Abstract
Untreated adult nonalcoholic fatty liver disease (NAFLD) is characterized by absent or mild portal chronic inflammation (CI); in the portal-based pediatric pattern of NAFLD, portal CI may be a predominant component. This study was undertaken to correlate clinical features with portal CI in the subjects enrolled in the NASH CRN.
Methods
Histology from central grading and clinical parameters temporally related to the biopsy were evaluated from 728 adults and 205 children.
Results
Sixty percent of adult biopsies had mild, 23% had more than mild, and 16% had no portal CI. In children, 76% had mild, 14% were more than mild, and 10% had no portal CI. In neither group were autoantibodies, elevated ALT, or generic use of “any” medications associated with the presence or degree of portal CI. Clinical features associated with “more than mild” in adults were older age (51 y v 44 y) (p<0.0001), female gender (p=0.001), higher BMI (p<0.0001), elevated insulin levels (median 20 v 14uU/ml) (p=0.001), higher HOMA-IR (median 5 v 3) (p<0.0001), and medications used for NAFLD (p=0.0004), diabetes (p<0.0001), and hypertension (p<0.0001). The same comparisons for “more than mild” v “none” in the pediatric biopsies showed only an association with younger age (12 y v 14 y) (p=0.01), but there was a trend favoring boys. There was no association with BMI, insulin or HOMA-IR. In both groups, lobular and portal inflammation scores had no association, but there was an association with a definite steatohepatitis diagnosis (p<0.0001 for both). Features in the adult biopsies associated with “more than mild” were steatosis amount (p=0.01and location (p<0.0001), presence of ballooning (p<0.0001), and advanced fibrosis (p<0.0001). In the pediatric biopsies, “more than mild” compared with “none” was associated with steatosis location (p=0.0008), and fibrosis score (p<0.0001), specifically, the pediatric (zone 1 accentuation) pattern (p<0.001) and portal/periportal fibrosis (or more advanced fibrosis) (p<0.01).
Conclusions
Increased portal CI is associated with many clinical and pathologic features of progressive NAFLD in both adults and children, but not with ALT, autoantibodies, or lobular inflammation. The presence of more than mild portal CI in liver biopsies of untreated NAFLD may be considered a marker of clinically and histologically advanced disease.
Introduction
Nonalcoholic fatty liver disease (NAFLD) remains a clinico-pathologic diagnosis; following certain clinical exclusions, NAFLD is traditionally histologically characterized in both adults and children by the presence of > 5% large droplet steatosis, with or without accompanying lobular inflammatory infiltrates. In adults, further findings may be zone 3 predominance of steatosis, and hepatocellular ballooning with or without intracytoplasmic inclusions, now known as Mallory-Denk bodies1. Collectively, the lesions of steatosis, hepatocyte injury (ballooning) and parenchymal inflammation are referred to as “nonalcoholic steatohepatitis” (NASH). When fibrosis occurs in adult NASH, the deposition of collagen occurs initially in the perisinusoidal space of Disse, also in zone 3; with progression, portal and periportal fibrosis may occur. In children (defined as up to 18 years of age) with clinically-defined NAFLD, three “broad” histologic patterns of injury have been described: an adult-like NASH (so-called “type 1”); “type 2” NASH with either zone 1 (periportal) predominance or panacinar large droplet steatosis, less or no ballooning, and presence of portal inflammation and fibrosis; and a pattern of “overlap” with features of both 2, 3. Thus, pediatric NAFLD may or may not manifest hepatocellular ballooning, zone 3 perisinusoidal fibrosis, and may have collagen deposition involving only the portal and periportal regions, prior to bridging fibrosis.
In contrast with the frequently mixed acute and chronic inflammatory infiltrates that may occur in the lobules in NAFLD, portal inflammation is comprised of cells referred to as “chronic”, i.e. lymphocytes, plasma cells, occasional eosinophils, monocytes 4. Investigators in NAFLD have noted that the “disproportionate” amount of portal chronic inflammation (CI) in NASH may be a harbinger of the possibility of another, concurrent chronic liver disease5, as recently reviewed 6. In fact, this was shown in large series that documented the co-existence of not only HCV 5, 7, 8, but also PBC, AIH and other forms of serologically diagnosed chronic liver disease with NAFLD or NASH 5.
A third group of NAFLD subjects in whom prominent portal chronic inflammation has been noted is successfully treated patients with either medical or surgical (bariatric) intervention in whom follow-up liver biopsies have been reported 9. This was specifically highlighted as a proportionate increase in portal:lobular inflammation in a blinded review of subjects’ biopsies in a trial of rosiglitazone10. No change or relatively less change in portal chronic inflammation compared with improvement of other components of steatohepatitis 11, 12, or notable portal inflammation in the setting of otherwise histologically successful outcomes 13, 14 have been reported.
The current study was undertaken to analyze contemporaneous clinical and biopsy data from a large cohort of well-characterized adult and pediatric NAFLD subjects, focused on portal CI. We assessed clinical correlations with the degree of portal CI, as had been documented by the central reviews of the Pathology Committee of the NASH CRN. Specifically, the study focused on factors that influenced the degree of portal CI. The study highlights clinical and histologic differences between adults and children, validates observations of pediatric pattern(s) of disease, and indicates that portal CI is a marker of advanced disease. In addition, the study found no correlation of the presence of portal CI with elevated ALT or with scores for lobular inflammation, indicating differing stimuli for portal and lobular inflammation in NAFLD.
Materials and Methods
The Pathology Committee of the NASH Cooperative Research Network (CRN) consists of one pathologist from each Clinical Center and one from the National Cancer Institute (NCI); the group meets on a quarterly basis for central, blinded assessment of all liver biopsies regularly submitted for the Database (Natural history) Study and the two treatment trials in the NASH CRN: the Pioglitazone Vitamin E for NASH (PIVENS) trial (adults) and the Treatment of NAFLD in Children (TONIC) trial (children). Biopsies are rigorously evaluated according to the published, validated feature based scoring system15. Criteria for portal CI evaluated during central review have been modified to “none”, “mild”, “more than mild”. Biopsies categorized as “none” had either no inflammation or only rare inflammatory cells identified at high magnification in any portal area. Mild portal CI is defined as a few mononuclear cells, usually, but not always, in more than one portal tract. Any lymphoid aggregates would disqualify as “mild”._“More than mild” was scored when at least one portal area showed a moderate to marked density of inflammation,and/or the presence of lymphoid aggregates. These categorizations of portal CI purposefully do not rely on absolute cell counts, as that is neither practical, nor how assessments are made in daily practice. Diagnostic categories utilized are: definite steatohepatitis, definitely not steatohepatitis, borderline steatohepatitis (zone 3 pattern) and borderline steatohepatitis (zone 1 pattern). The two borderline categories refer to histologic changes that fulfill some, but not all, for definite steatohepatitis, and further reflect a zonal accentuation of lesions.
Only laboratory values collected less than six months prior to or up to one month after the date of biopsy were used in the analysis. Recorded age was calculated for the age at the time of the biopsy . Body mass index (BMI) was categorized as <30 kg/m2, 30-34 kg/m2, and >35 kg/m2. ANA and ASMA were categorized as positive or negative, where ANA≥1:40 was positive and ASMA≥1:20 was positive. The homeostasis model assessment method for insulin resistance (HOMA-IR) was calculated as (fasting insulin (μU/mL)*fasting glucose (mmol/L)/22.5) 16
Information related to medication use was obtained from a baseline medical history collected at the time of entry into the CRN. Medication histories ranged from 3 months to 2 years prior to the biopsy. For the sake of assessment, not all medications were considered, but only those that could have had a potentially positive impact on treating NAFLD, those that may be predisposing to NAFLD, and multivitamins. These were then further considered in subgroups for ease of analysis: medications that have been historically considered related to and/or potentially causative of NAFLD 17; medications used to treat diabetes; medications that may be used to treat NAFLD; medications for cardiovascular disease, hypertension and hyperlipidemia; and medications used to treat obesity. These can be seen in Supplementary Table 1. Statistical analyses: P-values were determined by Fisher’s exact test for categorical variables and Wilcoxon rank sum test for continuous variables. Odds ratios and 95% confidence intervals were determined from multiple ordinal logistic regression analysis. Models were adjusted for age at biopsy, race and ethnicity, and gender. Analyses were performed using SAS statistical software (version 8, SAS Institute, Cary, NC) and Stata (Release 10, StataCorp, College Station, TX).
Results
Seven hundred twenty eight adult biopsies and 205 pediatric biopsies that had undergone Central review formed the subjects for this analysis. In the adult biopsies, 481 were from the Database study and 247 from PIVENS, while in the pediatric biopsies, 98 were from Database and 107 from TONIC.
In the adult biopsies, 16% had none, 60% had mild and 23% had more than mild portal chronic inflammation. In the pediatric biopsies, 10% had none, 76% had mild and 14% had more than mild portal chronic inflammation. Clinical and histologic features that were associated with portal chronic inflammation in adults are shown in Tables 1 and 2; those for pediatric biopsies are in Tables 3 and 4, respectively.
Table 1. Baseline patient characteristics by portal inflammation status, adults only (N=728).
| Portal Inflammation | P-value* | ||||
|---|---|---|---|---|---|
| None (N=117) | Mild (N=440) | More than mild (N=171) | None vs. Mild | None vs. more than mild | |
| Age at biopsy (yrs) | 44.0 ∀ 11.4 | 47.7 ∀ 11.7 | 51.4 ∀ 10.5 | 0.001 | <0.0001 |
| Gender | 0.03 | 0.001 | |||
| Male | 57 (48.7%) | 166 (37.7%) | 50 (29.2%) | ||
| Female | 60 (51.3%) | 274 (62.3%) | 121 (70.8%) | ||
| Race** | 0.13 | 0.004 | |||
| White | 94 (84.74) | 335 (83.5%) | 152 (92.1%) | ||
| Black | 0 (0%) | 11 (2.6%) | 5 (3.0%) | ||
| Asian or Pacific Islander | 12 (10.8%) | 25 (5.9%) | 4 (2.4%) | ||
| American Indian or Alaska Native | 2 (1.8%) | 17 (4.0%) | 3 (1.8%) | ||
| More than 1 race reported | 3 (2.7%) | 17 (4.0%) | 1 (0.6%) | ||
| Ethnicity | |||||
| Hispanic or Latino | 14 (12.0%) | 53 (12.1%) | 19 (11.1%) | 1.0 | 0.85 |
| BMI (kg/m2) | 0.02 | <0.0001 | |||
| <30 | 49 (41.9%) | 128 (29.2%) | 27 (15.8%) | ||
| 30-34 | 36 (30.8%) | 137 (31.3%) | 48 (28.1%) | ||
| >35 | 32 (27.4%) | 173 (39.5%) | 96 (56.1%) | ||
| ALT (U/L), median(IQR) | 73 (56-105) | 63 (45-96) | 71 (46-102) | 0.17 | 0.45 |
| ANAI | 0.19 | 0.66 | |||
| Negative | 89 (76.1%) | 351 (81.8%) | 131 (78.9%) | ||
| Positive | 28 (23.9%) | 78 (18.2%) | 35 (21.1%) | ||
| ASMA’ | 0.43 | 0.86 | |||
| Negative | 97 (85.1%) | 373 (88.0%) | 136 (86.1%) | ||
| Positive | 17 (14.9%) | 51 (12.0%) | 22 (13.9%) | ||
| Insulin (μU/mL),median (IQR) | 14 (10-23) | 19 (12-28) | 20 (12-32) | 0.001 | 0.001 |
| HOMA-IR&, median(IQR) | 3 (2-6) | 5 (3-7) | 5 (3-10) | 0.001 | <0.0001 |
| Use of any medications | 115 (98.3%) | 437 (99.3%) | 169 (98.8%) | 0.28 | 1.0 |
| Use of meds associated w/worsening of NAFLD | 10 (8.9%) | 50 (11.4%) | 24 (14.0%) | 0.50 | 0.19 |
| Medications used to treat/effect: | |||||
| Diabetes | 17 (14.5%) | 99 (22.5%) | 72 (42.1%) | 0.07 | <0.0001 |
| NAFLD | 13 (11.1%) | 92 (20.9%) | 49 (28.7%) | 0.02 | 0.0004 |
| Obesity | 0 (0%) | 15 (3.4%) | 2 (1.2%) | 0.05 | 0.52 |
| Cardiovascular/Hypertension | 42 (35.9%) | 232 (52.7%) | 106 (62.0%) | 0.001 | <0.0001 |
| Hyperlipidem ia | 33 (28.2%) | 143 (32.5%) | 61 (35.7%) | 0.43 | 0.20 |
| All other medical conditions | 113 (96.6%) | 430 (97.7%) | 168 (98.3%) | 0.51 | 0.45 |
Note: Values are means ∀ SD or N=s (%)
P-values calculated from Fisher=s Exact Test for categorical values and Wilcoxon rank sum test for continuous variables
27 patients did not report race
Only ALT values collected 6 months prior to liver biopsy or 1 month after liver biopsy were included in analysis (N=266)
ANA: Negative: <1:40, Positive: ∃1:40
ASMA: Negative: <1:20, Positive: ∃1:20
HOMA-IR: Homeostasis model assessment method for insulin resistance, calculated as (fasting insulin (μU/mL)*fasting glucose (mmol/L)/22.5
Table 2. Baseline histologic characteristics by portal inflammation status, adults only (N=728).
| Portal Inflammation | P-value* | ||||
|---|---|---|---|---|---|
| None (N=117) | Mild (N=440) | More than mild (N=171) | None vs. mild | None vs. more than mild | |
| Steatosis amount | 0.66 | 0.01 | |||
| <5% | 5 (4.3%) | 24 (5.5%) | 13 (7.6%) | ||
| 5-33% | 43 (36.8%) | 160 (36.4%) | 91 (53.2%) | ||
| 34-66% | 42 (35.9%) | 135 (30.7%) | 40 (23.4%) | ||
| >66% | 27 (23.1%) | 121 (27.5%) | 27 (15.8%) | ||
| Steatosis location | 0.003 | <0.0001 | |||
| Zone 3 (Central) | 67 (57.3%) | 169 (38.6%) | 28 (16.4%) | ||
| Zone 1 (Periportal) | 0 (0%) | 4 (0.9%) | 4 (2.3%) | ||
| Azonal | 23 (19.7%) | 103 (23.5%) | 89 (52.1%) | ||
| Panacinar | 27 (23.1%) | 162 (37.0%) | 50 (29.2%) | ||
| Lobular inflammation score | 0.47 | 0.28 | |||
| 0 | 2 (1.7%) | 2 (0.5%) | 0 (0%) | ||
| <2 under 20x mag | 57 (48.7%) | 220 (50.0%) | 75 (43.9%) | ||
| 2-4 under 20x mag | 44 (37.6%) | 171 (38.9%) | 69 (40.4%) | ||
| >4 under 20x mag | 14 (12.0%) | 47 (10.7%) | 27 (15.8) | ||
| Ballooning | <0.0001 | <0.0001 | |||
| None | 58 (49.6%) | 147 (33.4%) | 26 (15.2%) | ||
| Few | 36 (30.8%) | 121 (27.5%) | 43 (25.2%) | ||
| Many | 23 (19.7%) | 172 (39.1%) | 102 (59.7%) | ||
| Fibrosis score | <0.0001 | <0.0001 | |||
| None | 47 (40.2%) | 109 (25.2%) | 10 (6.0%) | ||
| Mild, zone 3 perisinusoidal | 32 (27.4%) | 73 (16.9%) | 6 (3.6%) | ||
| Moderate, zone 3, perisinusoidal | 21 (18.0%) | 44 (10.2%) | 5 (3.0%) | ||
| Portal/periportal only | 2 (1.7%) | 22 (5.1%) | 6 (3.6%) | ||
| Zone 3 and periportal, any combination | 10 (8.6%) | 87 (20.1%) | 38 (22.6%) | ||
| Bridging | 4 (3.4%) | 73 (16.9%) | 60 (35.7%) | ||
| Cirrhosis | 1 (0.9%) | 24 (5.6%) | 43 (25.6%) | ||
| Biopsy Diagnosis | 0.002 | <0.0001 | |||
| Definitely not Steatohepatitis | 41 (35.0%) | 93 (21.2%) | 20 (11.7%) | ||
| Suspicious/borderline/indeterminate: Zone 3 pattern | 29 (24.8%) | 89 (20.3%) | 22 (12.9%) | ||
| Suspicious/borderline/indeterminate: Zone 1, periportal pattern | 0 (0%) | 3 (0.7%) | 5 (2.9%) | ||
| Definite Steatohepatitis | 47 (40.2%) | 254 (57.9%) | 124 (72.5%) | ||
Note: Values are N=s (%)
P-values calculated using Fisher=s Exact test
Table 3. Baseline patient characteristics by portal inflammation status, children only (N=205).
| Portal Inflammation | P-value* | ||||
|---|---|---|---|---|---|
| None (N=20) | Mild (N=156) | More than mild (N=29) | None vs. Mild | None vs. more than mild | |
| Age at biopsy (yrs) | 13.6 ∀ 2.0 | 13.0 ∀ 2.5 | 11.9 ∀ 2.3 | 0.25 | 0.01 |
| Gender | 0.11 | 0.24 | |||
| Male | 11 (55.0%) | 116 (74.4%) | 21 (72.4%) | ||
| Female | 9 (45.0%) | 40 (25.6%) | 8 (27.6%) | ||
| Race ** | 0.32 | 0.002 | |||
| White | 17 (100%) | 107 (75.4%) | 16 (57.1%) | ||
| Black | 0 (0%) | 3 (2.1%) | 0 (0%) | ||
| Asian or Pacific Islander | 0 (0%) | 6 (4.2%) | 2 (7.1%) | ||
| American Indian or Alaska Native | 0 (0%) | 22 (15.5%) | 10 (35.7%) | ||
| More than 1 race reported | 0 (0%) | 4 (2.8%) | 0 (0%) | ||
| Ethnicity** | |||||
| Hispanic or Latino | 12 (60.0%) | 85 (54.8%) | 18 (62.1%) | 0.81 | 1.0 |
| BMI (kg/m2) | 0.46 | 0.57 | |||
| <30 | 7 (35.0%) | 56 (36.1%) | 6 (20.7%) | ||
| 30-34 | 8 (40.0%) | 43 (27.7%) | 14 (48.3%) | ||
| >35 | 5 (25.0%) | 56 (36.1%) | 9 (31.0%) | ||
| ALT(U/L)H, median (IQR) | 95 (71-152) | 101 (78-139) | 114 (69-188) | 1.0 | 0.58 |
| ANAI | 1.0 | 1.0 | |||
| Negative | 16 (84.2%) | 129 (84.9%) | 24 (82.8%) | ||
| Positive | 3 (15.8%) | 23 (15.1%) | 5 (17.2%) | ||
| ASMA’ | 0.81 | 1.0 | |||
| Negative | 11 (57.9%) | 71 (53.0%) | 14 (56.0%) | ||
| Positive | 8 (42.1%) | 63 (47.1%) | 11 (44.0%) | ||
| Insulin (μU/mL), median (IQR) | 23 (15-44) | 27 (17-41) | 24 (12-43) | 0.68 | 0.85 |
| HOMA-IR&, median (IQR) | 5 (3-11) | 6 (4-9) | 5 (2-9) | 0.76 | 0.70 |
| Use of any medications | 19 (95.0%) | 128 (82.1%) | 24 (82.8%) | 0.20 | 0.38 |
| Use of meds associated w/w orsening of NAFLD | 0 (0%) | 13 (8.3%) | 1 (3.5%) | 0.37 | 1.0 |
| Medications used to treat/effect: | |||||
| Diabetes | 5 (25.0%) | 8 (5.1%) | 1 (3.5%) | 0.008 | 0.03 |
| NAFLD | 5 (25.0%) | 12 (7.7%) | 1 (3.5%) | 0.03 | 0.03 |
| Obesity | 1 (5.0%) | 2 (1.3%) | 1 (3.5%) | 0.31 | 1.0 |
| Hyperlipidemia | 1 (5.0%) | 3 (1.9%) | 0 (0%) | 0.39 | 0.41 |
| All other medical conditions | 17 (85.0%) | 118 (75.6%) | 24 (82.8%) | 0.57 | 1.0 |
| Multivitamin | 8 (40.0%) | 22 (14.1%) | 6 (20.7%) | 0.008 | 0.20 |
Note: Values are means ∀ SD or N=s (%)
P-values calculated from Fisher=s Exact Test for categorical variables and Wilcoxon rank sum test for continuous variables
18 patients did not report race; 1 patient did not report Hispanic ethnicity
Only ALT values collected 6 months prior to liver biopsy or 1 month after liver biopsy were included in analysis (N=114)
ANA: Negative: <1:40, Positive: ∃1:40
ASMA: Negative: <1:20, Positive: ∃1:20
HOMA-IR: Homeostasis model assessment method for insulin resistance, calculated as (fasting insulin (μU/mL)*fasting glucose (mmol/L)/22.5
Table 4. Baseline histologic characteristics by portal inflammation status, children only (N=205).
| Portal Inflammation | P-value* | ||||
|---|---|---|---|---|---|
| None (N=20) | Mild (N=156) | More than mild (N=29) | None vs. mild | None vs. more than mild | |
| Steatosis amount | 0.20 | 1.0 | |||
| <5% | 1 (5.0%) | 1 (0.6%) | 1 (3.5%) | ||
| 5-33% | 8 (40.0%) | 44 (28.2%) | 11 (37.9%) | ||
| 34-66% | 5 (25.0%) | 51 (32.7%) | 9 (31.0%) | ||
| >66% | 6 (30.0%) | 60 (38.5%) | 8 (27.6%) | ||
| Steatosis location | <0.001 | 0.008 | |||
| Zone 3 (Central) | 12 (60.0%) | 34 (21.8%) | 5 (17.2%) | ||
| Zone 1 (Periportal) | 1 (5.0%) | 25 (16.0%) | 7 (24.1%) | ||
| Azonal | 4 (20.0%) | 12 (7.7%) | 5 (17.2%) | ||
| Panacinar | 3 (15.0%) | 85 (54.5%) | 12 (41.4%) | ||
| Lobular inflammation score | 0.37 | 0.55 | |||
| 0 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| <2 under 20x mag | 13 (65.0%) | 75 (48.1%) | 14 (48.3%) | ||
| 2-4 under 20x mag | 6 (30.0%) | 71 (45.5%) | 12 (41.4%) | ||
| >4 under 20x mag | 1 (5.0%) | 10 (6.4%) | 3 (10.3%) | ||
| Ballooning | 0.34 | 0.35 | |||
| None | 11 (55.0%) | 80 (51.3%) | 14 (48.3%) | ||
| Few | 8 (40.0%) | 49 (31.4%) | 9 (31.0%) | ||
| Many | 1 (5.0%) | 27 (17.3%) | 6 (20.7%) | ||
| Fibrosis score | 0.002 | <0.0001 | |||
| None | 13 (65.0%) | 43 (27.9%) | 2 (7.1%) | ||
| Mild, zone 3 perisinusoidal | 3 (15.0%) | 7 (4.6%) | 1 (3.6%) | ||
| Moderate, zone 3, perisinusoidal | 2 (10.0%) | 7 (4.6%) | 0 (0%) | ||
| Portal/periportal only | 0 (0%) | 48 (31.2%) | 13 (46.4%) | ||
| Zone 3 and periportal, any combination | 1 (5.0%) | 30 (19.5%) | 1 (3.6%) | ||
| Bridging | 1 (5.0%) | 18 (11.7%) | 10 (35.7%) | ||
| Cirrhosis | 0 (0%) | 1 (0.7%) | 1 (3.6%) | ||
| NASH diagnosis | 0.008 | <0.0001 | |||
| No NASH | 10 (50.0%) | 39 (25.2%) | 2 (6.9%) | ||
| Suspicious/borderline/indeterminate: Zone 3 pattern | 3 (15.0%) | 26 (16.8%) | 7 (24.1%) | ||
| Suspicious/borderline/indeterminate: Zone 1, periportal pattern | 0 (0%) | 43 (27.7%) | 14 (48.3%) | ||
| Yes, definite | 7 (35.0%) | 47 (30.3%) | 6 (20.7%) | ||
Note: Values are N=s (%)
P-values calculated using Fisher=s Exact Test
Demographic and Clinical Features
Adults
In comparison to patients with no portal CI, those with more than mild portal inflammation were older (51 ± 11y vs 44 ± 11y, p<0.0001), more often women (71% vs 51%, p=0.001), and had higher BMIs (56% vs. 27% had BMI> 35 kg/m2, p<0.0001). The patients with more than mild portal chronic inflammation had higher insulin values compared with those with none (median of 20 μU/ml vs 14 μU/ml, p = 0.001) as well as calculated index of insulin resistance, HOMA-IR score (median 5 vs 3, p < 0.0001), compared to those with none. Findings were similar when comparing patients with no portal inflammation to those with mild portal inflammation. (Table 1) There were no significant differences comparing none to mild or none to more than mild for serum ALT (p=0.17 and p=0.45, respectively), presence or absence of serum ANA (p=0.19 and p=0.66, respectively), or ASMA (p=0.43 and p=0.86, respectively).
Analysis of patients according to medication groups showed that those associated with more than mild portal inflammation v. none were medications used to treat diabetes (42% vs. 15%, p< 0.0001), NAFLD (29% vs. 11%, p= 0.0004), and cardiovascular/hypertensive conditions (62% vs. 36%, p<0.0001). No effect on the amount of portal CI was noted with use of medications associated with potentiating NAFLD (p = 0.19) or treating hyperlipidemia (p=0.20). In comparison, the medication groups associated with mild portal inflammation v none were medications used to treat NAFLD (21% vs. 11%, p=0.02), and cardiovascular/hypertensive conditions (53% vs. 36%, p=0.001). There was a trend toward significance for diabetic medications (p=0.07) and anti-obesity medications (p=0.05).
To explore the possibility that increased portal CI was a potential manifestation of use of “any” medication, analyses for the use of medications for all other, nonNAFLD-related medical conditions, as well as an analysis of “any medication use” were done. These failed to show differences in the spectrum of portal CI. The exception to this was the finding that all 17 of the individuals taking anti-obesity medications had at least mild portal CI; 15 had mild portal chronic inflammation (p=0.05), and two had greater than mild portal chronic inflammation, which was not statistically significant (p=0.52).
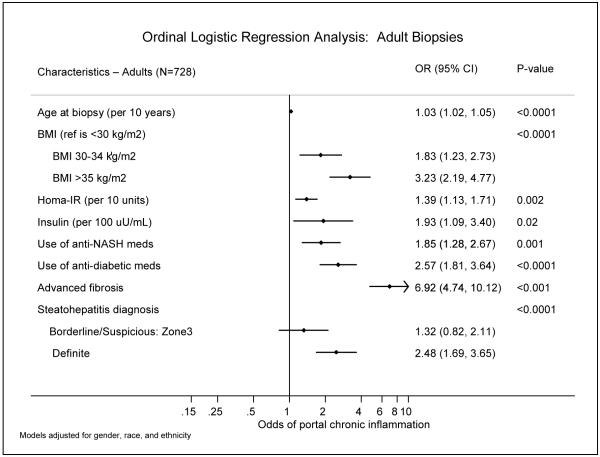
Figure 1 shows the demographic and clinical features associated with increased portal CI in adults from adjusted ordinal logistic regression models. Increased age (OR=1.03, p<0.0001), higher BMI (OR=3.12 for BMI>35 kg/m2, p<0.0001), higher insulin values (OR=2.06 per 100 uU/mL, p=0.01), increased HOMA-IR (OR=1.39 per 10 unit increase, p=0.002) were all associated with increased portal CI. In addition, use of anti-diabetic medications (OR=2.38, p<0.0001) and anti-NAFLD medications (OR=1.72, p=0.003) were associated with increased portal CI.
Figure 1.
Pediatrics
The age range for pediatric patients in the NASH CRN is 6-17 years; the mean age is 12.9 ±2.4 years. In comparison to patients with no portal CI, and in contrast with adults, pediatric patients with more than mild portal CI were younger (12 ± 2y vs 14 ± 2y, p=0.01). There was no difference in age comparing the patients with mild portal inflammation to those with none (p=0.25) Race was analyzed and categorized as follows: white, black, Asian or Pacific Islander, American Indian or Alaska Native, or more than one race. Hispanic ethnicity was also added as a separate variable. The results are shown in Tables 3 and and 4, and Figure 2.
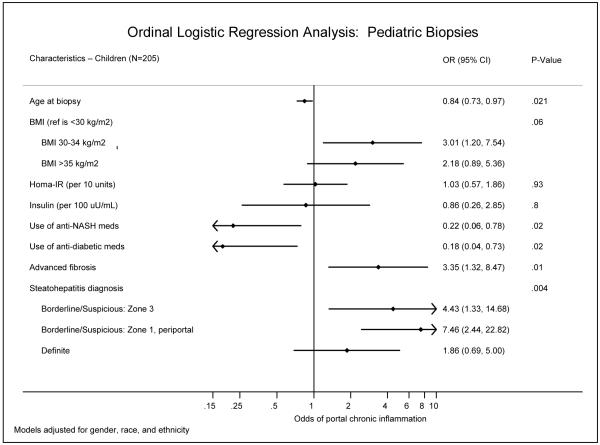
Figure 2.
There was not a significant association between Hispanic ethnicity and portal chronic inflammation in pediatric patients. However, the distribution of race was significantly different by portal CI category, with 36% of children with more than mild portal CI being American Indian or Alaska Native vs. 0% in the no portal CI group (p=0.002). The majority of patients who reported American Indian or Alaska Native race also reported Hispanic(vs non-Hispanic) ethnicity.
Although there was a clear trend of more than mild portal CI seen in boys compared with girls, this was not statistically significant (p=0.24). Also unlike adults, there were no differences among the biopsy groups in regard to BMI (p=0.57), insulin values (p=0.85) or calculated index of insulin resistance, HOMA-IR (p=0.70). Similarly, no differences were found for these characteristics when comparing the none v mild portal CI groups. As was found in the adult patients, the serum ALT values, ANA and ASMA tests had no relationship to the evaluation of none v more than mild portal CI in the corresponding biopsies (p=0.58, 0.1.0, 1.0 respectively) or none v mild portal CI (p=1.0, p=1.0, p=0.81, respectively).
The two other groups of pediatric patients in whom biopsies had significant differences were small, those using medications for either diabetes (n=14) or NAFLD (n=18). Unlike in adults, the use of these medications was inversely related to portal CI; 25% of the patients with no portal CI had a history of taking diabetic meds, compared to 5% of the mild portal CI group (p=0.008) and 4% of the more than mild group (p=0.03). Similarly, 25% of the no portal CI patients had a history of taking medications for NAFLD, compared to 8% of the mild portal CI group (p=0.03) and 4% of the more than mild group (p=0.03). Also, the adjusted odds of increased portal CI was lower among patients with a history of diabetes medications (OR=0.2, p=0.008) and NAFLD medications (OR=0.3, p=0.02) (Figure 2). Only 4 pediatric patients each were listed as taking anti-obesity medications or treatment for hyperlipidemia. This is considered too few for meaningful analysis, however, we observed that results for biopsy findings in the 4 were across the spectrum of portal CI. In patients with no portal inflammation, 40% were taking multivitamins as compared with 14% of those with mild portal inflammation (p=0.008). No associations were seen amongst the 14 pediatric patients taking medications that can exacerbate NAFLD, or among those taking “any” medications.
Histologic Correlations
Adults
Seventy-seven percent of the 171 biopsies with more than mild portal CI had 5-66% steatosis; 81% of the biopsies had steatosis that was either azonal or panacinar; none were without lobular inflammation, but only 16% had >4/20X; 60% had 2+ ballooning and 26% had cirrhosis. Thirty-six percent had bridging fibrosis and 23% had stage 2 (a combination of zone 3 perisinusoidal and periportal fibrosis).
Comparing more than mild v none portal CI in the 728 adults’ biopsies, significant histologic findings were decreased steatosis (16% vs. 23% had > 66% steatosis, p=0.01), azonal location (52% vs. 20%, p< 0.0001), increased ballooning (60% vs. 20% had many ballooned hepatocytes, p<0.0001), advanced fibrosis (60% vs. 4% had bridging fibrosis or cirrhosis, p<0.0001), and greater likelihood of definite NASH diagnosis (73% vs 40%, p<0.0001). Similar statistically significant associations were found when comparing patients with mild vs. none portal CI, except there was no association with the amount of steatosis.
Of note, lobular inflammation scores were evenly distributed among the portal CI categories and therefore had no correlative effects.
Figure 1 shows the adjusted odds ratios for the histologic features associated with increased portal CI, from ordinal logistic regression analysis. Advanced fibrosis was associated with increased portal CI (OR=6.5, p<0.001), as was steatohepatitis diagnosis (p<0.0001), where a diagnosis of definite steatohepatitis was significantly more frequent in biopsies with increased portal CI (OR=2.4).
Pediatrics
Of biopsies with more than mild portal CI, the steatosis grades were fairly evenly distributed (grade 1 38%, grade 2 31%, grade 3 28%); distribution, however favored panacinar (41%) and zone 1 (24%) over zone 3 (17%). None of the biopsies scored “0” for lobular inflammation; only 7% of the total 205 scored >4 foci/20x. The remaining 93% were in the <2/20x and 2-4/20x categories, and none of the categories had any correlation with presence or amount of portal inflammation. 10% of the 29 biopsies with more than mild portal CI were grade 3 (>4/20X). No hepatocellular ballooning was recorded in the majority of the biopsies with more than mild portal CI (48%); this was a similar score for ballooning in biopsies with an absence of portal CI (55%). In cases with “more than mild” or “mild” portal CI, 20% and 17% respectively had 2+ ballooning; (n.s.); thus, neither presence nor absence of portal CI was associated with presence or absence of ballooning in the pediatric biopsies.
Fifty eight (28%) of all the biopsies had no fibrosis and 30% had portal or periportal fibrosis without zone 3 perisinusoidal fibrosis. Five percent had only zone 3 perisinusoidal fibrosis, confirming the rarity of this pattern of fibrosis in pediatric NAFLD. 16% had stage 2 (both portal/periportal and zone 3 perisinusoidal fibrosis); 14% had bridging and 1% had cirrhosis. Thus, the majority of the cases did not have advanced (greater than stage 2) fibrosis. However, of the 31 patients with advanced fibrosis, all but one patient had mild or more than mild portal chronic inflammation (p<0.0001). The diagnostic category of borderline, zone 1 periportal pattern was associated with more than mild portal CI (48%, p<0.001)); 21% had definite steatohepatitis. In the 20 biopsies with no portal inflammation 35% were diagnosed as definite steatohepatitis and 50% as definitely not steatohepatitis; none were diagnosed as “indeterminate, zone 1,periportal pattern”.
Comparing none v more than mild showed a trend for panacinar steatosis location (p=0.06), significance for fibrosis stage > 1b (p<0.001), and diagnostic category of zone 1 periportal pattern (p<0.0001).
Figure 2 shows the adjusted odds ratios for the histologic features associated with increased portal CI. Similar to adults, advanced fibrosis was associated with increased portal CI (OR=4.5, p=0.001), as was steatohepatitis diagnosis (p=0.002), with an adjusted odds ratio for borderline zone 3 pattern of 4.5, 7.0 for the borderline, zone 1 periportal pattern and 2.2 for a definite steatohepatitis diagnosis. Further, the association between steatohepatitis diagnosis and Hispanic ethnicity showed that 35% of Hispanic patients had the borderline zone 1 pattern vs. 19% of non-Hispanic patients (p=0.03). (Table 5)
Table 5. Analysis of Diagnosis in Pediatric Biopsies by Ethnicity.
| Steatohepatitis Diagnosis | Hispanic (N=114) | Non-Hispanic (N=89) |
|---|---|---|
| None | 26 (23%) | 24 (27%) |
| Borderline, Zone 3 pattern | 22 (19%) | 14 (16%) |
| Borderline, Zone 1 pattern* | 40 (35%) | 17 (19%) |
| Definite | 26 (23%) | 34 (38%) |
P=0.03, from Fisher’s Exact Test
Borderline, zone 3 pattern and Borderline, zone 1 pattern have been defined in Materials and Methods
Discussion
Portal chronic inflammation is a lesion in nonalcoholic fatty liver disease that has not been well characterized, although it has been noted in three settings: concurrent chronic liver disease, following successful treatment intervention, and in biopsies from children. In the current study, we report results of clinico-pathologic correlations from biopsies of 728 adult and 205 pediatric subjects from the three studies of the NASH CRN. The clinical and demographic information of these well-characterized subjects was obtained within 6 months of the biopsies; all biopsy data was garnered from blinded Central Pathology review by the Pathology Committee of the NASH CRN. The primary finding of this study confirms that portal chronic inflammation may be present in liver biopsy in varying degrees from none, to mild, to “more than mild” and in both groups of patients, correlates with greater histologic severity of disease, including advanced stages of fibrosis. Further, increased portal chronic inflammation also correlated with clinical features associated with increased risk of progressive disease18-20: in adults, the lesion was more severe in women than men, and correlated with increased age, BMI and markers of insulin resistance. The fact that portal chronic inflammation did not correlate with increased BMI and markers of insulin resistance in children could be a reflection of the numbers of cases and the fact that the majority of biopsies (76%), in fact, had mild portal chronic inflammation. Significantly, in both adults and pediatric subjects, the presence of portal chronic inflammation could not be predicted by serum ALT levels, and was not associated a with, and therefore, not a spurious result of nonorgan specific autoantibodies that may occur in NAFLD21.
Significant portal chronic inflammation correlated with a definitive diagnosis of steatohepatitis in both adult and pediatric biopsies. This diagnosis, derived by a combination of the lesions of steatosis, ballooning, inflammation, and pattern of disease, is a separate exercise from the scoring of individual lesions for the NAFLD activity score. The fact that steatosis was no longer in a zone 3 distribution in adults in cases with increased portal chronic inflammation indicates the alterations that have been described with progression of disease, i.e. fibrosis, in nonalcoholic steatohepatitis. It is, in fact, well-described, that as fibrosis progresses, NASH may “lose” steatosis and lobular activity and result in cirrhosis that is otherwise considered “cryptogenic”22, 23. In children, the different histologic pattern of disease that has been formally codified as Type 22, was validated by the findings in this study: most portal chronic inflammation was associated with either panacinar or periportal, zone 1 steatosis, ballooning was not a commonly associated feature, and the lesion was most commonly associated with a diagnosis of either definite steatohepatitis or the zone 1 pattern of indefinite. In addition, when race and ethnicity were analyzed as discrete entities, children of self-reported Hispanic ethnicity did have the “borderline, zone 1 pattern” more often than those of non-Hispanic ethnicity. Furthermore, by racial analysis, children who also categorized themselves as “Hispanic ethnicity” had greater portal CI than other racial groups.
In both adults and children, lobular inflammatory scores had no association with those of portal chronic inflammation. From a mechanistic point, this observation implies distinct immunopathogenic processes in the lobules and portal tracts. This same separation can be inferred from the the semiquantitative scoring systems developed for chronic hepatitis, Knodell, Ishak, Scheuer. METAVIR, and Batts and Ludwig (reviewed in 24). Recently,there has been recognition of both the initiating and potentiating roles of the innate immune system in obesity, insulin resistance, and adipose tissue inflammation. Whether the effects of activation of the innate immune system are direct or occur through signaling pathways to result in liver tissue inflammation, is not known25. Further, to date, evaluation of portal inflammation has not been discussed distinctly from lobular inflammation in terms of innate immune system tissue injury in NAFLD/NASH.
Whether portal CI is associated with the ductular reaction and portal fibrosis, as recently shown for both chronic hepatitis C26 and NAFLD27, was not addressed in this study, however, separation of the ductular reaction and the associated inflammatory component of it makes this a challenge. Interestingly, in a study of alcoholic liver disease, portal chronic inflammation was found in 40% of biopsies, none of which were from patients with either hepatitis C or B. The strongest of 5 associations (gender, age, consumption, steatosis and fibrosis) was portal/septal fibrosis. The authors concluded that portal inflammation had a role in fibrosis in alcoholic liver disease.28
From a practical point of view, the finding does suggest a possible role for the inclusion of portal chronic inflammation in semiquantitative scoring, as proposed in an initial system29. Portal chronic inflammation was not included in the NAS15, as the feature was not statistically related to a NASH diagnosis; however, a possible reason for this is the relatively small number of cases in that study (52) compared to the current study (752).
Our study confirms some of the findings of previous clinico-pathologic studies that developed markers of prediction of fibrosis in adult NAFLD in that the predominance of increased BMI18, older age18, 20 and female gender18 were noted in our adult biopsies. However, our series did not confirm the usefulness of serologic assays for ALT.
In conclusion, the primary findings of this study confirm that the majority of biopsies with nonalcoholic fatty liver disease have at least some amount of portal chronic inflammation, either mild (60% and 76% respectively in adults and children) or more than mild (23% and 14%, respectively), and that more than mild portal inflammation correlates with advanced fibrosis. Thus, the presence of increased portal inflammation may be a marker of advanced disease in NAFLD. Interestingly, portal inflammation had no correlation with presence or amount of lobular inflammation and was not correlated to serum ALT, presence or absence of serum ANA or ASMA. Finally, in adults, “more than mild” portal chronic inflammation correlated with increased age, female gender, obesity, higher insulin levels and calculated insulin resistance, as well as use of medications for diabetes and hypertension, whereas in children, the correlations were with boys, younger children (12 y v 14 y), and lack of use of medications for diabetes, but not with obesity. The histologic findings in the children fit the increasingly recognized phenotype of zone1, portal-based injury in pediatric NAFLD30.
Supplementary Material
Acknowledgments
Source of funding
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713), and the National Institute of Child Health and Human Development (NICHD).
Several clinical centers use support from General Clinical Research Centers or Clinical and Translational Science Awards in conduct of NASH CRN Studies (grants UL1RR024989, M01RR000750, M01RR00188, RR02413101, M01RR000827, UL1RR02501401, M01RR000065, M01RR020359).
Appendix
Members of the Nonalcoholic Steatohepatitis Clinical Research Network:
Clinical Centers
Baylor College of Medicine, Houston, TX
Stephanie Abrams, MD; Diana Arceo, Denise Espinosa, Leanel Fairly
Case Western Reserve University Clinical Centers
MetroHealth Medical Center, Cleveland, OH: Arthur McCullough, MD, PI; Diane Bringman, RN, BSN; Srinivasan Dasarathy, MD; Carol Hawkins, RN; Yao-Chang Liu, MD; Nicholette Rogers, PhD, PA-C; Margaret Stager, MD
Cleveland Clinic Foundation, Cleveland, OH: Arthur McCullough, MD, PI; Srinivasan Dasarathy, MD; Kevin Edwards, NP; Ruth Sargent, LPN
Children’s Hospital & Regional Medical Center, Seattle, WA
Melissa Coffey, Karen Murray, MD; Melissa Young
Children’s National Medical Center, Washington DC
Parvathi Mohan, MD; Kavita Nair
Duke University Medical Center, Durham, NC
Manal Abdelmalek, MD; Anna Mae Diehl, MD; Marcia Gottfried, MD; Cynthia Guy, MD; Paul Killenberg, MD; Samantha Kwan, Yi-Ping Pan, Dawn Piercy, FNP; Melissa Smith
Indiana University School of Medicine, Indianapolis, IN
Prajakta Bhimalli, Naga Chalasani, MD; Oscar W. Cummings, MD; Lydia Lee, Linda Ragozzino, Raj Vuppalanchi, MD
Johns Hopkins Hospital, Baltimore, MD
Barbara Calabrese, BSN (2007); Debra Peglow, MSN, CPNP (2004-2006); Ann Scheimann, MD, MBA; Michael Torbenson, MD
Riley Hospital for Children, Indianapolis, IN
Ann Klipsch, RN; Jean Molleston, MD; Girish Subbarao, MD
St Louis University, St Louis, MO
Sarah Barlow, MD (2002-2007); Jose Derdoy, MD (2007-); Joyce Hoffmann, Debra King, RN; Joan Siegner, RN; Susan Stewart, RN; Brent A. Tetri, MD; Judy Thompson, RN
University of California San Diego, San Diego, CA
Cynthia Behling, MD; Manual Celedon, Lisa Clark, Janis Durelle, Tarek Hassanein, MD; Joel E. Lavine, MD, PhD; Susana Mendoza, Jeffrey B. Schwimmer, MD; Claude Sirlin, MD; Tanya Stein, Allison Tobin
University of California San Francisco, San Francisco, CA
Kiran Bambha, MD, Nathan M. Bass, MD, PhD; Linda D. Ferrell, MD; Danuta Filipowski, Raphael Merriman, MD (2002-2007); Mark Pabst, Monique Rosenthal, Philip Rosenthal, MD; Tessa Steel
Virginia Commonwealth University, Richmond, VA
Sherry Boyett, RN, Daphne Bryan, MD; Melissa J. Contos, MD; Michael Fuchs, MD; Martin Graham, MD; Amy Jones, Velimir AC Luketic, MD; Bimalijit Sandhu, MD; Arun J. Sanyal, MD; Carol Sargeant, RN, MPH; Kimberly Selph, Melanie White, RN
Virginia Mason Medical Center1, Seattle, WA
Grace Gyurkey, Kris V. Kowdley, MD; Jody Mooney, MS; James Nelson, PhD; Sarah Roberts, Cheryl Saunders, MPH; Alice Stead, Chia Wang, MD; Matthew Yeh, MD, PhD
Washington University, St. Louis, MO
Elizabeth Brunt, MD
Resource Centers
National Cancer Institute, Bethesda, MD
David Kleiner, MD, PhD
National Institute of Child Health and Human Development, Bethesda, MD
Gilman D. Grave, MD; Terry TK Huang, PhD, MPH
National Institute of Diabetes, Digestive and Kidney Diseases, Bethesda, MD
Edward Doo, MD; Jay Everhart, MD, MPH; Jay Hoofnagle, MD; Patricia R. Robuck, PhD (Project Scientist); Leonard Seeff, MD
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD
Patricia Belt, BS; Fred Brancati, MD, MHS; Jeanne Clark, MD, MPH; Ryan Colvin, MPH; Michele Donithan, MHS; Mika Green, MA; Rosemary Hollick (2003-2005), Milana Isaacson, Wana Kim, Alison Lydecker, Laura Miriel, Alice Sternberg, ScM; James Tonascia, PhD; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Laura Wilson, ScM; Katherine Yates, ScM
Footnotes
original grant with University of Washington
REFERENCES
- 1.Zatloukal K, Denk H, Stumptner C, et al. From Mallory to Mallory-Denk inclusion bodies: what, how and why? Experimental Cell Research. 2007;313:2033–49. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–9. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 3.Nobili V, Marcellini M, Devito R, et al. NAFLD in children: A prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–65. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 4.Brunt EM. Nonalcoholic steatohepatitis: pathologic features and differential diagnosis. Seminars in Diagnostic Pathology. 2005;22:330–8. doi: 10.1053/j.semdp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Brunt EM, Ramrakhiani S, Cordes BG, et al. Concurrence of histologic features of steatohepatitis with other forms of chronic liver disease. Mod Pathol. 2003;16:49–56. doi: 10.1097/01.MP.0000042420.21088.C7. [DOI] [PubMed] [Google Scholar]
- 6.Hubscher SG. Histological assessment of nonalcoholic fatty liver disease. Histopathology. 2006;49:450–65. doi: 10.1111/j.1365-2559.2006.02416.x. [DOI] [PubMed] [Google Scholar]
- 7.Sanyal AJ, Contos MJ, Sterling RK, et al. Nonalcoholic fatty liver disease in patients with hepatitis C is associated with features of the metabolic syndrome. Am J Gastroenterol. 2003;98:2064–71. doi: 10.1111/j.1572-0241.2003.07640.x. [DOI] [PubMed] [Google Scholar]
- 8.Ong JP, Younossi ZM, Speer C, Olano A, Gramlich T, Boparai N. Chronic hepatitis C and superimposed nonalcoholic fatty liver disease. Liver. 2001;21(4):266–71. doi: 10.1034/j.1600-0676.2001.021004266.x. [DOI] [PubMed] [Google Scholar]
- 9.Brunt EM, Clouston AD. Histologic features of fatty liver disease. In: Bataller R, Caballeria J, editors. Nonalcoholic steatohepatitis (NASH) Permanyer; Barcelona: 2007. pp. 95–110. [Google Scholar]
- 10.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improvement in nonoalcholic steatohepatitis following 48 weeks of treatment with the PPAR-g ligand rosiglitazone. Hepatology. 2003;38:1008–17. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 11.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment fo nonalcoholic steatohepatitis. Hepatology. 2004;39:188–96. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 12.Dixon JB, Bhatal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–54. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 13.Kral JG, Thung SN, Biron S, et al. Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery. 2004;135:48–58. doi: 10.1016/j.surg.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Barker KB, Palekar NA, Bowers SP, Goldberg JE, Pulcini JP, Harrison SA. Nonalcoholic steatohepatitis: effect of roux-en-y gastric bypass surgery. Am J Gastroenterol. 2006;101:368–73. doi: 10.1111/j.1572-0241.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Levy JC, Matthews DR, Herman MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 17.Farrell GC. Drugs and steatohepatitis. Semin Liver Dis. 2002;22:185–94. doi: 10.1055/s-2002-30106. [DOI] [PubMed] [Google Scholar]
- 18.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30(6):1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 19.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107(4):1103–9. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 20.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118(6):1117–23. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 21.Loria P, Lonardo A, Leonardi F, et al. Non-organ-specific autoantibodies in nonalcoholic fatty liver disease: prevalence and correlates. Digestive Disease and Science. 2003;48:2173–81. doi: 10.1023/b:ddas.0000004522.36120.08. [DOI] [PubMed] [Google Scholar]
- 22.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: Clinical characterization and risk factors for underlying disease. Hepatology. 1999;29(3):664–9. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 23.Abdelmalek M, Ludwig J, Lindor KD. Two cases from the spectrum of nonalcoholic steatohepatitis. Journal of Clinical Gastroenterology. 1995;20(2):127–30. doi: 10.1097/00004836-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Brunt EM. Grading and staging the histopathologcial lesions of chronic hepatitis: The Knodell histology activity index and beyond. Hepatology. 2000;31:241–6. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 25.Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: innate immunity in NASH. Hepatology. 2008;48:670–8. doi: 10.1002/hep.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–19. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 27.Richardson MM, Jonsson JR, Powell EE, et al. Progressive fibrosis in non-alcoholic steatohepatitis - altered regeneration and the ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Colombat M, Charlotte F, Ratziu V, Poynard T. Portal lymphcytic infiltrate in alcoholic liver disease. Hum Pathol. 2002;33:1170–4. doi: 10.1053/hupa.2002.129414. [DOI] [PubMed] [Google Scholar]
- 29.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 30.Patton HM, Sirlin C, Behling C, Middleton MS, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. Journal of Pediatric Gastroenterology & Nutrition. 2006;43:413–27. doi: 10.1097/01.mpg.0000239995.58388.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.