Abstract
The durability of HAART regimens is often limited by antiretroviral toxicity and nonadherence, which lead to virologic failure. We sought to determine sociodemographic and psychosocial patient factors predictive of short-term discontinuation of HAART regimens overall and stratified by the reason for discontinuation. A retrospective cohort study of the UAB 1917 Clinic Cohort evaluated short-term HAART regimen discontinuation (within 12 months of regimen initiation) between 1/1995 and 8/2004 classified as (1) gastrointestinal (GI) toxicity, (2) non-GI toxicity, (3) virologic failure or nonadherence (VF/NA), (4) loss to follow-up, and (5) other. Multivariable multinomial logistic regression models accounting for dependent observations were fit to assess the relationship between patient factors and type-specific regimen discontinuation. Among the 738 study participants, 1026 of 1852 HAART regimens (55%) were discontinued within 12 months of initiation. In multivariable analysis, discontinuation for GI toxicity was more common in patients lacking private health insurance and those with a history of intravenous (IV) drug use, whereas non-GI toxicity was more common in younger patients and females. African-American patients and those with a history of IV drug use were more likely to stop a regimen due to VF/NA. Loss to follow-up was more common in younger patients, individuals who were uninsured, and those with a history of IV drug use. Short-term discontinuation of HAART regimens is more common in vulnerable populations that bear a disproportionate burden of the U.S. HIV/AIDS epidemic. More vigilant monitoring of patient populations at higher risk of toxicity and virologic failure may allow for improved HAART regimen durability.
Introduction
Highly active antiretroviral therapy (HAART) is effective in decreasing plasma HIV viral load and reducing AIDS-related morbidity and mortality if taken correctly.1–6 However, toxicity associated with many of the antiretroviral drugs often leads to discontinuation or changes in treatment and also limits patient adherence and, therefore, virologic effectiveness.7–9 Several observational studies have shown that medication side effects are the most common cause of discontinuation of HAART regimens.1,7,9–14 Gastrointestinal (GI)-related side effects such as nausea, vomiting, and diarrhea have been among the most commonly reported medication toxicities leading to regimen discontinuation in numerous studies.1,7,12,13,15,16 Other studies have shown that certain patient characteristics such as race, gender, or socioeconomic status may predict higher rates of regimen discontinuation.
Most prior studies, however, have evaluated regimen status as “active” vs. “discontinued” without further investigating factors associated with specific, categorized reasons for HAART discontinuation. This information may be important for HIV providers to help identify reasons (e.g., GI toxicity) that certain patient populations (e.g., lower socioeconomic status) may be more likely to discontinue a HAART regimen within the first 12 months of initiation. Therefore, we evaluated sociodemographic and psychosocial patient factors associated with overall short-term HAART regimen discontinuation (within 12 months of regimen initiation) and then stratified these into five specific discontinuation categories: (1) GI toxicity, (2) non-GI toxicity, (3) virologic failure or non-adherence (VF/NA), (4) loss to follow-up, and (5) other.
Materials and Methods
Study population
The University of Alabama at Birmingham 1917 HIV/AIDS Clinic currently provides primary care and subspecialty services to over 1500 HIV-infected patients. The UAB 1917 HIV/AIDS Clinic Cohort Observational Database is an observational cohort study including over 6000 patients seen at the clinic since 1988.17,18 Here, we conduct a retrospective cohort study nested in the UAB 1917 Clinic Cohort to evaluate reasons for short-term HAART regimen discontinuation among patients starting HAART regimens between January 1995 and August 2004. HAART regimens were defined as a combination of three or more antiretroviral drugs. Individual patients could contribute multiple records to the present analysis if they initiated more than one HAART regimen during the study period. Detailed medical chart review was undertaken to determine specific reasons for HAART regimen discontinuation by reviewing provider notes from the date that regimens were stopped. Therefore, the identified reason for discontinuation reflects the impression of the medical provider as recorded in the medical record.
Regimen discontinuation was classified as follows: (1) discontinued due to GI toxicity, (2) discontinued due to non-GI toxicity, (3) discontinued due to virologic failure or nonadherence (VF/NA), (4) discontinued due to loss to follow-up, and (5) discontinued due to other reasons. A regimen that was continued for 12 months or longer was classified as “active.” The discontinuation classification categories were chosen based on the most common recorded reasons for regimen discontinuation. A hierarchy of discontinuation reasons was established as follows: if the regimen was discontinued within 12 months of initiation, the regimen was classified first as discontinuation for “GI toxicity” if certain predetermined GI signs or symptoms were recorded by the provider as a reason for stopping treatment. If a regimen was not discontinued for GI toxicity, it was then classified as “other toxicity” if any other signs of toxicity were recorded by the provider as a reason for stopping treatment. Next, if the discontinuation reason was not due to toxicity, it was classified as discontinuation for “VF/NA” if either of these reasons was documented by the provider. If a patient's last contact with the clinic occurred within 365 days of starting a regimen, this regimen was considered discontinued due to loss to follow-up since regimen status could not be ascertained at 12 months. Lastly, the discontinuation reason was classified as “other” if it did not meet any of these above mentioned criteria and other reasons were cited (e.g., financial considerations). The UAB Institutional Review Board approved the study protocol.
Statistical methods
Descriptive statistics were generated for all study variables to evaluate the distributions of these variables to ensure that assumptions of employed statistical tests were met. Categorical patient characteristics are presented as percentages and continuous characteristics as means and standard deviations.
To evaluate overall 12 month regimen status as active vs. discontinued, univariate logistic regression models were fit. Next a multivariable logistic regression model was fit with variable selection driven by information from univariate models and clinical judgment.
Subsequently, univariate and then multivariable multinomial logistic regression models were fit to assess the relationship between patient characteristics and the categorized 6-level 12-month discontinuation status: (1) active, (2) discontinued due to GI toxicity, (3) discontinued due to non-GI toxicity, (4) discontinued due to virologic failure or nonadherence, (5) discontinued due to loss to follow-up, and (6) discontinued due to other reasons; the “active” category served as the reference group. Because of the heterogeneous reasons captured in the “discontinued due to other reasons” category, we do not focus much attention on this group when discussing the results of these analyses.
For all regression models, the likely artificial deflation in standard errors due to multiple HAART regimens being contributed by the same patients was corrected by use of Huber–White robust variance estimates (Stata's “cluster” option on the “mlogit” procedure). As a sensitivity analysis, the final models were rerun using only each subject's initial regimen. Analyses were performed with SAS V9.1.3 and Stata V9.
Results
Among the 738 patients meeting study criteria and included in this analysis, 552 (75%) were male, 369 were black (50%), 322 were between the ages of 30 and 39 years (44%) at initiation, 347 (47%) were men who reported having sex with men (MSM), and 68 patients (9%) reported a history of IV drug use (Table 1). Over half of the patients had private health insurance (n = 389, 53%), with the other half almost equally divided between having public health insurance or no insurance. Two hundred and seventy-three patients (37%) were treated with a single HAART regimen during the study period, 191 patients were treated with two regimens (26%), 123 patients (17%) with three regimens, and another 151 patients (21%) received four or more regimens.
Table 1.
Baseline Characteristics of 738 HIV-Infected Patients Contributing 1852 HAART Regimens to the Study of Short-Term (12 Month) HAART Regimen Discontinuation at the UAB 1917 HIV/AIDS Clinic, 1995–2004
| Characteristics | N (%) or mean ± standard deviation |
|---|---|
| Patient characteristics (N = 738) | |
| Age | |
| <30 | 119 (16.1) |
| 30–39 | 322 (43.6) |
| 40–50 | 211 (28.6) |
| >50 | 86 (11.7) |
| Gender | |
| Female | 186 (25.2) |
| Male | 552 (74.8) |
| Race | |
| Black | 369 (50.0) |
| White | 369 (50.0) |
| ARV naive | |
| No | 145 (19.6) |
| Yes | 593 (80.4) |
| HAART naive | |
| No | 109 (14.8) |
| Tes | 629 (85.2) |
| Men who have sex with men (MSM) | |
| No | 391 (53.0) |
| Tes | 347 (47.0) |
| History of IV drug use | |
| No | 670 (90.8) |
| Tes | 68 (9.2) |
| Insurance classa | |
| None | 175 (23.9) |
| Private | 389 (53.2) |
| Public | 167 (22.9) |
| Depression | |
| No | 468 (63.4) |
| Tes | 270 (36.6) |
| Substance abuse | |
| No | 593 (80.4) |
| Tes | 145 (19.6) |
| Baseline CD4 (cells/mm3)a | 220 ± 232 |
| Baseline log10 plasma HIV RNA (copies/ml)a | 4.6 ± 1.1 |
| Number of regimens contributed to study | |
| 1 | 273 (37.0) |
| 2 | 191 (25.9) |
| 3 | 123 (16.7) |
| ≥4 | 151 (20.5) |
| HAART regimen characteristics (n = 1852) | |
| Year of regimen initiation | |
| 1995–2000 | 846 (45.7) |
| 2001–2004 | 1006 (54.3) |
| Number of antiretroviral drugs in regimen | |
| 3 | 1380 (74.5) |
| 4 | 407 (22.0) |
| ≥5 | 65 (3.5) |
| Number of NRTIs in regimen | |
| 0 | 19 (1.0) |
| 1 | 107 (5.8) |
| 2 | 1462 (78.9) |
| ≥3 | 264 (14.3) |
| Number of NNRTIs in regimen | |
| 0 | 1054 (56.9) |
| 1 | 798 (43.1) |
| Number of PIs in regimen | |
| 0 | 844 (45.6) |
| 1 | 595 (32.1) |
| ≥2 | 413 (22.3) |
| Reason for discontinuation by 12 months | |
| None (active regimen) | 826 (44.6) |
| GI-related toxicity | 233 (12.6) |
| Non-GI toxicity | 225 (12.2) |
| Virologic failure/non-adherence (VF/NA) | 172 (9.3) |
| Loss to follow-up (LTFU) | 95 (5.1) |
| Other | 301 (16.2) |
Seven patients had missing insurance class data, 154 patients had missing baseline CD4 data, and 183 patients had missing baseline plasma HIV RNA data
A total of 1852 HAART regimens among the 738 study participants were included in this analysis (Table 1). Most of the 1852 regimens contained three drugs (75%), 22% contained four drugs [including ritonavir-boosted protease inhibitor (PI) regimens], and 3% were composed of five or more drugs. The majority of regimens (80%) contained two nucleoside reverse transcriptase inhibitors (NRTIs) plus either a nonnucleoside reverse transcriptase inhibitor (NNRTI) or a PI. Twelve months after regimen initiation, 826 (45%) of the regimens were still active, while 1026 had been discontinued (55%). Of the 1026 discontinued regimens, nearly half were discontinued due to toxicity (n = 458, 45%); 233 regimens were discontinued due to GI toxicity (23%) and 225 due to non-GI toxicity (22%). VF/NA accounted for short-term discontinuation of 172 HAART regimens (17%), loss to follow-up was recorded for 95 discontinued regimens (9%), and 301 regimens were stopped due to other reasons (29%).
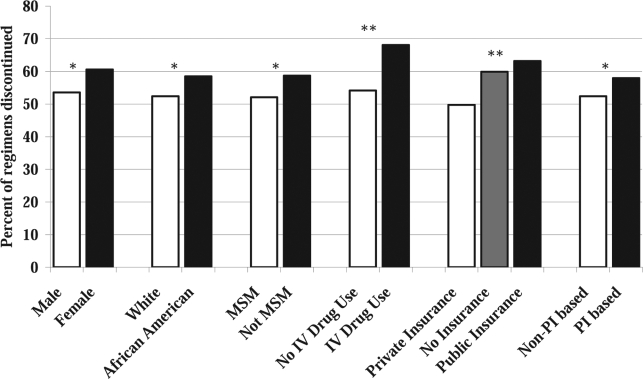
A breakdown of sociodemographic, psychosocial, and regimen characteristics associated with each category of HAART regimen discontinuation is presented in Table 2. First, all discontinued regimens (for any of the five reasons) were compared to regimens still active 1 year following initiation. Separate univariate logistic regression models accounting for dependent observations were used to evaluate factors associated with overall short-term HAART regimen discontinuation (Fig. 1 and Table 3). In these models, younger age, female sex, black race, risk group other than MSM, history of intravenous drug use (IVDU), no health insurance and public health insurance referent to private health insurance, PI-containing regimens, and the number of HAART regimens contributed to analysis were significantly associated with regimen discontinuation within 12 months following initiation.
Table 2.
Sociodemographic, Psychosocial, and Regimen Characteristics by Short-Term (12 Month) HAART Regimen Status for 1852 HAART Regimens Used in the Treatment of 738 HIV-Infected Patients at the UAB 1917 HIV/AIDS Clinic, 1995–2004a
| |
|
|
Discontinued regimens by reason |
||||
|---|---|---|---|---|---|---|---|
| Variable | Active (N = 826) | Discontinued (N = 1026) | GI toxicity (N = 233) | Non-GI toxicity (N = 225) | Virologic failure/nonadherence (N = 172) | Loss to follow-up (N = 95) | Other (N = 301) |
| Age (years) | |||||||
| <30 | 86 (10.4) | 134 (13.1) | 17 (7.3) | 32 (14.2) | 27 (15.7) | 14 (14.7) | 44 (14.6) |
| 30–39 | 307 (37.2) | 481 (46.9) | 116 (49.8) | 95 (42.2) | 78 (45.3) | 50 (52.6) | 142 (47.2) |
| 40–50 | 309 (37.4) | 289 (28.2) | 60 (25.8) | 76 (33.8) | 43 (25.0) | 25 (26.3) | 85 (28.2) |
| >50 | 124 (15.0) | 122 (11.9) | 40 (17.2) | 22 (9.8) | 24 (14.0) | 6 (6.3) | 30 (10.0) |
| Gender | |||||||
| Female | 194 (23.5) | 298 (29.0) | 76 (32.6) | 82 (36.4) | 43 (25.0) | 18 (18.9) | 79 (26.2) |
| Male | 632 (76.5) | 728 (71.0) | 157 (67.4) | 143 (63.6) | 129 (75.0) | 77 (81.1) | 222 (73.8) |
| Race | |||||||
| Black | 379 (45.9) | 534 (52.0) | 118 (50.6) | 110 (48.9) | 104 (60.5) | 50 (52.6) | 152 (50.5) |
| White | 447 (54.1) | 492 (48.0) | 115 (49.4) | 115 (51.1) | 68 (39.5) | 45 (47.4) | 149 (49.5) |
| Men who have sex with men (MSM) | |||||||
| No | 385 (46.6) | 547 (53.3) | 127 (54.5) | 124 (55.1) | 92 (53.5) | 51 (53.7) | 153 (50.8) |
| Yes | 441 (53.4) | 479 (46.7) | 106 (45.5) | 101 (44.9) | 80 (46.5) | 44 (46.3) | 148 (49.2) |
| History of IV drug use | |||||||
| No | 773 (93.6) | 913 (89.0) | 198 (85.0) | 206 (91.6) | 148 (86.0) | 81 (85.3) | 280 (93.0) |
| Yes | 53 (6.4) | 113 (11.0) | 35 (15.0) | 19 (8.4) | 24 (14.0) | 14 (14.7) | 21 (7.0) |
| Insuranceb | |||||||
| None | 166 (20.1) | 248 (24.2) | 60 (25.8) | 56 (24.9) | 26 (15.1) | 29 (30.5) | 77 (25.6) |
| Private | 497 (60.2) | 492 (48.0) | 99 (42.5) | 116 (51.6) | 90 (52.3) | 42 (44.2) | 145 (48.2) |
| Public | 162 (19.6) | 278 (27.1) | 74 (31.8) | 52 (23.1) | 54 (31.4) | 21 (22.1) | 77 (25.6) |
| Depression | |||||||
| No | 552 (66.8) | 721 (70.3) | 145 (62.2) | 159 (70.7) | 121 (70.3) | 71 (74.7) | 225 (74.8) |
| Yes | 274 (33.2) | 305 (29.7) | 88 (37.8) | 66 (29.3) | 51 (29.7) | 24 (25.3) | 76 (25.2) |
| Substance abuse | |||||||
| No | 695 (84.1) | 872 (85.0) | 193 (82.8) | 198 (88.0) | 145 (84.3) | 77 (81.1) | 259 (86.0) |
| Yes | 131 (15.9) | 154 (15.0) | 40 (17.2) | 27 (12.0) | 27 (15.7) | 18 (18.9) | 42 (14.0) |
| NNRTI | |||||||
| No | 453 (54.8) | 601 (58.6) | 150 (64.4) | 110 (48.9) | 108 (62.8) | 46 (48.4) | 187 (62.1) |
| Yes | 374 (45.2) | 425 (41.4) | 83 (35.6) | 115 (51.1) | 64 (37.2) | 49 (51.6) | 114 (37.9) |
| PI | |||||||
| No | 402 (48.7) | 442 (43.1) | 83 (35.6) | 108 (48.0) | 65 (37.8) | 49 (51.6) | 137 (45.5) |
| Yes | 424 (51.3) | 584 (56.9) | 150 (64.4) | 117 (52.0) | 107 (62.2) | 46 (48.4) | 164 (54.5) |
| HAART regimen number | |||||||
| 1 | 357 (43.2) | 381 (37.1) | 78 (33.5) | 96 (42.7) | 56 (32.6) | 40 (42.1) | 111 (36.9) |
| 2 | 222 (26.9) | 243 (23.7) | 51 (21.9) | 51 (22.7) | 39 (22.7) | 28 (29.5) | 74 (24.6) |
| 3 | 124 (15.0) | 150 (14.6) | 38 (16.3) | 25 (11.1) | 29 (16.9) | 13 (13.7) | 45 (15.0) |
| ≥4 | 123 (14.9) | 252 (24.6) | 66 (28.3) | 53 (23.6) | 48 (27.9) | 14 (14.7) | 71 (23.6) |
Data presented as N (column percent).
Insurance type unknown in 9 of the 1852 regimens.
FIG. 1.
Characteristics associated with short-term (12 month) HAART regimen discontinuation for 1852 regimens used in the treatment of 738 HIV-infected patients at the UAB 1917 HIV/AIDS Clinic, 1995–2004. Percents and p-values are based on six separate univariate logistic regression models using Huber-White robust standard errors, which account for the clustering of regimens within patients, but do not adjust for any additional covariates. Values plotted are the predicted probability of a regimen being discontinued within 12 months of initiation. *p < 0.05, **p < 0.01.
Table 3.
Univariate Logistic Regression Models Evaluating Characteristics Associated with Short-Term (12 Month) HAART Regimen Discontinuation for 1852 Regimens Used in the Treatment of 738 HIV-Infected Patients at the UAB 1917 HIV/AIDS Clinic Overall and Stratified by Discontinuation Reason, 1995–2004
| |
|
|
Discontinued regimens by reasonb |
|||
|---|---|---|---|---|---|---|
| Characteristic | Overall discontinueda | GI toxicity (N = 233) OR (95% CI) | Non-GI toxicity (N = 225) OR (95% CI) | Virologic failure/nonadherence (N = 172) OR (95% CI) | Loss to follow-up (N = 95) OR (95% CI) | Other (N = 301) OR (95% CI) |
| Age (per 10 years) | 0.81 (0.71–0.93) | 0.97 (0.80–1.19) | 0.81 (0.68–0.97) | 0.81 (0.61–1.07) | 0.64 (0.50–0.81) | 0.75 (0.63–0.89) |
| Female vs. male | 1.33 (1.03–1.73) | 1.58 (1.04–2.39) | 1.87 (1.32–2.64) | 1.09 (0.65–1.80) | 0.76 (0.44–1.32) | 1.16 (0.83–1.62) |
| Black vs. white race | 1.28 (1.02–1.61) | 1.21 (0.84–1.74) | 1.13 (0.81–1.57) | 1.80 (1.20–2.70) | 1.31 (0.85–2.02) | 1.20 (0.89–1.62) |
| Men who have sex with men (MSM) | 0.76 (0.61–0.96) | 0.73 (0.51–1.04) | 0.71 (0.51–0.99) | 0.76 (0.51–1.14) | 0.75 (0.49–1.16) | 0.84 (0.63–1.14) |
| History of IV Drug use | 1.81 (1.22–2.66) | 2.57 (1.55–4.29) | 1.35 (0.81–2.25) | 2.37 (1.34–4.18) | 2.52 (1.32–4.83) | 1.09 (0.57–2.10) |
| No insurance vs. private insurance | 1.51 (1.13–1.01) | 1.81 (1.12–2.93) | 1.45 (0.95–2.20) | 0.86 (0.51–1.47) | 2.07 (1.23–3.48) | 1.59 (1.11–2.28) |
| Public insurance vs. private insurance | 1.73 (1.32–2.28) | 2.29 (1.54–3.41) | 1.38 (0.92–2.06) | 1.84 (1.16–2.92) | 1.53 (0.88–2.67) | 1.63 (1.10–2.39) |
| Depression | 0.85 (0.67–1.08) | 1.22 (0.75–1.62) | 0.72 (0.46–1.13) | 0.99 (0.58–1.68) | 0.68 (0.42–1.11) | 0.68 (0.48–0.97) |
| Substance abuse | 0.94 (0.71–1.24) | 1.10 (0.58–1.36) | 0.73 (0.45–1.17) | 0.87 (0.50–1.51) | 1.24 (0.71–2.15) | 0.86 (0.56–1.32) |
| NNRTI | 0.86 (0.70–1.05) | 0.67 (0.49–0.93) | 1.27 (0.94–1.72) | 0.72 (0.51–1.01) | 1.29 (0.85–1.98) | 0.74 (0.56–0.99) |
| PI | 1.25 (1.03–1.52) | 1.71 (1.26–2.34) | 1.03 (0.76–1.40) | 1.56 (1.10–2.22) | 0.89 (0.58–1.36) | 1.13 (0.86–1.50) |
| HAART regimen number (range 1–12) | 1.14 (1.08–1.20) | 1.21 (1.13–1.29) | 1.10 (1.01–1.18) | 1.20 (1.09–1.31) | 0.96 (0.85–1.10) | 1.13 (1.06–1.21) |
Univariate logistic regression models accounting for dependent observations with active regimens as the reference group. Overall discontinued combines regimens stopped for GI toxicity, non-GI toxicity, virologic failure/nonadherence, and loss to follow-up.
Univariate multinomial logistic regression models accounting for dependent observations with active regimens as the reference group.
Next, a multivariable logistic regression model accounting for dependent observations was fit to evaluate patient factors associated with overall short-term HAART regimen discontinuation while adjusting for covariates (Table 4). In this model, younger age (OR 0.78 per 10 years; 0.69–0.89), history of IVDU (OR 1.63; 1.13–2.37), having no health insurance (OR 1.40; 1.07–1.83) or public health insurance (OR 1.64; 1.25–2.15) referent to private health insurance, and the number of HAART regimens contributed to analysis (OR 1.16 per additional regimen; 1.11–1.23) were significantly associated with regimen discontinuation within 12 months following initiation.
Table 4.
Multivariable Logistic Regression Models Evaluating Characteristics Associated with Short-Term (12 Month) HAART Regimen Discontinuation for 1852 Regimens Used in the Treatment of 738 HIV-Infected Patients at the UAB 1917 HIV/AIDS Clinic Overall and Stratified by Discontinuation Reason, 1995–2004
| |
|
|
Discontinued regimens by reasonb |
|||
|---|---|---|---|---|---|---|
| Characteristic | Overall discontinueda | GI toxicity (N = 233) OR (95% CI) | Non-GI toxicity (N = 225) OR (95% CI) | Virologic failure/nonadherence (N = 172) OR (95% CI) | Loss to follow-up (N = 95) OR (95% CI) | Other (N = 301) OR (95% CI) |
| Age (per 10 years) | 0.78 (0.69–0.89) | 0.93 (0.76–1.13) | 0.80 (0.67–0.96) | 0.77 (0.57–1.03) | 0.66 (0.51–0.86) | 0.72 (0.61–0.85) |
| Female vs. male | 1.19 (0.88–1.61) | 1.54 (0.94–2.52) | 1.96 (1.27–3.03) | 0.85 (0.48–1.52) | 0.59 (0.32–1.13) | 1.01 (0.66–1.54) |
| Black vs. white race | 1.09 (0.86–1.39) | 1.11 (0.77–1.60) | 0.86 (0.61–1.23) | 1.68 (1.08–2.60) | 1.19 (0.73–1.94) | 0.98 (0.70–1.38) |
| Men who have sex with men (MSM) | 0.90 (0.69–1.17) | 1.03 (0.67–1.58) | 1.00 (0.67–1.49) | 0.90 (0.56–1.44) | 0.66 (0.38–1.14) | 0.88 (0.60–1.28) |
| History of IV Drug use | 1.63 (1.13–2.37) | 2.32 (1.39–3.86) | 1.40 (0.81–2.46) | 2.22 (1.23–3.99) | 2.09 (1.06–4.13) | 0.92 (0.48–1.76) |
| No insurance vs. private insurance | 1.40 (1.07–1.83) | 1.69 (1.11–2.59) | 1.33 (0.88–2.04) | 0.79 (0.47–1.35) | 1.72 (1.00–2.93) | 1.52 (1.06–2.20) |
| Public insurance vs. private insurance | 1.64 (1.25–2.15) | 1.92 (1.30–2.84) | 1.41 (0.92–2.16) | 1.53 (0.93–2.52) | 1.37 (0.76–2.47) | 1.77 (1.21–2.60) |
| Depression | 0.83 (0.66–1.05) | 1.15 (0.82–1.61) | 0.81 (0.56–1.17) | 0.85 (0.55–1.32) | 0.76 (0.45–1.27) | 0.65 (0.46–0.93) |
| Substance abuse | 0.86 (0.65–1.14) | 0.89 (0.58–1.36) | 0.73 (0.45–1.17) | 0.87 (0.50–1.51) | 1.09 (0.61–1.94) | 0.86 (0.57–1.31) |
| NNRTI | 0.91 (0.68–1.21) | 0.84 (0.50–1.40) | 1.48 (0.96–2.28) | 0.88 (0.54–1.43) | 1.34 (0.74–2.42) | 0.60 (0.41–0.88) |
| PI | 1.13 (0.85–1.50) | 1.54 (0.93–2.55) | 1.27 (0.83–1.96) | 1.38 (0.83–2.29) | 1.05 (0.59–1.88) | 0.76 (0.52–1.12) |
| HAART regimen number (range 1–12) | 1.16 (1.11–1.23) | 1.21 (1.12–1.30) | 1.10 (1.02–1.19) | 1.23 (1.12–1.35) | 1.01 (0.88–1.15) | 1.17 (1.10–1.26) |
Multivariable logistic regression model accounting for dependent observations with active regimens as the reference group. Overall discontinued combines regimens stopped for GI toxicity, non-GI toxicity, virologic failure/nonadherence, and loss to follow-up.
Multivariable multinomial logistic regression model accounting for dependent observations with active regimens as the reference group.
Third, separate univariate multinomial logistic regression models accounting for dependent observations were used to evaluate factors associated with short-term HAART regimen discontinuation categorized by specific discontinuation reason relative to active regimens (those lasting more than 12 months) (Table 3). Females were more likely than males to discontinue a regimen for both GI toxicity and non-GI toxicity. Several other characteristics were also associated with discontinuation of a regimen for GI toxicity, including a history of IVDU, being uninsured (or having public health insurance referent to having private insurance), treatment with a regimen that included a PI, and successive HAART regimens received during the study period. Use of an NNRTI was inversely associated with discontinuation of a regimen for GI toxicity in univariate analysis. Younger patients and patients belonging to risk groups other than MSM were more likely to stop a regimen within 12 months of initiation for non-GI side effects. Factors associated with short-term regimen discontinuation secondary to VF/NA include black race, history of IVDU, public health insurance referent to having private insurance, as well as PI-containing regimens, and the number of regimens received during the study period. In univariate analyses, loss to follow-up was more common in younger patients, those with a history of IVDU, and patients who were uninsured.
Finally, a multivariable multinomial logistic regression model accounting for dependent observations was fit to evaluate patient factors associated with short-term HAART regimen discontinuation categorized by specific discontinuation reason relative to active regimens (those lasting more than 12 months) (Table 4). Patient characteristics associated with short-term discontinuation for GI toxicity include a history of IVDU (OR 2.32; 1.39–3.86) and having no health insurance (OR 1.69; 1.11–2.59) or public health insurance (OR 1.92;1.30–2.84) referent to having private insurance and successive HAART regimens received during the study period (OR 1.21 per additional regimen; 1.12–1.30). Regimen discontinuation for non-GI toxicity was observed more commonly among females (OR 1.96; 1.27–3.03) and with successive regimens during the study period (OR 1.10 per additional regimen; 1.02–1.19). Age was inversely associated with discontinuation for non-GI toxicity, being more common in younger patients (OR 0.80 per 10 years; 0.67–0.96). Black race (OR 1.68; 1.08–2.60), a history of IVDU (OR 2.22; 1.23–3.99), and successive HAART regimens (OR 1.23 per additional regimen; 1.12–1.35) were associated with discontinuation for VF/NA. Loss to follow-up, defined as the last contact with the clinic within 365 days of regimen initiation, was more common in younger patients (OR 0.66 per years older; 0.51–0.86), the uninsured (OR 1.72; 1.00–2.93), and patients with a history of IVDU (OR 2.09; 1.06–4.13).
Sensitivity analyses limited to each patient's initial HAART regimen yielded results similar to, albeit less precise than, the primary regression models (results not shown).
Discussion
Our results demonstrate that vulnerable populations including females, younger individuals, African-Americans, those with IVDU histories, and patients lacking private health insurance were more likely to experience short-term HAART regimen discontinuation. This study adds to the literature by evaluating patient characteristics associated with 12-month discontinuation of HAART regimens overall and further stratified into specific categories: (1) GI toxicity, (2) non-GI toxicity, (3) VF/NA, (4) loss to follow-up, or (5) other. Other studies have shown that indigent urban patients are less likely to maintain virologic suppression, usually secondary to nonadherence.19–21 However, our finding in a Southern U.S. cohort that the groups of patients who are disproportionately and increasingly impacted by the U.S. HIV/AIDS epidemic are also more likely to experience short-term HAART regimen discontinuation is cause for concern.
Prior studies have shown that medication side effects are the most common reason for change or discontinuation of a HAART regimen.1,7,9–14 The most frequently reported side effects are gastrointestinal, including nausea, vomiting, and diarrhea. The ICONA study demonstrated that 25% of participants discontinued a HAART regimen by 1 year due to toxicity.7 In the current study, 1026 of 1852 (55%) regimens were discontinued by 1 year and nearly half of these regimen discontinuations (458 of 1026 regimens) were due to toxicity attributed to antiretroviral medications.
Drug toxicity is a frequent cause of regimen nonadherence leading to virologic failure. Even subtle drug intolerance, such as mild or intermittent GI symptoms, can lead to skipped dose behavior. In our study, we could not determine how many patients who experienced virologic failure had subtle regimen intolerance that was not reported to the clinician. Concern regarding drug toxicity affects not only adherence to HAART regimens in patients being treated, but also may prevent some patients from initiating treatment. Bassetti and colleagues found that among their patients refusing treatment with HAART, 19% feared side effects and 18% thought that the treatment regimen was overly complicated.9 Improving patient education regarding the benefits of viral suppression and potential drug side effects and suggesting methods to help adhere to antiretroviral regimens are potential means of increasing initiation and decreasing discontinuation rates.
Previously, females have been shown to be among the populations at higher risk for HAART regimen discontinuation, particularly secondary to toxicity. Our data are consistent with these findings in demonstrating that female gender is significantly associated with short-term HAART regimen discontinuation for non-GI toxicity and of marginal significance for discontinuation due to GI toxicity. The ICONA study also showed that women were twice as likely as men to discontinue HAART due to toxicity.7 Other studies have shown that women experience a significantly higher number of side effects to PIs and also have a higher rate of PI discontinuation than do men.10,19,22
Several hypotheses have been postulated to explain why women may be less tolerant of antiretroviral medication side effects and have higher rates of HAART discontinuation when compared to men. Differences in pharmacokinetics in females may result in higher antiretroviral drug concentrations that could explain increased toxicity in this group. Proposed mechanisms for the alteration in pharmacokinetics include differences in weight, BMI, fat distribution, and hormone levels.23 Psychosocial factors, such as the position of many women as the primary caregiver for others, are other possible differences that could lead to higher rates of discontinuation in females.13,21,23–25 These findings highlight the need for further investigation regarding the pharmacokinetic differences and psychosocial factors leading females to be more likely to discontinue HAART regimens so that effective interventions may be developed.
A history of IV drug use was predictive of overall regimen discontinuation as well as discontinuation for GI toxicity, VF/NA, and loss to follow-up in our study. Other studies have found rates of HAART discontinuation as high as 44% in active IV drug users. Similar to the present study, antiretroviral medication side effects were a primary reason cited for discontinuation previously.26–28 Additional factors such as untreated addiction, poor living conditions, lack of social support, and poor access to medical care may also contribute to the high rate of VF/NA, loss to follow-up, and regimen discontinuation in this population.29
Lacking private health insurance, a marker of socioeconomic status, was associated with short-term regimen discontinuation for GI toxicity. While the reasons for this association cannot be determined in the current study, we hypothesize that patients lacking private health insurance may have less access to consistent medical care and greater difficulty in obtaining medications to treat GI side effects, resulting in higher discontinuation rates from GI toxicity. Uninsured patients were more likely to be lost to follow-up within 1 year of starting a HAART regimen, supporting our notion that these individuals may have greater difficulty consistently accessing outpatient HIV care, perhaps related to greater attention required by competing needs (e.g., housing and transportation).
African-American patients had higher rates of short-term HAART regimen discontinuation for VF/NA. These findings are in accordance with recently published clinical trial and observational cohort study results that found greater short-term virologic failure in African-Americans.30,31 Future research is needed to better understand the underlying factors contributing to these findings such that effective interventions may be implemented to improve health outcomes and overcome disparities in HIV-infected African-Americans.
Finally, younger patients were more likely to discontinue regimens due to non-GI toxicity and loss to follow-up, and also showed a trend for higher rates of discontinuation due to VF/NA. Previously, we demonstrated younger individuals were less likely to establish care at our clinic after calling for an initial appointment,32 and others have shown younger patients are more likely to miss scheduled outpatient HIV visits.33,34 The current study adds to this literature by demonstrating loss to follow-up shortly after starting a HAART regimen is more common in younger individuals. Collectively these studies highlight a priority population for interventions aimed at improving engagement in HIV care.
Our results should be interpreted with respect to the limitations of our study. As with all observational studies, we can identify associations but cannot attribute causality. As a single center study in the Southeast United States, our findings may or may not be generalizable to other regions of the country or nonacademic settings. Discontinuation reasons were determined from patients' medical records and reflect the impression of their medical provider; we did not specifically survey patients regarding the reason for HAART regimen discontinuation. Finally, some patients who were lost to follow-up at our clinic within 365 days of starting a HAART regimen may have remained on their regimen at 12 months, which would have led to inappropriate classification of these individuals.
In summary, our findings that short-term discontinuation of HAART regimens is more common in vulnerable patient populations that are disproportionately affected by the U.S. HIV/AIDS epidemic emphasize the need for increased education and patient-provider communication with these patient groups. We identified sociodemograpic characteristics of patients associated with short-term regimen discontinuation overall and further stratified by specific reasons for discontinuation. This information may help providers better assess the severity of side effects, the effect of toxicity on compliance, and also better identify patients who are at greater risk of being lost to follow-up shortly after starting a HAART regimen.35,36 Such knowledge may be used for better patient education regarding toxicity and the importance of retention in care, and for more vigilant monitoring for side effects and loss to follow-up, particularly for high-risk groups. Further research is needed to better understand the root causes of increased HAART regimen discontinuation among vulnerable patient populations in an effort to optimize regimen durability and improve clinical outcomes among these underserved groups.
Acknowledgments
We thank the University of Alabama at Birmingham 1917 Clinic Cohort management team for their assistance with this project (www.uab1917clinicalcohort.org). Financial support was provided by the University of Alabama at Birmingham Center for AIDS Research (Grant P30-AI27767), CFAR-Network of Integrated Clinical Systems, CNICS (Grant 1 R24 AI067039-1), and the Mary Fisher CARE Fund, K23MH082641 from the National Institute of Mental Health (M.J.M).
Disclosure Statement
M.J.M. has received grant support from Tibotec Therapeutics and Bristol-Myers Squibb. M.S.S. has received research or grant support from or is a consultant for Achillion, Avexa, Boehringer-Ingelheim, Bristol-Meyer Squibb, Gilead Sciences, GlaxSmithKlein, Merck, Monogram Biosciences, Panacos, Pfizer/Aragon, Progenics, Roche Laboratories, Seronon, Tanox, Tibotec, Trimeris, and Vertex. All other authors had no potential conflicts.
References
- 1.O'Brien ME. Clark RA. Besch CL. Myers L. Kissinger P. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J Acquir Immune Defic Syndr. 2003;34:407–414. doi: 10.1097/00126334-200312010-00008. [DOI] [PubMed] [Google Scholar]
- 2.Hogg RS. Heath KV. Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 3.Powderly WG. Landay A. Lederman MM. Recovery of the immune system with antiretroviral therapy: The end of opportunism? JAMA. 1998;280:72–77. doi: 10.1001/jama.280.1.72. [DOI] [PubMed] [Google Scholar]
- 4.Palella FJ., Jr Delaney KM. Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.Ledergerber B. Egger M. Opravil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: A prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353:863–868. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 6.Haubrich RH. Little SJ. Currier JS, et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. California Collaborative Treatment Group. AIDS. 1999;13:1099–1107. doi: 10.1097/00002030-199906180-00014. [DOI] [PubMed] [Google Scholar]
- 7.d'Arminio Monforte A. Lepri AC. Rezza G, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. AIDS. 2000;14:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 8.Vanhove GF. Schapiro JM. Winters MA. Merigan TC. Blaschke TF. Patient compliance and drug failure in protease inhibitor monotherapy. JAMA. 1996;276:1955–1956. [PubMed] [Google Scholar]
- 9.Bassetti S. Battegay M. Furrer H, et al. Why is highly active antiretroviral therapy (HAART) not prescribed or discontinued? Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 1999;21:114–119. [PubMed] [Google Scholar]
- 10.Bonfanti P. Valsecchi L. Parazzini F, et al. Incidence of adverse reactions in HIV patients treated with protease inhibitors: A cohort study. Coordinamento Italiano Studio Allergia e Infezione da HIV (CISAI) Group. J Acquir Immune Defic Syndr. 2000;23:236–245. doi: 10.1097/00126334-200003010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Dorrucci M. Pezzotti P. Grisorio B, et al. Time to discontinuation of the first highly active antiretroviral therapy regimen: A comparison between protease inhibitor- and non-nucleoside reverse transcriptase inhibitor-containing regimens. AIDS. 2001;15:1733–1736. doi: 10.1097/00002030-200109070-00020. [DOI] [PubMed] [Google Scholar]
- 12.Hansel A. Bucher HC. Nuesch R. Battegay M. Reasons for discontinuation of first highly active antiretroviral therapy in a cohort of proteinase inhibitor-naive HIV-infected patients. J Acquir Immune Defic Syndr. 2001;26:191–193. doi: 10.1097/00042560-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 13.Le Moing V. Chene G. Leport C, et al. Impact of discontinuation of initial protease inhibitor therapy on further virological response in a cohort of human immunodeficiency virus-infected patients. Clin Infect Dis. 2002;34:239–247. doi: 10.1086/324354. [DOI] [PubMed] [Google Scholar]
- 14.Cozzi-Lepri A. Phillips AN. d'Arminio Monforte A, et al. Virologic and immunologic response to regimens containing nevirapine or efavirenz in combination with 2 nucleoside analogues in the Italian Cohort Naive Antiretrovirals (I.Co.N.A.) study. J Infect Dis. 2002;185:1062–1069. doi: 10.1086/339821. [DOI] [PubMed] [Google Scholar]
- 15.Chen RY. Westfall AO. Mugavero MJ, et al. Duration of highly active antiretroviral therapy regimens. Clin Infect Dis. 2003;37:714–722. doi: 10.1086/377271. [DOI] [PubMed] [Google Scholar]
- 16.Staszewski S. Morales-Ramirez J. Tashima KT, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999;341:1865–1873. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 17.Chen RY. Accortt NA. Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42:1003–1010. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 18.Willig JH. Westfall AO. Allison J, et al. Nucleoside reverse-transcriptase inhibitor dosing errors in an outpatient HIV clinic in the electronic medical record era. Clin Infect Dis. 2007;45:658–661. doi: 10.1086/520653. [DOI] [PubMed] [Google Scholar]
- 19.Lucas GM. Chaisson RE. Moore RD. Highly active antiretroviral therapy in a large urban clinic: Risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 20.Visnegarwala F. Graviss EA. Sajja P. Lahart CJ. White AC., Jr Determinants of sustained virological suppression in indigent, HIV-infected patients: Is single protease inhibitor-based antiretroviral therapy truly highly active? HIV Clin Trials. 2004;5:117–124. doi: 10.1310/JQ8U-6KVB-9JCG-JL51. [DOI] [PubMed] [Google Scholar]
- 21.Visnegarwala F. Rodriguez-Barradass MC. Graviss EA. Caprio M. Nykyforchyn M. Laufman L. Community outreach with weekly delivery of anti-retroviral drugs compared to cognitive-behavioural health care team-based approach to improve adherence among indigent women newly starting HAART. AIDS Care. 2006;18:332–338. doi: 10.1080/09540120500162155. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Y. L'Italien G. Mukherjee J. Iloeje UH. Determinants of discontinuation of initial highly active antiretroviral therapy regimens in a US HIV-infected patient cohort. HIV Med. 2006;7:156–162. doi: 10.1111/j.1468-1293.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 23.Ofotokun I. Pomeroy C. Sex differences in adverse reactions to antiretroviral drugs. Top HIV Med. 2003;11:55–59. [PubMed] [Google Scholar]
- 24.Update: trends in AIDS incidence—United States, 1996. MMWR Morb Mortal Wkly Rep. 1997;46:861–867. [PubMed] [Google Scholar]
- 25.Hader SL. Smith DK. Moore JS. Holmberg SD. HIV infection in women in the United States: Status at the Millennium. JAMA. 2001;285:1186–1192. doi: 10.1001/jama.285.9.1186. [DOI] [PubMed] [Google Scholar]
- 26.Demas PSE. Hirky AE. Wills TA. Doll LS. Hartel DM. Klein RS. The relationship of HIV treatment acceptance and adherence to psychological factors among injection drug users. AIDS Behavior. 1998;2:283–292. [Google Scholar]
- 27.Kerr T. Marshall A. Walsh J, et al. Determinants of HAART discontinuation among injection drug users. AIDS Care. 2005;17:539–549. doi: 10.1080/09540120412331319778. [DOI] [PubMed] [Google Scholar]
- 28.Palepu A. Tyndall M. Li K. Yip B. Hogg RS. O'shaughnessy MV. Montaner JSG. Schecter M. Access and sustainability of antiretroviral therapy among injection drug users in Vancouver. Can J Infect Dis. 2005;(Suppl B):32B. [Google Scholar]
- 29.Lucas GM. Griswold M. Gebo KA. Keruly J. Chaisson RE. Moore RD. Illicit drug use and HIV-1 disease progression: A longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163:412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 30.Gulick RM. Ribaudo HJ. Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: A randomized controlled trial. JAMA. 2006;296:769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 31.Pence BW. Ostermann J. Kumar V. Whetten K. Thielman N. Mugavero MJ. The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:194–201. doi: 10.1097/QAI.0b013e31815ace7e. [DOI] [PubMed] [Google Scholar]
- 32.Mugavero MJ. Lin HY. Allison JJ, et al. Failure to establish HIV care: Characterizing the “no show” phenomenon. Clin Infect Dis. 2007;45:127–130. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- 33.Catz SL. McClure JB. Jones GN. Brantley PJ. Predictors of outpatient medical appointment attendance among persons with HIV. AIDS Care. 1999;11:361–373. doi: 10.1080/09540129947983. [DOI] [PubMed] [Google Scholar]
- 34.Israelski D. Gore-Felton C. Power R. Wood MJ. Koopman C. Sociodemographic characteristics associated with medical appointment adherence among HIV-seropositive patients seeking treatment in a county outpatient facility. Prev Med. 2001;33:470–475. doi: 10.1006/pmed.2001.0917. [DOI] [PubMed] [Google Scholar]
- 35.Ammassari A. Murri R. Pezzotti P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. 2001;28:445–449. doi: 10.1097/00042560-200112150-00006. [DOI] [PubMed] [Google Scholar]
- 36.Justice AC. Rabeneck L. Hays RD. Wu AW. Bozzette SA. Sensitivity, specificity, reliability, and clinical validity of provider-reported symptoms: A comparison with self-reported symptoms. Outcomes Committee of the AIDS Clinical Trials Group. J Acquir Immune Defic Syndr. 1999;21:126–133. [PubMed] [Google Scholar]