Abstract
AIM: To compare the impact of ErbB2 on cell invasion and proliferation in cholangiocarcinoma (CCA) cell lines.
METHODS: Level of endogenous ErbB2 expression in three CCA cell lines, namely HuCCA-1, KKU-100 and KKU-M213, was determined by real-time reverse-transcriptase polymerase chain reaction. Two ErbB2 inhibitory methods, a small molecule ErbB2 kinase inhibitor (AG825) and siRNA, were used to disrupt ErbB2 function in the cell lines. CCA cell invasion, motility and proliferation under ErbB2-disrupted conditions were detected using Transwell and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays. In addition, ErbB2 downstream effectors were investigated by Western blotting analysis.
RESULTS: Suppression of ErbB2 activity, using a specific kinase inhibitor (AG825), reduced invasion, motility and proliferation of all three CCA cell lines. The ability of this drug to inhibit neoplastic properties (invasion, motility and proliferation) increased concomitantly with the level of ErbB2 expression. Similarly, knockdown of ErbB2 level by siRNA inhibited cell invasion and proliferation of KKU-M213, a high-ErbB2-expressing cell, better than those of the lower-ErbB2-expressing cells, HuCCA-1 and KKU-100. Thus, both inhibitory methods indicated that there is more ErbB2-dependency for malignancy of the high-ErbB2-expressing cell, KKU-M213, than for that of low-ErbB2-expressing ones. In addition, interrupting ErbB2 activity decreased phosphorylation of AKT and p70S6K, but not extracellular signal-regulated kinase 1/2, in the high-ErbB2-expressing CCA cell line.
CONCLUSION: Our data indicated that high ErbB2 expression enhances CCA invasion, motility and proliferation via the AKT/p70S6K pathway, which suggests the possibility of targeting these molecules for CCA therapy.
Keywords: AKT, Cholangiocarcinoma, ErbB2, Invasion, p70S6K, Cell proliferation
INTRODUCTION
Cholangiocarcinoma (CCA) is an incurable and lethal cancer. Its incidence has been increasing worldwide during the past three decades and all cases have almost the same mortality rate[1]. Strikingly, the highest incidence of CCA has been found in Northeastern Thailand (96 per 100 000 men), followed by China and Japan[2]. As a result of the lack of a specific tumor marker and its silent symptoms, this tumor is difficult to diagnose and the majority of patients present at the late stage (stage III or IV) of disease progression[3]. From 70% to 80% of CCA patients are inoperable and are generally treated by chemotherapy and radiation, which unfortunately have no significant impact on long-term survival[3]. Thus, new diagnostic/prognostic markers and targeted therapies for CCA need to be investigated.
Human epidermal growth factor receptor (EGFR) 2, ErbB2 (also known as HER2/Neu), belongs to subclass I of receptor tyrosine kinases in the ErbB family. Unlike other members of the ErbB family, the conformation of the ErbB2 ectodomain is similar to the ligand-activated state of other ErbBs[4]. Therefore, activation of ErbB2 can occur without ligand binding[5]. Overexpression of ErbB2 results in receptor dimerization (either homodimerization with its own or heterodimerization with other ErbBs). Upon dimerization, cytoplasmic domains of ErbBs become autophosphorylated and act as docking sites for downstream proteins that bear an Src-homology 2 or a phosphotyrosine-binding domain, which recognizes specific phosphorylated tyrosine sites in the receptors, and leads to activation of such downstream pathways as mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/AKT[6]. Furthermore, ErbB2 can block internalization of EGFR for degradation, which results in an elevation in the membrane steady state level[7]. Thus, aberrant ErbB2 expression can lead to an extensive activation of its downstream signals and an aberration from the normal regulation, which promotes cancer development.
ErbB2 overexpression is found in approximately 30% of human breast carcinomas and in many other types of human malignancies, such as prostate, pancreas, colon and ovarian cancers[8]. Cancer patients with high ErbB2 expression tend to have a more aggressive disease, identified by clinical outcomes such as high metastasis and low response to treatment. Typically, ErbB2-positive tumors have high proliferation rates and more extensive invasion, with frequent metastasis[9]. In addition, clinical studies using ErbB2-targeted therapy with trastuzumab (a humanized monoclonal antibody against ErbB2) have shown that the response rates of breast cancer patients with ErbB2 overexpression are higher than those with normal ErbB2 expression[10].
Several lines of evidences have implicated the role of ErbB2 in aggressive forms of CCA. High expression of ErbB2 has been found in a variety of non-cancerous biliary proliferative diseases, such as in hepatolithiasis and primary sclerosing cholangitis, both of which are risk factors associated with CCA[11,12]. Immunohistochemical data have demonstrated that 20%-30% of tumor specimens from CCA patients show moderate to strong immunostaining for ErbB2[13], and most of them confer poor clinical outcomes (high metastasis and low survival rate)[13-16]. Moreover, about 30% of transgenic mice that constitutively express wild-type Neu develop gallbladder cancer and intrahepatic CCA within 8 mo[17]. Although many reports have indicated the involvement of ErbB2 in cholangiocarcinogenesis and CCA progression, the mechanism of aberrant ErbB2 expression in promoting CCA progression remains unclear.
In this study, the role of ErbB2 in governing the malignant phenotype (invasion and proliferation) of CCA was investigated by suppressing ErbB2 function in three human CCA cell lines that expressed different levels of ErbB2. Two strategies, inhibition of its kinase function and siRNA, were used to reduce ErbB2 activity. We demonstrated that downregulation of ErbB2 expression and activity suppressed CCA cell invasion, motility and proliferation, particularly in the high-ErbB2-expressing cells. Downstream signaling pathways of ErbB2 also were investigated.
MATERIALS AND METHODS
Cell culture
HuCCA-1 cell line was a generous gift from Professor Stitaya Sirisinha (Mahidol University, Thailand)[18] and KKU-100[19] and KKU-M213 cell lines were kindly provided by Dr. Banchob Sripa, (Khon Kaen University, Thailand). These three CCA cell lines were developed from Thai patients. The cells were maintained in HAM’s F-12 medium (Invitrogen, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen), 2 mmol/L glutamine, 15 mmol/L HEPES and 14 mmol/L sodium bicarbonate, 100 U/mL penicillin G and 100 U/mL streptomycin. All cell cultures were incubated at 37°C, in a 5% CO2 humidified atmosphere.
Quantitative polymerase chain reaction
Cells (80% confluent) were harvested with 0.1% trypsin/EDTA and RNA was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA, USA). Two micrograms of RNA were converted into cDNA using the Superscript™ RNase H Reverse Transcriptase kit (Invitrogen), which was amplified by quantitative polymerase chain reaction (PCR) (ABI 7500; Applied Biosystems, Foster City, CA, USA) in a 20-μL reaction volume that contained 0.5 U HotStart Taq polymerase (Qiagen), 1 × FastStart Universal SYBR Green Master cocktail (Roche, Germany) and 4 pmol of specific primer pairs (5'-CCAGGACCTGCTGAACTGGT-3' and 5'-TGTACGAGCCGCACATC-3' for ErbB2[20] and 5'-CTCTTCCAGCCTTCCTTCCT-3' and 5'-AGCACTGTGTTGGCGTACAG-3' for β-actin[21], used as internal control). The reactions were started with an initial heat activation step at 95°C for 15 min and the following thermal cycling conditions: 94°C for 30 s, 58°C for 30 s and 72°C for 1 min. ErbB2 mRNA levels among the test cells were determined using the 2-ΔCt method[22].
Immunoblot assay
Cells transfected with siRNA (for 72 h) or treated with AG825 (for 6 h) were washed twice with PBS and lysed on ice with freshly prepared lysis buffer that contained 150 mmol/L Tris-HCl pH 7.4, 150 mmol/L NaCl, 5 mmol/L EGTA, 5 mmol/L EDTA, 0.1% SDS, 1% sodium deoxycholate, 1% Nonidet P-40, 1 × protease inhibitor cocktail (Roche Diagnostics, Germany), 50 mmol/L NaF, 2 mmol/L Na3VO4, 40 mmol/L β-glycerophosphate, and 1 mmol/L dithiothreitol. Cells were centrifuged at 12 000 × g for 15 min. Protein lysate (80 μg) was separated by 8% SDS-PAGE and transferred to a nitrocellulose membrane (GE Healthcare, Munchen, Germany). After incubating with a blocking solution (5% skimmed milk/TBST), membranes were treated with primary antibodies specific for ErbB2, phospho-ErbB2 Y1248 (Labvision, Fremont, CA, USA), β-actin, AKT, phospho-AKT T308 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), ERK1/2, phospho-ERK1/2, p70S6K, and phospho-p70S6K T389 (Cell Signaling, Beverly, MA, USA), and then with horseradish-preoxidase-conjugated secondary antibodies (Santa Cruz Biotechnology). Signals were detected using enhanced chemiluminescence (ECL plus) (GE Healthcare, Little Chalfont, Bucks, UK) and quantified by Alpha Imager (Alpha Innotech, San Leandro, CA, USA).
siRNA transfection
Two Silencer® validated siRNAs against ErbB2 (Ambion, Austin, TX, USA) were used to target mRNA at different exons. CCA cells were transiently transfected with siRNA using Effectene (Invitrogen) following the manufacturer’s protocol. In brief, 3.25 μg of siErbB2 was mixed with Effectene and Enchancer (32.5 and 26.0 μL), incubated for 5 min, and then added to HAM’s F-12 medium that contained 10% FBS. The mixture was added to 80% confluent CCA cells in 60-mm dishes that contained 10% FBS medium. After 6 h of incubation, medium was removed, cells were washed with PBS and replenished with fresh medium. Cells transfected with Silencer® Cy™-3 labeled non-targeting siRNA (Ambion) were used as a negative control. Protein expression, cell invasion and motility were determined at 72 h post-transfection and cell proliferation was analyzed during 24-96 h post-transfection.
In vitro invasion and motility assay
Cell invasiveness was determined using a Transwell chamber (6.5-mm diameter polyvinylpyrrolidone-free polycarbonate filter of 8-μm pore size) (Corning, NY, USA) pre-coated with 30 μg Matrigel (BD Biosciences, San Jose, CA, USA). A 200-μL aliquot of cells (105) transfected with siRNA or treated with various concentrations of AG825 in 0.2% FBS medium was added to the upper compartment of the Transwell, and 10% FBS medium was added to the lower chamber. After 6 h of incubation at 37°C in a humidified CO2 incubator, non-invaded cells in the upper compartment were removed with a cotton swab, and the invaded cells were fixed and stained with 0.5% crystal violet in 25% methanol for 30 min, followed by washing twice with tap water. Finally, the invaded cells were counted under a microscope with a 10 × objective in five random fields. Cell motility assay was performed as described for the invasion assay but using a Matrigel-free system.
Cell viability assay
Cells (3000/well) transfected with siRNA or resuspended in various concentrations of AG825 were plated onto 96-well plates and incubated for 72 h. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as follows. Fifty micrograms of MTT were added to each well and the cells were incubated for a further 4 h. The supernatant was removed and insoluble formazan dye produced from MTT by living cells was solubilized by 200 μL DMSO. Percentage cell viability was quantified by measuring absorbance at 540 nm. Cell survival during the invasion assay was determined in the same way, except that 5000 cells/well were incubated for 6 h prior to addition of MTT.
Statistical analysis
Data are presented as the mean ± SE from three independent experiments conducted in triplicate. Comparison of data between groups was analyzed by one-way analysis of variance, followed by Newman-Keuls multiple comparison test (GraphPad Software, La Jolla, CA, USA).
RESULTS
ErbB2 mRNA expression level in human CCA cell lines and inhibition of its phosphorylation by AG825
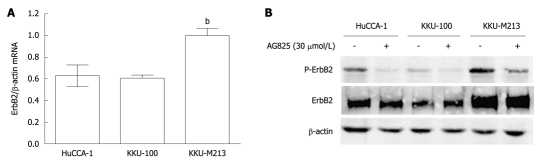
The role of ErbB2 in CCA was investigated in three cell lines that were established from tumor tissues of Thai CCA patients, namely, HuCCA-1, KKU-100 and KKU-M213. Steady-state level of ErbB2 mRNA was determined by quantitative reverse-transcriptase-PCR, normalized to β-actin mRNA. KKU-M213 cell line expressed the highest level of ErbB2 mRNA, with that of HuCCA-1 and KKU-100 being comparable at 60% (Figure 1A).
Figure 1.
Levels of ErbB2 expression in three cholangiocarcinoma cell lines and inhibition of ErbB2 phosphorylation by AG825. A: Cells cultured in 10% fetal bovine serum (FBS)-containing medium were lysed and the lysates were analyzed for ErbB2 mRNA by real-time reverse-transcriptase polymerase chain reaction. Data are presented as mean ± SE of ErbB2 mRNA level normalized with β-actin mRNA obtained from three independent experiments; B: Cells were incubated without and with 30 μmol/L AG825 in 10% FBS medium for 6 h, then lysed and analyzed for ErbB2, phospho-ErbB2 (Y1248) and β-actin by Western blotting. The results shown are representative of three separate experiments. bP < 0.01 vs HuCCA-1 and KKU-100.
Like other tyrosine kinase receptors, ErbB2 is activated by phosphorylation. Total and phosphorylated ErbB2 levels in the three CCA cell lines as analyzed by Western blotting showed correspondence with levels of ErbB2 mRNA, but in this case, the level of phosphorylated ErbB2 in KKU-100 cells was significantly lower than that in HuCCA-1 (Figure 1B). Inhibition of ErbB2 activity by treating for 6 h with its specific kinase inhibitor, AG825 (30 μmol/L), resulted in marked reduction ErbB2 phosphorylation at Y1248 in all three cell lines, without any significant effect on total ErbB2 levels (Figure 1B).
Invasive and proliferative abilities of CCA cell lines
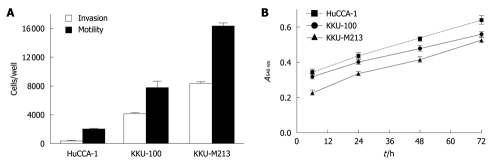
The abilities of the three CCA cell lines to migrate and invade were determined by Transwell in vitro assays. Among the three cell lines, KKU-M213 showed the highest invasive and motility abilities, which were related to its level of ErbB2 expression (Figure 2A). However, KKU-100, which contained the lowest phospho-ErbB2 level, still had a somewhat high invasive ability (about four fold higher than HuCCA-1, but still lower than KKU-M213). This suggested that other pathways might be involved in enhancing the invasive ability of KKU-100. However, the proliferative rates of the three CCA cell lines as assessed by MTT assay were comparable (Figure 2B), which suggested that ErbB2 is not the key player in regulating proliferation of these cell lines.
Figure 2.
Invasion motility and proliferation of three cholangiocarcinoma cell lines. A: Cells resuspended in 0.2% fetal bovine serum (FBS) medium were plated in the upper compartment of a Transwell chamber coated with and without Matrigel, for invasion and motility assays. The lower compartment was filled with 10% FBS medium. After 6 h incubation, numbers of invading/motile cells were counted; B: Cells were plated on 96-well plates in 10% FBS medium. After incubation for 12-72 h, cell survival was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data are presented as mean ± SE of the percentage relative to controls obtained from three separate experiments.
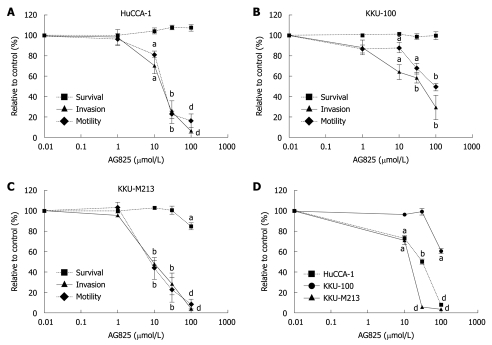
Effects of ErbB2 kinase inhibitor on CCA cell invasion and proliferation
As ErbB2 has been detected in CCA and other related cancers, and its expression level has been shown to correlate with highly proliferating diseases of the bile duct and with the degree of CCA local invasion and metastasis[11,23], the role of ErbB2 on invasion and motility of the CCA cell lines was investigated by examining the effects of AG825 on these properties using a Transwell assay. Treatment of AG825 (10-100 μmol/L) for 6 h suppressed cell invasion and motility (Figure 3A-C) in all three CCA cell lines with different IC50 values, with KKU-M213 being the most sensitive and KKU-100 the least. During the assay period, the drug marginally affected CCA cell survival (Figure 3A-C), which indicated that inhibition of invasion and motility were not due to drug cytotoxicity. However, incubation with AG825 for 72 h resulted in inhibition of cell proliferation, with KKU-M213 showing the most sensitivity and KKU-100 the least (Figure 3D).
Figure 3.
Effects of AG825 on cholangiocarcinoma cell invasion, motility and proliferation. A-C: Cells resuspended in medium that contained 0.2% fetal bovine serum (FBS) and indicated concentrations of AG825 were plated in the upper compartment of a Transwell chamber coated with and without Matrigel, for invasion and motility assays. After 6 h incubation, numbers of invading/motile cells were counted. Cells treated with the same vehicle were used as controls. Cell survival after 6 h of AG825 treatment was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay; D: Cells were treated with various concentrations of AG825 in 10% FBS medium for 72 h, and cell survival was determined using the MTT assay. Data are presented as mean ± SE of the percentage relative to the controls obtained from three separate experiments, each done in duplicate. aP < 0.05, bP < 0.01, dP < 0.001 vs control.
Effects of suppression of ErbB2 expression on CCA cell invasion, motility and proliferation
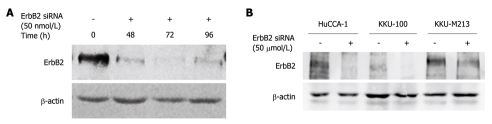
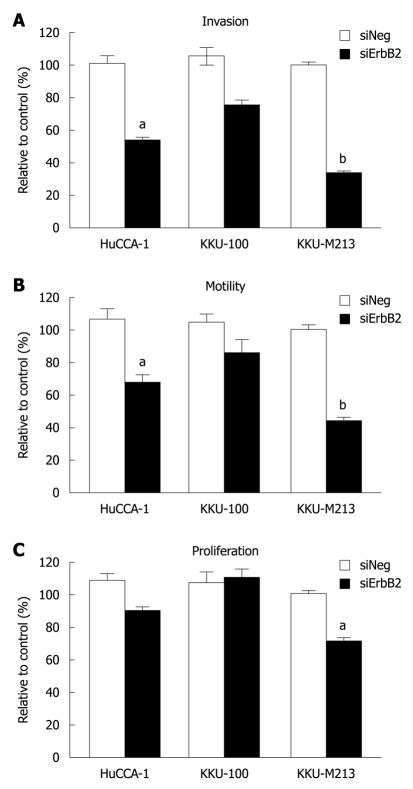
As ErbB2 kinase inhibitor experiments indicated the role of ErbB2 on malignant phenotypes (invasion, motility and proliferation) of CCA cell lines, we confirmed the specificity of this phenomenon using siRNA targeting of ErbB2 mRNA to suppress ErbB2 gene expression. In KKU-M213, the CCA cell line that expressed the highest ErbB2 level among the three cell lines, ErbB2 level was reduced in a time-dependent manner (up to 72 h), and was partially restored at 96 h post-transfection (Figure 4A). After 72 h of siRNA transfection, ErbB2 level in all three CCA cell lines was reduced by > 70% (Figure 4B). During this post-transfection period, there was attenuation of invasion and motility of KKU-M213 and HuCCA-1 cells, but not of KKU-100 cells (Figure 5A and B). Although proliferation of KKU-M213 cells was suppressed, that of HuCCA-1 and KKU-100 cells was marginally affected (Figure 5C).
Figure 4.
Inhibition of ErbB2 expression by siRNA against ErbB2. A: KKU-M213 cells were transfected with siErbB2 and the levels of ErbB2 at 0-96 h after transfection were determined by Western blotting; B: After 72 h of siRNA transfection, ErbB2 expression in HuCCA-1, KKU-100, and KKU-M213 cells was determined. Scramble siRNA (siNeg) was used as a negative control. The results shown are representatives of two (A) and three (B) separate experiments.
Figure 5.
Effects of ErbB2 knockdown on cell invasion motility and proliferation of the three cholangiocarcinoma cell lines. Cells transfected with siRNA (negative control and ErbB2 targeted siRNA) for 72 h were analyzed for in vitro invasion (A) and motility (B) by using a Transwell chamber coated with and without Matrigel. After 6 h of incubation, numbers of invading/motile cells were counted. Cell proliferation (C) was analyzed by determining the number of viable cells during 24-96 h post-transfection by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data are expressed as mean ± SE of the percentage relative to the controls obtained from three independent experiments. aP < 0.05, bP < 0.01 vs controls.
These results on suppression of ErbB2 gene expression, together with those conducted on inhibition of ErbB2 kinase activity, indicated that a threshold level of ErbB2 is necessary to induce properties associated with malignancy (invasion, motility and proliferation) in CCA cells (viz. KKU-M213).
Involvement of ErbB2 in activation of downstream effectors, AKT, p70S6K and ERK1/2
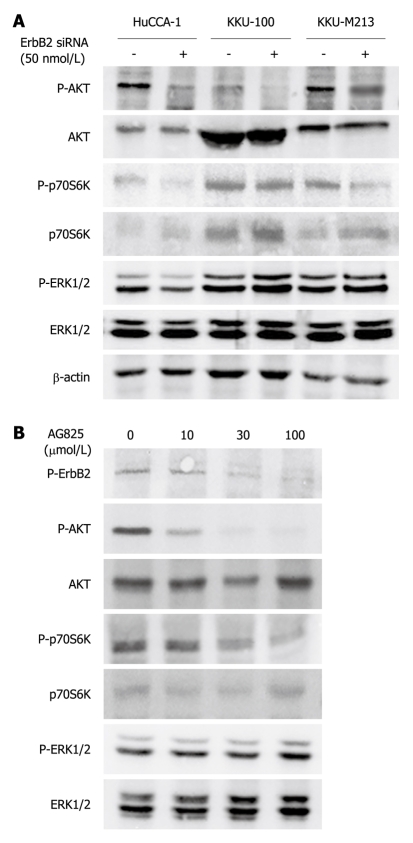
ERK1/2 and PI3K/AKT are the two major signaling pathways that regulate cell proliferation, motility and invasion in response to a variety of growth factors/receptor tyrosine kinases[24]. To investigate the pathways by which ErbB2 promoted invasion and proliferation of CCA cells, we examined the effects of ErbB2 inhibition (by kinase inhibitor or siRNA) on effectors activated by ErbB2 in these two main signaling pathways. Suppression of ErbB2 gene expression by siRNA transfection (72 h) reduced phospho-AKT level in all three CCA cell lines, but without affecting total AKT level (Figure 6A). Phosphorylation of its downstream effector, p70S6K, was similarly affected in KKU-M213 and HuCCA-1 cells, but not in KKU-100 cells (Figure 6A). On the other hand, ERK1/2 phosphorylation was marginally, if at all, affected in all three CCA cell lines (Figure 6A). Similarly, treatment of KKU-M213 cells with AG825 (30 μmol/L, 6 h) suppressed AKT and p70S6K phosphorylation but did not affect phospho-ERK1/2 level (Figure 6B). Thus, the PI3K/AKT/p70S6K and not ERK1/2 pathway is responsive to ErbB2 activity related to CCA cell invasion, motility and proliferation.
Figure 6.
Effects of ErbB2 inhibition (using siRNA and specific kinase inhibitor) on ERK1/2, AKT, and p70S6K phosphorylation in the cholangiocarcinoma cell lines. A: Cholangiocarcinoma cell lines were transfected with siErbB2 and siNeg, as a control, for 72 h and the collected cell lysates were analyzed by Western blotting; B: KKU-M213 cells were treated without and with AG825 in medium that contained 10% fetal bovine serum for 6 h, then lysed and analyzed by Western blotting. The results shown are representatives of three separate experiments.
DISCUSSION
ErbB2 is overexpressed in 20%-30% of epithelial bile duct cancers[13]. Its overexpression is associated with lymph node metastasis in intrahepatic CCA[23]. Unlike other ErbBs, ErbB2 is not expressed in normal adult liver (both hepatocytes and cholangiocytes) or in fetal liver[11,25] which makes it an interesting candidate for molecular targeting therapy for liver and bile duct cancers.
The mechanism by which ErbB2 exerts tumorigenicity in CCA is not completely understood. Here, the role of ErbB2 involved in controlling critical characteristics of CCA, namely, invasion, motility and proliferation, was investigated in three human CCA cell lines that expressed different levels of ErbB2, as shown by measurements of mRNA and phosphorylated (P-Y1248) ErbB2 levels. Data showed that ErbB2 was more important for these properties in the highest ErbB2-expressing cell line (KKU-M213), through activation of the AKT/p70S6K pathway.
Upregulation of ErbB2 is implicated in cancer aggressiveness. Studies in transgenic mice have revealed that animals bearing either an activated form of Neu (ErbB2) or overexpressing wild-type Neu frequently develop mammary tumors and lung metastases[26,27]. ErbB2 overexpression plays an important role in cell proliferation and invasion of many cancers, especially those of breast, ovary, stomach and bladder[28,29]. In breast cancer, application of antisense RNA or siRNA against ErbB2 inhibits growth of high-ErbB2-expressing cells, but has only a little effect on lower-ErbB2-expressing cells[30,31]. In the present study, disruption of ErbB2, by both inhibition of ErbB2 kinase activity using AG825 and knock-down of ErbB2 expression using siRNA, suppressed the neoplastic phenotype of the high-ErbB2-expressing CCA cell line, KKU-M213, to a greater extent than that of the low-ErbB2-expressing cell line, KKU-100. This indicated that the neoplastic phenotype of the high-ErbB2-expressing cell line, KKU-M213, was highly dependent on ErbB2 compared to the low-ErbB2-expressing cells.
There are several possible explanations for the increase in the requirement of ErbB2 in cancer malignancy. Firstly, in cancer cells that have evolved high ErbB2 expression, this pathway has become predominant in regulating cell proliferation and invasion, which results in cells with dependency on this protein. Moreover, Kaelin[32] has suggested that an increase in the requirement of a given protein for cancer cell survival is due either to intrinsic (genetic/epigenetic) or extrinsic (microenvironment) changes. In this case, accumulation of genetic/epigenetic alteration during cancer development might cause a loss of proteins with functional redundancy to ErbB2, or a gain of those with opposing functions. However, cancer cells with ErbB2 overexpression are able to overcome the selective pressures and therefore survive and propagate, thus yielding cells with high dependency on ErbB2. Therefore, the malignant behavior of such ErbB2-dependent cells responds to inhibition of ErbB2 function, whereas those with low or no ErbB2 expression depend on other pathways for their survival, and hence are refractory to such treatment. This has provided the rationale for application of ErbB2-targeted therapy in ErbB2-overexpressing cancer. In fact, trastuzumab, a monoclonal antibody that targets ErbB2, has been used successfully for the treatment of ErbB2-positive metastatic breast cancer as well as for adjuvant therapy of early breast cancer[33]. Although ErbB2-targeted therapy has not been studied in CCA, our data on the inactivation of ErbB2 suggest that this is a crucial oncogene for maintaining the malignant phenotype of CCA, thus becoming a potential target for future therapy.
In the CCA cells, inhibition of ErbB2 by both kinase inhibitor and siRNA suppressed phosphorylation (activation) of AKT and its downstream effector, p70S6K, but not that of ERK1/2. This might be because the regulation of ERK1/2 by other signals overrides the importance of ErbB2 for its activation, or because signaling pathways downstream of ErbB2 are diverse. This diversity depends partly on dimerization partners of ErbB2. For instance, PI3K/AKT is the most important oncogenic signal downstream of ErbB2-ErbB3 heterodimerization[34]. In this case, overexpressed ErbB2 might dimerize with ErbB3, thereby activates PI3K/AKT as a major pathway. The role of AKT in the malignant characteristics of CCA has also been previously reported. Upregulation of AKT pathway has been reported to be related to low survival rate[35]. Moreover, LY294002, a specific inhibitor of PI3K/AKT pathway, attenuates CCA cell proliferation and promotes apoptosis in 10% serum-containing medium[36], and suppresses KKU-M213 and HuCCA-1 cell invasion induced by HGF[37]. In other systems such as ovarian cancer[38,39] and chick embryo fibroblasts[40], both AKT and p70S6K have been reported to regulate cell invasion, motility and proliferation. Therefore, this implies that ErbB2 regulates invasion and proliferation via the PI3K/AKT/p70S6K pathway.
In summary, the results presented here demonstrate that ErbB2 is particularly important for malignant properties (invasion, motility and proliferation) of human high-ErbB2-expressing CCA cells. ErbB2 acts through activation of the AKT/p70S6K pathway. These findings lend support for the therapeutic targeting of ErbB2 and/or its effector molecules in CCA with ErbB2 overexpression.
COMMENTS
Background
Cholangiocarcinoma (CCA), a malignancy of the biliary tract, is an aggressive and currently incurable cancer. The incidence is high in Thailand, China and Japan. Disease progression, especially metastasis, is correlated with ErbB2 overexpression.
Research frontiers
ErbB2 promotes many oncogenic properties, including cell growth, survival, adhesion, motility, invasion, and metastasis. Although many reports have shown the involvement of ErbB2 in CCA, the mechanism that underlies ErbB2-promoted CCA progression is not clearly understood.
Innovations and breakthroughs
Suppression of ErbB2 activity/expression reduced invasion, motility and proliferation in CCA cell lines. This is believed to be the first report to show that, in CCA cell lines with high ErbB2 expression, such neoplastic behavior is more sensitive to ErbB2 inhibition than that in cells with lower ErbB2 expression. Moreover, AKT and p70S6K, but not extracellular signal-regulated kinase 1/2, play important roles as ErbB2 downstream effectors in high-ErbB2-expressing CCA cells.
Applications
As ErbB2 is overexpressed in 20%-30% of CCA, our finding that the malignant phenotypes of CCA cells with high ErbB2 expression are highly dependent on ErbB2 supports the potential use of ErbB2-targeted therapy for the treatment of patients with ErbB2-overexpressing CCA.
Terminology
ErbB2/HER2, human epidermal growth factor receptor 2, is a member of the epidermal growth factor receptor family. Both AKT and p70S6K are downstream effectors of many receptor tyrosine kinases including ErbB2. Their activations induce proliferation and invasion of cancer cells, which leads to metastasis. This study shows that aberrant ErbB2 expression is involved in CCA cell proliferation and invasion via AKT/p70S6K.
Peer review
This study provides evidence that high ErbB2 expression in CCA cell lines might be an indicator that blockage of this molecule could be beneficial in cancer therapy. This was a well performed and clearly presented study.
Acknowledgments
The authors thank Professor Stitaya Sirisinha and Dr. Banchob Sripa for providing HuCCA-1 and KKU-100 and KKU-M213 cell lines respectively, and Prof. Prapon Wilairat for critical reading of the manuscript.
Footnotes
Supported by A grant from Mahidol University, Thailand (to Suthiphongchai T); and a scholarship from the Royal Golden Jubilee Ph.D. Program, the Thailand Research Fund (to Treekitkarnmongkol W)
Peer reviewer: Christa Buechler, PhD, Regensburg University Medical Center, Internal Medicine I, Franz Josef Strauss Allee 11, 93042 Regensburg, Germany
S- Editor Wang JL L- Editor Kerr C E- Editor Lin YP
References
- 1.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 2008;10:77–82. doi: 10.1080/13651820801992641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandhu DS, Roberts LR. Diagnosis and management of cholangiocarcinoma. Curr Gastroenterol Rep. 2008;10:43–52. doi: 10.1007/s11894-008-0008-9. [DOI] [PubMed] [Google Scholar]
- 4.Badache A, Hynes NE. A new therapeutic antibody masks ErbB2 to its partners. Cancer Cell. 2004;5:299–301. doi: 10.1016/s1535-6108(04)00088-1. [DOI] [PubMed] [Google Scholar]
- 5.Penuel E, Akita RW, Sliwkowski MX. Identification of a region within the ErbB2/HER2 intracellular domain that is necessary for ligand-independent association. J Biol Chem. 2002;277:28468–28473. doi: 10.1074/jbc.M202510200. [DOI] [PubMed] [Google Scholar]
- 6.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 8.Vernimmen D, Gueders M, Pisvin S, Delvenne P, Winkler R. Different mechanisms are implicated in ERBB2 gene overexpression in breast and in other cancers. Br J Cancer. 2003;89:899–906. doi: 10.1038/sj.bjc.6601200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, Panageas KS, Arroyo C, Valero V, Currie V, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587–2595. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- 11.Terada T, Ashida K, Endo K, Horie S, Maeta H, Matsunaga Y, Takashima K, Ohta T, Kitamura Y. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325–331. doi: 10.1046/j.1365-2559.1998.00496.x. [DOI] [PubMed] [Google Scholar]
- 12.Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439–450. doi: 10.1053/jhep.2002.34435. [DOI] [PubMed] [Google Scholar]
- 13.Sirica AE. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2008;14:7033–7058. doi: 10.3748/wjg.14.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng J, Zhu YM. Expression of c-erbB-2 proto-oncogene in extrahepatic cholangiocarcinoma and its clinical significance. Hepatobiliary Pancreat Dis Int. 2007;6:412–415. [PubMed] [Google Scholar]
- 15.Kim HJ, Yoo TW, Park DI, Park JH, Cho YK, Sohn CI, Jeon WK, Kim BI, Kim MK, Chae SW, et al. Gene amplification and protein overexpression of HER-2/neu in human extrahepatic cholangiocarcinoma as detected by chromogenic in situ hybridization and immunohistochemistry: its prognostic implication in node-positive patients. Ann Oncol. 2007;18:892–897. doi: 10.1093/annonc/mdm006. [DOI] [PubMed] [Google Scholar]
- 16.Settakorn J, Kaewpila N, Burns GF, Leong AS. FAT, E-cadherin, beta catenin, HER 2/neu, Ki67 immuno-expression, and histological grade in intrahepatic cholangiocarcinoma. J Clin Pathol. 2005;58:1249–1254. doi: 10.1136/jcp.2005.026575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiguchi K, Ruffino L, Kawamoto T, Ajiki T, Digiovanni J. Chemopreventive and therapeutic efficacy of orally active tyrosine kinase inhibitors in a transgenic mouse model of gallbladder carcinoma. Clin Cancer Res. 2005;11:5572–5580. doi: 10.1158/1078-0432.CCR-04-2603. [DOI] [PubMed] [Google Scholar]
- 18.Sirisinha S, Tengchaisri T, Boonpucknavig S, Prempracha N, Ratanarapee S, Pausawasdi A. Establishment and characterization of a cholangiocarcinoma cell line from a Thai patient with intrahepatic bile duct cancer. Asian Pac J Allergy Immunol. 1991;9:153–157. [PubMed] [Google Scholar]
- 19.Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C, Miwa M. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100) World J Gastroenterol. 2005;11:3392–3397. doi: 10.3748/wjg.v11.i22.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Specht K, Richter T, Müller U, Walch A, Werner M, Höfler H. Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol. 2001;158:419–429. doi: 10.1016/S0002-9440(10)63985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg R, Schwartz I, Skornick Y, Kaplan O. Detection of hepatocyte growth factor/scatter factor receptor (c-Met) in axillary drainage after operations for breast cancer using reverse transcriptase-polymerase chain reaction. Breast Cancer Res. 2003;5:R71–R76. doi: 10.1186/bcr588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Aishima SI, Taguchi KI, Sugimachi K, Shimada M, Sugimachi K, Tsuneyoshi M. c-erbB-2 and c-Met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinoma. Histopathology. 2002;40:269–278. doi: 10.1046/j.1365-2559.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 24.Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer. 2001;8:161–173. doi: 10.1677/erc.0.0080161. [DOI] [PubMed] [Google Scholar]
- 25.Voravud N, Foster CS, Gilbertson JA, Sikora K, Waxman J. Oncogene expression in cholangiocarcinoma and in normal hepatic development. Hum Pathol. 1989;20:1163–1168. doi: 10.1016/s0046-8177(89)80006-1. [DOI] [PubMed] [Google Scholar]
- 26.Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 27.Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohnishi Y, Nakamura H, Yoshimura M, Tokuda Y, Iwasawa M, Ueyama Y, Tamaoki N, Shimamura K. Prolonged survival of mice with human gastric cancer treated with an anti-c-ErbB-2 monoclonal antibody. Br J Cancer. 1995;71:969–973. doi: 10.1038/bjc.1995.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokuda Y, Ohnishi Y, Shimamura K, Iwasawa M, Yoshimura M, Ueyama Y, Tamaoki N, Tajima T, Mitomi T. In vitro and in vivo anti-tumour effects of a humanised monoclonal antibody against c-erbB-2 product. Br J Cancer. 1996;73:1362–1365. doi: 10.1038/bjc.1996.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roh H, Pippin J, Boswell C, Drebin JA. Antisense oligonucleotides specific for the HER2/neu oncogene inhibit the growth of human breast carcinoma cells that overexpress HER2/neu. J Surg Res. 1998;77:85–90. doi: 10.1006/jsre.1998.5353. [DOI] [PubMed] [Google Scholar]
- 31.Faltus T, Yuan J, Zimmer B, Krämer A, Loibl S, Kaufmann M, Strebhardt K. Silencing of the HER2/neu gene by siRNA inhibits proliferation and induces apoptosis in HER2/neu-overexpressing breast cancer cells. Neoplasia. 2004;6:786–795. doi: 10.1593/neo.04313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaelin WG Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 33.Dean-Colomb W, Esteva FJ. Her2-positive breast cancer: herceptin and beyond. Eur J Cancer. 2008;44:2806–2812. doi: 10.1016/j.ejca.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung JY, Hong SM, Choi BY, Cho H, Yu E, Hewitt SM. The expression of phospho-AKT, phospho-mTOR, and PTEN in extrahepatic cholangiocarcinoma. Clin Cancer Res. 2009;15:660–667. doi: 10.1158/1078-0432.CCR-08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Sario A, Bendia E, Omenetti A, De Minicis S, Marzioni M, Kleemann HW, Candelaresi C, Saccomanno S, Alpini G, Benedetti A. Selective inhibition of ion transport mechanisms regulating intracellular pH reduces proliferation and induces apoptosis in cholangiocarcinoma cells. Dig Liver Dis. 2007;39:60–69. doi: 10.1016/j.dld.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Menakongka A, Suthiphongchai T. Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2010;16:713–722. doi: 10.3748/wjg.v16.i6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006;18:2262–2271. doi: 10.1016/j.cellsig.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Zhou HY, Wong AS. Activation of p70S6K induces expression of matrix metalloproteinase 9 associated with hepatocyte growth factor-mediated invasion in human ovarian cancer cells. Endocrinology. 2006;147:2557–2566. doi: 10.1210/en.2005-1404. [DOI] [PubMed] [Google Scholar]
- 40.Qian Y, Corum L, Meng Q, Blenis J, Zheng JZ, Shi X, Flynn DC, Jiang BH. PI3K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am J Physiol Cell Physiol. 2004;286:C153–C163. doi: 10.1152/ajpcell.00142.2003. [DOI] [PubMed] [Google Scholar]