Abstract
T cell factor 1 (TCF-1) is a transcription factor known to act downstream of the canonical Wnt pathway and is essential for normal T cell development. However, its physiological roles in mature CD8+ T cell responses are unknown. Here we showed that TCF-1 deficiency limited proliferation of CD8+ effector T cells and impaired their differentiation towards a central memory phenotype. Moreover, TCF-1-deficient memory CD8+ T cells were progressively lost over time, exhibiting reduced expression of the anti-apoptotic molecule Bcl-2, interleukin-2 receptor β chain and diminished IL-15-driven proliferation. TCF-1 was directly associated with the Eomes allele and the Wnt-TCF-1 pathway was necessary and sufficient for optimal Eomes expression in naïve and memory CD8+ T cells. Importantly, forced expression of Eomes partly protected TCF-1-deficient memory CD8+ T cells from time-dependent attrition. Our studies thus identify TCF-1 as a critical player in a transcriptional program that regulates memory CD8 differentiation and longevity.
CD8+ T cells are critical in controlling infection by intracellular pathogens including viruses and intracellular bacteria. Upon encountering antigen, naïve CD8+ T cells are activated and clonally expand to a large quantity of effector cells equipped with cytokines and cytolytic molecules. Most of the effectors succumb to apoptosis during the contraction phase, and only a small portion of them transition into memory CD8+ T cells, capable of providing enhanced protection against the same pathogen. The transition of effector to memory CD8+ T cells is affected by extracellular stimuli including the strength and timing of stimulatory signals derived from T cell receptor (TCR)-antigen interactions, costimulation, inflammatory cytokines including interferons and IL-12 (Harty and Badovinac, 2008; Kaech and Wherry, 2007; Williams and Bevan, 2007). Memory CD8+ T cells are heterogeneous, consisting of at least two phenotypically and functionally distinct subsets, i.e., effector memory (Tem) and central memory (Tcm) (Sallusto et al., 1999). Whereas Tem cells patrol peripheral tissues, Tcm cells migrate through secondary lymphoid organs and are capable of more efficient homeostatic self-renewal and secondary proliferation than Tem (Lefrancois and Marzo, 2006; Wherry et al., 2003). Generation and differentiation of memory T cells are stipulated by intrinsic transcriptional programs. Inactivation of T-bet, Blimp-1, and Id2 and forced expression of Bcl-6 increased formation of Tcm cells (Cannarile et al., 2006; Ichii et al., 2004; Intlekofer et al., 2007; Kallies et al., 2009; Rutishauser et al., 2009). The longevity of memory T cells, in contrast, depends on IL-15 (Schluns and Lefrancois, 2003), and the IL-15 responsiveness is supported by eomesodermin-mediated positive regulation of IL-2 receptor β chain (IL-2Rβ) (Intlekofer et al., 2005).
T cell factor 1 (TCF-1) is a known effector transcription factor downstream of the canonical Wnt pathway, functioning as either transcriptional activator or repressor depending on its interacting partners (Staal et al., 2008). The most studied co-activator, β-catenin, is post-transcriptionally regulated by a multi-molecular “destruction complex” containing two scaffolding proteins, adenomatous polyposis coli and axis inhibition protein (Axin), and two protein kinases, casein kinase I and glycogen synthase kinase 3β (GSK3β). Phosphorylation of β-catenin by the kinases marks it for proteosome-mediated degradation. Wnt stimulation leads to inhibition of GSK3β and hence β-catenin stabilization. The accumulated β-catenin translocates into the nucleus where it displaces corepressor TLE-GRG (transducin-like enhancer-Groucho-related gene) proteins and complexes with TCF-1 to activate Wnt downstream genes (Staal et al., 2008). TCF-1 is required for normal T cell development as inactivation of TCF-1 partly blocked thymocyte maturation at several early stages (Verbeek et al., 1995), whereas TCF-1 deficiency did not affect proliferation and cytolytic activity of mature T cells when assayed in vitro (Schilham et al., 1998). However, several studies demonstrated that TCF-1-β-catenin pathway is operative in naïve or activated T cells (Jeannet et al., 2008; Wu et al., 2007) and can be modulated by TCR signaling (Xu et al., 2003). We and others have recently shown that during CD8+ T cell responses, TCF-1 is dynamically regulated, being downregulated in effectors and partly restored in memory T cells (Willinger et al., 2006; Zhao et al., 2010). Simultaneous activation of TCR and the TCF-1-β-catenin Wnt pathways in vitro, however, prevented TCF-1 downregulation and promoted a CD8+ memory stem cell phenotype (Gattinoni et al., 2009). In line with this, constitutive activation of the TCF-1-β-catenin pathway in vivo favored generation of memory CD8+ T cells (Zhao et al., 2010). These observations suggest that TCF-1-β-catenin activity can be manipulated to positively regulate CD8+ memory. In contrast to its well-elucidated roles in T cell development, it remains unknown what physiological roles TCF-1 may play in regulating mature CD8+ T cells. This study revealed the critical requirements of TCF-1 for CD8+ effector T cell expansion, Tcm differentiation, and persistence of CD8+ memory T cells.
RESULTS
TCF-1 deficiency limited CD8+ T cell response to Listeria monocytogenes infection
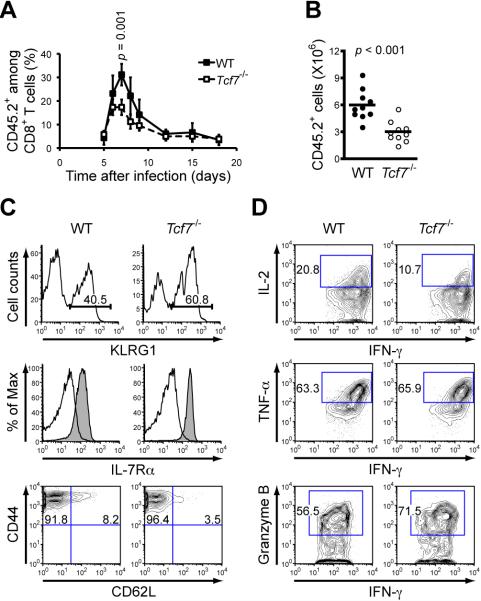
To circumvent potential alterations in TCR repertoire and precursor frequency caused by TCF-1 (encoded by Tcf7) deficiency (Verbeek et al., 1995), we crossed Tcf7-/- to OT-I TCR transgenic mice whose TCR recognizes the SIINFEKL epitope from chicken ovalbumin. To mimic physiological precursor frequency (Badovinac et al., 2007), we transferred low numbers of WT or Tcf7-/- OT-I CD8+ T cells (expressing CD45.2) into CD45.1+ B6.SJL recipients, followed by infection with attenuated Listeria monocytogenes expressing Ova (actA-LM-Ova). WT OT-I cells exhibited peak proliferation on day 7 after infection as tracked in peripheral blood leukocytes (PBLs), however, proliferation of Tcf7-/- OT-I cells reached peak on day 6 and was reduced in magnitude by approximately 50% (Figure 1A). Tcf7-/- OT-I effectors in the spleens were also decreased by about 50% (Figure 1B). Both WT and Tcf7-/- OT-I effectors were all positive for Ova-specific MHC-I tetramer and had similar capacity to produce interferon-γ (IFN-γ) when stimulated in vitro (data not shown). Compared with WT controls, Tcf7-/- OT-I effectors exhibited similar downregulation of IL-7Rα and CD62L, similar CD44 upregulation, and moderately increased upregulation of KLRG1 (Figure 1C). Further, Tcf7-/- effectors showed similar tumor necrosis factor-α (TNF-α) production, moderately increased granzyme B and decreased IL-2 production (Figure 1D). The perturbed effector proliferation due to TCF-1 deficiency was further substantiated in experiments transferring Thy1.2+ WT or Tcf7-/- OT-I (as test cells) together with Thy1.1+ OT-I cells (as reference cells) into CD45 disparate recipients where the test and reference cells were exposed to the same host environment (Figure S1A and S1B). Taken together, Tcf7-/- CD8+ T cells can be activated to acquire effector function but showed reduced magnitude of proliferation.
Figure 1. TCF-1 deficiency limits expansion of antigen-specific effector CD8+ T cells.
(A) Five hundreds of WT or Tcf7-/- OT-I T cells (CD45.2+) were injected into CD45.1+ B6.SJL recipients, followed by i.v. infection with 5 × 106 CFU actA-LM-Ova. Kinetics of early responses of WT or Tcf7-/- OT-I cells were tracked in the PBLs, and their percentages in CD8+ T cells are shown. Data are representative of 3 independent experiments with similar results (n ≥ 3 for each time point). All p-values, including those in following figures, were determined using Student's t-test.
(B) Numbers of effector OT-I T cells in the spleen on day 7 after infection. Data are pooled from 4 independent experiments.
(C) Cell surface phenotypes of effector OT-I T cells. Day 7 effector T cells were analyzed for KLRG1, IL-7Rα, CD62L, and CD44 expression. Percentages for KLRG1+, CD44+CD62Llo, and CD44+CD62Lhi populations are shown. Shaded histogram in IL-7Rα denotes its expression in naïve CD8+ T cells for a direct comparison with the effector T cells.
(D) Production of effector molecules by effector OT-I T cells. Splenocytes were isolated on day 7 after infection and incubated with Ova peptide for 6 hrs in vitro. The cells were sequentially surface-stained, fixed and permeabilized, and intracellularly stained for IL-2, TNF-α, and granzyme B. Gating of positive populations was based on respective isotype controls. For (C) and (D), data are representative from at least 3 independent experiments with similar results.
By measuring 5’-bromo-2’-dexoxy-uridine (BrdU) uptake, we found that Tcf7-/- OT-I effectors proliferated similarly to WT during days 4-5 after infection but their proliferation was not sustained during days 6-7 (Figure S1C and S1D). On the other hand, WT and Tcf7-/- effectors at days 5 and 7 exhibited similar rates of apoptosis, as measured by caspase-3 and -7 activation (Figure S1E), in key contrast to increased caspase-3 and -7 activity in Tcf7-/- thymocytes (Figure S1F). Our results demonstrate that TCF-1 is essential for sustained proliferation of effector T cells, highlighting a distinct regulatory role of TCF-1 in activated mature T cells.
Tcf7-/- memory T cells were impaired in secondary expansion but not cytotoxity
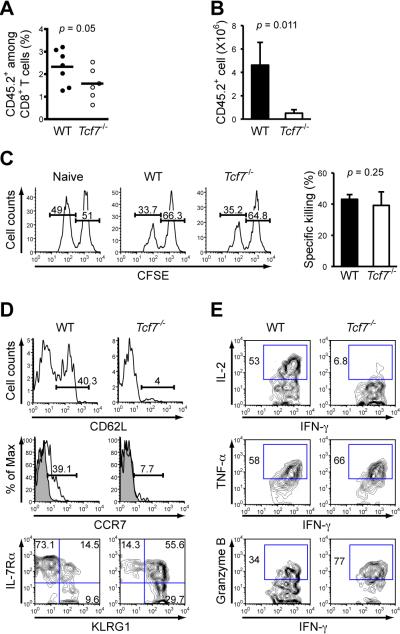
Tcf7-/- effector OT-I T cells contracted similarly to WT effectors, giving rise to proportionally lower frequency of early memory CD8+ T cells in the PBLs (Figure 2A). Memory T cells are characteristic of enhanced proliferation upon reencounter of the same pathogen. To measure the secondary expansion on a per cell basis, we transferred the same number of sorted WT or Tcf7-/- OT-I memory T cells into naïve B6.SJL hosts. After infection with virulent LM-Ova, secondary proliferation of Tcf7-/- memory T cells was greatly diminished in the spleens (Figure 2B), indicating that secondary effectors are more dependent on TCF-1 for expansion compared with the primary response.
Figure 2. Tcf7-/- memory CD8+ T cells are impaired in secondary expansion and in differentiation to a Tcm phenotype.
(A) Frequency of early memory OT-I cells in PBLs. B6.SJL recipients of either WT or Tcf7-/- OT-I cells were infected with actA-LM-Ova, and the percentage of CD45.2+ OT-I in CD8+ T cells was determined on days 34-44 after infection. Data are pooled results from 2 independent experiments.
(B) Secondary expansion of memory CD8+ T cells in the spleens. Sorted WT or Tcf7-/- memory OT-I T cells (6 × 103) were transferred into naïve B6.SJL hosts, followed by infection with 2 × 105 CFU of virulent LM-Ova. The numbers of CD45.2+ OT-I cells in the spleens were determined 6 days later. Data are means ± s.d. (n = 3). Similar results were obtained when total splenocytes containing equivalent numbers of WT or Tcf7-/- memory OT-I cells were transferred without sorting separation (not shown).
(C) In vivo killing capacity of memory CD8+ T cells. CD45.2+ splenocytes were differentially labeled with CFSE. Ova peptide-pulsed CFSElo and non-peptide pulsed CFSEhi cells were injected at 1:1 ratio into naïve or immune chimeras (35 days after infection). The spleens were harvested 4 hrs later, and percentages of CFSElo and CFSEhi cells in CD45.2+ splenocytes were determined. Data are representative of 2 independent experiments with similar results.
(D) Surface staining for CD62L, CCR7, IL-7Rβ, and KLRG1 on antigen-specific memory CD8+ T cells. During days 75-85 after infection with actA-LM-Ova, splenocytes from WT or Tcf7-/- OT-I recipients were stained. Percentages of CD62Lhi and CCR7+ subsets were shown in histograms, with shaded histogram denoting isotype control. Data are representative of 2-3 independent experiments (n ≥ 4).
(E) Intracellular detection of IFN-γ, IL-2, TNF-α, and granzyme B in memory CD8+ T cells. Splenocytes were stimulated with Ova peptide for 6 hrs, followed by surface and intracellular staining. Gating of positive populations was based on respective isotype controls. All data are representative of 3 independent experiments (n ≥ 5).
To assess the cytolytic activity of WT and Tcf7-/- memory CD8+ T cells, we used a short-term in vivo killing assay which does not involve extensive secondary expansion (Barber et al., 2003). To this end, we transferred 2,500 WT or Tcf7-/- OT-I cells prior to LM-Ova infection so that the endogenous antigen-specific T cells in the B6.SJL hosts were outcompeted (Badovinac et al., 2007), chose the recipients containing similar frequencies of WT and Tcf7-/- early memory OT-I T cells for the assay. Whereas the killing target cells that were Ova peptide-pulsed and labeled with low concentration of carboxyfluorescein succinimidyl diester (CFSE) were not affected in naïve mice, they were substantially eliminated in the immune recipients of either WT or Tcf7-/- OT-I cells (Figure 2C). Thus, lack of TCF-1 did not detectably alter the cytolytic activity of memory CD8+ T cells.
Tcf7-/- memory CD8+ T cells exhibited predominantly a Tem phenotype
Effector CD8+ T cells are generally IL-7Rαlo and KLRG1hi, but IL-7Rα upregulation and KLRG1 downregulation are associated with differentiation of effectors to long-lived protective memory CD8+ T cells (Joshi et al., 2007). Tcm and Tem memory T cells have distinct expression of homing receptors including CD62L and CCR7 (Sallusto et al., 1999). Analysis of Tcf7-/- memory CD8+ T cells at 75-85 days after infection revealed that substantially fewer cells expressed CD62L and CCR7 than WT controls and that most of them retained high expression of KLRG1 (Figure 2D). In contrast, IL-7Rα was upregulated similarly in both WT and Tcf7-/- memory T cells (Figure 2D). Upon peptide stimulation in vitro, WT and Tcf7-/- memory T cells were both capable of producing IFN-γ and TNF-α, and increased portion of Tcf7-/- CD8+ memory expressed granzyme B (Figure 2E). Importantly, IL-2 production, another characteristic feature of Tcm cells (Wherry et al., 2003), was impaired in Tcf7-/- memory T cells (Figure 2E). Thus, Tcf7-/- memory T cells predominantly manifested an effector memory phenotype even at >75 days after infection, indicating an essential role of TCF-1 in differentiation of Tcm cells. The intrinsic requirements of TCF-1 for memory CD8+ differentiation were further substantiated using a viral infection model, where Tcf7-/- memory OT-I T cells generated in response to infection with vaccinia virus expressing Ova (VacV-Ova) exhibited similarly predominant Tem phenotypes (Figure S2A and S2B).
Tcf7-/- mice are moderately lymphopenic, and Tcf7-/- T cells show increased portion of CD44hi subset, probably resulting from increased homestatic proliferation (Schilham et al., 1998). Tcf7-/- OT-I T cells retained this phenotype and were positive for CD62L, IL-7Rα and CCR7 but negative for KLRG1 (Figure S2C). Whereas CD44hi CD8+ T cells produced IFN-γ after brief TCR stimulation (Cho et al., 2000; Goldrath et al., 2000), the TCR-stimulated IFN-γproduction was negligible in both WT and Tcf7-/- CD44lo CD8+ T cells (Figure S2D). Thus, Tcf7-/- CD44lo T cells manifested phenotypic and functional features that resembled naïve T cells. To exclude the possibility that the increased CD44hi subset in Tcf7-/- T cells may account for the defects observed above, we sorted CD62L+CD44lo naïve OT-I cells from WT and Tcf7-/- spleens and repeated the adoptive transfer and LM-Ova infection experiments. Tcf7-/- OT-I effectors in PBLs derived from the naïve precursors were also limited in proliferation (Figure S2E). Further, the Tcf7-/- memory CD8+ T cells derived from naïve precursors were also predominantly Tem cells, showing reduced CD62L expression and IL-2 production (Figures S2F and S2G). These findings further corroborated an intrinsic requirement of TCF-1 for Tcm cell differentiation, in spite of previous exposure to a lymphopenic environment.
Progressive loss of Tcf7-/- CD8+ memory T cells due to diminished responsiveness to IL-15
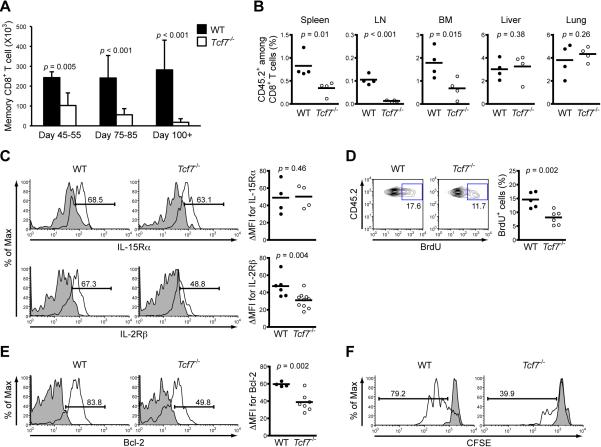
In addition to defective secondary expansion and Tcm differentiation, we noted that Tcf7-/- memory CD8+ T cells in the spleens underwent progressive loss over time, in contrast to relatively stably maintained WT CD8+ memory (Figure 3A). The decrease was not due to skewed tissue distribution, because Tcf7-/- memory CD8+ T cells were diminished similarly in bone marrow cells and did not accumulate in livers or lungs (Figure 3B). In fact, the decrease in Tcf7-/- CD8+ memory was most evident in lymph nodes (Figure 3B), consistent with its reduced CD62L and CCR7 expression and hence homing defects to secondary lymphoid tissues. IL-7 and IL-15 are known to be critical for maintaining memory CD8+ T cells (Schluns and Lefrancois, 2003). Although no apparent differences in IL-7Rα and IL-15Rα were observed, IL-2Rβ, a shared receptor subunit between IL-2 and IL-15, was decreased in expression in Tcf7-/- memory T cells (Figure 3C). Consistent with the reported roles of IL-15 in homeostatic proliferation and promoting survival of memory T cells (Berard et al., 2003; Prlic et al., 2002), Tcf7-/- memory T cells showed diminished BrdU uptake (Figure 3D) and Bcl-2 expression (Figure 3E), and exhibited substantially reduced IL-15-driven proliferation compared with WT cells (Figure 3F). Importantly, reduced expression of IL-2Rβ and Bcl-2 was observed in Tcf7-/- memory OT-I cells that were generated in response to VacV-Ova infection (Figure S3A) and from naïve precursors (Figure S3C), supporting that TCF-1 is intrinsically required for sustaining IL-15 responsiveness in CD8+ memory.
Figure 3. Long-term maintenance and IL-15 responsiveness of memory CD8+ T cells depend on TCF-1.
(A) Progressive loss of Tcf7-/- memory CD8+ T cells. B6.SJL recipients of WT or Tcf7-/- OT-I cells were sacrificed on indicated days post-infection and memory OT-I T cells in the spleens were enumerated. Data are pooled from at least 3 independent experiments.
(B) Tissue distribution of memory CD8+ T cells. During 75-85 days after infection, frequency of memory OT-I in CD8+ T cells was determined in indicated tissues. Data are pooled from 2 independent experiments.
(C) Expression of IL-15Rα and IL-2Rβ on memory CD8+ T cells. Memory OT-I cells were surface-stained, and percentages of positive cells were shown in representative histograms based on isotype staining (shaded). Cumulative results from 2-4 experiments were shown on the right panel as ΔMFI, the difference of MFI (mean fluorescent intensity) values of antibody- and isotype-stained entire cell populations without positivity gating. The same approach was used to present data in Figures 3E, 4B, 5E, and 7C.
(D) BrdU uptake in memory CD8+ T cells. The B6.SJL recipients were i.p. injected with BrdU on day 70 after infection and fed with BrdU in drinking water for 1 week. The percentage of BrdU+ population was shown in representative contour plots (on the left) or cumulative data from 3 independent experiments (on the right).
(E) Expression of Bcl-2 in memory CD8+ T cells. Memory OT-I cells were intracellularly stained for Bcl-2. Data are either representative of or pooled from 3 independent experiments.
(F) Proliferation of memory CD8+ T cells in response to IL-15. Memory OT-I cells were labeled with CFSE and cultured in the presence of IL-15 (50 ng/ml). The shaded histogram denotes the CFSE level in cells cultured without IL-15, and the percentage denotes actively dividing cells. Representative data after 4-day culture are shown for 2 independent experiments with similar results (n = 3).
Wnt-TCF-1 pathway was necessary and sufficient to induce eomesodermin expression
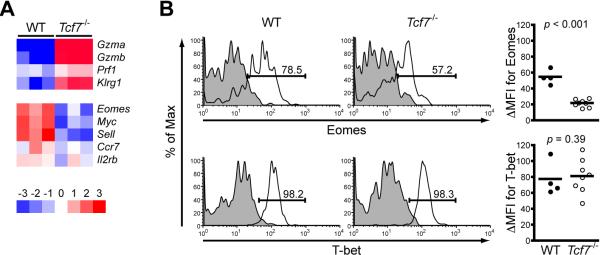
To gain mechanistic insights into the role of TCF-1 in maintaining memory CD8+ T cells, we performed transcriptomic analysis on sorted WT and Tcf7-/- memory OT-I cells. By the setting of p < 0.05 for significantly differential expression, 526 genes were upregulated and 776 genes downregulated for >1.5 fold due to TCF-1 deficiency. Functional annotation using DAVID (the Database for Annotation, Visualization, and Integrated Discovery) bioinformatics resources (Huang da et al., 2009) revealed that TCF-1 controls a wide spectrum of biological functions in memory T cells, including transcriptional regulation, cell cycle progression, and apoptosis, in addition to CD8+ effector molecules, cytokines, chemokines, and their receptors (Supplemental Table I). In line with our functional and phenotypic analyses, granzymes A and B, and KLRG1 were among the top 20 upregulated genes, and perforin transcript was increased by 1.45-fold in Tcf7-/- memory T cells (p = 0.024, Figure 4A). Additionally, Tcf7-/- memory T cells had decreased transcripts of CD62L (encoded by Sell), CCR7, and IL-2Rβ (Figure 4A, Il2rb transcript reduced by 30%, p = 0.038). Eomesodermin (Eomes) and c-Myc were among the most downregulated genes in Tcf7-/- memory CD8+ T cells (Figure 4A). Compared with another T-box factor T-bet, Eomes has been shown to directly bind to 5’-regulatory regions in the Il2rb gene, more effectively enhance IL-2Rβ expression, and confer IL-15 responsiveness to memory CD8+ T cells (Intlekofer et al., 2007). By intranuclear staining, we found that the protein expression of Eomes but not T-bet was substantially diminished in Tcf7-/- memory CD8+ T cells (Figure 4B). Similarly diminished Eomes expression was observed in Tcf7-/- memory OT-I T cells generated in response to VacV-Ova infection (Figure S3B) or from naïve precursors (Figure S3D).
Figure 4. TCF-1 is necessary for optimal Eomes expression.
(A) Transcriptomic analysis revealed expression changes in genes that are critical for regulating CD8+ T cell activities. Day 70-80 memory CD8+ T cells were sorted from 3 WT or 3 Tcf7-/- OT-I recipients and subjected to microarray analysis using Mouse GENE 1.0 ST arrays. Heatmaps of select genes and colour-coded scales are shown.
(B) Eomes but not T-bet was expressed at lower levels in Tcf7-/- memory CD8+ T cells. Memory CD8+ T cells (>70 days after infection) were intranuclearly stained for Eomes and T-bet. Shown on left are representative histograms with percentages of positive subsets, and on right are cumulative ΔMFI data from 3 independent experiments.
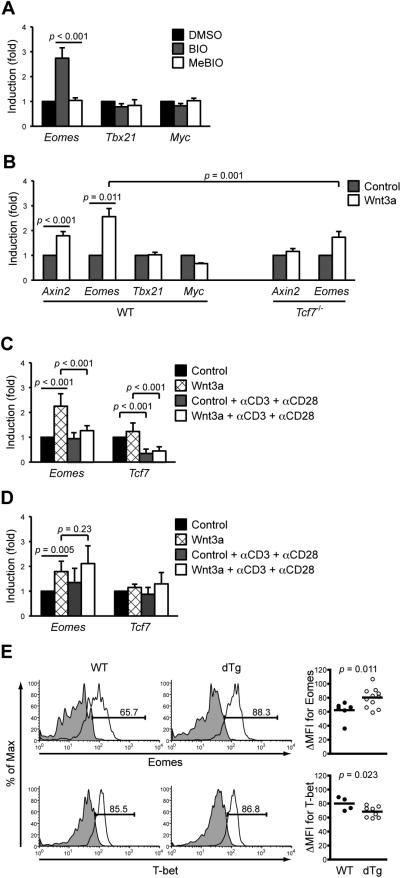
To determine if Eomes expression is responsive to activation of Wnt signaling, we used 6-bromo-substituted indirubin-acetoxime (BIO-acetoxime), a specific inhibitor of GSK-3β, to block β-catenin phosphorylation and degradation (Meijer et al., 2004). BIO-acetoxime treatment stabilized β-catenin in naïve CD8+ T cells as expected and induced the expression of Eomes but not T-bet (encoded by Tbx21) (Figure S4A and S4B). c-Myc acts at least partly downstream of IL-15 in regulating homeostasis of memory CD8+ T cells (Bianchi et al., 2006). Although reported to be a Wnt target gene in human colorectal cancers (He et al., 1998), c-Myc did not respond to Wnt activation in mature T cells, suggesting that Wnt responsive genes could be highly cell context-dependent (Figure S4B). Treatment of sorted memory OT-I cells with BIO-acetoxime also induced Eomes expression but had little effects on T-bet and c-Myc (Figure 5A). In contrast, treatment with N-methylated BIO (MeBIO), an inactive analog of BIO-acetoxime, neither stabilized β-catenin nor apparently altered Eomes and other genes’ expression (Figures S4 and 5A).
Figure 5. Activation of the canonical Wnt/TCF-1/β-catenin pathway is sufficient to induce Eomes expression.
(A) Inhibition of GSK3β induces Eomes expression in memory CD8+ T cells. Day 60 memory OT-I T cells were sorted from LM-Ova-infected B6.SJL recipients and treated with DMSO, BIO-acetoxime (BIO), or N-methylated BIO (MeBIO) for 12 hrs. The expression of select genes was quantitatively determined, and all normalized to the samples treated with DMSO.
(B) Induction of Axin2 and Eomes by Wnt3a in naïve CD8+ T cells. Freshly isolated naïve CD8+ T cells from WT or Tcf7-/- mice were exposed to Wnt3a conditioned or control medium for 3 hrs. Gene expression was quantitatively determined and then normalized to the control medium-treated samples.
(C) Effect of Wnt and TCR stimulation in naïve CD8+ T cells. WT naïve CD8+ T cells were stimulated with Wnt3a and/or plate-bound anti-CD3 (5 μg/ml) + soluble anti-CD28 (1 μg/ml) for 3 hrs, and gene expression was quantitatively determined and normalized as in (B).
(D) Effect of Wnt and TCR stimulation in memory CD8+ T cells. Memory CD8+ T cells were isolated as in (A) and stimulated as in (C), and gene expression was quantitatively determined. For (A)-(D), data are representative of at least 2 independent experiments with similar results (n ≥ 4).
(E) Increased Eomes expression in memory CD8+ T cells in the presence of constitutively active Wnt signalling. Double transgenic (dTg) mice with forced expression of p45 TCF-1 and stabilized β-catenin and their WT littermates were infected with actA-LM-Ova. During days 45-55 after infection, memory CD8+ T cells were identified by intracellular detection of IFN-γ after 6-hr Ova peptide stimulation, and Eomes or T-bet expression was determined in IFN-γ+ CD8+ T cells. Data shown are representative histograms (on the left) and cumulative ΔMFI from 2-3 independent experiments (on the right).
We next investigated if naïve and memory CD8+ T cells are responsive to bona fide Wnt ligands. Consistent with previous observations that both resting and activated human T cells expresses multiple Fzd receptors (Wu et al., 2007), our microarray analysis showed that WT memory CD8+ T cells expressed high amounts of Fzd5 and Fzd9, Lrp5 and Lrp6 co-receptors, and Dvl molecules connecting Fzd-Lrp receptor and the β-catenin destruction complexes (Figure S4C), most of which were not apparently affected by TCF-1 deficiency. Thus, memory CD8+ T cells are equipped with Wnt-responsive receptors. Axin2 has been suggested to be a universal Wnt-induced target gene as a negative regulator of the Wnt pathway (Jho et al., 2002; Lustig et al., 2002). We stimulated naïve CD8+ T cells with Wnt3a, which has been shown to promote proliferation of hematopoietic stem cells via accumulation of β-catenin (Willert et al., 2003). We confirmed induction of Axin2 by Wnt3a and found that Wnt3a induced Eomes but not T-bet or c-Myc expression in naïve CD8+ T cells (Figure 5B). Further, the induction of Axin2 and Eomes by Wnt3a was diminished in Tcf7-/- CD8+ T cells, indicating its TCF-1 dependency (Figure 5B). The remaining induction of Eomes in Tcf7-/- CD8+ cells may be ascribed to the presence of another Wnt effector transcription factor, lymphoid enhancer-binding factor 1 (LEF-1).
Eomes expression was reported to increase in effector T cells (Intlekofer et al., 2007). We found that TCR stimulation of naïve T cells did not detectably alter Eomes expression, at least during the observation period when the induction by Wnt3a was apparent (Figure 5C), suggesting that Eomes can be induced rapidly by Wnt ligand stimulation in the absence of TCR-derived signals. Interestingly, Wnt3a-induced Eomes upregulation was diminished when naïve T cells were stimulated by the combination of Wnt and TCR (Figure 5C). This is likely due to downregulation of TCF-1 in naïve T cells by TCR stimulation, regardless if Wnt3a is present (Figure 5C).
We further validated that Eomes can be induced by Wnt3a in memory CD8+ T cells (Figure 5D), albeit the induction was somehow smaller compared with that seen in naïve T cells. This is likely explained by the fact that memory CD8+ T cells express higher amounts of Eomes (Intlekofer et al., 2007) but lower amounts of TCF-1 and LEF-1 than naïve T cells (Willinger et al., 2006; Zhao et al. 2010), or alternatively Wnt3a may not be the most potent Wnt ligand acting on memory T cells. In key contrast to naïve T cells, TCR stimulation of memory CD8+ T cells did not apparently downregulate TCF-1 or diminish Wnt-mediated induction of Eomes (Figure 5D). These findings suggest distinct cross-talk between Wnt and TCR signaling pathways in naïve and memory T cells, which merits future investigations.
We recently demonstrated that constitutive activation of Wnt signaling pathway, via transgenic expression of p45 TCF-1 and stabilized β-catenin, favors generation of CD8+ memory T cells (Zhao et al. 2010). In memory T cells generated in the double transgenic mice (dTg), protein expression of Eomes was elevated and T-bet expression was slightly decreased, if any (Figure 5E). These in vivo data further support the notion that in memory CD8+ T cells, the Wnt-TCF-1 pathway is necessary and sufficient for inducing the optimal expression of Eomes transcription factor, which in turn positively regulates IL-2Rβ expression and IL-15 responsiveness.
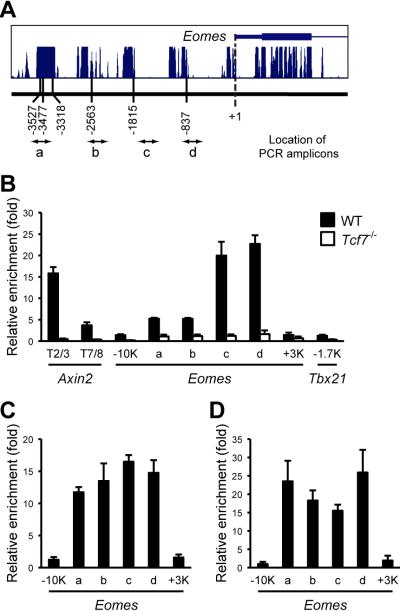
TCF-1 bound to regulatory sequences in the Eomes gene in vivo
To investigate if TCF-1 is directly associated with the Eomes gene, we surveyed a 6 kb non-coding DNA sequence flanking the Eomes transcription initiation site (- 5 kb to + 1 kb) for the core consensus TCF-1 binding motif “CTTTG” (van de Wetering and Clevers, 1992). Among a total of 20 TCF-1 motifs found in the 5’-regulatory region, 6 were conserved among different species (Figure 6A and S5). Eight TCF-1 motifs (termed T1 to T8) within –1.5 kb to +2.6 kb of the Aixn2 gene were previously defined to contribute to its Wnt responsiveness (Jho et al., 2002). Because Axin2 was induced by Wnt3a stimulation in mature CD8+ T cells, we used 2 clusters of TCF-1 motifs (T2/3 and T7/8) in the Axin2 gene (corresponding to its promoter and an intron region, respectively) as potential positive controls. Rag2 is silenced in mature T cells and T-bet does not respond to Wnt, we therefore used the Rag2 promoter (-0.6 ~ +0.7 kb) and a Tbx21 5’-regulatory region (-2.6 ~ -1.2 kb) containing no conserved TCF-1 motifs as negative controls. Because Eomes is inducible by activated Wnt signaling in both naïve and memory T cells as shown above, we used naïve CD8+ T cells to obtain sufficient cells for chromatin immunoprecipitation (ChIP). As shown in Figure 6B, enriched TCF-1 binding was found in the 2 Axin2 regulatory clusters, 6 conserved 5’ consensus “CTTTG” motifs in Eomes (with first 3 in “cluster a” and the other 3 as “elements b-d”), but not in the Tbx21 gene or -10 kb and +3 kb regions in the Eomes gene, although both locations in the Eomes allele harbored conserved TCF-1 motifs. Importantly, all the enriched TCF-1 bindings were abrogated when Tcf7-/- CD8+ T cells were used (Figure 6B). Additionally, ChIP with another irrelevant antibody against transcription factor GA binding protein did not enrich TCF-1-associated sequences, consistent with ChIP-seq data showing no binding of GA binding protein in Eomes or Axin2 gene loci (data not shown). These results demonstrate direct and specific binding of TCF-1 with multiple cis-regulatory sequences in the Eomes and Axin2 loci.
Figure 6. TCF-1 is directly associated with regulatory sequences in the Eomes gene.
(A) Schematic showing locations of conserved consensus TCF-1 binding motifs in the 5’-regulatory region of the Eomes gene. Multiple species conservation from the UCSC genome browser is shown on the top, and locations of each conserved TCF-1 motif and corresponding PCR amplicon are marked below. The 3 TCF-1 motifs found in -3.5 kb were collectively defined as “cluster a”, and the other 3 relatively scattered motifs were referred to as “element b to d”. See Figure S5 for the sequence alignments among different species.
(B) TCF-1 binds to the Eomes regulatory sequences in vivo. Chromatin fragments from WT or Tcf7-/- splenic CD8+ T cells were immunoprecipitated with an anti-TCF-1 antibody. Enrichment of each segment was determined with quantitative PCR and normalized to the Rag2 promoter region. Data are representative of 3 independent ChIP experiments with each sample measured in duplicates or triplicates.
(C) TLE-GRG and (D) β-catenin co-occupy Eomes regulatory sequences with TCF-1. Chromatin fragments from splenic CD8+ T cells of WT or β-catenin transgenic mice were immunoprecipitated with TLE-GRG or β-catenin antibody, respectively. Enrichment of each segment was quantitatively measured, and data are representative of 2 independent experiments with similar results.
To further substantiate direct regulation of Eomes by the Wnt-TCF-1 pathway, we performed ChIP using an antibody recognizing all 4 isoforms of TLE-GRG, the corepressor proteins interacting with TCF-1 in the absence of active Wnt signaling. We also performed ChIP with a β-catenin antibody on transgenic CD8+ T cells expressing stabilized β-catenin. All the TCF-1-bound motifs were enriched by either TLE-GRG or β-catenin antibody (Figure 6C and 6D), indicating the co-occupancy of TLE-GRG or β-catenin with TCF-1 on regulatory sequences in the Eomes gene. Taken together, these findings identified Eomes as a direct Wnt target gene in mature CD8+ T cells.
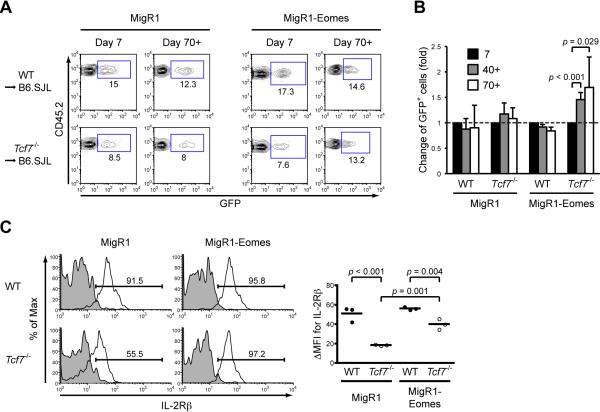
Forced expression of Eomes protected time-dependent loss of Tcf7-/- memory T cells
To demonstrate that Eomes is functionally important in mediating regulation of memory T cell maintenance by TCF-1, we introduced the Eomes cDNA into primed WT or Tcf7-/- OT-I T cells using MigR1 bicistronic retroviral vector with GFP as an expression indicator. The retrovirally transduced OT-I T cells were then adoptively transferred into naïve mice, followed by LM-Ova infection (Figure S6A) (Joshi et al., 2007). We hypothesized that if Eomes is a key mediator of TCF-1-dependent persistence of memory CD8+ T cells, forced expression of Eomes should confer protection to Tcf7-/- memory OT-I T cells. Throughout effector to early and late memory phases, when infected with MigR1 control retrovirus, the percentages of GFP-positive WT or Tcf7-/- OT-I T cells remained relatively stable, indicating that retroviral infection itself or GFP expression did not detectably alter the behavior of effector and memory CD8+ T cells (Figure 7A and 7B). The GFP+ subset was also stably sustained in WT OT-I cells infected with MigR1-Eomes retrovirus. In key contrast, the portion of GFP+ Tcf7-/- OT-I cells that had forced expression of Eomes was elevated in early memory T cells compared with effector phase and continued to increase in late memory stage (Figure 7A and 7B), indicating that Eomes-expressing Tcf7-/- memory T cells gained a relative self-renewal and/or survival advantage whereas those without Eomes continues to disappear over time. We confirmed increased expression of Eomes in GFP+ WT or Tcf7-/- memory OT-I T cells infected with MigR1-Eomes (Figure S6B). We further determined that forced expression of Eomes substantially increased IL-2Rβ expression in Tcf7-/- memory CD8+ T cells, albeit it did not have an evident effect on IL-2Rβ expression in WT memory CD8+ T cells (Figure 7C). Additional phenotypic and functional analyses showed that increased Eomes expression in Tcf7-/- T cells did not improve generation of IL-2-producing CD62L+ Tcm cells (Figure S6C). Nonetheless, forced expression of Eomes can at least partly restore IL-2Rβ expression in TCF-1-deficient memory T cells and protect them from time-dependent attrition.
Figure 7. Forced expression of Eomes protects Tcf7-/- memory CD8+ T cells from attrition.
(A) Representative flow profiles of retrovirally transduced effector (day 7) and memory (day 70+) CD8+ T cells. Percentages of GFP+ subsets, infected with either MigR1 control retrovirus or Eomes-expressing retrovirus, in CD8+CD45.2+ cells are shown from 2 independent experiments (n ≥ 4).
(B) Fold changes of GFP+ cells during OT-I response. GFP+ percentage in each recipient mouse on day 7 after infection was arbitrarily set to 1, and that in each mouse during the memory phase was normalized to day 7 to calculate the fold changes. Data are means ± s.d. of at least 4 individual recipients for each group (pooled from 2 independent experiments).
(C) IL-2Rβ expression in retrovirally infected WT and Tcf7-/- memory CD8+ T cells. Splenocytes were isolated from recipients of retrovirally infected WT or Tcf7-/- OT-I T on day 40 after LM-Ova infection, and IL-2Rβ expression was determined on GFP+CD45.2+CD8+ T cells with representative histograms and cumulative ΔMFIs shown from 2 independent experiments.
DISCUSSION
TCF-1 is known to be important for normal thymocyte development, and loss of TCF-1 partly blocked T cell development at several early stages (Verbeek et al., 1995). Whereas deficiency in LEF-1, another Wnt effector transcription factor, did not cause detectable T cell defects, a combination of hypomorphic TCF-1 alleles and LEF-1 null alleles completely arrested T cell development at the immature single positive stage due to failure of TCRα rearrangements (Okamura et al., 1998). These observations indicated a partially overlapping function of TCF-1 and LEF-1 in developing T cells. Our current studies revealed that TCF-1 is a key player in regulating mature T cell responses at several different phases and that TCF-1 may have both overlapping and non-redundant roles with LEF-1 during these processes. TCF-1 deficiency limited primary expansion of effector T cells, but this effect was relatively moderate. In addition, TCF-1 appeared to be dispensable for cytolytic activity of memory CD8+ T cells. During revision of the current manuscript, Jeannet et al (2010) reported that the proliferation of Tcf7-/- CD8+ effectors and cytolytic activities of Tcf7-/- memory CD8+ T cells were relatively unaffected using a lymphocytic choriomeningitis (LCMV) infection model. These are likely explained by compensatory effect by LEF-1. In contrast, the requirements of TCF-1 in Tcm cell differentiation and longevity are non-redundant with LEF-1, although TCF-1 and LEF-1 are both expressed in memory T cells (Zhao et al. 2010). Additionally, the secondary expansion of Tcf7-/- memory CD8+ T cells was more severely impaired in both LM and LCMV infection models (Jeannet et al. 2010), indicating a unique and strong dependence on TCF-1 in memory CD8+ T cells. In line with this, a distinct function of TCF-1 has also been recently described in differentiation of CD4+ T cells to a Th2 cell fate (Yu et al., 2009).
A transcriptional regulatory network has been elucidated that determines the fate of activated B cells to antibody-secreting plasma cells and long-lived memory B cells. In the network, B lymphocyte-induced maturation protein-1 (Blimp-1) and Bcl-6 are two key transcription factors that mutually repress each other (Martins and Calame, 2008). A similar transcription network regulating CD8+ effectors to central memory fate is beginning to emerge from several recent studies. Inactivation of T-bet, Blimp-1, and Id2 and forced expression of Bcl-6 increased formation of Tcm cells (Cannarile et al., 2006; Ichii et al., 2004; Intlekofer et al., 2007; Rutishauser et al., 2009). Gene expression analysis showed that T-bet difficiency is associated with increased expression of Eomes and that loss of Blimp-1 resulted in downregulation of T-bet and Id-2 and upregulation of Eomes and Bcl-6 (Intlekofer et al., 2007; Kallies et al., 2009; Rutishauser et al., 2009). Our current study revealed that ablation of TCF-1 impaired Tcm formation. Coupled with our previous finding that forced expression of TCF-1 and stabilized β-catenin gave rise to increased numbers of IL-2-producing memory CD8+ T cells (Zhao et al., 2010), TCF-1 has a positive regulatory role in Tcm cell differentiation. In line with this notion, TCF-1 expression was upregulated approximately 10-fold in Blimp-1-deficient CD8+ effector cells (Rutishauser et al., 2009). Collectively, along with other transcription factors, TCF-1 contributes to an intricate yet interactive gene regulatory network to collaboratively program Tcm cell formation.
Wnt responsive genes were previously reported in double negative thymocytes, including transcription factors c-Jun and c-Fos (Staal et al., 2004), and direct binding of TCF-1 to regulatory elements of these genes in vivo has not been demonstrated in thymocytes. In mature T cells, information on Wnt targets is scarce. Recently, it has been shown using ChIP that TCF-1 can bind to the proximal promoter of Gata3 in CD4 T cells to promote a Th2 cell fate (Yu et al., 2009), and to the promoters of matrix metalloproteinases 2 and 9 genes in human T cells to facilitate T cell transmigration through epithelial cells (Wu et al., 2007). All the aforementioned Wnt-responsive or TCF-1-β-catenin-bound genes were not markedly altered in expression between WT and TCF-1-deficient memory CD8+ T cells. In addition, c-Myc, another known Wnt target in human colorectal cancers (He et al., 1998), was decreased in Tcf7-/- memory T cells but its expression did not respond to stimulation by Wnt3a or β-catenin stabilization. These findings highlight that TCF-1-regulated genes are highly dependent on cell types and their developmental stages for T cells in particular. Axin2 has been characterized to be a Wnt target gene in many other tissue and cell types (Jho et al., 2002; Lustig et al., 2002). In the current study of mature CD8+ T cells, we found that Axin2 was induced by Wnt3a stimulation in a TCF-1 dependent manner and further showed direct association of TCF1 with the Axin2 allele in vivo. Using the Axin2 gene as a positive control, we demonstrated direct and specific binding of TCF-1 to 6 conserved consensus sequences in the Eomes 5’-regulatory region. The relevance of these TCF-1 binding sites in Wnt signaling was supported by the co-occupancy by TLE-GRG corepressor proteins in the absence of Wnt stimulation and by β-catenin upon stabilization. Along with TCF-1-dependent induction of Eomes by Wnt, these findings collectively identified Eomes as a direct target of the Wnt-TCF-1 pathway in mature CD8+ T cells.
It is well known that TCF-1 activity is regulated by Wnt ligands which stabilize the coactivator β-catenin and lead to displacement of TLE-GRG corepressors (Staal et al., 2008). Recent studies suggest that the expression of TCF-1 in T cells can be modulated by signals derived from TCR and cytokines. In vivo activation of T cells downregulated TCF-1 in both mice and humans (Willinger et al., 2006; Zhao et al., 2010). This is also true for in vitro TCR-stimulated T cells, however, when combined with pharmacologically stabilized β-catenin, TCF-1 downregulation was blocked (Gattinoni et al., 2009). In addition, TCF-1 expression can be influenced by cytokines when primed in vitro, being induced by IL-21 but sustained at a low level by IL-2 and IL-15 (Hinrichs et al., 2008). Furthermore, β-catenin stabilization by TCR stimulation was described in thymocytes and CD4 T cells (Xu et al., 2003). Collectively, it is reasonable to assume that TCF-1-β-catenin pathway can integrate signals from Wnt ligands, TCR engagement, and various cytokines during T cell responses, especially during effector expansion and transition to memory T cells. Detailed dissection of the contribution of individual pathways to regulating TCF-1 activity and expression awaits future investigations. Nevertheless, data from the current study suggest a direct Wnt involvement in the persistence of memory CD8+ T cells. We demonstrated that activation of the Wnt pathway by either GSK3β inhibition or Wnt3a stimulation, in the absence of signals from TCR or cytokines, induced the expression of Eomes in both naïve and memory CD8+ T cells. In vivo, forced expression of TCF-1 and stabilized β-catenin expanded the CD8+ memory T cell pool (Zhao et al., 2010). By the time when memory T cells are generated in response to acute viral or bacterial infection, inflammatory cytokines produced during early immune responses have subsided, and it has been demonstrated that persistence of memory CD8+ T cells does not depend on signaling from MHC class I ligands but rather on IL-15 (Jabbari and Harty, 2005; Murali-Krishna et al., 1999; Schluns and Lefrancois, 2003; Williams and Bevan, 2007). In the absence of TCR stimulation, the endogenous IL-15 signal alone may not affect TCF-1 expression in memory CD8+ T cells in vivo, and this notion is supported by our recent observation that memory T cells express higher amounts of TCF-1 than effectors (Zhao et al., 2010). Thus, TCF-1-β-catenin-mediated regulation of Eomes expression in memory CD8+ T cells is more likely ascribed to Wnt, rather than TCR or cytokine-derived signals. Whereas the identity and source of Wnt ligands that contribute to the longevity of CD8+ memory remain to be elucidated, it has been shown that macrophages and in vitro differentiated dendritic cells can produce Wnt7b and Wnt5a, respectively (Lehtonen et al., 2007; Lobov et al., 2005) and that multiple Wnt transcripts including Wnt 1, 2B, 4, 5A, and 8B were detected in vascular endothelial cells (Wu et al., 2007). Our findings, along with other studies, raised an intriguing possibility that Wnt morphogenic proteins may act upstream of the known Eomes-IL-2Rβ axis to confer cytokine responsiveness to memory CD8+ T cells. The potential roles of Wnt agonists in regulating immunological memory may be explored to improve vaccination regimens and enhance tumor immunotherapy.
EXPERIMENTAL PROCEDURES
Animals and Infectious Agents
TCF-1-null mice, p45 TCF-1 isoform transgenic mice, and stabilized β-catenin transgenic mice were as described (Ioannidis et al., 2001; Verbeek et al., 1995; Xie et al., 2005). Thy1.1+ or Thy1.2+ OT-I TCR transgenic mice, and B6.SJL mice were from Jackson Laboratories. Attenuated or virulent LM-Ova, VacV-Ova, and their usage were as described (Zhao et al., 2010). All the animals were housed and handled following protocols approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Cell Isolations and Flow cytometry
Isolation and adoptive transfer of WT or Tcf7-/- OT-I cells, infection of B6.SJL recipients were described previously (Badovinac and Harty, 2007). Isolation of lymphocytes from the livers and lungs was carried out using an established protocol as described (Masopust et al., 2001). Identification and characterization of antigen-specific T cells were performed using cell surface, intracellular, or intranuclear staining following standard protocols (Intlekofer et al., 2007; Zhao et al., 2010). Detection of BrdU uptake and activated Caspsase-3/7 was as described (Zhao et al., 2010). Detailed methods are in Supplemental Experimental Procedures.
Chromatin immunoprecipitation
Splenic CD8+ T cells were isolated from WT, Tcf7-/-, or β-catenin transgenic mice, cross-linked, and sonicated to generate chromatin fragments as previous described (Yu et al., 2010). The chromatin was immunoprecipitated with antibodies against TCF-1 (C63D9) and TLE1/2/3/4 (both from Cell Signaling Technology), β-catenin (clone 14, BD Biosciences), or respective normal rabbit or mouse IgG. Each DNA segment of interest was quantitatively determined by real-time PCR (Yu et al., 2010), and abundance of the segment by an antibody was first normalized to that by control IgG, followed by normalization to the Rag2 promoter region to calculate the relative enrichment for the segment.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs Hans Clevers (Hubrecht Institute, the Netherlands), Werner Held (Ludwig Institute for Cancer Research, Switzerland), and Zuomin Sun (University of Illinois) for providing Tcf7-/-, p45 Tg, and β-catenin Tg mice, respectively. We thank Dr. Steven Reiner (University of Pennsylvania) for providing Eomes cDNA, Dr. Justin Fishbaugh and George Rasmussen (Flow Cytometry Core) for advice and assistance with cell sorting, Garry Hauser and Tom Bair (DNA Facility) for microarray and data analysis, Dr. Susan Kaech (Yale University) for sharing the protocol for retroviral infection of effector T cells, and Dr. Kevin Legge and Jodi McGill (University of Iowa) for advice on IL-15Rα staining. This study is supported in part by NIH grants to H.H.X. (AI077504, AI080966, and HL095540), to V.P.B (AI083286), and to J.T.H. (AI046653, AI042767, AI050073, and AI059752). The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL DATA
Supplemental Data include Supplemental Experimental Procedures, 1 Supplemental Table, and 6 Supplemental Figures.
DATA ACCESSION NUMBERS
Microarray data analyzing differential gene expression in WT and Tcf7-/- memory T cells have been deposited at the NCBI Gene Expression Omnibus (GEO), accession number GSE20754.
REFERENCES
- Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179:53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- Bianchi T, Gasser S, Trumpp A, MacDonald HR. c-Myc acts downstream of IL-15 in the regulation of memory CD8 T-cell homeostasis. Blood. 2006;107:3992–3999. doi: 10.1182/blood-2005-09-3851. [DOI] [PubMed] [Google Scholar]
- Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Ioannidis V, Beermann F, Clevers H, Held W. The beta-catenin--TCF-1 pathway ensures CD4(+)CD8(+) thymocyte survival. Nat Immunol. 2001;2:691–697. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]
- Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. Cutting edge: differential self-peptide/MHC requirement for maintaining CD8 T cell function versus homeostatic proliferation. J Immunol. 2005;175:4829–4833. doi: 10.4049/jimmunol.175.8.4829. [DOI] [PubMed] [Google Scholar]
- Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0914127107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Lefrancois L, Marzo AL. The descent of memory T-cell subsets. Nat Rev Immunol. 2006;6:618–623. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]
- Lehtonen A, Ahlfors H, Veckman V, Miettinen M, Lahesmaa R, Julkunen I. Gene expression profiling during differentiation of human monocytes to macrophages or dendritic cells. J Leukoc Biol. 2007;82:710–720. doi: 10.1189/jlb.0307194. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–169. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- Prlic M, Lefrancois L, Jameson SC. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J Exp Med. 2002;195:F49–52. doi: 10.1084/jem.20020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Weerkamp F, Baert MR, van den Burg CM, van Noort M, de Haas EF, van Dongen JJ. Wnt target genes identified by DNA microarrays in immature CD34+ thymocytes regulate proliferation and cell adhesion. J Immunol. 2004;172:1099–1108. doi: 10.4049/jimmunol.172.2.1099. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Clevers H. Sequence-specific interaction of the HMG box proteins TCF-1 and SRY occurs within the minor groove of a Watson-Crick double helix. EMBO J. 1992;11:3039–3044. doi: 10.1002/j.1460-2075.1992.tb05374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176:1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Huang Z, Sadim MS, Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. J Immunol. 2005;175:7981–7988. doi: 10.4049/jimmunol.175.12.7981. [DOI] [PubMed] [Google Scholar]
- Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- Yu S, Zhao DM, Jothi R, Xue HH. Critical requirement of GABPa for normal T cell development. J. Biol. Chem. 2010;285:10179–10188. doi: 10.1074/jbc.M109.088740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat Immunol. 2009;10:992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DM, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue HH. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.