for the real-time PCR assay; (circle) the PC9 cell line was used as a negative control; (diamond) 1000 ng of H1975 genomic DNA; (square) 100 ng of H1975 genomic DNA; (asterisk) 10 ng of H1975 genomic DNA; (triangle) 1 ng of H1975 genomic DNA; (dashed line) 0.5 ng of H1975 genomic DNA.
The sensitivity of heterogeneous detection by the molecular beacon-based real-time PCR assay was investigated to determine whether the MB-based real-time PCR method was more sensitive in detecting the T790M mutation in the presence of normal genomic DNA, namely a mixed DNA population. We used different mixtures of purified genomic DNA carrying the T790M mutation over a range of 100%, 50%, 10%, 2%, and 0% in each tube. As shown in Figure 5A, the presence of T790M was detected by real-time PCR with the T790M-MB in a thermal cycler. Using the molecular beacon-based real-time PCR, mutations could be detected in dilutions containing as little as 2% of genomic DNA from the H975 cells, whereas direct sequencing was not able to distinguish the peak signal from that of the background noise signal t ratios as low as 1:10 (10%; Figure 5B).
Figure 5.
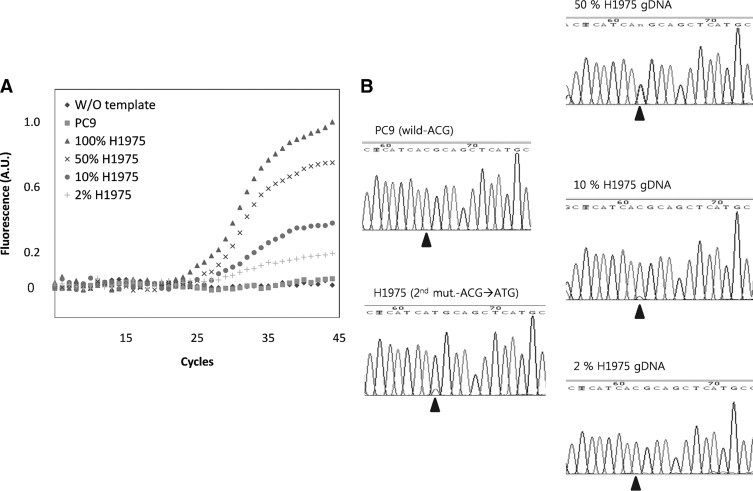
A: PCR with a different portion of genomic DNA from H1975 with the secondary T790M mutation. A total of 200 ng of a mixture of genomic DNAs from PC9 and H1975 were used as template. The portion of genomic DNA from H1975 was as follows: 100% (triangle); 50% (asterisk); 10% (circle); 2% (+); PCR without template (diamond); and with PC9 genomic DNA (square) as negative controls. B: Standard sequencing results of PCR products from A. Arrowheads indicate the mutation site.
MB Based Real-Time Analysis of the EGFR T790M of Samples from Patients with Cancer
Detection of the T790M mutation by using real-time PCR with the MB was also evaluated in samples from patients with lung cancer. The characteristics of the two tissue samples obtained from patients that initially had an activating mutation of EGFR (L858R) and eventually developed an acquired resistance to EGFR TKIs are shown in Table 2. The secondary T790M mutation in the two samples was confirmed by sequencing analysis. Genomic DNA was extracted from frozen lung cancer biopsy samples of patients, and used for real-time PCR with the T790M-MB. As shown in Figure 6, distinct fluorescence signals were observed from the patients with acquired resistance to gefitinib. These findings demonstrate that this method provides a simple and effective way to detect the secondary mutation in EGFR conferring acquired resistance to EGFR TKIs.
Figure 6.
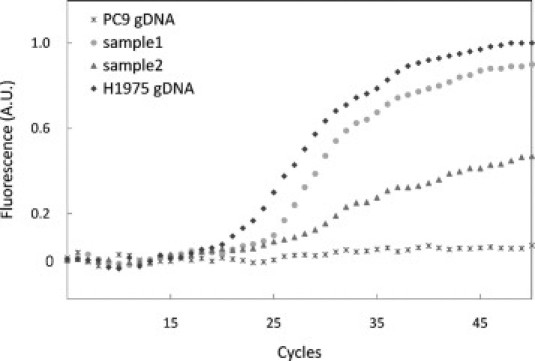
Molecular beacon-based real-time PCR for patient samples with acquired resistance: PC9 genomic DNA (asterisk), H1975 genomic DNA (diamond), sample 1 genomic DNA (square), and sample 2 genomic DNA (triangle) were used as a template.
Detection of the Point Mutation Using in Situ Fluorescence Imaging with the MB
We further examined fluorescence imaging of the cells with the T790M mutation by binding to specific mRNA targets on fixed cells. Acetone fixed cells were incubated with a mixture of 50 nmol/L of either the T790M specific molecular beacon or scrambled molecular beacon at 50°C for 40 minutes. Figure 7, A and D, clearly shows that a strong fluorescent signal was produced in the H1975 cells that contained an ACG to ATG mutation compared with the PC9 cells with no secondary mutation in the EGFR mRNA. We also tested both cell lines with a scrambled molecular beacon labeled with a Cy5 fluorophore as a control. In Figure 7, B and E, there were no fluorescent signals in either cancer cell line. As shown in Figure 7, C and F, the molecular beacon probe was bound to its specific target mRNA in the cytoplasm, resulting in distinct fluorescence signals. These results demonstrate that molecular beacons bind only to the specific mRNA of their targets.
Figure 7.
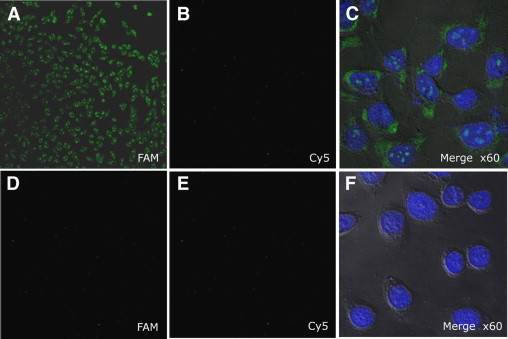
Fluorescence microscope imaging of cells using T790M specific molecular beacons: Target T790M-MB was labeled with FAM fluorophore and control-MB (scrambled molecular beacon) was conjugated with Cy5 fluorophore. Two types of molecular beacons were incubated with H1975 cancer cells that had the secondary mutation in EGFR mRNA (top row) and with the PC9 cancer cells without the secondary mutation (bottom row). FAM: fluorescent images filtered by 550 to 560 nm wavelength band pass (A and D), and Cy 5 fluorescence images were obtained filtered by 650 nm wave length long pass (B and E). DAPI (4’,6-diamidino-2-phenylindole) fluorescent image after DAPI staining; all images were magnified ×10, except Merge ×60, which were magnified ×60 (C and F).
Discussion
We used two approaches to detect the T790M secondary point mutation in the EGFR tyrosine kinase domain: a real-time PCR based assay and in situ fluorescence imaging. The first used the molecular beacon for real-time measurement with tissue genomic DNA as a template. During the PCR cycles, molecular beacons undergo a number of changes in their conformation. In the presence of a complementary target, the molecular beacon is able to bind at the annealing temperature and thus generate fluorescence. At the primer extension temperature or during polymerization, the molecular beacons are no longer able to bind to their complementary amplicon and they dissociate. The second approach is in situ fluorescence imaging of target mRNA in a fixed cell or tissue. The brief fixation and incubation of the designed molecular beacons produce a strong fluorescent signal in the presence of the target mRNA in the fixed cells. Unlike a linear probe, molecular beacon probes do not require extensive washing steps due to their structural properties.
To test the molecular beacon for the detection of the secondary mutation (T790M) of EGFR in the real-time PCR setting, the PCR and detection conditions were first optimized, with respect to varying parameters such as different concentrations of molecular beacon probes, different annealing temperatures and amplicon size. The experimental results indicated that a longer amplicon causes signals to diminish, possibly due to a greater hindrance to the molecular beacons positioning in-between the double strands during the annealing step.
Since cancer cells are mixed with surrounding normal stromal cells in the biopsy samples from patients, it is clinically important to be equipped with detection tools that successfully identify target molecules in the presence of mixed unwanted specimen. Direct sequencing of PCR amplified fragments is the standard method of detecting the T790M mutation. However, there exists a practical limitation in obtaining an adequate amount of tumor sample from patients and the need for microdissection to remove excess amounts of mixed stromal cell populations, which often hinders the genotyping analyses. With a direct sequencing method, this can lead to false negative results with regard to the genotype status of the patients. Thus, it is essential to accurately extract relevant clinical information from the limited amount of tumor samples with improved sensitivity. With the development of a more sensitive MB-based T790M mutation detection system, we were able to detect T790M at as low as 2% from mixed samples. Other methods including the mutant-enrichment PCR assay, high-resolution melting analysis, smart amplification process, Surveyor, and scorpion amplification refractory mutation system have been shown to be more sensitive than direct sequencing. The detection limits of these methods have been reported to range from 0.1% to 10% of mutant cells in mixed samples.6,7,15,16,17 Given that the molecular beacon-based real-time PCR can detect a mutation in dilutions containing as little as 2% of mutant genomic DNA, as demonstrated in this study, the molecular beacon-based real-time PCR presented here appears to offer comparable sensitivity to other methods and a protocol that is practical for clinical use.
The development of other noninvasive methods is clinically important to follow the evolution of cancer genotypes. It is notable that we were able to detect the T790M mutation from a 0.5-ng sample of genomic DNA template. This is roughly equivalent to the amount of genomic DNA extracted from 150 human cells. Recent development of circulating tumor cell (CTC)-chip technology has led to the specific isolation of CTCs from NSCLC samples, with the mean value of isolated CTCs around 155 cells/ml of blood.18 Thus, routine blood sampling and processing with the CTC-chip might yield enough tumor cells amenable to quantitative detection of T790M by MB-based genotype screening. The serum genomic DNA fragment is another noninvasive source of tumor derived DNA. It is of note that serum DNA of patients is collected at a concentration of 1 ng/μl to 10 ng/μl, which is within the dynamic range of MB based T790M detection.
The fluorescence imaging technique using the molecular beacon also provides a novel method for identifying cancer cells and evaluating the effects of therapeutic reagents in clinical samples.12,19,20,21 Recently, Peng et al22 detected mRNA expression levels of the survivin gene in frozen tissues by using a molecular beacon. Even though molecular beacons have been used for the detection of mutations, there is no prior report on its application to the analysis of secondary point mutations in the tyrosine kinase domain for the monitoring of drug resistance in lung cancer. Here, we developed a simple and rapid approach for the detection of a point mutation, which is a marker for drug resistance, using a T790M specific molecular beacon. Since cells with the T790M mutation would emit fluorescence signals on encountering T790M MB probes, it is possible to detect the presence of T790M mutation at the single cell resolution directly on fixed cells without DNA preparation. By contrast, any of the pre-existing EGFR mutation detection methods, such as WAVE/Surveyor or scorpion amplification refractory mutation system, do not provide the technical capability for identifying a specific mutation at the intact single cell level. This raises the promising possibility of MB imaging in samples with a very limited number of tumor cells, such as CTCs isolated from patient blood samples.
Lung cancer is the most common cause of cancer death, but only a few clinical studies have been done with small numbers of patients so as to address the secondary mutation related to drug resistance. It was reported that only a couple of patient samples have been tested with acquired resistance.23 As far as we know, it is very far and between to obtain patient sample with acquired resistance because second biopsy of patients with lung cancer is not routinely performed. It is expected that accumulated clinical data with developed method here will help to be considered as an effective direction for cancer treatment.
In summary, our results show that molecular beacon technology is a very powerful approach for the detection of mutations in a very small number of tumor cells or intact single cells by real-time-PCR and in situ fluorescence imaging. The high sensitivity and simplicity of the MB-based method allows for the follow-up of the genotype of patients, possibly circumventing many of the practical limitations of obtaining sufficient tumor tissue to perform genotype analysis. The principle of MB-based mutation detection can be easily generalized to the detection of other mutations. The appropriate choice of detection probes could lead to a multiplex mutation detection system, allowing for the simultaneous detection of the EGFR activating mutation and the EGFR secondary mutation and/or other de novo resistance markers such as k-ras mutations.
Acknowledgements
We thank Drs. Jinseok Ahn and Jeeyun Lee for their advice and comments.
Footnotes
Supported by the Korea Health 21C R&D Project (0405-MN01-0604-0007) of Ministry for Health, Welfare and Family Affairs and the Nano Science and Technology Program (M10503000868-08M0300-86810), the Nano/Bio Science and Technology Program (20090065621), Pioneer Research Program for Converging Technology (M10711300001-08M1130-00110), the Brain Korea 21 Program, and the World Class University program (R31-2008-000-10071-0) of Ministry of Education, Science and Technology.
Y.-H.O. and Y.K. contributed equally to this work.
Contributor Information
Keunchil Park, Email: kpark@skku.edu.
Hak-Sung Kim, Email: hskim76@kaist.ac.kr.
References
- 1.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wing PA, Howe HL, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Ogino A, Kitao H, Hirano S, Uchida A, Ishiai M, Kozuki T, Takigawa N, Takata M, Kiura K, Tanimoto M. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non small cell lung cancer cell line. Cancer Res. 2007;67:7807–7814. doi: 10.1158/0008-5472.CAN-07-0681. [DOI] [PubMed] [Google Scholar]
- 6.Kimura H, Fujiwara Y, Sone T, Kunitoh H, Tamura T, Kasahara K, Nishio K. High sensitivity detection of epidermal growth factor receptor mutations in the pleural effusion of non-small cell lung cancer patients. Cancer Sci. 2006;97:642–648. doi: 10.1111/j.1349-7006.2006.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janne PA, Borras AM, Kuang Y, Rogers AM, Joshi VA, Liyanage H, Lindeman N, Lee JC, Halmos B, Maher EA, Distel RJ, Meyerson M, Johnson E. A rapid and sensitive enzymatic method for epidermal growth factor receptor mutation screening. Clin Cancer Res. 2006;12:751–758. doi: 10.1158/1078-0432.CCR-05-2047. [DOI] [PubMed] [Google Scholar]
- 8.Yatabe Y, Hida T, Horio Y, Kosaka T, Takahashi T, Mitsudomi T. A rapid, sensitive assay to detect EGFR mutation in small biopsy specimens from lung cancer. J Mol Diagn. 2006;8:335–341. doi: 10.2353/jmoldx.2006.050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyagi S, Bratu DP, Kramer FR. Multicolor molecular beacons for allele discrimination. Nature Biotechnol. 1998;16:49–53. doi: 10.1038/nbt0198-49. [DOI] [PubMed] [Google Scholar]
- 10.Tan L, Li Y, Drake TJ, Moroz L, Wang K, Li J, Munteanu A, Chaoyong JY, Martinez K, Tan W. Molecular beacons for bioanalytical applications. Analyst. 2005;130:1002–1005. doi: 10.1039/b500308n. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet G, Tyagi S, Libchaber A, Kramer FR. Thermodynamic basis of the enhanced specificity of structured DNA probes. Proc Natl Acad Sci USA. 1999;96:6171–6176. doi: 10.1073/pnas.96.11.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marras SA, Tyagi S, Kramer FR. Real-time assays with molecular beacons and other fluorescent nucleic acid hybridization probes. Clin Chim Acta. 2006;363:48–60. doi: 10.1016/j.cccn.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 13.Takacs T, Jeney C, Kovacs L, Mozes J, Benczik M, Sebe A. Molecular beacon-based real-time PCR method for detection of 15 high-risk and 5 low-risk HPV types. J Virol Methods. 2008;149:153–162. doi: 10.1016/j.jviromet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Wells D. Use of real-time polymerase chain reaction to measure gene expression in single cells. Methods Mol Med. 2007;132:125–133. doi: 10.1007/978-1-59745-298-4_11. [DOI] [PubMed] [Google Scholar]
- 15.Asano H, Toyooka S, Tokumo M, Ichimura K, Aoe K, Ito S, Tsukuda K, Ouchida M, Aoe M, Katayama H, Hiraki A, Sugi K, Kiura K, Date H, Shimizu N. Detection of EGFR gene mutation in lung cancer by mutant-enriched polymerase chain reaction assay. Clin Cancer Res. 2006;12:43–48. doi: 10.1158/1078-0432.CCR-05-0934. [DOI] [PubMed] [Google Scholar]
- 16.Hoshi K, Takakura H, Mitani Y, Tatsumi K, Momiyama N, Ichikawa Y, Togo S, Miyagi T, Kawai Y, Kogo Y, Kikuchi T, Kato C, Arakawa T, Uno S, Cizdziel PE, Lezhava A, Ogawa N, Hayashizaki Y, Shimada H. Rapid detection of epidermal growth factor receptor mutations in lung cancer by the SMart-Amplification Process. Clin Cancer Res. 2007;13:4974–4983. doi: 10.1158/1078-0432.CCR-07-0509. [DOI] [PubMed] [Google Scholar]
- 17.Takano T, Ohe Y, Tsuta K, Fukui T, Sakamoto H, Yoshida T, Tateishi U, Nokihara H, Yamamoto N, Sekine I, Kunitoh H, Matsuno Y, Furuta K, Tamura T. Epidermal growth factor receptor mutation detection using high-resolution melting analysis predicts outcomes in patients with advanced non small cell lung cancer treated with gefitinib. Clin Cancer Res. 2007;13:5385–5390. doi: 10.1158/1078-0432.CCR-07-0627. [DOI] [PubMed] [Google Scholar]
- 18.Wong KK, Fracasso PM, Bukowski RM, Lynch TJ, Munster PN, Shapiro GI, Janne PA, Eder JP, Naughton MJ, Ellis MJ, Jones SF, Mekhail T, Zacharchuk C, Vermette J, Abbas R, Quinn S, Powell C, Burris HA. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–2558. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 19.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res. 2005;33:5394–5403. doi: 10.1093/nar/gki863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perlette J, Tan W. Real-time monitoring of intracellular mRNA hybridization inside single living cells. Anal Chem. 2001;73:5544–5550. doi: 10.1021/ac010633b. [DOI] [PubMed] [Google Scholar]
- 22.Peng XH, Cao ZH, Xia JT, Carlson GW, Lewis MM, Wood WC, Yang L. Real-time detection of gene expression in cancer cells using molecular beacon imaging: new strategies for cancer research. Cancer Res. 2005;65:1909–1917. doi: 10.1158/0008-5472.CAN-04-3196. [DOI] [PubMed] [Google Scholar]
- 23.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to Gefitinib or Erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]


