Abstract
Diabetes mellitus (DM) is closely related to cardiovascular morbidity and mortality, but the specific molecular basis linking DM with increased vulnerability to cardiovascular injury remains incompletely understood. Methylglyoxal (MG), a precursor to advanced glycation end products (AGEs), is increased in diabetic patient plasma, but its role in diabetic cardiovascular complications is unclear. Thioredoxin (Trx), a cytoprotective molecule with antiapoptotic function, has been demonstrated to be vulnerable to glycative inhibition, but whether Trx is glycatively inhibited by MG, thus contributing to increased cardiac injury, has never been investigated. Cultured H9c2 cardiomyocytes were treated with MG (200 μM) for 6 days. The following were determined pre- and post-simulated ischemia-reperfusion (SI-R; 8 h of hypoxia followed by 3 h of reoxygenation): cardiomyocyte death/apoptosis, Trx expression and activity, AGE formation, Trx-apoptosis-regulating kinase-1 (Trx-ASK1) complex formation, and p38 mitogen-activated protein kinase (MAPK) phosphorylation and activity. Compared with vehicle, MG significantly increased SI-R-induced cardiomyocyte LDH release and apoptosis (P < 0.01). Prior to SI-R, Trx activity was reduced in MG-treated cells, but Trx expression was increased moderately. Moreover, Trx-ASK1 complex formation was reduced, and both p38 MAPK activity and phosphorylation were increased. To investigate the effects of MG on Trx directly, recombinant human Trx (hTrx) was incubated with MG in vitro. Compared with vehicle, MG incubation markedly increased CML formation (a glycation footprint) and inhibited Trx activity. Finally, glycation inhibitor aminoguanidine administration during MG treatment of cultured cells reduced AGE formation, increased Trx activity, restored Trx-ASK1 interaction, and reduced p38 MAPK phosphorylation and activity, caspase-3 activation, and LDH release (P < 0.01). We demonstrated for the first time that methylglyoxal sensitized cultured cardiomyocytes to SI-R injury by posttranslational modification of Trx via glycation. Therapeutic interventions scavenging AGE precursors may attenuate ischemic-reperfusion injury in hyperglycemic state diseases such as diabetes.
Keywords: hypoxia and reoxygenation, apoptosis
diabetes mellitus (DM) is a leading metabolic disorder in developed society and causes devastating systemic consequences if poorly managed in the clinical setting. Considerable experimental and clinical data have demonstrated the close association between diabetes and significant cardiovascular morbidity and mortality. Recent studies have demonstrated that DM is a major risk factor for ischemic heart disease development (3), directly adversely affecting ischemic cardiomyocytes, resulting in larger infarct size and more severe heart failure after ischemia-reperfusion. Although many signaling pathways relating diabetic cellular injury and cardiac dysfunction have been reported, the specific molecular basis linking DM with increased vulnerability to ischemia-reperfusion injury and resultant mortality has not been established.
Methylglyoxal (MG), a highly reactive dicarbonyl, is a natural metabolite in glucose metabolism. It is capable of inducing the nonenzymatic reaction glycation, or glycosylation, between reducing sugars and proteins and other biomolecules, yielding irreversible advanced glycation end products (AGEs) (5, 28). The concentration of MG is increased not only in diabetic animal tissues (37) but also in the plasma of diabetic patients (4, 11). Elevated MG levels are believed to contribute to complications seen in poorly controlled diabetic states. Indeed, recent investigations have demonstrated that MG induces apoptosis of rat Schwann cells (12) and human vascular endothelial cells (2), buttressing evidence to the significant role MG plays in the etiology of diabetic complications. However, the role of MG in ischemic injury endured in the diabetic cardiomyocyte and any potentially involved mechanisms (apoptotic or otherwise) remain unidentified.
Thioredoxin (Trx), a 12-kDa protein ubiquitously expressed in all living cells, fulfills a variety of biological functions related to regulation of cellular proliferation and apoptosis (41) and cytoprotection against oxidative stress (46). Clinical and experimental results have demonstrated that inhibition of Trx promotes apoptosis (24). Recent in vitro studies have demonstrated that Trx interacts directly with and inhibits the activity of apoptosis-regulating kinase-1 (ASK1), a mitogen-activated protein kinase (MAPK) that activates two proapoptotic kinases, p38 MAPK and JNK (26). These results give mechanistic insight as to how Trx may critically regulate the balance between cell proliferation and cell death.
Recent studies have demonstrated that besides upregulation or downregulation of Trx expression at the gene level, Trx activity is regulated by posttranslational modification. Five forms of posttranslational modifications of Trx have been identified previously, with each modification affecting Trx differently. These include oxidation, glutathionylation, S-nitrosylation, nitration, and glycation. We have demonstrated recently that Trx is susceptible to nonenzymatic glycation via lipopolysaccharide (LPS) exposure (47) and consequent inactivation and is furthermore unable to provide protection against LPS-induced liver toxicity. However, whether Trx activity is altered in the presence of prolonged MG exposure and any functional consequence of such alteration with respect to cardiomyocyte protection against simulated ischemia-reperfusion has never been investigated.
Therefore, the aims of the present study were 1) to determine whether long-term treatment with MG can enhance the injury of cultured H9c2 cardiomyocytes subjected to simulated ischemia-reperfusion and, if so, 2) to investigate whether Trx activity was reduced after long-term treatment with MG, 3) to determine the signaling mechanism(s) by which reduced Trx activity leads to increased cardiomyocyte death after prolonged MG exposure, and 4) to investigate whether administration of the glycation inhibitor aminoguanidine (AG) might be a therapeutic intervention reversing the observed phenomena related to MG exposure of cardiomyocytes.
MATERIALS AND METHODS
Cell culture and experimental protocol.
H9c2 cardiomyoblast cells (referred to as cardiomyocytes thereafter), an embryonic rat heart-derived cell line (American Type Culture Collection, Manassas, VA), were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% calf bovine serum (CBS; MP Biomedical, Solon, OH), penicillin (100 U/ml), and streptomycin (100 μg/ml). The cells were maintained at 37°C under a water-saturated atmosphere of 95% ambient air and 5% CO2 (normoxic conditions). Stock cultures were passaged at 2- to 3-day intervals. Cells were seeded at a density of 3 × 105 cells/35-mm well of six-well plates for 24-h culture and were made quiescent by overnight serum starvation (0% CBS). After 6 days of treatment with MG (200 μM; Sigma-Aldrich, St. Louis, MO) or MG (200 μM) plus AG hemisulfate (100 μM; Sigma-Aldrich), the cells were subjected to simulated ischemia-reperfusion, as described previously (30). Briefly, the cells were incubated in slightly hypotonic Hanks’ balanced saline solution (1.3 mm CaCl2, 5 mm KCl, 0.3 mm KH2PO4, 0.5 mm MgCl2, 0.4 mm MgSO4, 69 mm NaCl, 4 mm NaHCO3, and 0.3 mm Na2HPO4) without glucose or serum and transferred in an airtight incubator from which oxygen was removed and replaced by nitrogen for 8 h at 37°C. The incubator oxygen concentration (1%) was adjusted to simulate hypoxic conditions. Following hypoxia, the cells were reoxygenated for 3 h in DMEM with 1% serum at 37°C. Sham cells incubated in DMEM with 1% serum were not subjected to hypoxia. MG and AG were dissolved in double-distilled H2O. Cells received double-distilled H2O as vehicle in control experiments. MG or AG was present only during the 6-day preincubation period and not during SI-R period. Concentrations of MG and incubation time were established on the basis of dose- and time-dependent pilot experiments.
Lactate dehydrogenase activity assay.
Post-treatment completion, all conditioned media were collected, and the cells were lysed (21). In short, a 100-μl medium was added to 100 μl of a solution containing 100 mM Tris buffer, pH 8.2, 1.35 mM tetrazolium salt, 0.58 mM phenazine methosulfate, and 2.7 mM NADH. The optical density values were read at 0 and 30 min using a SpectraMax-Plus microplate spectrophotometer (Molecular Devices, Sunnyvale, CA) at 490 nm. The percentage of lactate dehydrogenase (LDH) release was calculated as (A − B)/(C − B) × 100, where A = LDH activity in conditioned media, B = LDH activity in culture media (without cells), and C = LDH activity in cell lysates.
Assessment of cardiomyocyte apoptosis.
Cardiomyocyte apoptosis was determined by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining and caspase-3 activity, as we reported previously (41). TUNEL assay was performed utilizing the In Situ Cell Death Detection Kit (Roche, Palo Alto, CA). Briefly, cells were fixed with 10% paraformaldehyde and incubated with the TUNEL reaction mixture containing TdT-mediated dUTP. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Samples were visualized on an Olympus BX51 fluorescence microscope, and digital images were acquired with IP Lab Imagine Analysis Software (version 3.5; Scanalytics, Fairfax, VA). Apoptotic index (number of TUNEL positively stained nuclei/total number of nuclei × 100) was automatically calculated and exported for further analysis. Assays were performed in a blinded manner. The caspase-3 activity assay utilized the fluorogenic substrate DEVD-7-amino-4-trifluoromethyl-coumarin (AFC). Briefly, cells were lysed using caspase-3 lysis buffer {50 mM HEPES, pH 7.4, 0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 5 mM DTT, 0.1 mM EDTA, 0.1% Triton X-100}, and total protein concentration was determined by the Bradford method. To each well of a 96-well plate, a supernatant containing 50 μg of protein was loaded and incubated with 3.645 μg of Ac-DEVD-AFC at 37°C for 1.5 h. AFC was cleaved from DEVD by activated caspase-3, and the free AFC was quantified with a Biotek FL600 microplate fluorescence reader (excitation wavelength, 400 nm; emission wavelength, 508 nm). Caspase-3 activity was expressed as nanomoles of AFC formation per hour per milligram of protein.
Trx activity assay.
Trx activity was determined via the insulin disulfide reduction assay (17). In brief, 40 μg of cellular protein extracts were preincubated at 37°C for 15 min with 2 μl of activation buffer (100 mM HEPES, 2 mM EDTA, 1 mg/ml BSA, and 2 mM DTT) to reduce Trx. After addition of 20 μl of reaction buffer (100 mM HEPES, 2.0 mM EDTA, 0.2 mM NADPH, and 140 μM insulin), the reaction was initiated by addition of mammalian Trx reductase (1 μl, 15 mU; Sigma), and samples were incubated for 30 min at 37°C (the controls received water only). The reaction was terminated by adding 125 μl of stopping solution (0.2 M Tris-Cl, 10 M guanidine-HCl, and 1.7 mM 3-carboxy-4-nitrophenyl disulfide). Absorption measurement occurred at 412 nm.
Immunocytochemical detection of AGEs.
Cardiomyocytes, seeded on glass coverslips in six-well plates, were treated as described above. Cells were fixed with polyformaldehyde (4% in PBS) for 1 h, washed with PBS, blocked with 10% normal goat serum, and incubated with rabbit anti-AGE polyclonal antibody (Abcam, Cambridge, MA). Immunostaining was developed with a Vectastain ABC kit (Vector Laboratories, Burlingame, CA), and examined under light microscopy.
Detection of Trx-ASK1 interaction.
Cells were homogenized with lysis buffer. Immunoprecipitation and immunoblotting were performed using a procedure described by Vadseth et al. (43). In brief, endogenous Trx was immunoprecipitated with a monoclonal anti-murine Trx antibody (Redox Bioscience). After sample separation, the Trx-ASK1 interaction was determined by Western blot analysis using a monoclonal antibody against ASK1 (Upstate Biotechnology, Lake Placid, NY) and horseradish peroxidase (HRP)-conjugated anti-mouse IgG antibody (Cell Signaling Technology, Danvers, MA).
p38 MAPK activity assay.
The p38 MAPK activity assay was performed utilizing a p38 MAPK assay kit (Cell Signaling Technology) with substrate activating transcription factor-2 (ATF-2) per the manufacturer's instructions (14). In brief, cells were homogenized in ice-cold lysis buffer. Lysates were sonicated on ice and centrifuged at 12,000 g for 10 min at 4°C. Immunoprecipitation was performed by adding 20 μl of resuspended immobilized monoclonal antibody against phospho-p38 MAPK (Thr180/Tyr182) to 100 μl of cell lysate containing 150 μg of protein. The mixture was incubated with gentle rocking overnight at 4°C. After a 10,000-g centrifugation at 4°C for 2 min, the pellets were washed twice with lysis buffer and twice with kinase buffer. The kinase reactions occurred in the presence of 200 μM ATP and 2 μg ATF-2 fusion protein at 30°C for 30 min. After incubation the samples were separated by SDS-PAGE, and ATF-2 phosphorylation was measured by Western immunoblotting using a monoclonal antibody against phosphorylated ATF-2, followed by enhanced chemiluminescent detection.
In vitro incubation of recombinant Trx with MG or MG ± AG.
To investigate the effects of MG on Trx directly, 1 μg of recombinant human Trx (hTrx; Sigma-Aldrich) was incubated with 500 μM MG at 37°C for 8 days. Activity of treated Trx (0.3 μg) was then determined as described above. Control Trx was incubated with deionized water under the same conditions. For the antiglycation treatment, AG (300 μM) was added at the initiation of incubation.
Western blot analysis.
Cultured cardiomyocyte cells were collected in lysis buffer after treatment. Aliquots containing 30–60 μg of protein were separated by electrophoresis through 8–12% SDS-polyacrylamide gel and transferred to positively charged nylon membranes. The membranes were blocked with 5% dry fat-free milk in Tris-buffered saline containing 0.1% Tween-20 and then incubated with primary antibodies against Trx (Redox Bioscience), p38, phospho-p38, GAPDH (Cell Signaling Technology), and Nε-carboxymethyl lysine (CML; Abcam). Positively charged nylon membranes were then incubated with HRP-conjugated anti-rabbit immunoglobulin G antibody (Cell Signaling) for 1 h. The blot was developed with a Supersignal Chemiluminescence Detection Kit (Pierce, Rockford, IL). Bands were visualized with a Kodak Images Station 400 (Rochester, NY), and the band densities were analyzed with Kodak 1-Dimensional software (version 3.6).
Statistical analysis.
All values in the text, table, and figures are presented as means ± SE of n independent experiments. All data (except Western blot density) were subjected to ANOVA followed by Bonferroni correction for post hoc t-test. Western blot densities were analyzed with the Kruskal-Wallis test followed by Dunn's posttest. Probabilities of 0.05 or less were considered to be statistically significant.
RESULTS
Preculturing cardiomyocytes with high MG increased their susceptibility to SI-R injury.
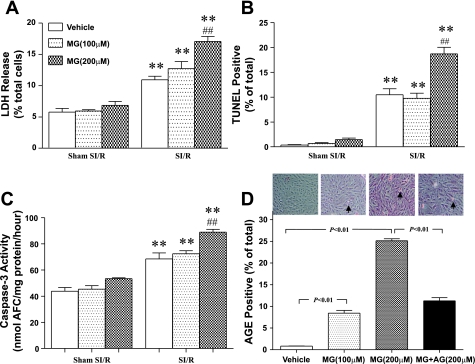
Cardiomyocytes subjected to SI-R injury manifested significant cellular injury including necrotic and apoptotic cell death, as evidenced by increased LDH release, TUNEL staining, and caspase-3 activity (Fig. 1). Under basal conditions, cardiomyocytes treated with MG developed normally without apparent injury (MG + Sham SI-R groups in Fig. 1). However, when subjected to SI-R, the cells treated with MG for 6 days (200 μM) exhibited significantly greater cellular injury compared with control in the parameters listed above.
Fig. 1.
Simulated ischemia-reperfusion (SI-R) injury is increased significantly in methylglyoxal (MG)-precultured cardiomyocytes, as measured by lactate dehydrogenase (LDH) release (A), apoptotic index by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-positive staining cells (B), and caspase-3 activity (C). D: MG preculture results in cardiomyocyte glycation prior to being subjected to SI-R, as measured by total advanced glycation end product (AGE) content; n = 6 independent experiments. **P < 0.01 vs. Sham SI-R; ##P < 0.01 vs. vehicle + SI-R. AFC, 7-amino-4-trifluoromethyl-coumarin.
Preculturing cardiomyocytes with high MG caused significant protein glycation.
As a highly reactive α-oxoaldehyde, MG may modify proteins and other substrates via glycation. To determine whether preculturing cardiomyocytes with MG may cause cardiomyocyte protein glycation, AGE content in vehicle or MG-cultured cardiomyocytes was determined by immunohistological staining. As shown in Fig. 1D, exposure of cardiomyocytes to MG caused a concentration-dependent increase in AGE content, with a 30.4-fold increase in AGE formation observed at 200 μM MG. Moreover, cotreatment of cardiomyocytes with AG, a potent protein glycation inhibitor, markedly reduced high-concentration MG-induced protein glycation (Fig. 1D, far right bar).
Treatment with AG protected MG-treated cardiomyocytes from SI-R injury.
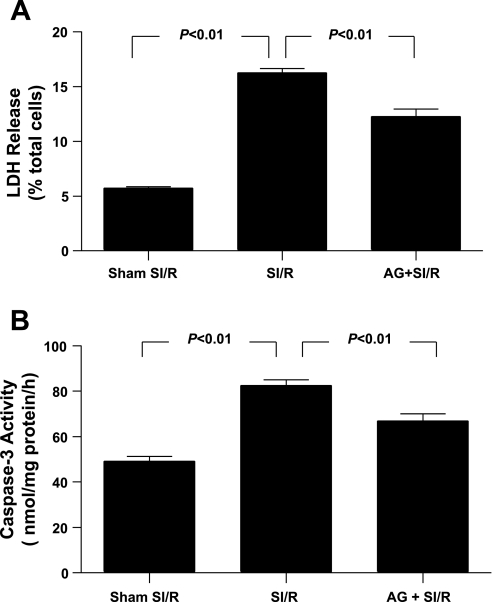
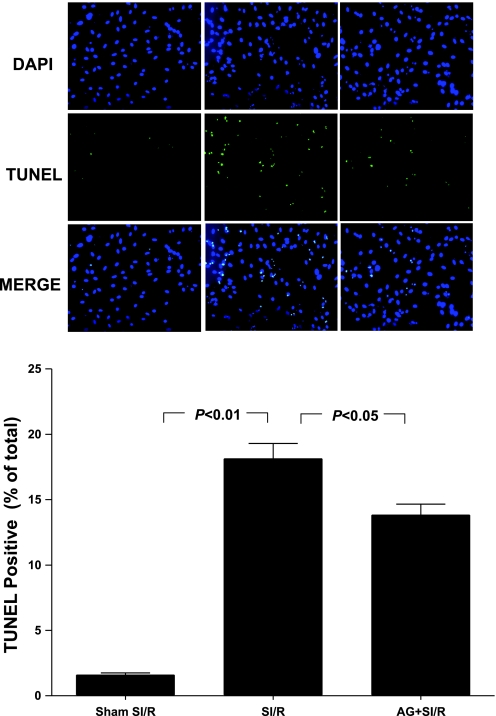
Having demonstrated that preculturing cardiomyocytes with high concentration of MG significantly increased AGE formation and enhanced SI-R injury, we further determined whether increased protein glycation may play a causative role in enhanced cardiomyocyte SI-R injury. As summarized in Fig. 2, cotreatment of cardiomyocytes with AG, an AGE formation inhibitor (33), significantly reduced LDH release (Fig. 2A), TUNEL staining (Fig. 2B), and caspase-3 activation (Fig. 3) in high MG (200 μM) pretreated cells subjected to SI-R. These results demonstrated that blockade of MG-induced protein glycation significantly protected cardiomyocytes from SI-R injury.
Fig. 2.
Effect of aminoguanidine (AG) treatment on MG-enhanced LDH release (A) and caspase-3 activity (B) after SI-R; n = 5–6 independent experiments.
Fig. 3.
Effect of AG treatment on MG-enhanced apoptotic cell death determined by TUNEL-positive staining after SI-R; n = 5–6 independent experiments. DAPI, 4,6-diamino-2-phenylindole.
MG incubation induced recombinant hTrx glycation and significantly decreased its activity.
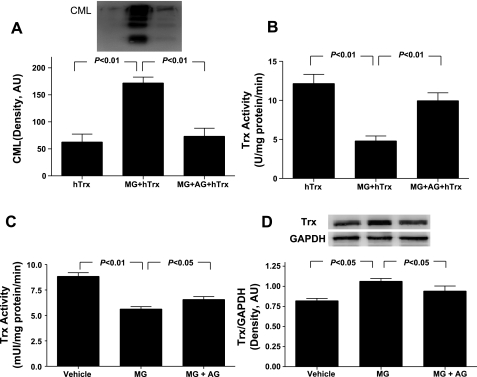
Our experimental results presented above demonstrated that MG is capable of initiating protein glycation reactions and that blockade of protein glycation reduced SI-R injury in MG-pretreated cardiomyocytes. However, the specific proteins glycated by MG that are potentially contributive to increased SI-R injury remain unknown. After a thorough literature review identifying feasible protein candidates, Trx was selected because 1) Trx is a critical antiapoptotic and cell survival molecule, and its inactivation has been causatively related to cardiovascular injury, and 2) we recently demonstrated that the Trx is susceptible to glycative modification by LPS and consequent activity inhibition. To directly test a novel hypothesis that MG may cause Trx-glycative inactivation, rendering cardiomyocytes more susceptible to SI-R injury, we first determined whether Trx can be glycatively modified by MG with subsequent activity inhibition. Recombinant hTrx (human thioredoxin-1) was incubated with MG in a cell-free system, and Trx glycation and activity were determined. As summarized in Fig. 4A, in vitro incubation of hTrx caused Trx glycation, as evidenced by abundant production of CML, a biomarker of AGE formation. MG incubation markedly inhibited Trx activity (60.4% reduction, P < 0.01 vs. vehicle-incubated Trx; Fig. 4B). More importantly, addition of AG in the system completely abolished MG-induced Trx glycation (Fig. 4A) and significantly attenuated MG-induced Trx inactivation (Fig. 4B).
Fig. 4.
Effects of MG on Nϵ-carboxymethyl lysine (CML) formation (A) and thioredoxin (Trx) activity of recombinant human Trx (hTrx) (B) in the absence and presence of AG, an AGE formation inhibitor; n = 6 independent experiments. Effect of AG treatment on MG-exposed Trx inactivation (C) and Trx expression (D) in cardiomyocytes prior to being subjected to SI-R. A and D, top: representative Western blots. Bar graphs represent density analysis (n = 5–8 independent experiments). AU, arbitrary units.
Trx is glycatively inhibited in MG-pretreated cardiomyocytes prior to SI-R injury.
Having demonstrated that MG is capable of causing Trx-glycative inhibition in a cell-free system, we further determined whether cellular Trx activity/expression might be reduced after MG exposure, leaving the cells more vulnerable to reperfusion injury. Compared with control, Trx activity was significantly decreased in the MG-treated cells prior to SI-R (Fig. 5A). However, Trx expression was slightly increased in MG-treated cells (Fig. 5B), indicating that the observed reduction in Trx activity in the MG-treated cells is not from reduced expression of the protein but rather its posttranslational modification. More importantly, cotreatment with AG significantly attenuated MG inhibition of Trx (Fig. 5A).
Fig. 5.
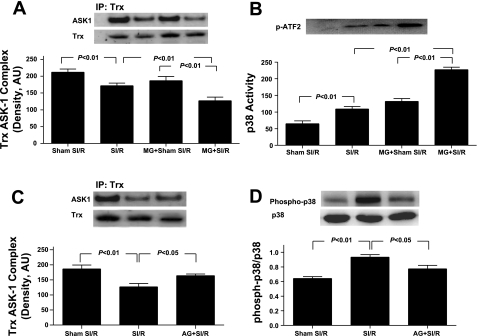
MG preculture decreases Trx-apoptosis-regulating kinase-1 (ASK1) binding (A) and increases p38 MAPK activation (B). A and B, top: representative Western blots. Bar graphs represent density analysis (n = 5–7 independent experiments). Effect of AG treatment on MG enhanced Trx-ASK1 dissociation (C) and p38 MAPK phosphorylation (D) in cardiomyocytes subjected to SI-R. C and D, top: representative Western blots. Bar graphs represent density analysis (n = 5–6 independent experiments).
Preculturing cardiomyocytes with MG promoted SI-R-induced Trx-ASK1 dissociation and subsequent p38 MAPK activation, which were attenuated by cotreatment with AG.
Recent in vitro studies have demonstrated that the binding and resultant inhibition of ASK1 is the primary mechanism by which Trx exerts its antiapoptotic effect (38). Moreover, the increased ratio of ASK1/Trx-ASK1 correlates with the increased basal activity of the p38 MAPK pathway (18). To determine whether MG inhibition of Trx may alter the Trx-ASK1 interaction and consequently activate downstream proapoptotic kinases, two additional experiments were performed. Via anti-Trx-1 immunoprecipitation and anti-ASK1 immunoblotting, Fig. 5A illustrates that Trx is physically associated with ASK1 in normal cultured cardiomyocytes, and this protein-protein interaction was significantly decreased after SI-R. Consequently, the activity of p38 MAPK, a proapoptotic downstream molecule for ASK1, was significantly enhanced in the MG-treated cardiomyocyte compared with control (Fig. 5B). More importantly, this SI-R-induced disassociation of Trx-ASK1 was further significantly enhanced when cells were precultured with MG, and p38 MAPK activity was further significantly increased (Fig. 5B, far right bar). Treatment with aminoguanidine restored Trx-ASK1 interaction (Fig. 5C) and reduced p38 MAPK phosphorylation (Fig. 5D) in MG-treated cardiomyocytes.
DISCUSSION
Ischemic heart disease continues to gain prevalence as a cause of disability and death in the United States and is costly in terms of patient morbidity and mortality as well as financial resources utilized in acute and chronic treatment. The specific molecular mechanisms underlying why diabetes mellitus directly increases ischemic heart disease risk remain elusive. Accumulating evidence has indicated that MG, a reactive dicarbonyl compound produced mainly from cellular glycolytic intermediates, is often found at high circulating blood levels in diabetic patients (4, 11, 39). Evidence suggests elevated MG levels may play a role in the development of a number of diabetic complications (32). Elucidation of the effects of MG and other AGE precursors upon the preischemic heart, and the involved underlying mechanisms, could yield improved preventative and therapeutic treatment of the diabetic heart at risk for and undergoing ischemic injury, respectively.
Our current study provided evidence that protein glycation is a new mechanism through which MG aggravates SI-R injury. This notion is supported by our observations that 1) preculturing cardiomyocytes with MG for 6 days caused a greater than 30-fold increase in AGE production, which was dramatically reduced by cotreatment with AG, a strong AGE formation inhibitor, and 2) preculturing cardiomyocytes with MG for 6 days made cardiomyocytes more susceptible to SI-R, as evidenced by increased LDH release, more cardiac caspase-3 activation, and greater percentage of TUNEL-positive staining, all of which were also markedly inhibited by AG cotreatment. Two experimental limitations should be discussed. First, the MG concentration present in the culture medium is much higher than that found in diabetic patient plasma (31, 35). However, it must be indicated that clinical situations are much more complicated, and actual MG concentrations to which in vivo cells are exposed remain uncertain. The intracellular MG level is likely much higher than the plasma MG level in the diabetic condition because diabetic tissues are chronically (months to years) exposed to high MG levels, which can cause dramatic intracellular MG accumulation (≤300 μM) (7). In contrast, cultured cells were only transiently (days) exposed to high concentrations of MG, which may limit intracellular MG accumulation (8). Additionally, actual diabetic tissues are concomitantly exposed to high levels of plasma glucose, whereas cultured cells in this study were exposed to normal glucose concentration. Furthermore, because MG is formed during glycolysis, clinical diabetes, often causing tissue hypoperfusion and hypoxia, may stimulate intracellular MG production. In contrast, normal oxygen was present during the 6-day preculturing period of our study, and cellular glycolysis was minimal. For these reasons, high concentrations of MG ranging from 200 μM to 1.5 mM were typically used in previously published studies by many investigators (10, 19, 23, 25, 45). Second, besides its strong antiglycation property, AG is also a potent inducible nitric oxide synthase (iNOS) inhibitor. Therefore, the protective effect of AG against MG-enhanced SI-R injury could be attributed to its anti-iNOS effect. Although theoretically possible, our experimental results do not support this possibility because 1) we have demonstrated previously that significant iNOS upregulation begins 2 h after reperfusion, but AG was washed out from the culturing system before the cells were subjected to SI-R; and 2) MG preculturing did not cause any significant cell injury (sham SI-R group; Fig. 1) unless the cells were subjected to SI-R (SI-R group; Fig. 1), and pretreatment with AG alone during the preculturing period (washed out before SI-R) had no effect on cellular injury before or after SI-R (data not shown).
Discovered 40 years ago in bacteria, Trx's influence in human cells has only recently begun to be appreciated as the diverse gamut of processes (including cellular redox balance, cell growth promotion, apoptosis inhibition, and inflammation modulation) regulated by Trx continue to be discovered (34). Therefore, it is not surprising to behold the role Trx plays in a wide range of human diseases and conditions, including cancer, viral pathology, and ischemia-reperfusion injury (9). Emerging evidence suggests that Trx plays critical roles in promoting cell proliferation/survival and reducing cell death. Trx and its reductase protein (TrxR) are upregulated in cancer tissues; molecules inhibiting Trx or TrxR promote apoptosis and reduce cancer development (36). In contrast, Trx activity is reduced in diseased tissues where pathological apoptosis is increased (27). Recent studies have demonstrated that besides upregulation or downregulation of Trx expression at the gene level, Trx activity is differentially regulated by posttranslational modifications. Oxidation of the thiol groups of Cys32 and -35 forms a disulfide bond and reversibly inhibits Trx's antioxidative activity. Glutathionylation, occurring at Cys73, significantly inhibits Trx's antioxidant activity (6). S-nitrosylation occurs at Cys69 or Cys73 and has been shown to markedly enhance Trx's antioxidant, antiapoptotic, and organ-protective activity (15, 16, 41). Nitration, occurring at Ty49, causes significant irreversible inhibition of Trx's antioxidative and cellular protection.
Protein glycation, also know as nonenzymatic glycosylation, is a protein modification reaction between proteins and reducing sugars (42). Glycation occurs in several steps. In an initial step that is completed in a short period of time (minutes to hours), the reducing sugar reacts with the protein chain and produces Schiff reaction primary-glycated products (e.g., fructosamine). After several days or weeks, amadorial rearrangement commences, and AGEs are formed (22). In recent years, the pathogenic roles of AGEs have been investigated extensively. Increased AGE accumulation and subsequent tissue injury have been found in many human diseases, such as type 2 diabetes, and during the aging process (1, 13, 44). However, whether the early modification of protein by sugar prior to AGE formation may alter protein function remains largely unknown. A study by McCarthy et al. (29) reported that incubation of alkaline phosphatase with reducing sugars reduced enzyme activity associated with an increase in fructosamine levels, indicating that early glycation may alter protein function. In two more recent studies, it was reported that human Cu-Zn-superoxide dismutase (20) and esterase (40) can be glycated by MG, and their activities are subsequently inhibited. MG reacts with the free amino groups of lysine and arginine and with cysteine thiol groups to form AGEs. In a recent study, we have demonstrated that Trx is susceptible to nonenzymatic glycation via LPS exposure (47) and consequent inactivation, and furthermore, it is unable to provide protection against LPS-induced liver toxicity.
Our current study demonstrated for the first time that preculturing cardiomyocytes significantly inhibited cellular Trx activity before the cells were subjected to SI-R and caused greater dissociation of Trx-ASK1 and p38 MAPK activation after SI-R. The MG inhibition of Trx is likely attributable to Trx-glycative modification and partially responsible for MG enhancement of SI-R injury. This novel hypothesis is supported by the following observations. First, MG preculture slightly increased Trx expression, indicating that posttranslational modification, rather than Trx gene expression, is responsible for reduced Trx activity in MG-precultured cardiomyocytes (Fig. 5). Second, MG caused significant recombinant hTrx-1 glycation and inactivation in a cell-free incubation system, and cotreatment with AG blocked Trx glycation and preserved Trx activity (Fig. 4). Third, addition of AG significantly attenuated the inhibitory effect of MG on cellular Trx activity (Fig. 5). Finally, treatment with AG only during the preculturing period significantly improved Trx-ASK1 association and inhibited proapoptotic p38 MAPK activation after SI-R (Fig. 7).
It should be indicated that Trx is also susceptible to nitrative inhibition. AG, as an iNOS inhibitor, may preserve Trx activity in MG-treated cells by blocking iNOS expression. However, our current study supports that Trx-glycative modification is a more likely mechanism responsible for MG inactivation of Trx because 1) preculturing cells with MG significantly reduced Trx activity even before cells were subjected to SI-R, whereas significant iNOS expression was not observed until 2 h after reperfusion; 2) treatment with AG only during the preculturing period where no significant iNOS is present significantly attenuated MG inactivation of Trx; and 3) in a cell-free system where no iNOS is present, AG blocked Trx glycation and preserved Trx activity after MG incubation.
Finally, some limitations should be addressed. First, the specific amino acid residues of Trx-1 responsible for glycative modification remain unknown and are currently under investigation. However, our preliminary data indicated that cysteine residues are not involved in glycative modification, because mutations of any or all of the five Trx cysteine residues failed to block Trx-1 glycation. Second, we were unable to directly measure cardiomyocyte Trx glycation after MG incubation, because a method sensitive enough to detect early protein glycation in cells is currently unavailable. Nonetheless, our cell-free experimental results demonstrating that Trx function is glycatively inhibited, together with our cellular experimental results showing that cardiomyocyte Trx-1 activity is reduced in MG-treated cells and preserved by AG, summarily suggest that glycative Trx inactivation may contribute to MG enhancement of cardiomyocyte SI-R injury. Third, H9c2 cells are neonatal myoblasts and may have some differences from the adult cardiomyocytes. However, this cell line has been used extensively as an experimental cardiomyocyte model, especially in those experiments where cells need to be cultured for a long period of time.
In conclusion, our results demonstrated that Trx activity was decreased due to posttranslational glycative modification in the cardiomyocytes treated with MG. Blocking AGE production inhibited Trx inactivation and significantly protected the cardiomyocytes from SI-R injury. These results suggest that clinical therapeutic interventions preserving Trx activity or scavenging MG in the diabetic setting may be novel modalities for attenuating injury endured in myocardial ischemia-reperfusion processes.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-63828 and HL-096686, American Diabetes Association Research Award 7-08-RA-98, American Heart Association Grant-in-Aid 0855554D (to X. L. Ma), and National Natural Science Foundation of China Grant 30700276 (to X. L. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab 9: 233–245, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Akhand AA, Hossain K, Mitsui H, Kato M, Miyata T, Inagi R, Du J, Takeda K, Kawamoto Y, Suzuki H, Kurokawa K, Nakashima I. Glyoxal and methylglyoxal trigger distinct signals for map family kinases and caspase activation in human endothelial cells. Free Radic Biol Med 31: 20–30, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Alpert JS. Diabetes mellitus and the risk for cardiovascular disease. Curr Cardiol Rep 5: 337, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes 54: 3274–3281, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes 43: 836–841, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Casagrande S, Bonetto V, Fratelli M, Gianazza E, Eberini I, Massignan T, Salmona M, Chang G, Holmgren A, Ghezzi P. Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proc Natl Acad Sci USA 99: 9745–9749, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaplen FW, Fahl WE, Cameron DC. Evidence of high levels of methylglyoxal in cultured Chinese hamster ovary cells. Proc Natl Acad Sci USA 95: 5533–5538, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Che W, Asahi M, Takahashi M, Kaneto H, Okado A, Higashiyama S, Taniguchi N. Selective induction of heparin-binding epidermal growth factor-like growth factor by methylglyoxal and 3-deoxyglucosone in rat aortic smooth muscle cells. The involvement of reactive oxygen species formation and a possible implication for atherogenesis in diabetes. J Biol Chem 272: 18453–18459, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Das DK. Thioredoxin regulation of ischemic preconditioning. Antioxid Redox Signal 6: 405–412, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Du J, Suzuki H, Nagase F, Akhand AA, Yokoyama T, Miyata T, Kurokawa K, Nakashima I. Methylglyoxal induces apoptosis in Jurkat leukemia T cells by activating c-Jun N-terminal kinase. J Cell Biochem 77: 333–344, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Fosmark DS, Torjesen PA, Kilhovd BK, Berg TJ, Sandvik L, Hanssen KF, Agardh CD, Agardh E. Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated with retinopathy in patients with type 2 diabetes mellitus. Metabolism 55: 232–236, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga M, Miyata S, Higo S, Hamada Y, Ueyama S, Kasuga M. Methylglyoxal induces apoptosis through oxidative stress-mediated activation of p38 mitogen-activated protein kinase in rat Schwann cells. Ann NY Acad Sci 1043: 151–157, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Furber JD. Extracellular glycation crosslinks: prospects for removal. Rejuvenation Res 9: 274–278, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Gao F, Yue TL, Shi DW, Christopher TA, Lopez BL, Ohlstein EH, Barone FC, Ma XL. p38 MAPK inhibition reduces myocardial reperfusion injury via inhibition of endothelial adhesion molecule expression and blockade of PMN accumulation. Cardiovasc Res 53: 414–422, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Haendeler J, Hoffmann J, Tischler V, Berk BC, Zeiher AM, Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat Cell Biol 4: 743–749, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Haendeler J, Hoffmann J, Zeiher AM, Dimmeler S. Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells: a novel vasculoprotective function of statins. Circulation 110: 856–861, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Holmgren A, Björnstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol 252: 199–208, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Hsieh CC, Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J 20: 259–268, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia X, Olson DJ, Ross AR, Wu L. Structural and functional changes in human insulin induced by methylglyoxal. FASEB J 20: 1555–1557, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kang JH. Modification and inactivation of human Cu,Zn-superoxide dismutase by methylglyoxal. Mol Cells 15: 194–199, 2003 [PubMed] [Google Scholar]
- 21.Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods 64: 313–320, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Lapolla A, Traldi P, Fedele D. Importance of measuring products of non-enzymatic glycation of proteins. Clin Biochem 38: 103–115, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Li SY, Sigmon VK, Babcock SA, Ren J. Advanced glycation endproduct induces ROS accumulation, apoptosis, MAP kinase activation and nuclear O-GlcNAcylation in human cardiac myocytes. Life Sci 80: 1051–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Lincoln DT, Ali Emadi EM, Tonissen KF, Clarke FM. The thioredoxin-thioredoxin reductase system: over-expression in human cancer. Anticancer Res 23: 2425–2433, 2003 [PubMed] [Google Scholar]
- 25.Liu BF, Miyata S, Hirota Y, Higo S, Miyazaki H, Fukunaga M, Hamada Y, Ueyama S, Muramoto O, Uriuhara A, Kasuga M. Methylglyoxal induces apoptosis through activation of p38 mitogen-activated protein kinase in rat mesangial cells. Kidney Int 63: 947–957, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res 90: 1259–1266, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lovell MA, Xie C, Gabbita SP, Markesbery WR. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer's disease brain. Free Radic Biol Med 28: 418–427, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Ma H, Li SY, Xu P, Babcock SA, Dolence EK, Brownlee M, Li J, Ren J. Advanced glycation endproduct (AGE) accumulation and AGE receptor (RAGE) up-regulation contribute to the onset of diabetic cardiomyopathy. J Cell Mol Med 13: 1751–1764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.McCarthy AD, Cortizo AM, Gimenez SG, Bruzzone L, Etcheverry SB. Non-enzymatic glycosylation of alkaline phosphatase alters its biological properties. Mol Cell Biochem 181: 63–69, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Mestril R, Chi SH, Sayen MR, O'Reilly K, Dillmann WH. Expression of inducible stress protein 70 in rat heart myogenic cells confers protection against simulated ischemia-induced injury. J Clin Invest 93: 759–767, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirza MA, Kandhro AJ, Memon SQ, Khuhawar MY, Arain R. Determination of glyoxal and methylglyoxal in the serum of diabetic patients by MEKC using stilbenediamine as derivatizing reagent. Electrophoresis 28: 3940–3947, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med 314: 403–408, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Moriyama T, Kemi M, Okumura C, Yoshihara K, Horie T. Involvement of advanced glycation end-products, pentosidine and N(epsilon)-(carboxymethyl)lysine, in doxorubicin-induced cardiomyopathy in rats. Toxicology 268: 89–97, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Nakamura H. Thioredoxin as a key molecule in redox signaling. Antioxid Redox Signal 6: 15–17, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Odani H, Shinzato T, Matsumoto Y, Usami J, Maeda K. Increase in three alpha,beta-dicarbonyl compound levels in human uremic plasma: specific in vivo determination of intermediates in advanced Maillard reaction. Biochem Biophys Res Commun 256: 89–93, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Powis G, Mustacich D, Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic Biol Med 29: 312–322, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Randell EW, Vasdev S, Gill V. Measurement of methylglyoxal in rat tissues by electrospray ionization mass spectrometry and liquid chromatography. J Pharmacol Toxicol Methods 51: 153–157, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17: 2596–2606, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM. Glucosepane is a major protein cross-link of the senescent human extracellular matrix. Relationship with diabetes. J Biol Chem 280: 12310–12315, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Sen S, Bose T, Roy A, Chakraborti AS. Effect of non-enzymatic glycation on esterase activities of hemoglobin and myoglobin. Mol Cell Biochem 301: 251–257, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation. Proc Natl Acad Sci USA 101: 11471–11476, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids 25: 275–281, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Vadseth C, Souza JM, Thomson L, Seagraves A, Nagaswami C, Scheiner T, Torbet J, Vilaire G, Bennett JS, Murciano JC, Muzykantov V, Penn MS, Hazen SL, Weisel JW, Ischiropoulos H. Pro-thrombotic state induced by post-translational modification of fibrinogen by reactive nitrogen species. J Biol Chem 279: 8820–8826, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, Ulrich P, Cerami A, Lévy BI. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci USA 95: 4630–4634, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamawaki H, Saito K, Okada M, Hara Y. Methylglyoxal mediates vascular inflammation via JNK and p38 in human endothelial cells. Am J Physiol Cell Physiol 295: C1510–C1517, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Yoshida T, Oka S, Masutani H, Nakamura H, Yodoi J. The role of thioredoxin in the aging process: involvement of oxidative stress. Antioxid Redox Signal 5: 563–570, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Yuan Y, Jiao X, Lau WB, Wang Y, Christopher TA, Lopez BL, Ramachandrarao SP, Tao L, Ma XL. Thioredoxin glycation: a novel posttranslational modification that inhibits its antioxidant and organ protective actions. Free Radic Biol Med. In press [DOI] [PMC free article] [PubMed] [Google Scholar]