Introduction
Neurological injury still occurs in association with pediatric cardiac surgery, despite major progress in reducing mortality over the past two decades. Forty to 50% of 5 year olds who underwent complex open cardiac surgery as neonates and young infants have impaired neurodevelopmental outcomes; including fine and gross motor deficits, attention deficit hyperactivity disorder, and speech and language impairment.(1,2) Recent research has provided important insights into the etiologies of brain injury in this population, including brain immaturity and abnormal brain development before surgery, chromosomal and genetic influences, and a pattern of brain injury on postoperative magnetic resonance imaging (MRI) that includes a significant incidence of white matter injury as a major mechanism.(3–5) There is general agreement that the perioperative period in pediatric cardiac surgery may be responsible for a significant burden of lifetime neurological injury in patients with congenital heart disease, so there is a pressing need to develop and study neuroprotective strategies and treatments in these patients.(6) This chapter will first discuss the existing and potential markers of acute neurological injury. Then some of the newer established neuroprotection methods will be briefly discussed, and finally novel future potential neuroprotective strategies will be reviewed.
Markers/Surrogates for Neurological Injury
In order to apply neuroprotective strategies, it is necessary to develop markers for acute neurological injury, and early surrogates that predict later abnormal neurodevelopmental outcomes. It is only with this early indication of which patients are injured, and the severity of the injury, that protective strategies can be applied soon enough to maximally improve the eventual outcome. Monitoring modalities including near infrared spectroscopy (NIRS), and electroencephalogram (EEG), as well as brain MRI injuries will be discussed. Biomarkers of cerebral injury will also be reviewed.
Near-Infrared Spectroscopy
The use of NIRS to measure cerebral oxygenation was first described by Jobsis in 1977.(7) This technology exploits the distinctive light absorption spectra of oxy- and deoxhyhemoglobin in the 700–1000 nm wavelength range to directly measure or derive a venous-weighted regional tissue oxyhemoglobin saturation (rSO2) that is real-time, reliable, and non-invasive. It is intuitive to accept that if cerebral rSO2 is below some threshold for hypoxic-ischemic injury for a prolonged period of time, that the patient would have an acute brain injury. Indeed, in several pediatric cardiac surgery studies this is the case, the largest of which is Austin et al’s study of 250 infants and children undergoing surgery with cardiopulmonary bypass (CPB).(8) One hundred and two of these patients had cerebral desaturation, defined as rSO2 more than 20% relative decrease below baseline for greater than 3 minutes. In the historical control phase of the study, these patients had a 26% incidence of acute neurological change including seizures, hemiparesis, and coma; versus a 6% incidence for patients without cerebral desaturation. In the subsequent treatment phase of the trial, if low rSO2 successfully was restored to baseline values, the acute neurological injury incidence was identical to patients with no cerebral desaturation. Dent et al (9) reported a series of 22 patients with hypoplastic left heart syndrome undergoing Norwood Stage I palliation, where prolonged low rSO2 <45% for greater than 180 minutes in the perioperative period was associated with new hypoxic ischemic lesions on MRI. Despite this potential utility as a marker for new brain injury, Andropoulos et al.(3) did not find an association of prolonged low rSO2 with new brain injury in a series of 68 neonatal patients studied with MRI after cardiac surgery.
Novel uses of NIRS as a marker for brain injury include measurement of cerebral autoregulation. Brady and colleagues (10) have described an index of cerebral autoregulation called the cerebral oximetry index (COx), which is derived from time-domain analysis correlating low-frequency changes in arterial blood pressure to changes in cerebral oximetry. The COx is an index of autoregulation, which approaches 1 when autoregulation is impaired (rSO2 is correlated to blood pressure), and approaches 0 when autoregulation is intact (rSO2 is preserved with changes in blood pressure). As impaired autoregulation is frequently seen after infant cardiac surgery with bypass (11) and may be a marker of brain injury, this method has promise as a potential way to identify patients at risk.
Electroencephalographic Seizures
EEG seizures after an acute neurological insult have been generally accepted indicators of significant injury with an association with later adverse neurodevelopmental effect, particularly in the neonate. (12) In earlier reports, EEG seizures were observed in 14–20% of neonates undergoing cardiac surgery. Rappaport et al (13) reported an association between EEG seizures and adverse neurological outcomes at age 1 year after arterial switch operations between 1988 and 1992. However, Gaynor et al (14) did not find such an association in a later era with surgery between 1998 and 2003. Andropoulos et al (15) observed only one patient with 2 brief EEG seizures in a cohort of 69 neonates undergoing cardiac surgery from 2005 to 2008 with a high flow CPB strategy that avoids deep hypothermic circulatory arrest (DHCA). Thus, it would appear that EEG seizures have minimal utility as a marker of acute brain injury.
Magnetic Resonance Imaging
Brain MRI is an accepted method for early detection of brain injury in infants and children. In preterm infants and full term infants with hypoxic ischemic encephalopathy (HIE), lesions such as white matter injury (WMI) and watershed infarction correlate with later adverse neurodevelopmental outcomes. New hypoxic-ischemic brain injury is detected in 36–73% of neonates after cardiac surgery with CPB on standard T1, T2, and diffusion-weighted imaging.(3,4,9,16) Brain injury on standard MRI sequences is associated with delayed maturation in the pyramidal tract of neonates after cardiac surgery(17), possibly explaining the later difficulties with psychomotor tasks at 1 year follow-up.(5) However despite the intuitive relationship between early MRI changes and later outcome, more data is needed to definitively make this connection.(18) In a recent report, small foci of hemosiderin consistent with resolved tiny hemorrhages in multiple areas of the brain, were found in 38% of patients returning for MRI at age 1 year, after infant cardiac surgery. These foci were associated with lower psychomotor development index scores.(19) New MRI injury typically requires several days to appear on diffusion weighted imaging, and the logistical difficulty and time required to obtain MRI data in unstable infants after cardiac surgery limits its utility as a rapid surrogate test for brain injury. Recently, antenatal MRI and preoperative MRI after delivery have been performed in the fetus and infant with congenital heart disease. These studies have given new insights into the brain substrate in CHD that makes these patients more vulnerable to injury in the perioperative period. In the third trimester, brains in CHD patients grow more slowly and exhibit delayed morphologic and microcellular development (20), changes which persist after delivery (21, 22) and lead to increased susceptibility of MRI injury after cardiac surgery in the neonate. (3) It is possible that altering timing of delivery and approach to CPB, when possible, could serve as a neuroprotective strategy in patients with vulnerable features on brain MRI. Quantitative MRI measurements, such as diffusion tensor imaging, which measures fractional anisotropy and average diffusion coefficient; as well as MR spectroscopy, diffusion tensor tracking, and volumetric analysis, have promise to determine both acute injury, and the progression of growth and development of the brain in response to injury.(17,20,23)
Biomarkers
Developing valid biomarkers of neural injury is critical to determining which patients are injured, the extent and etiology of injury, and prognosis. In addition, to effectively study any neuroprotective therapy, biomarkers would be necessary to identify patient subsets that would benefit from the therapy, and to serve as surrogate outcomes to evaluate the effects of the therapy. A good biomarker is a test that can be done rapidly at most centers, to determine within hours which patients are injured, would be elevated in proportion to the injury, is capable of predicting longer term outcomes early after the course of an injury, and is easily obtainable, i.e. serum or urine.(24) Cerebrospinal fluid (CSF) has also been used to measure biomarkers of brain injury, but the risk of obtaining CSF in anticoagulated cardiac surgery patients precludes its use in this population. The limitations of previously studied biomarkers, e.g. creatine kinase brain band (CK-BB), neuron specific enolase (NSE), and S100β protein are that they have not proved to be specific enough to brain tissue. These biomarkers will be discussed, along with novel biomarkers which have potential for more specificity for the brain.
CK-BB, Neuron –Specific Enolase, S100β
Creatine kinase is a ubiquitous enzyme involved in energy metabolism found in all cell types; the brain band (CK-BB) is an isoenzyme found in neurons and astrocytes. The S100 protein is dimeric acidic calcium-binding protein found as a major component of the cytosol of many cell types; protein S100β consists of the isotype with ββ subunits predominantly found in astrocytes and Schwann cells. Neuron-specific enolase is a dimeric isoenzyme of the glyocytic enzyme enolase, found in the cytoplasm of neurons and cells with neuroendocrine differentiation. Despite apparent specificity for neural tissue injury, elevations in these biomarkers have not been consistently found to correlate with neurological outcomes after brain injury. Nagdyman et al (25) studied time course of elevation of CK-BB, NSE, and S100β in 29 full term infants with birth asphyxia and 20 control infants, measuring serum levels of these markers at 2, 6, 12, and 24 hr after birth, and then performing neurodevelopmental examinations at 20 months of age. Protein S100β and CK-BB levels were elevated in the asphyxiated infants, while NSE levels were not. None of these biomarkers had any predictive value for death or mental retardation at age 20 months.
There are few cardiac surgery studies of these biomarkers. Schmidtt et al (26) investigated serum and CSF NSE levels in 27 children undergoing cardiac surgery with CPB. Many of the serum samples (40%) had to be discarded because of hemolysis (erythrocytes contain NSE). On the first and second day, the median NSE values were significantly higher than levels in later samples; however in the 11 patients with CSF NSE levels, all were within normal limits. The two children with neurological sequelae had normal CSF NSE levels. The authors questioned the specificity of NSE for neural tissue, and concluded that the value of serum NSE to ascertain brain injury was highly questionable.
S100β levels may have better specificity for brain injury in pediatric cardiac surgery. Lardner et al reported data from 43 children undergoing surgery with CPB. (27) Neonates had elevated S100β levels preoperatively, and all patients had elevated levels immediately postbypass. There was no association between elevated S100β levels 24 hr after bypass and outcomes; however in the two patients with acute neurological injury and later developmental delay, the increase at 48 hr was significantly higher than the remaining patients without brain injury. The authors noted that S100β is not specific to brain, and may be found in adipose tissue, thymus, pleural drainage fluid, and skeletal muscle. They noted that the small sample size precluded definitive conclusions about the value of S100β. Other published studies of S100β levels after CPB surgery in infants and children do not report intermediate or longer term neurological outcomes.
A search for published data about CK-BB elevation in pediatric cardiac surgery reveals only the early data from the Boston Circulatory Arrest Study of 171 infants comparing DHCA to low flow CPB.(28) DHCA was associated with higher levels of CK-BB; the longer term predictive value of elevated CK-BB levels has not been reported.
Newer Biomarkers of Brain Injury
There are several novel serum biomarkers that have promise for more specificity for CNS injury. Glial fibrillary acidic protein (GFAP) is a cytoskeletal protein found in the astroglia of the CNS, first studied in CNS tumors. It is not constitutively expressed and thought to be released only after cell death. Specificity for white matter may be valuable in patients with CHD because of the known association with white matter injury before and after neonatal cardiac surgery.(3,4) In a study of 51 trauma patients, 39 of whom had computed tomography (CT) scan documented traumatic brain injury, GFAP levels on day 2 after injury were highly predictive of death from head trauma.(29) In a study of 24 children with septic shock(30), Hsu et al determined that serum NSE and S100β were elevated in all children compared to controls, yet only 5 of 24 had elevated GFAP levels. There was no description of neurological outcomes. Studies specific for cardiac surgery have yet to be published.
Inflammatory cytokine markers such as interleukins (IL) 1β, 6, 8, and tumor necrosis factor (TNF), taken together with markers more specific for CNS injury, have promise to help predict adverse outcomes after a brain injury. Inflammation is an important component of brain injury after birth asphyxia, cardiac arrest, cardiac surgery, sepsis, and other critical illness. Elevations in serum and CSF levels of IL-1β, 6, 8 have been shown to correlate with long term adverse neurodevelopmental outcomes in some studies of birth asphyxia in full term neonates. (24) In a study of 12 neonates undergoing CPB with DHCA, (31) elevations in IL-6 and 8 levels in the serum were associated with higher protein S100β levels; this was not found in older children undergoing standard CPB without DHCA. No neurological outcomes were reported.
Ubiquitin C-terminal hydrolase 1 (UHCL1) and phosphorylated axonal neurofilament heavy chain (pNF-H) are two novel biomarkers that show promise for better specificity in brain injury, especially in neonates after HIE from birth asphyxia. pNF-H is one of a family of neurofilament proteins found specifically in neurons, as the major cytoskeletal element in nerve axons and dendrites. pNF-H was consistently elevated 3–4 times higher than controls in a study of 9 severe traumatic brain injury patients.(32) They are also elevated in CSF in adults after DHCA for thoracoabdominal aortic aneurysm; but not in these cases without DHCA unless an acute CNS injury occurred.(33) UCHL1 is an enzyme heavily and specifically concentrated in neuronal cytoplasm and dendrites, and could provide a complementary measure of neuronal injury with pNF-H. UCHL1 was also elevated in this study of thoracoabominal aneurysm surgery in the same patients. In a study of 41 severe traumatic brain injury patients, serial measurements of CSF UHCL1 were significantly more elevated in patients with lower Glasgow Coma Scale scores, death, post injury complications, and worse 6 month neurological outcomes. (34)
These new and novel biomarkers of brain injury give rise to the potential that better, more specific tests could discriminate patients at high risk for long term adverse outcomes, and thus are candidates for early neuroprotective therapies. Also, these tests could potentially discriminate between neuronal and white matter injury. A panel of multiple biomarkers, measured serially, will likely prove to be more predictive of injury; this will be an important field for future study in the pediatric cardiac surgery population. Previous study limitations of small single center studies without neurodevelopmental follow-up will need to be overcome, with multicenter studies with routine long term outcome follow-up. Table 1 summarizes the currently available indicators, and biomarkers of acute brain injury available in the pediatric cardiac surgery population.
Table 1.
Potential Markers of Acute Brain Injury in Pediatric Cardiac Surgery
| Marker | Source | Potential Predictive Value for Long Term Neuro Outcomes | Difficulty Factor to Obtain Data | References |
|---|---|---|---|---|
| NIRS; including autoregulation | Patient monitor | +++ | + | 3,8–10 |
| EEG | 10–20 lead or aEEG | + | ++ | 12–15 |
| MRI | Research or clinical data | ++++ | +++ | 3,4,9,16–23 |
| Biomarkers--older: CK-BB, NSE, S100β | Blood | + | + | 24–28 |
| Biomarkers--inflammation: IL-1B,6,8, TNF | Blood | − | + | 24,31 |
| Biomarkers--newer CNS specific: GFAP, UHCL1, pNF-H | Blood | ++ | ++ | 29–34 |
| Biomarker panels: multiple specific, with inflammatory markers | Blood | +++ | ++ | 24 |
Abbreviations: NIRS, near-infrared spectroscopy; EEG, electroencephalogram; aEEG, amplitude-integrated electroencephalogram; CK-BB, creatine kinase brain band; NSE, neuron-specific enolase; S100β, protein S100β; IL, interleukin; TNF, tumor necrosis factor; CNS, central nervous system; GFAP, glial fibrillary acidic protein ;UHCL1, ubiquitin C-terminal hydrolase 1; pNF-H, phosphorylated axonal neurofilament heavy chain.
Potential Predictive Value for Long Term Neuro Outcomes: −, not predictive; +, minimal predictive value; ++, some potential for predictive value; +++, good potential for predictive value; ++++, high potential for predictive value. Difficulty to obtain data: +, easy to obtain; ++, some difficulty to obtain; +++, moderate difficulty to obtain.
Neuroprotective Strategies and Treatments
Neuroprotective strategies and treatments can be classified as preventive, reactive, or reparative. Preventive strategies are therapies given in advance of an anticipated neurological injury or insult, such as an obligate long period of DHCA. They are designed to prevent neuronal and white matter loss. Reactive strategies are therapies given in response to a recent neurological insult or injury, for example diagnosed by perioperative brain MRI or rise in neuronal biomarkers. They are designed to limit the extent of neuronal or white matter loss. Reparative strategies are treatments given after a known neurological injury, for example one which produces late MRI injury and clinical neurological deficits, in order to repair and restore neuronal or white matter loss by growing new neural tissue. Table 2 categorizes the strategies reviewed in the following sections according to this classification scheme.
Table 2.
Potential Strategies and Treatments for Neuroprotection in Pediatric Cardiac Surgery
| Treatment/Strategy | Preventive or Reactive/Reparative | Status of Clinical Research Trials | Feasibility | References |
|---|---|---|---|---|
| CPB techniques: DHCA vs. ACP | Preventive | 2 published; low ACP flow rates | +++ | 35–40 |
| NIRS monitoring and treatment of low rSO2;autoregulation | Preventive | No prospective studies | +++ | 3, 8–10 |
| Anesthetic regimens: dexmedetomidine vs. GABA/NMDA agents | Preventive | Retrospective data; no prospective studies | +++ | 41–47 |
| Therapeutic hypothermia | Preventive/reactive | None in cardiac surgery; complete neonatal HIE, ongoing pediatric cardiac arrest | ++++ | 49–53 |
| Remote ischemic preconditioning | Preventive | One controlled study ongoing | ++++ | 57 |
| Erythropoietin | Preventive/reactive/reparative | One phase I/II trial underway | ++ | 58–63 |
| Neurotrophic factors: BDNF | Preventive/reactive/reparative | None | ++ | 66,67 |
| Umbilical cord stem cells | Preventive/reactive/reparative | None | + | 69,70 |
Abbreviations: CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; ACP, antegrade cerebral perfusion; NIRS, near-infrared spectroscopy; rSO2, regional cerebral oxygen saturation; GABA, γ-aminobutryric acid receptor agonist; NMDA, N-methyl-D-aspartate receptor antagonist; HIE, hypoxic-ischemic encephalopathy; BDNF, brain-derived neurotrophic factor. Feasibility: ++++, very feasible with current practices; +++, feasible with current practices; ++, less feasible; experimental therapy that would alter current practices; +, not feasible; experimental therapy for future study.
Cardiopulmonary Bypass Strategies
Cardiopulmonary bypass (CPB) techniques have long been considered an important potential determinant of acute and long term neurological injury, and optimizing CPB techniques could serve as important acute neuroprotection. Despite this intuitive association, there is limited controlled study data as to which strategies lead to improved short and longer term outcomes. It is well established that prolonged DHCA, with a cutoff point of 40 minutes, is associated with long term adverse neurodevelopmental outcomes at 8 years after the neonatal arterial switch operation.(35) pH stat management of CPB is associated with some early outcome advantages after cardiac surgery in 2-ventricle infants, but neurodevelopmental outcomes in survivors were not substantially different at age 1 year.(36) Higher hematocrit on CPB for infant cardiac surgery is associated with better psychomotor development index at age 1 year. Combining the results of two hematocrit trials, Newberger et al determined that a cut point existed for a hematocrit below 24% on CPB.(37) Antegrade cerebral perfusion (ACP) has been touted as a neuroprotective strategy that can replace DHCA as the primary strategy during aortic arch reconstruction. However, the results of two recent studies do not reveal a difference in Bayley Scales of Infant Development scores at age 1 year after Norwood Stage I palliation for HLHS.(38,39) These studies have been criticized, however, because ACP flow rates were significantly lower than needed to provide full oxygenation to both cerebral hemispheres.(40) With high flow ACP, the incidence of new MRI brain injury after neonatal cardiac surgery was not different than with full flow CPB.(3) Well designed studies of DHCA versus ACP at adequate flow rates are important to address this question. In the future, larger multicenter trials and data collection of much larger numbers of patients will be important to address questions of which CPB techniques offer the best neuroprotection.
Anesthetic Agents
In recent years there has been significant concern in the pediatric anesthesia community concerning animal data, mostly in rodents, that commonly used anesthetic agents cause increased neuroapoptosis in the developing brain.(41) This has been observed with agents that produce their anesthetic and sedative effects by interacting with γ-aminobutyric acid (GABA) receptors as agonists: anesthetic gases isoflurane, sevoflurane, desflurane; benzodiazepines, nitrous oxide, propofol, and barbiturates. In addition, ketamine, an N-methyl-D-aspartate (NMDA) antagonist, has the same effect. The mechanism is thought to be interference with the actions of the GABA and glutamate receptors as neurotransmitter mediators during the period of rapid synaptogenesis. This research has been criticized for being conducted in the absence of surgery or other painful stimulus, and because the exposure was very prolonged. In fact, ketamine is protective against neuroapoptosis in rodent models of repetitive painful stimulus. (42) In addition, Loepke et al (43) demonstrated that desflurane given in the sweep gas of the CPB circuit in a model of low flow CPB in neonatal piglets, has profound neuroprotective effects, including significant reduction in apoptosis and excitatory cell death, as well as improved neurobehavioral examination in recovered animals. In addition, McAuliffe et al (44) studied neonatal mice with preconditioning with desflurane, sevoflurane, or isoflurane for a 3 hr period, and 24 hr later subjected them to 1 hr of hypoxia. All 3 anesthetic gases protected against HIE when compared with no preconditioning on a battery of neurobehavioral tests.
Ketamine may also have neuroprotective properties in pediatric cardiac surgery. Ketamine antagonizes the NMDA receptor, which can block the influx of Ca2+ in response to neuronal ischemia. The influx of Ca2+ rapidly initiates apoptosis and cellular necrosis; thus ketamine potentially has important effects that would offset any adverse effect of increased apoptosis in the developing brain. (45) This data has led to the potential that ketamine, in the setting of cardiac surgery, could actually be neuroprotective in infants and children, and is a subject of active study at this time. (CinicalTrials.gov; NCT00556361, NCT00598195).
Dexmedetomidine is a centrally acting α2 receptor antagonist that produces sedation and analgesia by reducing flux of the excitatory neurotransmitter norepinephrine in the CNS. This agent does not produce apoptosis in the developing brain, and has profound neuroprotective effects in animal models of focal cerebral ischemia, and even isoflurane neurotoxicity.(46,47) The mechanisms of dexmedetomidine neuroprotection are not completely understood, but this agent does reduce isoflurane-induced apoptosis by reversing the reduction in the anti-apoptotic signaling pathways caused by isoflurane.
It will be important in future studies to control for the exposure to anesthetic gases, and other agents such as benzodiazepines and ketamine. Studies randomizing patients to different anesthetic regimens, especially comparing dexmedetomidine against GABA agonists or NMDA antagonists would be important to clarify the neuroprotective and neurodegenerative impacts of these agents.
Therapeutic Hypothermia
Deep hypothermia has been utilized during CPB for more than 40 years to protect the brain during DHCA, low flow bypass, and now ACP. The limitations of different levels of hypothermia to prevent brain injury during bypass have been well studied by Greeley and colleagues.(48) Deep hypothermia to 15–18° C appears to induce a state of vasoparesis, elevated cerebral vascular resistance, and altered responsiveness of the cerebral vasculature to CO2.(11) It may be that avoiding deep hypothermia if possible would better preserve cerebral vascular reactivity and autoregulation, and this strategy should be studied as a neuroprotective strategy.
In contrast to temperature management during CPB, the inflammatory response to CPB often results in hyperthermia in the first 24 hr postoperatively, and standard measurements such as rectal temperature will significantly underestimate brain temperature in many patients. Bissonnette et al (49) studied 25 children after CPB surgery with serial temperature measurements with probes in the rectum, esophagus, tympanic membrane, and the jugular venous bulb, the latter as a surrogate for brain temperature. By 6 hr post-CPB, the mean rectal temperature was 37.8° C; however the mean jugular venous bulb temperature was 39.6° C. Because cerebral metabolic rate for oxygen (CMRO2) increases by 7–10% for every 1° C increase in temperature, cerebral watershed areas could be vulnerable to further injury after an insult during CPB.
Therapeutic hypothermia has been studied as a treatment strategy for cerebral protection after neonatal HIE, and pediatric cardiac arrest. Core temperature is lowered 2–5° C after the insult, to reduce CMRO2 and thus ongoing metabolism that contributes to further neuronal loss. In two large published controlled trials in neonatal HIE of over 400 full term infants, hypothermia was induced for 72 hr by means of a circulating water blanket or cooling cap, with servo controlled cooling of temperature to 33.5–35° C. Control infants received standard care to maintain temperature 36.5–37° C. The primary outcome variable in both trials was death or disability at age 18–22 months, defined on the Bayley Scales of Infant Development. Death or severe/moderate disability was reduced by 30–40% with hypothermia. (50) These and other data have conclusively proven the benefit of therapeutic hypothermia in neonatal HIE, and this therapy is now becoming standard of care in this population.
Therapeutic hypothermia in the range of 33–34° C is currently recommended in the American Heart Association cardiopulmonary resuscitation guidelines after cardiac arrest in children.(51) To this point retrospective studies in this population have not showed benefit but are flawed by increased severity of illness in the hypothermia groups in these non-randomized studies.(52) There are currently several ongoing prospective randomized controlled trials enrolling pediatric patients suffering in-hospital or out of hospital cardiac arrest. (ClinicalTrials.gov, NCT00880087. NCT00878644)
Therapeutic hypothermia in the early postoperative period in patients at high risk for new brain injury after CPB, such as neonates undergoing complex surgery with long CPB times, such as Norwood Stage I palliation for HLHS, would be a relatively simple and important therapy with potential for significant benefit. In a study in a neonatal piglet model of DHCA, mild hypothermia in the post CPB period significantly reduced neuronal cell death due to apoptosis.(53) Adequate numbers of patients, likely in a multicenter study, would be crucial to adequately address the question as to whether this therapy would be beneficial.
Hypoxic-Ischemic Preconditioning
Hypoxic-ischemic preconditioning refers to the intriguing finding that a mild, short lived period of ischemia and/or hypoxia, experienced in advance of a major hypoxic ischemic insult, confers neuroprotection by limiting the extent of brain injury, compared to patients who did not have preconditioning. (54, 55) This phenomenon is nearly ubiquitous across all tissues, and is highly conserved across a wide variety of species. One of the first clinical observations was that stroke patients who experience transient ischemic attacks before a stroke have better longer term outcomes. The mechanisms of ischemic preconditioning are extremely complex, and involve many changes in transmembrane and intracellular signaling that result in protection from excitotoxic and apoptotic cell death. Molecules and receptors involved include GABA receptors, nitric oxide, protein kinase C, glutamate, brain derived neurotrophic factor (BDNF), nuclear factor kappa B (NFκB), heat shock proteins, hypoxia-inducible factor, erythropoietin, vascular endothelial growth factor (VEGF), and many others. A cascade of upregulation of genes producing protective effects occurs, and the final common pathway involves modification of mitochondrial function via opening of ATP-sensitive potassium channels and the closure of mitochondrial permeability transition pores. Also, the classic initial preconditioning effect is short lived, not persisting beyond a few hours; however a second window of protection recurs after 24–48 hours and can persist for up to 3–4 days. It is possible that mild degrees of cerebral desaturation, as can be seen in preoperative neonates with dextrotransposition of the great arteries (d-TGA), may have a protective effect during the subsequent CPB and perioperative period. Andropoulos et al (56) reported that cerebral oxygenation was low in d-TGA patients with mean rSO2 55 ± 10%, with 31% of readings below 50%. This was significantly different (p<0.001) from the single ventricle patients, all of whom had hypoplastic left heart syndrome, who had a mean rSO2 of 61 ± 8%, with only 1% of values below 50%. In a later study, the HLHS patients had a significantly higher burden of new MRI injury 7 days postoperatively, assessed by brain injury score and incidence of white matter injury. (3) It may be that hypoxic ischemic preconditioning contributed to the lower burden of injury in the arterial switch patients. Although intentionally producing mild cerebral desaturation is not an option, hypoxic ischemic preconditioning needs further elucidation as a mechanism of neuroprotection, and indeed this concept gives rise to two of the therapeutic options discussed below, remote ischemic preconditioning, and the use of erythropoietin for neuroprotection.
Remote Ischemic Preconditioning
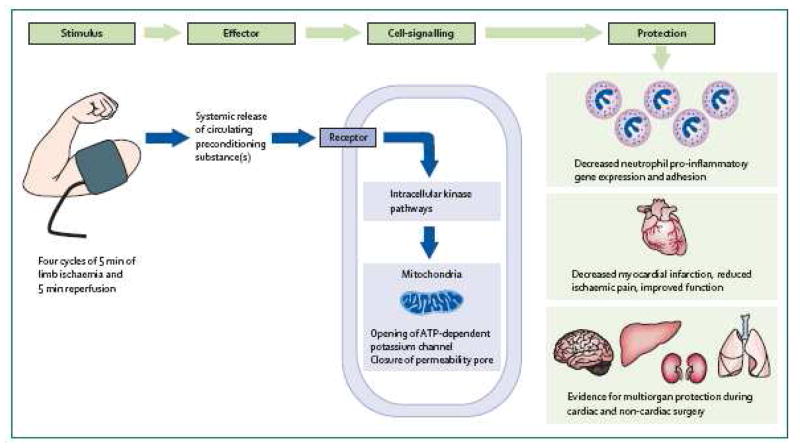
Within a decade of the elucidation of the phenomenon of ischemic preconditioning, the concept that ischemia in a remote organ can produce protective effects, similar to those observed in classic ischemic preconditioning, was discovered. (57)(Figure 1) Although the exact nature of the signal transduction or humoral factors involved in protecting a remote organ, i.e. myocardium or brain, after preconditioning by inducing ischemia in, for example an extremity, are not fully understood, the phenomenon has been applied to pediatric cardiac surgery patients. In a controlled, blinded study of 37 children undergoing cardiac surgery with CPB, the study group had four 5-minute inflations of a blood pressure cuff on a lower limb, under anesthesia before CPB, vs. standard therapy for the control group. Troponin I release was significantly lower in the remote ischemic preconditioning group, and inotrope score and airway resistance were lower as well. This proof of concept study led to a larger study powered to achieve clinical endpoints, including neurocognitive outcomes as a measure of neuroprotection. Data collection is underway and also includes genomic responses to this treatment. (ClinicalTrials.gov; NCT00650507) This therapy has significant potential for neuroprotection and would be easy to adopt clinically because of its relatively non-invasive simple nature, and ease of acceptance by practitioners, patients, and their families.
Figure 1.
Biological effects of remote ischemic preconditioning. Transient ischemia of the arm or leg liberates a circulating effector that induces remote cellular adaptation to a subsequent, extended, and potentially lethal period of ischemia in remote tissues. Reproduced with permission from reference 57: Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–65.
Erythropoietin
Erythropoietin (EPO) is a 30.4 kilodalton glycoprotein which, until the last 15 years, had been thought only to be necessary for erythropoiesis, and to be released by the kidney in response to anemia. Recombinant human EPO has been U.S. FDA approved and available since 1985, and has been used for over 15 years in premature neonates to treat the anemia of prematurity. It has been shown to be safe and effective when utilized in the setting of elective pediatric cardiac, craniofacial, and orthopedic surgery preoperatively to raise hemoglobin, in order to reduce the need for heterologous blood administration. In the past 15 years, new information about the multiple biological roles of EPO has become available, specifically the neurological and cardioprotective effects of EPO.(58) Besides the kidneys, organs discovered to secrete EPO include liver, uterus, vascular smooth muscle, peripheral endothelial cells, insulin producing cells, heart, and, most intriguing, central nervous system tissue.(59)
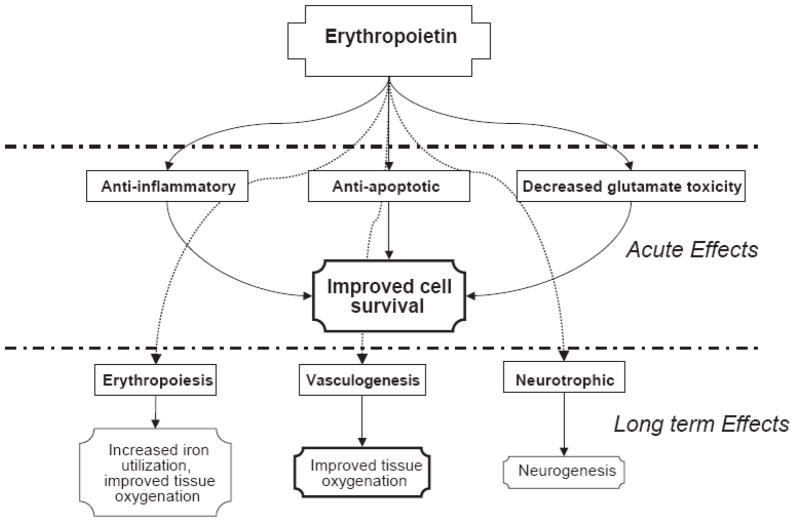
EPO and its receptor, EPOR, are produced by neurons, glial cells, and endothelial cells in response to tissue hypoxia and other stimuli, including anemia, hypoglycemia, reactive oxygen species and insulin; in part via expression of hypoxia-inducible factor-1α (HIF-1 α). (60) The EPO gene has been demonstrated to be a target for HIF-1α, and EPO mRNA increases proportionally to the degree of tissue hypoxia, as does EPO itself. EPO has a dose-dependent protective effect on neurons in tissue culture. EPO and EPOR production change significantly during development, with high expression found in the late fetal and neonatal periods in a fetal sheep model, as well as human tissue culture model. The EPO system is presumably a protective mechanism against perinatal asphyxia. This body of novel information has given rise to the notion that EPO could be used therapeutically in a variety of clinical settings involving CNS insults. Multiple animal models of neonatal hypoxic-ischemic brain insult, including the rat, mouse, and primate, demonstrate a 50–70% reduction in neuronal cell death, and neurological and behavioral damage when high dose EPO is given prior to the insult.(58) Recently, a porcine model using CPB and 60 minutes of DHCA demonstrated an improvement in neurobehavioral score with 3 doses of 500 units/kg EPO the day before, day of and the day after surgery.(61) EPO neuroprotective mechanisms are complex, and include anti-apoptotic, anti-inflammatory, and decreased glutamate toxicity actions.(62) (Figure 2). EPO prevents apoptotic processes by binding to its receptor, which then activates Janus-tyrosine kinase 2, phosphoinositide 3 kinase, and protein kinase B, which result in transcription of anti-apoptotic genes, and direct inhibition of proapoptotic factors including glycogen-synthase kinase 3β and the fork head family member FOXO3a. EPO prevents cellular inflammation by inhibiting the proinflammatory cytokines IL-6, TNF-α, and monocyte chemoattractant protein 1. (59) EPO reduces excitotoxic cell death by decreasing both glutamate-induced neuronal death, and release of glutamate from neurons injured by excitotoxic mechanisms. Thus EPO provides a robust protection against neuronal injury and death by a number of cellular mechanisms.
Figure 2.
Schematic diagram of the beneficial acute and longer term effects of erythropoetin administration for neuroprotection. Reproduced with permission from reference 62: Juul S. Recombinant erythropoietin as a neuroprotective treatment: in vitro and in vivo models. Clin Perinatol. 2004;31:129–42.
EPO treatment has potential to ameliorate CNS injury and promote repair of injured gray and white matter in a number of clinical settings, including white matter injury of prematurity, inflammatory and apoptotic cell death in both premature and full term infants, and watershed infarction in full term infants with HIE. Recent clinical studies in the neonatal period have demonstrated improved outcomes after EPO treatment in premature infants. (58) In a study of 167 full term neonates with moderate to severe HIE, Zhu et al (63) randomized them to receive either EPO 300 or 500 units/kg IV every other day for two weeks, or placebo. Death and/or moderate/severe disability was assessed at 18 months, and was reduced from 44% in the control group to 25% in the treatment group (p=0.017)
Neonates undergoing cardiac surgery, have a 30–40% incidence of preoperative MRI brain injury, and approximately 40% experience new injury after surgery consisting of WMI, infarction in an arterial distribution, or small intraparenchymal hemorrhagic infarctions. (3) These patients are ideal candidates for EPO treatment because the timing of the CNS insult is known, allowing hours for the transcriptional effects of EPO to occur beforehand. EPO is already FDA approved and frequently administered to neonates. At Texas Children’s Hospital there is an active 80 patient phase I/II, prospective, randomized, controlled, blinded trial of EPO neuroprotection in neonates undergoing hypothermic CPB for repair or palliation of TGA, HLHS, interrupted aortic arch, or other 2 ventricle lesions. (ClinicalTrials.gov; NCT NCT00513240). Patients are randomized to receive 3 doses of EPO 500 units/kg IV or saline placebo, the day before surgery, on POD #1, and POD #3. A brain MRI is performed before surgery, at 7 days after surgery, and a third MRI done at 3–6 months. Neurodevelopmental outcomes are assessed at 1, 3, and 5 years. If this trial yields results consistent with improvement in EPO patients without significant side effects, the plan is to expand this to a larger multicenter trial.
Neurotrophic Factors
There has been a recent explosion of knowledge about the protein growth factors necessary for normal development of the central nervous system, and also of the factors involved in response to hypoxic ischemic injury and other brain insults, that both protect neurons from injury and go on to promote repair and growth of new neurons. (64) Neonatal HIE has been the model for potential application of these growth factors in therapeutic uses. Neuroimaging has demonstrated that brain injury after a perinatal insult evolves over days to weeks; this has been substantiated by animal models. (65). The neuronal cell death begins immediately and continues over a period of days to weeks. Early on, necrosis is the major pathology but soon changes primarily to apoptosis. Oxidative stress and excitotoxicity occur early, and lead to both inflammation and repair of neurons through downstream intracellular signaling. (Figure 3). Stimulation of post-lesion plasticity by growth factors may have important benefits, separate from acute brain protection drugs, i.e. EPO. Although the mechanisms of neonatal brain injury before and after cardiac surgery are not completely analogous to the watershed and basal ganglia infarction pattern often seen in neonatal HIE, there are some overlapping mechanisms in these populations. There are also important similarities between the neonatal CHD population and the premature infant’s brain injury, including a predominance of WMI. It would appear that growth factor treatment could be useful in brain injury in CHD, especially with the evidence for brain immaturity and vulnerability to injury in this population.
Figure 3.
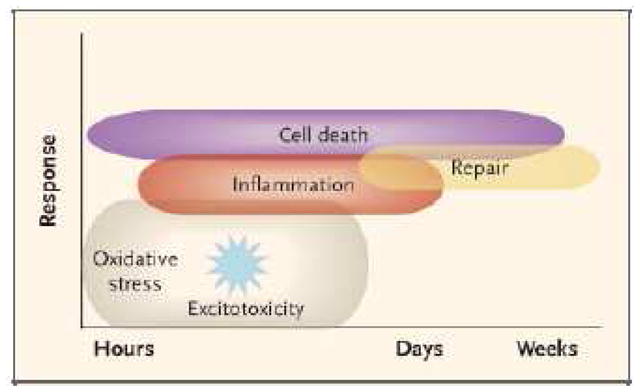
Mechanisms of brain injury in the term neonate. Oxidative stress and excitotoxicity, through downstream intracellular signaling, produce both inflammation and repair. Cell death begins immediately and continues during a period of days to weeks. The cell-death phenoytype changes from and early necrotic morphology go a pathology resembling apoptosis. This evolution is called the necrosis-apoptosis continuum. Reproduced with permission from reference 65: Ferriero DM. Neonatal brain injury. N Engl J Med 2004;351:1985–95.
Brain-Derived Neurotrophic Factor
Brain-Derived Neurotrophic Factor (BDNF) is one of the most widely distributed neurotrophic protein factors, which acts specifically via high affinity cell surface receptor (TrkB receptor), which in turn activates intracellular protein kinase B, mitogen activated protein kinases, and extracellular signal regulated kinases, and prevents acute cell death after an hypoxic-ischemic insult. BDNF also promotes synaptic and axonal plasticity associated with learning, memory, and sensorimotor recovery. (66) In a neonatal rat model, BDNF treatment followed by hypoxia-ischemia resulted in improved performance on the Morris Water Maze, a standard test of spatial memory. In addition, BDNF treated rats had significantly less tissue loss in the hippocampus, cortex, and striatum. (67) Daily BDNF doses for 5 days after stroke in a rat model resulted in significantly improved sensorimotor scores during the 6 week study period. At the structural level, BDNF significantly increases neurogenesis in the dentate gyrus and enhanced migration of subventricular zone progenitor cells to the striatum of the ischemic hemisphere. (66) The significant number of published studies of BDNF neuroprotection in animal models would appear to make it one of the leading candidates for future human clinical trials.
A number of other neurotrophins, such as glial cell line derived neurotrophic growth factor (GDNF), ciliary neurotrophic factor (CNTF), vascular endothelial growth factor (VEGF), nerve growth factor, (NGF), and many others, play roles in both neurodevelopment, and repair in response to neuronal injury. (68) Study in this area is very complex and has stimulated much interest in assessing these factors as pharmacologic treatments in brain injury. To date, there are no published clinical trials of these factors in human brain injury.
Stem Cell Treatment
Stem cells are undifferentiated cells which possess extensive proliferative capacity, are capable of long term self renewal, and are multipotent.(69) The substantial therapeutic potential for stem cells in brain injury in congenital heart disease make this an important area of study for the future, especially as the mechanisms for this injury are better understood, including brain immaturity. There are a number of potential sources of stem cells, including embryonic stem cells, neural stem cells (both fetal and postnatal), and mesenchymal stem cells. Sources of mesenchymal stem cells include bone marrow, peripheral blood, umbilical cord blood, and umbilical cord matrix. (Figure 4)
Figure 4.
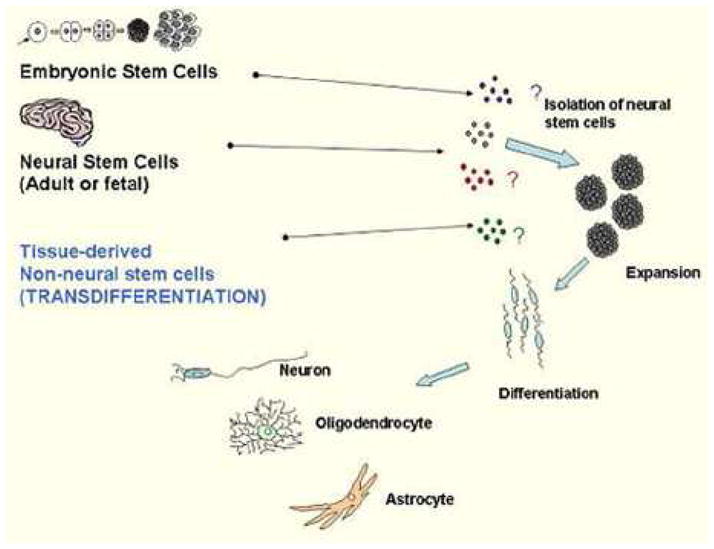
Sources and strategies using stem cells for neural cell replacement. Tissue-derived non-neural stem cells include umbilical cord blood stem cells. Reproduced with permission from reference 69: Vawda R, Woodbury J, Covey M, Levison SW, Mehmet H. Stem cell therapies for perinatal brain injuries. Semin Fetal Neonatal Med. 2007;12:259–72.
Human umbilical cord blood cells contain stem cells and are an appealing candidate for stem cell therapy in neonatal diseases because they are relatively plentiful, there are no ethical concerns, and are not immunogenic in the case of autotransplantation of the patient’s own cord blood. Cord-blood stem cell transplants have been a part of reconstitution of cell lines after treatment for hematologic malignancies for over 15 years. (70) There are now a number of animal studies in rodent models of stroke and traumatic brain injury, which demonstrate that cord blood cells migrate into and accumulate in the injured areas of the brain and improve functional recovery. However, the exact mechanisms underlying this recovery are yet to be elucidated. In a recent study of human umbilical cord blood mononuclear cells from 15 normal pregnancies, the neurotrophic factors (see above) BDNF, GDNF, NGF, neurotrophin-3, and neurotrophin 4/5 were present in levels 4–5 times greater than in peripheral blood mononuclear cells. The authors concluded that since umbilical cord blood is a known rich source of hematopoietic stem cells and the neural differentiation capacity of cord blood cells is a relatively new finding, that these high levels of neurotrophic factors might explain some of the therapeutic effects, and insight into neural differentiation potential, of these cells.
In the setting of neonatal surgery for CHD, umbilical cord blood stem cell infusion could have potential utility in the prevention and treatment of brain injury. Forty to 70% of patients with CHD have a prenatal diagnosis. (71) This allows time to plan a comprehensive delivery and postnatal course, including delivery in a referral center, delaying delivery when feasible, and collection of cord blood for tissue culture growth of stem cells, which could be multiplied and differentiated toward neural cells, and infused back into the infant either before or after surgery, or both. Clearly there is a tremendous amount of work to be done in this area before any human trials would be undertaken, but if high risk patients could be identified, umbilical cord blood stem cell treatment would have potential utility as an “over the horizon” neuroprotective strategy.
Conclusion
The field of neuroprotection for neonatal and pediatric cardiac surgery is still clearly in its infancy. Table 2 summarizes the potential neuroprotective strategies and treatments discussed in this chapter. The incidence, risk factors, and pathophysiology of brain injury in the perioperative period are still incompletely understood. There is still much work to be done in the area of valid markers or predictors of acute neurological injury which correlate with long term outcomes, and strategies or treatments capable of reducing the burden of neurological injury from the perioperative period in pediatric cardiac surgery. These treatments range from the simple, such as therapeutic hypothermia, remote ischemic preconditioning, or administering different anesthetic agents or EPO, to the very complex such as umbilical cord stem cell therapy. The timeframe for treatment may range from the prenatal period, to the postnatal/preoperative period, to the perioperative period, and beyond. (Figure 5) After proper animal study validation, pilot studies to prove concepts in patients will be needed, likely followed by multicenter studies to determine whether treatments have wider applicability. This will require many years, and ideally coordinated multicenter approaches to accrue sufficient patient numbers for valid conclusions. Any trials will have to be planned carefully to account for the multitude of confounding variables affecting neurological injury and outcome in congenital heart surgery.
Figure 5.
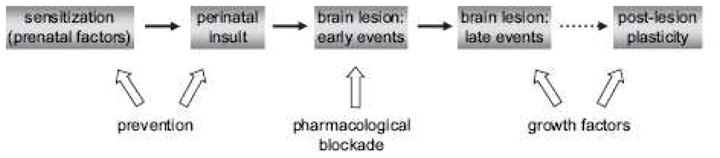
Schematic representation of the timeframe of potential strategies for the neuroprotection of perinatal brain injury, including that seen in congenital heart disease. Reproduced with permission from reference 62: Sizonenko SV, Bednarek N, Gressens P. Growth factors and plasticity. Semin Fetal Neonatal Med. 2007;12:241–9.
Acknowledgments
Dr. Andropoulos is supported in part by an NIH grant from NICHD R21RHD055501A, a Charles A. Dana Foundation Neuroimaging Grant, and Baylor NIH GCRC M01 RR00188 Grant #0942. He reports no potential financial conflicts of interest.
Dr. Brady has a licensing agreement with Somanetics, Inc. for the cerebral autoregulation technology described in this manuscript, which is managed by Johns Hopkins University.
Drs. Easley and Fraser report no funding or potential financial conflicts of interests in conjunction with this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Creighton DE, Robertson CM, Sauve RS, et al. Neurocognitive, functional, and health outcomes at 5 years of age for children after complex cardiac surgery at 6 weeks of age or younger. Pediatrics. 2007;120:e478–86. doi: 10.1542/peds.2006-3250. [DOI] [PubMed] [Google Scholar]
- 2.Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. 2006;148:72–7. doi: 10.1016/j.jpeds.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 3.Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2009 Nov 10; doi: 10.1016/j.jtcvs.2009.08.022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli KK, Zimmerman RA, Jarvik GP, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Fuller S, Nord AS, Gerdes M, et al. Predictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur J Cardiothorac Surg. 2009;36:40–7. doi: 10.1016/j.ejcts.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Nelson DP, Andropoulos DB, Fraser CD., Jr Perioperative neuroprotective strategies. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2008:49–56. doi: 10.1053/j.pcsu.2008.01.003. Review. [DOI] [PubMed] [Google Scholar]
- 7.Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–7. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 8.Austin EH, 3rd, Edmonds HL, Jr, Auden SM, et al. Benefit of neurophysiologic monitoring for pediatric cardiac surgery. J Thorac Cardiovasc Surg. 1997;114:707–15. doi: 10.1016/S0022-5223(97)70074-6. [DOI] [PubMed] [Google Scholar]
- 9.Dent CL, Spaeth JP, Jones BV, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2005;130:1523–30. doi: 10.1016/j.jtcvs.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38:2818–25. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassan H, Gauvreau K, Newburger JW, et al. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res. 2005;57:35–41. doi: 10.1203/01.PDR.0000147576.84092.F9. [DOI] [PubMed] [Google Scholar]
- 12.Silverstein FS, Jensen FE. Neonatal seizures. Ann Neurol. 2007;62:112–20. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- 13.Rappaport LA, Wypij D, Bellinger DC, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. 1998;97:773–9. doi: 10.1161/01.cir.97.8.773. [DOI] [PubMed] [Google Scholar]
- 14.Gaynor JW, Jarvik GP, Bernbaum J, et al. The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2006;131:181–9. doi: 10.1016/j.jtcvs.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andropoulos DB, Mizrahi E, Hrachovy RA, et al. Electroencephalographic seizures after neonatal cardiac surgery with high flow cardiopulmonary bypass. Anesth Analg. 2010 doi: 10.1213/ANE.0b013e3181dd5a58. in press. [DOI] [PubMed] [Google Scholar]
- 16.McQuillen PS, Barkovich AJ, Hamrick SE, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38(2 Suppl):736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 17.Partridge SC, Vigneron DB, Charlton NN, et al. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol. 2006;59:640–51. doi: 10.1002/ana.20772. [DOI] [PubMed] [Google Scholar]
- 18.McQuillen PS. Magnetic resonance imaging in congenital heart disease: what to do with what we see and don’t see? Circulation. 2009;119:660–2. doi: 10.1161/CIRCULATIONAHA.108.835744. [DOI] [PubMed] [Google Scholar]
- 19.Soul JS, Robertson RL, Wypij D, et al. Subtle hemorrhagic brain injury is associated with neurodevelopmental impairment in infants with repaired congenital heart disease. J Thorac Cardiovasc Surg. 2009;138:374–81. doi: 10.1016/j.jtcvs.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 22.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherlock RL, McQuillen PS, Miller SP aCCENT. Preventing brain injury in newborns with congenital heart disease: brain imaging and innovative trial designs. Stroke. 2009;40:327–32. doi: 10.1161/STROKEAHA.108.522664. [DOI] [PubMed] [Google Scholar]
- 24.Ramaswamy V, Horton J, Vandermeer B, Buscemi N, Miller S, Yager J. Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediatr Neurol. 2009;40:215–26. doi: 10.1016/j.pediatrneurol.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Nagdyman N, Grimmer I, Scholz T, Muller C, Obladen M. Predictive value of brain-specific proteins in serum for neurodevelopmental outcome after birth asphyxia. Pediatr Res. 2003;54:270–5. doi: 10.1203/01.PDR.0000072518.98189.A0. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt B, Bauersfeld U, Schmid ER, et al. Serum and CSF levels of neuron-specific enolase (NSE) in cardiac surgery with cardiopulmonary bypass: a marker of brain injury? Brain Dev. 1998;20:536–9. doi: 10.1016/s0387-7604(98)00046-1. [DOI] [PubMed] [Google Scholar]
- 27.Lardner D, Davidson A, McKenzie I, Cochrane A. Delayed rises in serum S100B levels and adverse neurological outcome in infants and children undergoing cardiopulmonary bypass. Paediatr Anaesth. 2004;14:495–500. doi: 10.1111/j.1460-9592.2004.01230.x. [DOI] [PubMed] [Google Scholar]
- 28.Jonas RA. Review of current research at Boston Children’s Hospital. Ann Thorac Surg. 1993;56:1467–72. doi: 10.1016/0003-4975(93)90732-w. [DOI] [PubMed] [Google Scholar]
- 29.Lumpkins KM, Bochicchio GV, Keledjian K, Simard JM, McCunn M, Scalea T. Glial fibrillary acidic protein is highly correlated with brain injury. Glial fibrillary acidic protein is highly correlated with brain injury. J Trauma. 2008;65:778–82. doi: 10.1097/TA.0b013e318185db2d. [DOI] [PubMed] [Google Scholar]
- 30.Hsu AA, Fenton K, Weinstein S, Carpenter J, Dalton H, Bell MJ. Neurological injury markers in children with septic shock. Pediatr Crit Care Med. 2008;9:245–51. doi: 10.1097/PCC.0b013e3181727b22. [DOI] [PubMed] [Google Scholar]
- 31.Ashraf S, Bhattacharya K, Tian Y, Watterson K. Cytokine and S100B levels in paediatric patients undergoing corrective cardiac surgery with or without total circulatory arrest. Eur J Cardiothorac Surg. 1999;16:32–7. doi: 10.1016/s1010-7940(99)00136-0. [DOI] [PubMed] [Google Scholar]
- 32.Siman R, Toraskar N, Dang A, et al. A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. J Neurotrauma. 2009;26:1867–77. doi: 10.1089/neu.2009.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siman R, Roberts VL, McNeil E, et al. Biomarker evidence for mild central nervous system injury after surgically-induced circulation arrest. Brain Res. 2008;1213:1–11. doi: 10.1016/j.brainres.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papa L, Akinyi L, Liu MC, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010;38:138–44. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 36.Jonas RA, Bellinger DC, Rappaport LA, et al. Relation of pH strategy and developmental outcome after hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1993;106:362–68. [PubMed] [Google Scholar]
- 37.Wypij D, Jonas RA, Bellinger DC, et al. The effect of hematocrit during hypothermic cardiopulmonary bypass in infant heart surgery: results from the combined Boston hematocrit trials. J Thorac Cardiovasc Surg. 2008;135:355–60. doi: 10.1016/j.jtcvs.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg CS, Bove EL, Devaney EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg. 2007 Apr;133(4):880–7. doi: 10.1016/j.jtcvs.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 39.Visconti KJ, Rimmer D, Gauvreau K, et al. Regional low-flow perfusion versus circulatory arrest in neonates: one-year neurodevelopmental outcome. Ann Thorac Surg. 2006;82:2207–11. doi: 10.1016/j.athoracsur.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 40.Fraser CD, Jr, Andropoulos DB. Principles of antegrade cerebral perfusion during arch reconstruction in newborns/infants. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2008:61–8. doi: 10.1053/j.pcsu.2007.12.005. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 42.Anand KJ, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, Hall RW. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res. 2007;62:283–90. doi: 10.1203/PDR.0b013e3180986d2f. [DOI] [PubMed] [Google Scholar]
- 43.Loepke AW, Priestley MA, Schultz SE, McCann J, Golden J, Kurth CD. Desflurane improves neurologic outcome after low-flow cardiopulmonary bypass in newborn pigs. Anesthesiology. 2002;97:1521–7. doi: 10.1097/00000542-200212000-00026. [DOI] [PubMed] [Google Scholar]
- 44.McAuliffe JJ, Loepke AW, Miles L, Joseph B, Hughes E, Vorhees CV. Desflurane, isoflurane, and sevoflurane provide limited neuroprotection against neonatal hypoxia-ischemia in a delayed preconditioning paradigm. Anesthesiology. 2009;111:533–46. doi: 10.1097/ALN.0b013e3181b060d3. [DOI] [PubMed] [Google Scholar]
- 45.Hudetz JA, Pagel PS. Neuroprotection by ketamine: a review of the experimental and clinical evidence. J Cardiothorac Vasc Anesth. 2010;24:131–42. doi: 10.1053/j.jvca.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Sato K, Kimura T, Nishikawa T, Tobe Y, Masaki Y. Neuroprotective effects of a combination of dexmedetomidine and hypothermia after incomplete cerebral ischemia in rats. Acta Anaesthesiol Scand. 2009 Oct 26; doi: 10.1111/j.1399-6576.2009.02139.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Sanders RD, Sun P, Patel S, Li M, Maze M, Ma D. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010 doi: 10.1111/j.1399-6576.2009.02177.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Greeley WJ, Kern FH, Ungerleider RM, et al. The effect of hypothermic cardiopulmonary bypass and total circulatory arrest on cerebral metabolism in neonates, infants, and children. J Thorac Cardiovasc Surg. 1991;101:783–94. [PubMed] [Google Scholar]
- 49.Bissonnette B, Holtby HM, Davis AJ, Pua H, Gilder FJ, Black M. Cerebral hyperthermia in children after cardiopulmonary bypass. Anesthesiology. 2000;93:611–8. doi: 10.1097/00000542-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Laptook AR. Use of therapeutic hypothermia for term infants with hypoxic-ischemic encephalopathy. Pediatr Clin North Am. 2009;56:601–16. doi: 10.1016/j.pcl.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 51.American Heart Association. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric advanced life support. Pediatrics. 2006;117:e1005–28. doi: 10.1542/peds.2006-0346. [DOI] [PubMed] [Google Scholar]
- 52.Manole MD, Kochanek PM, Fink EL, Clark RS. Postcardiac arrest syndrome: focus on the brain. Curr Opin Pediatr. 2009;21:745–50. doi: 10.1097/MOP.0b013e328331e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pastuszko P, Pirzadeh A, Reade E, et al. The effect of hypothermia on neuronal viability following cardiopulmonary bypass and circulatory arrest in newborn piglets. Eur J Cardiothorac Surg. 2009;35:577–81. doi: 10.1016/j.ejcts.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steiger HJ, Hänggi D. Ischaemic preconditioning of the brain, mechanisms and applications. Acta Neurochir (Wien) 2007;149:1–10. doi: 10.1007/s00701-006-1057-1. [DOI] [PubMed] [Google Scholar]
- 55.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andropoulos DB, East DL, Eble BK, Stapleton GE, Chang AC. Preoperative cerebral oxyhemoglobin saturation in neonates undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2005;103:A1341. (Abstract) [Google Scholar]
- 57.Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–65. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 58.McPherson RJ, Juul SE. Recent trends in erythropoietin-mediated neuroprotection. Int J Dev Neurosci. 2008 Feb;26(1):103–11. doi: 10.1016/j.ijdevneu.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maise K, Li F, Chiong ZZ. New avenues of exploration for erythropoetin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu R, Suzuki A, Guo Z, et al. Intrinsic and extrinsic erythropoetin enhances neuroprotection against ischemia and reperfusion injury in vitro. J Neurochem. 2006;96:1101–10. doi: 10.1111/j.1471-4159.2005.03597.x. [DOI] [PubMed] [Google Scholar]
- 61.Givehchian M, Beschorner R, Ehmann C, et al. Neuroprotective effects of erythropoietin during deep hypothermic circulatory arrest. Eur J Cardiothorac Surg. 2010;37:662–668. doi: 10.1016/j.ejcts.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 62.Juul S. Recombinant erythropoietin as a neuroprotective treatment: in vitro and in vivo models. Clin Perinatol. 2004;31:129–42. doi: 10.1016/j.clp.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Zhu C, Kang W, Xu F, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:e218–26. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- 64.Sizonenko SV, Bednarek N, Gressens P. Growth factors and plasticity. Semin Fetal Neonatal Med. 2007;12:241–9. doi: 10.1016/j.siny.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 66.Schäbitz WR, Steigleder T, Cooper-Kuhn CM, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–72. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 67.Almli CR, Levy TJ, Han BH, Shah AR, Gidday JM, Holtzman DM. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp Neurol. 2000;166:99–114. doi: 10.1006/exnr.2000.7492. [DOI] [PubMed] [Google Scholar]
- 68.Abe K. Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J Cereb Blood Flow Metab. 2000;20:1393–408. doi: 10.1097/00004647-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 69.Vawda R, Woodbury J, Covey M, Levison SW, Mehmet H. Stem cell therapies for perinatal brain injuries. Semin Fetal Neonatal Med. 2007;12:259–72. doi: 10.1016/j.siny.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 70.Fan CG, Zhang QJ, Tang FW, Han ZB, Wang GS, Han ZC. Human umbilical cord blood cells express neurotrophic factors. Neurosci Lett. 2005;380:322–5. doi: 10.1016/j.neulet.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 71.Levey A, Glickstein JS, Kleinman CS, et al. The impact of prenatal diagnosis of complex congenital heart disease on neonatal outcomes. Pediatr Cardiol. 2010 Feb 18; doi: 10.1007/s00246-010-9648-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]