Abstract
We report localization of a cytosolic protein histidine phosphatase (PHP; ∼16 kDa) in INS 832/13 cells, normal rat islets, and human islets. siRNA-mediated knockdown of PHP markedly reduced glucose- or mitochondrial fuel-induced but not KCl-induced insulin secretion. siRNA-mediated knockdown of PHP also attenuated mastoparan-induced insulin secretion, suggesting its participation in G protein-sensitive signaling steps, leading to insulin secretion. Functional assays revealed that the β-cell PHP catalyzes the dephosphorylation of ATP-citrate lyase (ACL). Silencing of PHP expression markedly reduced ACL activity, suggesting functional regulation of ACL by PHP in β-cells. Coimmunoprecipitation studies revealed modest effects of glucose on the interaction between PHP and ACL. Confocal microscopic evidence indicated that glucose promotes association between ACL and nm23-H1, a known kinase histidine kinase, but not between PHP and ACL. Furthermore, metabolic viability of INS 832/13 cells was resistant to siRNA-PHP, suggesting no regulatory roles of PHP in cell viability. Finally, long-term exposure (24 h) of INS 832/13 cells or rat islets to high glucose (30 mM) increased the expression of PHP. Such increases in PHP expression were also seen in islets derived from the Zucker diabetic fatty rat compared with islets from the lean control animals. Together, these data implicate regulatory roles for PHP in a G protein-sensitive step involved in nutrient-induced insulin secretion. In light of the current debate on putative regulatory roles of ACL in insulin secretion, additional studies are needed to precisely identify the phosphoprotein substrate(s) for PHP in the cascade of events leading to nutrient-induced insulin secretion.
Keywords: nm23-H1, adenosine 5′-triphosphate-citrate lyase
it is well established that in the majority of cell types transduction of extracellular signals involves ligand binding to a receptor, often followed by the activation of one or more GTP-binding proteins (G proteins) and their effector systems (7). The pancreatic β-cell is unusual in that glucose, the major physiological agonist, lacks an extracellular receptor. Instead, events consequent to glucose metabolism promote insulin secretion via the generation and/or altered distribution of diffusible second messengers such as calcium, cAMP, and lipid hydrolytic products of various phospholipases (5, 28, 32, 34, 36). It is noteworthy that a selective increase in intracellular calcium not only initiates insulin secretion but also regulates various enzymes such as protein kinases, phosphodiesterases, adenylyl cyclases, and phospholipases, thereby facilitating insulin secretion. In the context of protein kinases, in addition to calcium-dependent protein kinase(s), several other kinases, including calmodulin-, cyclic nucleotide-, and phospholipid-dependent protein kinases, tyrosine kinases, and mitogen-activated protein kinases have been described in β-cells (6, 10, 13, 33).
To date, the most phosphorylated amino acids identified include serine (p-Ser), threonine (p-Thr), and tyrosine (p-Tyr). Phosphoamino acids exhibit differential sensitivities to acidic and alkaline pH conditions. p-Ser and p-Thr, which form O-p (alcoholic O-monoester) linkages, are stable at acidic pH and fairly unstable under alkaline conditions. p-Tyr, which forms O-p (phenolic O-monoester), is stable under both acidic and alkaline conditions. Therefore, due to their stability under acidic conditions, p-Ser, p-Thr, and p-Tyr are readily identified after acid hydrolysis of phosphorylated proteins. However, acid-labile phosphoramidate linkage has been reported (1, 2, 25, 30) in histidine (p-His), arginine (p-Arg), and lysine (p-Lys). It is not surprising that very little information is available on the number of proteins with p-His, since its phosphate is rapidly lost during identification of phosphoamino acids under standard acid hydrolysis conditions or under conditions used for SDS-PAGE (1, 2, 21, 25, 30). It is important to note that p-His accounts for 6% total protein phosphorylation in eukaryotes, which makes it nearly 100-fold more abundant than p-Tyr but less abundant than p-Ser or p-Thr (30).
Several earlier studies have reported the localization, characterization, and regulation of protein histidine kinases in multiple cell types (15, 38). Wei and Matthews (42) first reported a filter paper-based protein kinase assay that selectively quantitated acid-labile and alkali-stable phosphorylation reactions. Employing this assay, these investigators reported the purification and characterization of a protein histidine kinase from Saccharomyces cerevisiae, using histone 4 as the substrate (42). These studies were the first to describe not only a convenient and reliable method for the quantitation of p-His phosphorylation but also the characterization of a novel protein histidine kinase in cellular preparations. Using two different assay methods (i.e., stringent electrophoretic conditions to retain p-His and the Nytran filter paper assay), we reported the localization, characterization, and regulation of protein histidine kinases in lysates derived from normal rat islets, human islets, and pure β-cells (HIT-T15 and INS-1). Some examples of the phosphoprotein substrates endogenous to islet β-cells that undergo histidine phosphorylation include three isoforms of nucleoside diphosphate kinases, mitochondrial succinyl thiokinase, the β-subunit of heterotrimeric G proteins, and a histone 4-phosphorylating histidine kinase (25).
Accumulating evidence suggests the existence of protein histidine phosphatases (PHP) in many cell types (14–16, 31, 35, 44, 45). Specifically, observations by Klumpp and Krieglstein (15, 16) have provided evidence on the identification and functional characterization of PHP in rabbit liver-soluble extracts. In followup studies, including amino acid analysis and inhibitor sensitivity measurements, these investigators concluded that this PHP is distinct from other known phosphatases acting on p-Ser, p-Thr, or p-Tyr. On the basis of this evidence and as a logical extension to our ongoing work in the area of understanding putative regulatory roles of protein histidine phosphorylation and dephosphorylation in the stimulus-secretion coupling of glucose-stimulated insulin secretion (GSIS), we undertook the current investigation to immunologically identify and study the roles of PHP in physiological insulin secretion from the pancreatic β-cell. Data accrued from this investigation clearly implicate novel roles for PHP in glucose- and mitochondrial fuel-induced but not KCl-induced insulin secretion.
MATERIALS AND METHODS
Reagents and antibodies.
Small interfering RNA (siRNA) designed to deplete the expression of endogenous PHP and scrambled siRNA (negative control) were purchased from Ambion (Foster City, CA). Mastoparan (Mas), α-ketoisocaproic acid (KIC), monomethyl succinate (MMS), malate dehydrogenase, reduced coenzyme A (CoASH), NADH, and ATP were purchased from Sigma Chemical (St. Louis, MO). The [γ-32P]ATP and ECL reagents were from GE Healthcare. HiPerFect transfection reagent was from Qiagen, Valencia, CA. The rat insulin ELISA kit was from American Laboratory Products (Windham, NH). Rac1 activation assay kit was from Cytoskeleton (Denver, CO). BrdU cell proliferation kit was from Calbiochem (Darmstadt, Germany). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) cell viability kit was from Roche Diagnostics (Indianapolis, IN). Pierce Classic IP kit was from Thermo Scientific (Rockford, IL). Purified polyclonal antibody against PHP was generated as described previously by Bäumer et al. (4). Affinity purified polyclonal antibody against ACL and nm23-H1 were from Santa Cruz Biotechnology (Santa Cruz, CA).
Insulin-secreting INS 832/13 cells, control and diabetic rat islets, and human islets.
INS 832/13 cells were kindly provided by Dr. Chris Newgard (Duke University Medical Center, Durham, NC). The cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum supplemented with 100 IU/ml penicillin and 100 IU/ml streptomycin, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 11 mM glucose, and 10 mM HEPES (pH 7.4). The medium was changed twice weekly, and cells were subcloned following trypsinization. Islets were isolated from pancreata of 3-mo-old male Sprague-Dawley rats (Harlan Laboratories, Oxford, MI), using collagenase digestion and a ficoll gradient as we described previously (22). All experiments were reviewed and approved by the Wayne State University Institutional Animal Care and Use Committee (protocol no. A 09-04-09). Soleus muscle, liver, and abdominal fat were also removed, washed free of blood with PBS, and homogenized. Homogenates were centrifuged at 2,600 g for 10 min to remove tissue debris, and the supernatant was saved to study the expression of the PHP.
Human pancreatic islets were obtained by L. K. Olson from the Juvenile Diabetes Research Foundation Human Islet Distribution Program at the University of Minnesota and University of Miami. Human islet sample 1 was from a 36-yr-old female donor (purity >90%) and was cultured for 2 wk in keratinocyte serum-free medium (Invitrogen) supplemented with 2 mM N-acetylcysteine. Human islet sample 2 was from a 20-yr-old male donor (purity ≥50%) and was cultured for 6 days in neurobasal medium containing 1% N2 supplement (Invitrogen). After culturing, the islets were cryopreserved at −80°C in 10% dimethyl sulfoxide, 40% FBS, and 50% culture medium. Upon thawing, the islets were washed once with PBS and homogenized with Tris·HCl buffer (50 mM, pH 7.4) containing sucrose (250 mM), EDTA (1 mM), DTT (1 mM), and protease inhibitor cocktail. Protein content was measured, resolved on 12% SDS-PAGE, and immunoblotted for PHP protein.
To study the PHP expression pattern during diabetes, male (12-wk-old) Zucker diabetic fatty rats (ZDF-LeprFa/Ctr) with age- and sex-matched lean controls (lean +/?) were purchased from Charles River Laboratories International, (Wilmington, MA). They were maintained on Purina diet no. 5008 to induce programmed and consistent development of type 2 diabetes. Hyperglycemia in diabetic rats was confirmed prior to euthanization by tail vain puncture using Glucometer Elite from Bayer (Leverkusen, Germany). Pancreatic islets were isolated employing the collagenase digestion method as described above.
Isolation of subcellular fractionations from pancreatic β-cells.
Subcellular fractions were isolated by differential centrifugation method, as described earlier (23).
INS 832/13 cells were homogenized with Tris·HCl buffer (50 mM, pH 7.4) containing sucrose (250 mM), EDTA (1 mM), DTT (1 mM), and protease inhibitor cocktail and centrifuged at 900 g for 5 min to obtain nuclear pellet. The supernatant was subjected to centrifugation at 5,500 g for 10 min to obtain the mitochondria-enriched fraction. The postmitochondrial supernatant was spun at 25,000 g for 25 min to obtain pellet rich in secretory granules. Microsomes were isolated by centrifugation of supernatant obtained in the previous step at 100,000 g for 1 h; the clear supernatant obtained thereof served as cytosol. All centrifugation procedures were carried out at 4°C. Proteins from individual fraction were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The blots were then probed with antibody raised against PHP (1:500 dilution) and with rabbit secondary antibody conjugated to horseradish peroxidase. Immune complexes were detected using the enhanced chemiluminescence kit and developed by autoradiography.
Triton X-114 partition protocol for the isolation of total hydrophilic and hydrophobic compartments.
Total hydrophobic and hydrophilic phases of lysates derived from INS 832/13 cells and pancreatic islets were separated using Triton X-114 according to method described earlier by us (22). Briefly, ∼400 μg of cell (INS 832/13 cell or islet) homogenate protein prepared in 400 μl of buffer (20 mM Tris·HCl, pH 7.5, 0.5 mM EGTA, 2 mM MgCl2, 10 μg/ml leupeptin, and 2 μg/ml aprotinin) and supplemented with 1% (wt/vol) Triton X-114 was overlaid on 400 μl of 6% sucrose cushion (wt/vol) prepared in 20 mM Tris·HCl buffer (pH 7.4) containing 0.06% (wt/vol) Triton X-114. Following brief incubation at 30°C, samples were centrifuged at 300 g for 3 min, and the aqueous phase was mixed with 0.5% (wt/vol) fresh Triton X-114 at 4°C. Following dissolution, the mixture was again overlaid on the same sucrose cushion, incubated for 3 min at 30°C, and centrifuged at 300 g for 3 min. The lower hydrophobic phase was diluted to a final volume of 400 μl with homogenization buffer, whereas the aqueous phase was transferred into a separate tube supplemented with 2% fresh Triton X-114, incubated for 3 min at 30°C, and centrifuged at 300 g without sucrose cushion. The supernatant obtained thereof served as total hydrophilic phase. The relative abundance of PHP in hydrophilic and hydrophobic phases was determined by Western blotting, as described above.
siRNA-mediated knockdown of PHP.
Endogenous expression of PHP was depleted by transfecting INS 832/13 cells with siRNA, a 21-oligonucleotide RNA forming a 19-base pair duplex core. INS 832/13 cells were plated on 24-well plates, and transfection with PHP-siRNA was performed at 50–60% confluence at a final concentration of 100 nmol/l using HiPerFect transfection reagent. To assess specificity of siRNA, cells were transfected in parallel (as above) with nontargeting siRNA that included at least four nucleotide mismatches with all known mouse, rat, and human gene (i.e., scrambled siRNA) duplexes. Transfected cells were maintained in complete growth medium for 24 h, and efficiency of PHP knockdown was determined by Western blot analysis.
Cell viability and proliferation assays.
INS 832/13 cells were transfected with PHP-specific or scrambled siRNA as described above.
Cell viability was determined by quantitating the reduction of MTT to purple-colored formazan by metabolically active cells at 570 nm using an ELISA plate reader. For cell proliferation assays, cells transfected with either control or siRNA-PHP were labeled for 6 h with bromodeoxyuridine (BrdU; 100 nmol/l). Incorporated BrdU was probed with peroxidase-coupled anti-BrdU antibody, and the immunocomplexes were quantified, following the instructions provided in the kit.
Insulin release studies.
INS 832/13 cells were cultured overnight in RPMI 1640 medium containing 2.5 mM glucose and 2.5% fetal bovine serum for 24 h following transfection with PHP or scrambled siRNA. The cells were incubated with Krebs-Ringer bicarbonate buffer for 2 h prior to stimulation with low (2.5 mM) or high glucose (20 mM), mitochondrial fuels (MMS; 15 mM), and KIC (5 mM), KCl (40 mM), or Mas (0–15 μM) for 45 min at 37°C. Insulin released into the medium was quantified by ELISA as described earlier (22, 39).
Effect of glucose on the expression and subcellular distribution of PHP in pancreatic β-cells.
INS-832/13 cells were incubated overnight with low serum (2.5%) and low glucose (2.5 mM) medium prior to stimulation with low (2.5 mM) and high (20 mM) glucose for 45 min. Cells were homogenized in mannitol-HEPES buffer (250 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EGTA, and 1 mM DTT) containing protease inhibitor cocktail. An aliquot of the homogenate was saved for Triton X-114 phase partition studies, as described earlier. The remaining homogenate was subjected to a single-step centrifugation at 105,000 g for 60 min to separate membrane and soluble fractions (as demonstrated in Ref. 23) and used for detection of PHP by Western blotting.
Glucose-induced Rac1 activation.
The extent of Rac1 activation (i.e., GTP-bound form) was determined using a commercially available kit (Cytoskeleton, Denver, CO) described earlier (22, 39). Scrambled or PHP-siRNA transfected INS 832/13 cells were incubated with low (2.5 mM) or high glucose (20 mM) for 15 min at 37°C. Cell lysates (500 μg protein/ml) were clarified by centrifugation at 4,800 g for 5 min, and 20 μl of PAK-PBD beads (p21-activated kinase-binding domain) were added to the supernatant and gently mixed using rotator for 90 min at 4°C. The beads were pelleted out by centrifugation at 4,000 g for 3 min, rinsed three times with wash buffer (25 mM Tris, pH 7.5, 30 mM MgCl2, 40 mM NaCl, and 150 mM EDTA), and then reconstituted in 3× Laemmli buffer. Proteins were separated on 12% SDS-PAGE and immunoblotted for Rac1 protein.
Determination of PHP enzyme activity.
PHP activity was determined by measuring dephosphorylation of (32P-His) ATP-citrate lyase (ACL) in rat liver homogenate (4, 17). Prior to activity determination, autophosphorylation of ACL-His760 was carried out in a reaction volume of 5 μl containing 100 μg of rat liver homogenate protein, 25 mM Tris·HCl (pH 7.5), 5 mM EDTA, and 1 μM ATP, including 2 μCi [γ-32P]ATP at 37°C for 15 min. The assay was stopped by placing the tube on ice. Unincorporated ATP was removed using CenTrisep spin columns (Princeton Separations). Thereafter, dephosphorylation of the rat liver-phosphorylated ACL protein was carried out immediately at 37°C for 45 min in a total volume of 15 μl with 600 ng of recombinant wt-PHP (4) or 100 μg of β-cell lysate protein in a buffer containing 25 mM Tris·HCl, pH 7.5, and 5 mM EDTA. The reaction was stopped by addition of 4 μl of sample buffer (130 mM Tris·HCl, pH 6.8, 10% SDS, 10% 2-mercaptoethanol, 20% glycerol, 0.06% bromphenol blue) without heating, and protein was separated on 12.5% SDS-PAGE minigels, followed by autoradiography. Densitometric evaluation of the band intensities was carried out using AIDA Image Analyzer software version 3.21 (Raytest, Straubenhardt, Germany).
Effects of PHP depletion on ACL activity in pancreatic β-cells.
ACL activity was measured using the malate dehydrogenase-coupled method without any modifications (37). In brief, INS 832/13 cell lysates were prepared from either control or cells in which expression of endogenous PHP was depleted, using siRNA as described above. Fifty micrograms of whole cell lysate protein was added to the reaction mixture containing 100 mM Tris·HCl (pH 8.7), 20 mM potassium citrate, 10 mM MgCl2, 10 mM DTT, 0.5 U/ml malate dehydrogenase, 0.33 mM CoASH, 0.14 mM NADH, and 5 mM ATP. The absorbance was monitored at 340 nm for 5 min in a PerkinElmer Lambda 25 spectrophotometer. Enzyme activity was calculated using molar extinction coefficient (6.22 mM/cm) and expressed as μmol NADH oxidized·min−1·mg protein−1.
Coimmunoprecipitation of PHP with ACL antibody.
INS 832/13 cells were cultured overnight in low-glucose/low-serum medium and incubated in the presence of either basal (2.5 mM) or high glucose (20 mM) for 45 min. Following cell lysis, the lysate protein was processed for immunoprecipitation with 4 μg of ACL antibody per the instructions provided in the Pierce Classic immunoprecipitation kit. The sample obtained in the final step was separated by 12% SDS-PAGE, transferred to a nitrocellulose membrane, and probed with antibody for the detection of PHP.
Immunofluorescence studies of PHP, nm23-H1, and ACL in pancreatic β-cells.
INS 832/13 cells were cultured on a coverslip for 48 h and then incubated with low-serum (2.5%) and low-glucose (2.5 mM) medium for 12 h prior to stimulation with glucose (low: 2.5 mM; high: 20 mM). Cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 5% horse serum in PBS for 1 h. Following incubation with either primary rabbit anti-PHP or anti-nm23-H1 polyclonal antibody (1:150 dilution) for 1 h, cells were washed three times (TBS, 10 min each) and incubated with goat anti-ACL polyclonal antibody (1:200 dilution) for an additional 1 h. Following three 10-min washes with TBS, the cells were probed with anti-rabbit secondary antibody conjugated to Alexa fluor 480 (1:200; Molecular Probes, Eugene, OR) for 1 h for the detection of the PHP/nm23-H1 and anti-goat secondary antibody conjugated with Alexa fluor 546 (1:200; Molecular Probes) for localization of ACL. The coverslip was mounted in an antifading mounting medium (DAKO, Carpinteria, CA), and the localization of PHP/nm23-H1 and ACL was photographed using a confocal imaging LSM 510 microscope with a 63× oil immersion lens at the Research Core Facility, Wayne State University School of Medicine, Detroit, MI.
Effect of long-term exposure of glucose on the expression of PHP in pancreatic β-cells.
INS-832/13 cells were cultured to attain 80% confluence and were incubated in the presence of either 5, 11.1, or 30 mM glucose for 24 h at 37°C. Male Sprague-Dawley rat islets were isolated by collagenase digestion method and cultured overnight in islet medium and further incubated in the presence of either 5 or 30 mM glucose for 24 h at 37°C. Subsequently, cells/islets were homogenized by sonication, and lysate proteins were separated with 12% SDS-PAGE and immunoprobed for PHP protein.
Protein assay.
Protein concentration in the lysate from islets and INS 832/13 cells and in subcellular fractions was determined by Bradford's dye-binding method, whereas the bicinchonic acid method was employed for estimation of protein content in hydrophobic and hydrophilic phases. Bovine serum albumin was used as internal standard.
Statistical analysis.
The statistical significance of the data was determined by ANOVA, and a P value <0.05 was considered significant.
RESULTS
Immunological identification and subcellular distribution of PHP in INS 832/13 cells, normal rodent islets, and human islets.
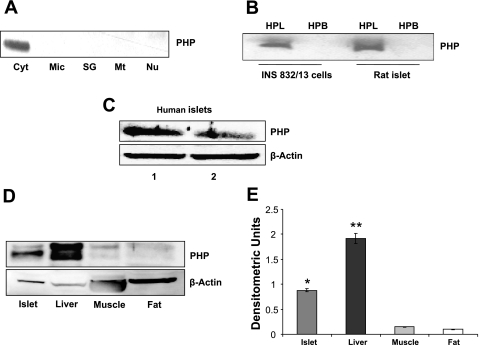
At the outset, we determined the subcellular localization of PHP in various subcellular fractions derived from insulin-secreting INS 832/13 cells. To accomplish this, individual fractions were isolated by the differential centrifugation method, and relative abundance of PHP in those fractions was determined by Western blotting. Data in Fig. 1A indicated that PHP is predominantly cytosolic in distribution. Next, we isolated total hydrophobic and hydrophilic phases from homogenates of INS 832/13 cells and normal rat islets by the Triton X-114 phase separation method to determine the relative association of PHP with these fractions. Data in Fig. 1B suggested distinct association of PHP with the hydrophilic compartment in both INS 832/13 cells and primary rat islets. Last, Western blot analysis suggested that PHP is also present in human islets (Fig. 1C). Together, data shown in Fig. 1 suggested that PHP is predominantly soluble in nature and highly expressed in islet tissue. We next studied localization of PHP in other tissues that are relevant for the maintenance of glucose homeostasis. Data in Fig. 1D showed that PHP is highly expressed in liver, with very little PHP detected in muscle and fat. The relative rank order of tissue distribution appears to be liver > islets > muscle > adipose tissue (Fig. 1E). In the next series of studies, we investigated potential regulatory roles for PHP in insulin secretion from isolated pancreatic β-cells (see below).
Fig. 1.
Immunological identification and subcellular distribution of protein histidine phosphatase (PHP) in INS 832/13 cells, normal rodent islets, human islets, and various tissues relevant for glucose homeostasis A: PHP was identified by Western blotting in individual subcellular fractions isolated from INS 832/13 cells by the differential centrifugation method (see materials and methods for additional details). Data are representative of 2 separate experiments yielding identical results. Cyt, cytosol; Mic, microsomes; SG, secretory granules; Mt, mitochondria; Nu, nuclear fractions. B: total hydrophobic (HPB) and hydrophilic (HPL) compartments were isolated from INS 832/13 cells and normal rat islets by the Triton X-114 phase separation method (see materials and methods for additional details). PHP was identified by Western blotting. Data are representative of 2 separate experiments yielding identical results. C: homogenates of human islets from 2 donors were probed for PHP protein by Western blotting. Actin was used as a loading control. D: lysates from islets, liver, soleus muscle, and abdominal fat were resolved by SDS-PAGE, and relative abundance of PHP was determined by Western blotting method. Actin was used as a loading control. A representative blot from 2 experiments is shown here. E: densitometric ratios of PHP to actin were presented. *P < 0.05, difference between islets vs. muscle or fat tissues; **P < 0.05, liver vs. islets.
siRNA-mediated depletion of PHP significantly reduces glucose- and mitochondrial fuel- but not KCl-induced insulin secretion in INS 832/13 cells.
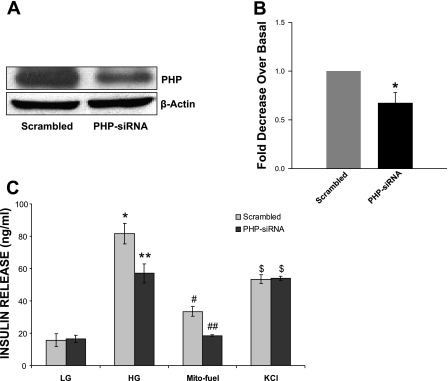
Data in Fig. 2A represent a Western blot to demonstrate that transfection of INS 832/13 cells with siRNA-PHP, but not the scrambled siRNA, markedly reduced the endogenous expression of PHP. Data from multiple experiments (Fig. 2B) suggested an ∼40% reduction in the expression of PHP under these conditions. We then quantitated glucose-, mitochondrial fuel-, or KCl-induced insulin secretion in INS 832/13 cells in which the expression of endogenous PHP was reduced via siRNA-PHP or retained using the scrambled siRNA. Data in Fig. 2C suggested a significant reduction in glucose (∼30%) and mitochondrial fuel-mediated (∼44%) but not KCl-induced insulin secretion in PHP-depleted cells. Together, these data suggest regulatory roles of PHP in glucose- or mitochondrial fuel-induced metabolic events leading to insulin secretion.
Fig. 2.
siRNA-mediated depletion of PHP significantly reduces glucose- and mitochondrial fuel- but not KCl-induced insulin secretion in INS 832/13 cells. INS 832/13 cells were transfected with PHP-siRNA or scrambled siRNA at a final concentration of 100 nmol/l using HiPerFect transfection reagent and cultured overnight in low-glucose/low-serum medium. PHP expression was determined by Western blotting. A: representative blot of 3 independent experiments is shown. B: pooled data from multiple studies to depict reduction in the expression of PHP in cells transfected with PHP-siRNA. Data are means ± SE from 3 independent experiments. *P < 0.05 vs. scrambled siRNA-transfected cells. C: PHP-siRNA or scrambled siRNA-transfected cells were incubated in the presence of low glucose (LG; 2.5 mM), high glucose (HG; 20 mM), a mixture of monomethyl succinate (20 mM) plus α-ketoisocaproic acid (5 mM), or KCl (40 mM) for 45 min at 37°C. Insulin released into the medium was quantitated by ELISA. Data are means ± SE from 8 independent determinations. Bars with different symbols differed significantly (P < 0.05) from their respective controls.
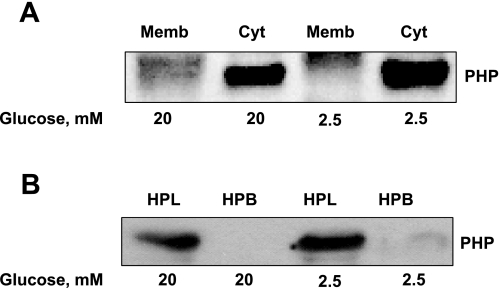
Glucose fails to affect the subcellular distribution of PHP.
Herein, we determined whether glucose promotes translocation of cytosolic PHP to the membrane fraction. To accomplish this, INS 832/13 cells were incubated with either basal (2.5 mM) or stimulatory (25 mM) glucose concentrations for 45 min, after which total membrane and cytosolic fractions were isolated (as in Fig. 1) and relative abundance of PHP in these fractions was assessed by Western blotting. Data in Fig. 3A indicate no significant alterations in the subcellular distribution of PHP in INS 832/13 cells when they are stimulated with glucose. Compatible with these findings, there was no redistribution of PHP between hydrophilic and hydrophobic phases of INS 832/13 cells following exposure to high glucose (Fig. 3B). Together, data in Fig. 4 indicate no significant effects of glucose on the subcellular distribution of PHP.
Fig. 3.
Glucose fails to elicit significant effects on the subcellular distribution of PHP in INS 832/13 cells. INS 832/13 cells were cultured overnight in low-glucose/low-serum medium. Cells were incubated further in the presence of either basal (2.5 mM; LG) or HG (20 mM) for 45 min. A: total membrane and cytosolic fractions from LG- and HG-treated INS 832/13 cells were isolated by a single-step centrifugation method and separated by SDS-PAGE, transferred to a membrane, and probed with PHP antibody. Data are representative of 2 separate experiments yielding identical results. Memb, membrane. B: HPL and HPB compartments from LG- and HG-treated INS 832/13 cells were isolated by the Triton X-114 phase separation method. Fractions were separated by SDS-PAGE, transferred to a membrane, and probed with PHP antibody. Data are representative of 2 separate experiments yielding identical results.
Fig. 4.
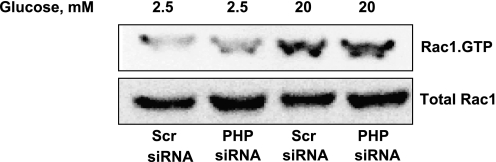
siRNA-mediated depletion of endogenous PHP exerts no significant effects on glucose-induced activation of Rac1 in INS 832/13 cells. INS 832/13 cells transfected with either scrambled siRNA or PHP-siRNA were exposed to either low (2.5 mM) or high (20 mM) glucose for 15 min, as indicated in the text. Relative degree of Rac1 activation in these cells was quantitated by PAK-PBD pulldown assay. For the purpose of controls, total Rac1 from lysates was also determined, and the data suggest no significant effects of PHP depletion on total Rac1 content. Data are representative of 2 separate experiments yielding identical results.
PHP is necessary for Mas-induced insulin secretion in INS 832/13 cells.
Previous studies from multiple laboratories have suggested regulatory roles for both heterotrimeric and small molecular mass G proteins in GSIS (26). In addition, Mas, a tetradecapeptide from wasp venom, has been shown to elicit direct stimulatory effects on protein histidine phosphorylation, G protein activation, and insulin secretion (11, 20, 25, 26) in a variety of insulin-secreting cells. Therefore, we next determined potential regulatory roles for PHP in Mas-induced insulin secretion from INS 832/13 cells. Data in Table 1 show that Mas significantly stimulated (i.e., ∼5-fold at 5 μM and ∼9-fold at 15 μM) insulin secretion in these cells. siRNA-mediated knockdown of PHP markedly inhibited (i.e., ∼39% at 5 μM and ∼28% at 15 μM) insulin secretion elicited by Mas at both concentrations studied without significantly affecting the basal insulin secretion (Table 1). Together, these data suggest that G protein-mediated insulin secretion may require the intermediacy of PHP in INS 832/13 cells.
Table 1.
Mas-induced insulin secretion is significantly impaired in PHP depleted in INS 832/13 cells
| Mas, μM | Scrambled siRNA | PHP-siRNA | P Value |
|---|---|---|---|
| 0 | 13.0 ± 0.98 | 16.4 ± 0.85 | Not significant |
| 5 | 68.7 ± 5.95 | 41.6 ± 2.85 | <0.05 |
| 15 | 123.5 ± 16.62 | 89.4 ± 9.99 | <0.05 |
Data are means ± SE from 8 independent determinations. Mas, mastoparan; PHP, protein histidine phosphatase. INS 832/13 cells were transfected with either PHP-siRNA or scrambled siRNA using HiPerFect transfection reagent and cultured overnight in low-glucose/low-serum medium (see matierals and methods for additional details). Cells were incubated further in the presence of either low glucose (2.5 mM) or Mas (0–15 μM) for 45 min at 37°C. Insulin released into the medium was quantitated by ELISA.
PHP does not mediate glucose-induced activation of Rac1 in INS 832/13 cells.
Existing evidence suggests a requirement of the activation of Rac1, a small-molecular-weight G protein, in glucose- and Mas-induced insulin secretion; such effects of Rac1 are mediated via its regulation of cytoskeletal remodeling in the β-cell (26). Recent studies by Xu et al. (44) have suggested that the 14-kDa PHP might be involved in lung cancer cell migration and invasion of lung cancer cells through the regulation of actin cytoskeletal rearrangements. Therefore, as a logical extension to the studies described under Fig. 2, we asked whether signaling steps involved in glucose-induced activation of Rac1 require the intermediacy of PHP. To accomplish this, we quantitated glucose-induced activation of Rac1 in INS 832/13 cells transfected with either scrambled siRNA or PHP-siRNA. As expected, stimulatory glucose concentrations markedly activated Rac1 in these cells (Fig. 4, lanes 1 vs. 3). However, we observed no significant effects of PHP depletion on glucose-induced activation of Rac1 under these conditions (Fig. 4, lanes 2 vs. 3). Taken together, our findings (Figs. 3 and 5 and Table 1) implicate roles for PHP in G protein-mediated signaling events, but not at the level of Rac1 activation leading to glucose- or Mas-induced insulin secretion.
Fig. 5.
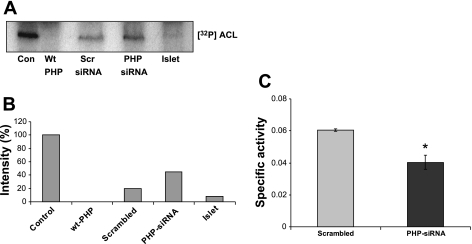
Dephosphorylation of ATP-citrate lyase (ACL) by PHP endogenous to INS 832/13 cells and normal rat islets. A: a soluble extract from rat liver was phosphorylated with [γ-32P]ATP in the presence of 5 mM EDTA and used as the phosphoprotein substrate for PHP endogenous to pancreatic β-cells (see materials and methods for additional details). An autoradiogram representing the relative degrees of dephosphosphorylation of labeled ACL [lane 1; marked as control (Con)] by recombinant wild-type (Wt) PHP (lane 2), lyastes derived from INS 832/13 cells transfected with the scrambled siRNA (lane 3), or PHP-siRNA (lane 4). Lane 5 represents the dephosphorylation of labeled ACL by PHP endogenous to normal rat islet lysates. A representative blot from 2 independent experiments is shown. B: means from 2 independent studies depicted in A. C: ACL activity was measured in lysates derived from either scrambled or PHP-siRNA-transfected cells by the malate dehydrogenase-coupled method. ACL activity was expressed as μmol NADH oxidized·min−1·mg protein−1. Data are means ± SE from 3 independent determinations. PHP-siRNA decreased specific activity significantly (*P < 0.05).
Phosphohistidine dephosphorylation of ACL by PHP endogenous to β-cells.
Recent evidence in other cell types indicates that ACL undergoes phosphorylation and dephosphorylation at histidine-760, although putative effects of this modification on the catalytic function of the enzyme remain unclear (18). Nucleoside diphosphate kinase-A (also refered to as nm23-H1) has been shown to catalyze the histidine phosphorylation of ACL (41), which is dephosphorylated by PHP (18). Herein, we assessed the ability of PHP endogenous to INS 832/13 cell and normal rat islets to dephosphorylate 32P-labeled ACL. This was accomplished according to the published method of Klumpp et al. (18). In brief, the ACL endogenous to liver-soluble extracts was allowed to autophosphorylate at histidine-760 in the presence of [γ-32P]ATP (Fig. 5A, lane 1), followed by quantitation of dephosphorylation of the labeled ACL by the β-cell PHP (see materials and methods for additional details). Data in Fig. 5A depict the dephosphorylation profile of [32P]ACL by the recombinant PHP (Wt; lane 2) and by lysates derived from INS 832/13 cells transfected with either scrambled siRNA (lane 3) or siRNA-PHP (lane 4). As expected, the recombinant PHP completely dephosphorylated the phosphorylated ACL. A much lesser degree of dephosphorylation of ACL was demonstrable in INS 832/13 cell lysates in which PHP expression was reduced by siRNA-PHP compared with the scrambled siRNA-transfected cell lysates, indicating that the β-cell PHP is the putative phosphatase that mediates the dephosphorylation of ACL. Furthermore, PHP endogenous to islet lysates potently dephosphorylated phospho-ACL (Fig. 5A, lane 5). Pooled data from multiple studies are provided in Fig. 5B. Together, these data suggest that PHP endogenous to INS 832/13 cells and normal rodent islets mediates the dephosphorylation of ACL. As a logical extension to the above studies, we quantitated ACL activity in lysates derived from INS 832/13 cells transfected with either scrambled siRNA or siRNA-PHP. Data in Fig. 5C demonstrated a significant reduction (−33%) in the total ACL activity in PHP-depleted cells, suggesting that PHP might play a positive modulatory role in islet β-cell function and insulin secretion (compatible with data in Fig. 2).
Evidence to suggest physical association of PHP with ACL in insulin-secreting cells.
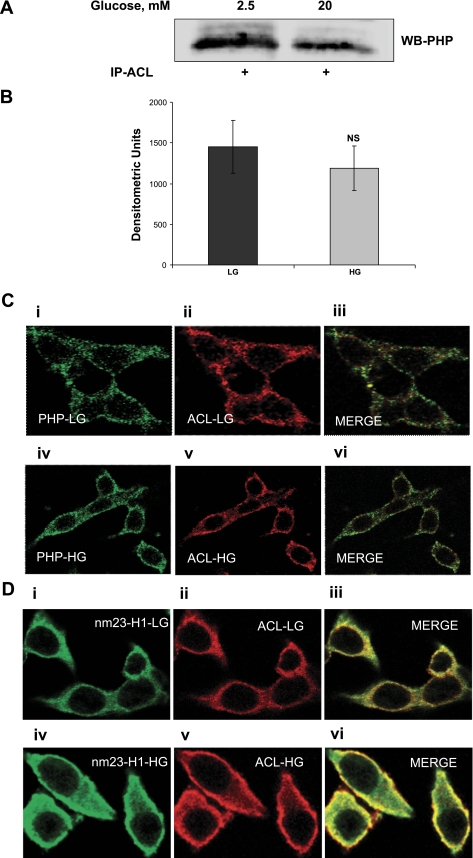
Herein, potential association or complexation between PHP and ACL under basal and stimulatory conditions was examined by a coimmunoprecipitation approach. In this study, INS 832/13 cells were incubated in the presence of low (2.5 mM) or high (20 mM) glucose, and those cell lysates were immunoprecipitated using the ACL antiserum. Relative abundance of PHP in the immunoprecipitates was determined by Western blotting. Data in Fig. 6A indicated detectable levels of PHP in the immunoprecipitates, suggesting that these two proteins stay complexed under basal conditions. However, incubation of these cells with glucose did not result in any significant increase in the amount of PHP in the immunoprecipitates (Fig. 6, A and B). Together, these data suggest that PHP stays associated with ACL in an unstimulated cell and that glucose treatment does not promote further association between the two proteins.
Fig. 6.
Coimmunoprecipitation and confocal microscopic evidence for association of ACL with PHP in INS 832/13 cells. A: INS 832/13 cells were were incubated in the presence of either basal (2.5 mM; LG) or HG (20 mM) for 45 min. Samples were immunoprecipitated with ACL, as described in materials and methods. The immunoprecipitates (IP) were resolved by SDS-PAGE and probed with PHP antibody. A representative Western blot (WB) from 2 studies is shown. B: quantification of PHP levels from 2 studies, as described above. C: INS 832/13 cells were cultured on a coverslip for 24 h and then maintained overnight in low-glucose/low-serum medium. Cells were further incubated in the presence of either LG (2.5 mM; i and ii) or HG (20 mM; iv and v) for 45 min. Localization of PHP and ACL proteins was determined using respective antibodies (see materials and methods for additional details). PHP was stained green, whereas ACL was stained red. C, iii: merged images of i and ii; C, vi: merged images of iv and v. Data are representative of 2 experiments. D: INS 832/13 cells were cultured on a coverslip for 24 h and then maintained overnight in low-glucose/low-serum medium. Cells were incubated further in the presence of either LG (2.5 mM; i and ii) or HG (20 mM iv and v) for 45 min. Localization of nm23-H1 and ACL proteins was determined using respective antibodies (see materials and methods for additional details). nm23-H1 was stained green, whereas ACL was stained red. D, iii: merged images of i and ii; D, vi: merged images of iv and v. Data are representative of 2 experiments.
We next addressed the question of association between ACL and PHP by confocal microscopy. Data in Fig. 6C demonstrate localization of PHP (Fig. 6C, i) and ACL (Fig. 6C, ii) in INS 832/13 cells under basal conditions. Distribution pattern of these proteins under glucose stimulatory conditions is provided in Fig. 6C, iv, for PHP and Fig. 6C, v, for ACL. These data indicate no significant redistribution of these proteins in a stimulated β-cell. Furthermore, merged images under basal (Fig. 6C, iii) or stimulatory conditions (Fig. 6C, vi) suggest no further increase in the colocalization of these two proteins in a glucose-stimulated β-cell. Taken together, these data are suggestive of no additional association between PHP and ACL under stimulatory glucose conditions, although PHP catalyzed the histidine dephosphorylation of ACL in these cells (Fig. 5).
Evidence to suggest potential colocalization of nm23-H1 and ACL in pancreatic β-cells.
Studies from the laboratory of Wagner and Vu (41) have provided the first evidence to indicate histidine phosphorylation of ACL by nm23-H1, suggesting potential regulation of this metabolically important enzyme by histidine phosphorylation. Furthermore, we previously reported histidine phosphorylation of an ∼120-kDa protein (similar to the molecular size of ACL) in normal rat islets (21), based on which we postulated that function of ACL might be regulated by nm23-H1 (as histidine kinase) and PHP (as histidine phosphatase). As a followup to studies described under Figs. 5 and 6, we investigated potential interaction between ACL and nm23-H1 in INS 832/13 cells under basal and stimulatory conditions by confocal microscopy. Data in Fig. 6D show the localization and distribution of nm23-H1 in INS 832/13 cells under basal (Fig. 6D, i) and stimulatory (Fig. 6D, iv) conditions; localization of ACL under these conditions is shown in Fig. 6D, ii and v, respectively. These data suggested a significant redistribution of nm23-H1 in these cells under stimulatory conditions, as evidenced by translocation of this protein toward the cell periphery. Analysis of merged images of Fig. 6D, i and ii (in Fig. 6D, iii), as well as Fig. 6D, iv and v (Fig. 6D, vi), appears to indicate an increased association between nm23-H1 and ACL in the stimulated β-cell.
PHP is not involved in metabolic cell viability and cell proliferation.
Data in Fig. 5 indicated that PHP mediates the dephosphorylation of ACL in INS 832/13 cells and normal rat islet. It also suggested that siRNA-mediated depletion of PHP markedly reduces ACL activity in INS 832/13 cells. Published evidence suggests regulatory roles of ACL in cell survival (3, 9, 19). Therefore, we asked whether reduction in ACL activity via PHP knockdown manifests in reduced cell viability and proliferation. To address this, metabolic cell viability and cell proliferation rate (see materials and methods for additional details) were quantitated in INS 832/13 cells transfected with either scrambled siRNA or siRNA-PHP. Data from these studies indicated no significant differences in the metabolic cell viability of INS 832/13 cells expressing either scrambled siRNA (0.53 ± 0.03 units; n = 10) or PHP-specific siRNA (0.54 ± 0.04 units; n = 10). Likewise, we failed to detect any significant differences in cell proliferation rates between the control and PHP knocked-down INS 832/13 cells (155 ± 4 units in control vs. 153 ± 3 units in PHP knocked-down cells; n = 10 in each case). Taken together, these findings indicate that PHP and its phosphoprotein substrate ACL do not play regulatory roles in cell survival and proliferation in isolated β-cells.
PHP expression is increased in β-cells under in vitro conditions of glucolipotoxicity and in islets derived from the Zucker diabetic fatty rat, a model for type 2 diabetes.
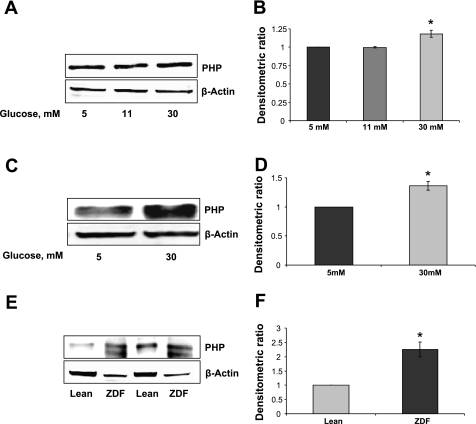
We next investigated whether long-term exposure of isolated β-cells to glucose in vitro or in vivo leads to abnormalities in the expression of PHP. To address this expression, levels of PHP were quantitated by Western blotting in INS 832/13 cells (Fig. 7, A and B) or normal rat islets (Fig. 7, C and D) following exposure to glucose (5–30 mM) for 24 h. Data from these studies suggested a modest increase (∼15%) in PHP expression in cells exposed to glucose (Fig. 7, A and B). A much higher increase (∼30%) in PHP expression was noticed in rat islets following exposure to glucose. Together, these data suggest increased expression of PHP in INS 832/13 cells and rat islets under the duress of glucotoxicity. In the last set of studies, we quantitated PHP expression in islets derived from the Zucker diabetic fatty (ZDF) rat, a model for type 2 diabetes, and compared it with PHP expression levels in islets from lean control rats. Data in Fig. 7, E and F, indicated a marked increase (∼2-fold) in the expression of PHP in islets from 12-wk-old ZDF animals (with mean blood glucose of 385 ± 7 mg/dl) compared with the control (with mean blood glucose of 85 ± 7 mg/dl) rat islets. Together, data in Fig. 7 suggest marked increase in the expression of PHP in in vitro and in vivo models of β-cell dysfunction. Potential links between the increase in the expression of this protein and the corresponding increase in the dephosphorylation of key signaling proteins in the onset of insulin secretory abnormalities remain to be verified (see discussion).
Fig. 7.
A modest but significant increase in the expression of PHP in INS 832/13 cells and normal rat islets following chronic exposure to high glucose; evidence for increased expression of PHP in islets from the Zucker diabetic fatty (ZDF) rat. INS-832/13 cells (A) or normal rat islets (C) were incubated in the presence of glucose (5–30 mM) for 24 h, after which PHP levels were determined by Western blot. Actin was used as a loading control. B and D: densitometric ratios of PHP to actin. Data are means ± SE from 3 independent experiments. *P < 0.05 vs. 5 mM glucose. E: PHP levels were determined by lysates from islets of lean control and ZDF rats by immunoblot. Actin was used as a loading control. A representative blot from 2 experiments is shown here. F: densitometric ratios of PHP to actin; data are means ± SD from 2 experiments. *P < 0.05 vs. lean control islets.
DISCUSSION
The overall objective of this study was to determine the localization and understand the involvement of PHP in the stimulus-secretion coupling of nutrient-induced insulin secretion from the pancreatic β-cell. Salient findings of this study are that 1) PHP is localized in INS 832/13 cells, normal rat islets, and human islets; 2) siRNA-mediated knockdown of PHP markedly attenuates glucose- or mitochondrial fuel- but not KCl-induced insulin secretion; 3) siRNA-mediated depletion of PHP also inhibits Mas-induced insulin secretion, suggesting that PHP-mediated signaling events underlie G protein activation; 4) siRNA-mediated knockdown of PHP markedly reduces dephosphorylation and catalytic function of ACL; 5) ACL is complexed with nm23-H1 (histidine kinase) and PHP (histidine phosphatase); 6) metabolic cell viability and cell proliferation are not affected in PHP-depleted cells; and 7) the expression of PHP is increased in INS 832/13 cells and normal rat islets following long-term exposure to glucose and in islets derived from the ZDF rat compared with islets from the lean controls.
Available evidence suggests expression of different types of PHPs in prokaryotes as well eukaryotes (1, 14–16, 25, 31, 35). The SixA protein, consisting of 161-amino acid chain length with an arginine-histidine-glycine signature at the NH2 terminus from E. coli, was the first bacterial histidine phosphatase, which has been implicated in the histidine-aspartate phosphorelay mechanisms (29). The localization of a 150-kDa protein in rat brain with dual 6-p-Lys and 3-p-His phosphatase properties was reported by Ohmori et al. (35). Protein phosphatases with similar catalytic functional properties were also reported in rat tissues by Wong et al. (43). Multiple lines of evidence from Kim et al. (14) have identified protein phosphatases 1, 2A, and C as protein p-His phosphatases. These studies also determined inhibitory constants for various known inhibitors of phosphatases (e.g., inhibitors 1 and 2, okadaic acid, and mictocystin-LR) against p-His dephosphorylation using phosphorylated histone 4 as the substrate. Furthermore, immunohistochemical studies by Zhang et al. (45) have suggested expression of PHP in several mouse and human tissues. Our current findings provide the evidence for expression of PHP in insulin-secreting INS 832/13 cells, normal rat islets, and human islets. Directly relevant to the current study are the observations by Klumpp and Krieglstein (15) demonstrating the identification and functional characterization of vertebrate PHP from rabbit liver. On the basis of additional studies, including amino acid analysis and inhibitor sensitivity, these investigators concluded that this PHP is distinct from other known phosphatases acting on p-Ser, p-Thr, or p-Tyr.
Recent investigations by Klumpp et al. (18) have suggested that the 16-kDa PHP mediates the dephosphorylation of ATP-citrate lyase. Our current findings further substantiate a regulatory role for this PHP in the dephosphorylation and functional regulation of ACL in the islet β-cell. We have demonstrated that the β-cell PHP dephosphorylates ACL and that silencing of PHP results in reduction of ACL activity and inhibition of glucose- and mitochondrial fuel-induced insulin secretion. Although our findings identify ACL as one of the phosphoprotein substrates in the islet, the physiological significance of these findings in context remains to be determined. Considerable debate still exists with regard to roles of ACL in physiological insulin secretion. For example, MacDonald et al. (27) have reported minimal effects of shRNA-mediated knockdown of ACL on GSIS in INS 832/13 cells. Along these lines, studies by Joseph et al. (12) have provided convincing evidence to support the observation that silencing of ACL in primary rat islets had no effects on GSIS. In contrast to the above two reports, recent investigations by Guay et al. (8) have reported a marked inhibition of GSIS in INS 832/13 cells by shRNA-ACL. Therefore, due to the aforementioned controversy surrounding the roles of ACL in GSIS, we asked whether reduced ACL activity following the knockdown of PHP would affect cell viability and proliferation. This is based on the published evidence that implicates ACL in cell viability and survival (3, 9, 19). However, we failed to detect any significant effects of PHP depletion (and the associated inhibition of ACL activity) on metabolic cell viability and proliferation in INS 832/13 cells, although it should be pointed out that studies by Klumpp et al. (19) in neuronal cells suggested that overexpression of PHP resulted in decreased phosphorylation and catalytic activation of ACL culminating in loss in cell viability. Interestingly, downregulation of PHP failed to affect ACL phosphorylation state, activity, and cell viability in these cells. The underlying reasons for these contrasting effects remain unknown at the present time. Additional studies, including overexpression of PHP and measurements of phosphorylation state, function of ACL, and metabolic cell viability, may be necessary prior to ruling out the regulatory effects of ACL in β-cell viability and proliferation.
Previous studies have suggested that Mas- and glucose-induced insulin secretion involve activation of G proteins via the stimulation of histidine kinases endogenous to the islet β-cell. They demonstrated that Mas mediated activation of a novel histidine kinase that catalyzes the histidine phosphorylation of the β-subunit of trimeric G proteins in a variety of insulin-secreting cells (25). Our current findings (Table 1) also suggest key roles for PHP in Mas-induced and G protein-mediated insulin secretion, since it was markedly attenuated in cells in which PHP expression was knocked down by siRNA-PHP. Along these lines, recent studies by Mäurer et al. (31) have provided evidence to suggest dephosphorylation of Gβ-subunit by PHP. Using an antiserum directed against PHP, these investigators demonstrated colocalization of Gβ-subunit and the PHP in various rat tissues, including brain, heart, kidney, spleen, and lung. In subsequent experiments, they were able to demonstrate marked dephosphorylation of the Gβ-subunit in H10 and C3 cells overexpressing the PHP (31). It remains to be seen whether the PHP that we have identified in β-cells mediates the dephosphorylation of Gβ-subunit. This is likely, since we reported previously that the Gβ-subunit undergoes histidine phosphorylation in the plasma membrane and secretory granule fractions isolated from a variety of insulin-secreting cells. Therein, we have also demonstrated a time-dependent loss in the phosphate from the Gβ-subunit on which we postulated the intermediacy of a putative PHP in such a dephosphorylation step (21). Further studies are needed to determine whether the currently identified PHP mediates the dephosphorylation of the Gβ-subunit in the pancreatic β-cell.
In the current study, we have also presented evidence to indicate a significant increase in the expression of PHP in INS 832/13 cells and primary rat islets under glucotoxic conditions in vitro. We also observed a marked increase in the expression of this protein in islets derived from the ZDF rat, a model for type 2 diabetes. Significance of these findings in the onset of metabolic dysfunction of the islet in these models will remain elusive until we are able to identify the phosphoprotein targets of this phosphatase. It may be germane to point out that the expression levels of nucleoside diphosphate kinase/nm23-H1-like proteins are significantly reduced in isolated β-cells under glucolipotoxic conditions in vitro (40) and in islets derived from the Goto-Kakizaki rat (24) and the ZDF rat (Kowluru A, unpublished observations), both of which are established models of type 2 diabetes. Although speculative, it is likely that the observed increase in the expression of a histidine phosphatase (current study) and a decrease in the expression of a histidine kinase in the diabetic β-cell (40) might retain key regulatory proteins in their dephosphorylated states, leading to the metabolic dysfunction of the islet in type 2 diabetes.
GRANTS
This research was supported by a Merit Review Award (to A. Kowluru) from the Department of Veterans Affairs and the National Institute of Diabetes and Digestive and Kidney Diseases (DK-74921). A. Kowluru is also the recipient of the Senior Research Career Scientist Award from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Brandon Koch, Carmel Harkins, and Mary Olive (Wayne State University Confocal Facility) for their help with these studies.
REFERENCES
- 1.Alex LA, Simon MI. Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet 10: 133–138, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Attwood PV, Piggott MJ, Zu XL, Besant PG. Focus on phosphohistidine. Amino Acids 32: 145–156, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene 24: 6314–6322, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bäumer N, Mäurer A, Krieglstein J, Klumpp S. Expression of protein histidine phosphatase in escherichia coli, purification, and determination of enzyme activity. Methods Mol Biol 365: 247–260, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Deeney JT, Prentki M, Corkey BE. Metabolic control of beta-cell function. Semin Cell Dev Biol 11: 267–275, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Easom RA. CaM kinase II: a protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes 48: 675–684, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Guay C, Madiraju SR, Aumais A, Joly E, Prentki M. A role for ATP-citrate lyase, malic enzyme, and puruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem 282: 35657–35665, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thomson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8: 311–321, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Jones PM, Persaud SJ. Protein kinases, protein phosphorylation, and the regulation of insulin secretion from pancreatic beta-cells. Endocr Rev 19: 429–461, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Jones PM, Mann FM, Persaud SJ, Wheeler-Jones CP. Mastoparan stimulates insulin secretion from pancreatic beta-cells by effects at a late stage in the secretory pathway. Mol Cell Endocrinol 94: 97–103, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Joseph JW, Odegaard ML, Ronnebaum SM, Burgess SC, Muehlbauer J, Sherry AD, Newgard CB. Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J Biol Chem 282: 31592–31600, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Khoo S, Gibson TB, Arnette D, Lawrence M, January B, McGlynn K, Vanderbilt CA, Griffen SC, German MS, Cobb MH. MAP kinases and their roles in pancreatic beta-cells. Cell Biochem Biophys 40: 191–200, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Huang J, Cohen P, Matthews HR. Protein phosphatases 1, 2A, and 2C are protein histidine phosphatases. J Biol Chem 268: 18513–18518, 1993 [PubMed] [Google Scholar]
- 15.Klumpp S, Krieglstein J. Phosphorylation and dephosphorylation of histidine residues in proteins. Eur J Biochem 269: 1067–1071, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Klumpp S, Krieglstein J. Reversible phosphorylation of histidine residues in proteins from vertebrates. Sci Signal 2: pe13, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Klumpp S, Hermesmeier J, Krieglstein J. Detection of protein histidine phosphatase in vertebrates. Methods Enzymol 366: 56–64, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Klumpp S, Bechmann G, Mäurer A, Selke D, Krieglstein J. ATP-citrate lyase as a substrate of protein histidine phosphatase in vertebrates. Biochem Biophys Res Commun 306: 110–115, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Klumpp S, Faber D, Fischer D, Litterscheid S, Krieglstein J. Role of protein histidine phosphatase for viability of neuronal cells. Brain Res 1264: 7–12, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Komatsu M, McDermott AM, Gillison SL, Sharp GW. Mastoparan stimulates exocytosis at a Ca(2+)-independent late site in stimulus-secretion coupling. Studies with the RINm5F beta-cell line. J Biol Chem 268: 23297–23306, 1993 [PubMed] [Google Scholar]
- 21.Kowluru A, Seavey SE, Rhodes CJ, Metz SA. A novel regulatory mechanism for trimeric GTP-binding proteins in the membrane and secretory granule fractions of human and rodent beta cells. Biochem J 313: 97–107, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowluru A, Veluthakal R. Rho guanosine diphosphate-dissociation inhibitor plays a negative modulatory role in glucose-stimulated insulin secretion. Diabetes 54: 3523–3529, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kowluru A, Metz SA. Characterization of nucleoside diphosphokinase activity in human and rodent pancreatic beta cells: evidence for its role in the formation of guanosine triphosphate, a permissive factor for nutrient-induced insulin secretion. Biochemistry 33: 12495–12503, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Kowluru A. Defective protein histidine phosphorylation in islets from the Goto-Kakizaki diabetic rat. Am J Physiol Endocrinol Metab 285: E498–E503, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kowluru A. Emerging roles for protein histidine phosphorylation in cellular signal transduction: lessons from the islet beta-cell. J Cell Mol Med 12: 1885–1908, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev 31: 52–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald MJ, Smith AD, Hasan NM, Sabat G, Fahien LA. Feasibility of pathways for transfer of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta-cell. J Biol Chem 282: 30596–30606, 2007 [DOI] [PubMed] [Google Scholar]
- 28.MacDonald MJ. Elusive proximal signals of beta-cells for insulin secretion. Diabetes 39: 1461–1466, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Matsubara M, Mizuno T. The SixA phospho-histidine phosphatase modulates the ArcB phosphorelay signal transduction in Escherichia coli. FEBS Lett 470: 118–124, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Matthews HR. Protein kinases and phosphatases that act on histidine, lysine, or arginine residues in eukaryotic proteins: a possible regulator of the mitogen-activated protein kinase cascade. Pharmacol Ther 67: 323–350, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Mäurer A, Wieland T, Meissl F, Niroomand F, Mehringer R, Krieglstein J, Klumpp S. The beta-subunit of G proteins is a substrate of protein histidine phosphatase. Biochem Biophys Res Commun 334: 1115–1120, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Metz SA. The pancreatic islet as Rubik's Cube. Is phospholipid hydrolysis a piece of the puzzle? Diabetes 40: 1565–1573, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Nesher R, Anteby E, Yedovizky M, Warwar N, Kaiser N, Cerasi E. Beta-cell protein kinases and the dynamics of the insulin response to glucose. Diabetes 51: S68–S73, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem 64: 689–719, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Ohmori H, Kuba M, Kumon A. Two phosphatases for 6-phospholysine and 3-phosphohistidine from rat brain. J Biol Chem 268: 7625–7627, 1993 [PubMed] [Google Scholar]
- 36.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 67: 1185–1248, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Srere PA. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem 234: 2544–2547, 1959 [PubMed] [Google Scholar]
- 38.Tan E, Besant PG, Attwood PV. Mammalian histidine kinases: do they REALLY exist? Biochemistry 41: 3843–3851, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Veluthakal R, Kaur H, Goalstone M, Kowluru A. Dominant-negative alpha-subunit of farnesyl- and geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells. Diabetes 56: 204–210, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Veluthakal R, Suresh MV, Kowluru A. Down-regulation of expression and function of nucleoside diphosphate kinase in insulin-secreting beta-cells under in vitro conditions of glucolipotoxicity. Mol Cell Biochem 329: 121–129, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Wagner PD, Vu ND. Phosphorylation of ATP-citrate lyase by nucleoside diphosphate kinase. J Biol Chem 270: 21758–21764, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Wei YF, Matthews HR. A filter-based protein kinase assay selective for alkali-stable protein phosphorylation and suitable for acid-labile protein phosphorylation. Anal Biochem 190: 188–192, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Wong C, Faiola B, Wu W, Kennelly PJ. Phosphohistidine and phospholysine phosphatase activities in the rat: potential protein-lysine and protein-histidine phosphatases? Biochem J 296: 293–296, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu A, Hao J, Zhang Z, Tian T, Jiang S, Hao J, Liu C, Huang L, Xiao X, He D. 14-kDa phosphohistidine phosphatase and its role in human lung cancer cell migration and invasion. Lung Cancer 67: 48–56, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Zhang XQ, Sundh UB, Jansson L, Zetterqvist O, Ek P. Immunohistochemical localization of phosphohistidine phosphatase PHPT1 in mouse and human tissues. Ups J Med Sci 114: 65–72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]