Abstract
The ATDC5 cell line exhibits a multistep process of chondrogenic differentiation analogous to that observed during endochondral bone formation. Previous investigators have induced ATDC5 cells to differentiate by exposing them to insulin at high concentrations. We have observed spontaneous differentiation of ATDC5 cells maintained in ascorbic acid-containing α-MEM. A comparison of the differentiation events in response to high-dose insulin vs. ascorbic acid showed similar expression patterns of key genes, including collagen II, Runx2, Sox9, Indian hedgehog, and collagen X. We took advantage of the action of ascorbic acid to examine signaling events associated with differentiation. In contrast to high-dose insulin, which downregulates both IGF-I and insulin receptors, there were only minimal changes in the abundance of these receptors during ascorbic acid-induced differentiation. Furthermore, ascorbic acid exposure was associated with ERK activation, and ERK inhibition attenuated ascorbic acid-induced differentiation. This was in contrast to the inhibitory effect of ERK activation during IGF-I-induced differentiation. Inhibition of collagen formation with a proline analog markedly attenuated the differentiating effect of ascorbic acid on ATDC5 cells. When plates were conditioned with ATDC5 cells exposed to ascorbic acid, ATDC5 cells were able to differentiate in the absence of ascorbic acid. Our results indicate that matrix formation early in the differentiation process is essential for ascorbic acid-induced ATDC5 differentiation. We conclude that ascorbic acid can promote the differentiation of ATDC5 cells by promoting the formation of collagenous matrix and that matrix formation mediates activation of the ERK signaling pathway, which promotes the differentiation program.
Keywords: ATDC5 cells, chondrocyte differentiation, collagen, extracellular matrix
endochondral bone growth occurs in a predictable pattern that is reflected in the complex molecular events that regulate chondrocyte proliferation and maturation (6, 20). The sequential processes of mesenchymal condensation, chondrocyte proliferation, and chondrocyte hypertrophy involve the choreographed expression of many genes and the induction of numerous transcription factors, matrix proteins, and cell-matrix interaction mediators (46). These events occur in a spatially defined relationship within the growth plate. When round, proliferative chondrocytes form a columnar layer, they stop proliferating and become prehypertrophic chondrocytes, which subsequently differentiate into postmitotic hypertrophic cells. This is followed by apoptosis of hypertrophic chondrocytes, blood vessel invasion, and finally, the replacement of the cartilaginous matrix by bone (6). In recent years the molecular process of chondrogenesis within the growth plate has been characterized in detail, utilizing various in vitro and in vivo model systems (41).
The ATDC5 cell line is a well-characterized chondrogenic cell line derived from mouse teratocarcinoma cells (4). It exhibits a multistep process similar to that observed during chondrocyte differentiation, making it a useful model for in vitro studies. ATDC5 cells are widely used to study chondrocyte growth and differentiation. To date, there are more than 200 studies where ATDC5 cells were utilized. Atsumi et al. (4) originally described these cells and demonstrated that insulin at high concentrations could effectively induce their chondrogenic differentiation. We demonstrated previously that not only could ATDC5 differentiation be induced by high concentrations of insulin but that physiological concentrations of IGF-I and insulin can do so (28, 29). We (16), along with others (38), have also observed ATDC5 cell differentiation upon exposure to ascorbic acid-containing α-MEM medium without any requirement for growth factors. We speculated that this observation would be potentially useful in studying growth factor signaling during ATDC5 differentiation. The ability to induce ATDC5 cells without growth factors might eliminate the confounding effects of continuous growth factor exposure. The present studies were undertaken to characterize the mechanisms by which ascorbic acid induces ATDC5 cell differentiation.
Ascorbic acid is a critical factor in the processes of cartilage and bone development. In humans, ascorbic deficiency results in scurvy. At the growth plate, ascorbic acid deficiency can result in decreased chondrocyte proliferation, impaired matrix synthesis, and a reduction in osteoblast cell number (17, 47). This is likely due to the changes in collagen metabolism that accompany ascorbate deficiency. Ascorbic acid is an essential cofactor for prolyl lysyl hydroxylase (33, 35, 36), a key enzyme in collagen biosynthesis. In addition, ascorbic acid is necessary for the differentiation of many mesenchymal-derived cell types, including adipocytes (43), osteoblasts (3, 9, 26, 40, 42), myoblasts (15, 18), and chondrocytes (12, 21). In ATDC5 cells, ascorbic acid has been shown to enhance the differentiation process in the presence of high-dose insulin (2). In the MC3T3-E1 osteoblastic cell line, exposure to ascorbic acid in the presence of a collagen synthesis inhibitor not only blocks type I collagen synthesis but also decreases ascorbic acid-induced osteoblastic differentiation (9). The importance of collagen in mesenchymal cell differentiation is further supported by the observation that collagen deficiency results in an inability of embryonic avian muscle myoblasts to form myotubes in vitro (25). Taken together, these observations indicate that ascorbic acid has an important role in mesenchymal differentiation that is, at least in part, accounted for by its role in collagen synthesis.
Our laboratory has focused on the signal transduction events involved in the chondrogenic process and, in particular, the differentiation of ATDC5 cells (16, 27–29). We have found that IGF-I and insulin can promote differentiation via their cognate receptors (28). We further demonstrated the critical role of the mitogen-activated protein kinase pathway involving ERK (ERK1 and ERK2) kinases in chondrocyte differentiation. IGF-I-induced differentiation was enhanced by inhibition of ERK signaling (29), indicating that the proproliferative activity of this pathway attenuated differentiation. However, other studies on the role of ERK signaling in chondrocyte growth and differentiation showed that inhibition of ERK signaling with U-0126 blocked insulin-induced chondrogenesis (24). This was further supported by our own work demonstrating that even low concentrations of insulin that are specific for the insulin receptor can induce ERK phosphorylation, proliferation, and chondrocyte differentiation in ATDC5 cells (28).
ERK activation can be mediated by the cross-linking of extracellular matrix with integrins (32, 39). In human chondrocytes, the interaction of collagen II with the β1-integrin receptor has been shown to activate ERK (37). In the present series of experiments, we hypothesized that ERK signaling is involved in ascorbic acid-induced ATDC5 differentiation. To test this hypothesis, we first characterized the differentiation process that ATDC5 cells undergo in response to the traditional condition for ATDC5 cell induction, i.e., high-dose insulin, vs. ascorbic acid in the absence of insulin or IGF-I. We went on to explore the relationship between the role of ascorbic acid in matrix protein synthesis and its ability to modulate ERK activity.
MATERIALS AND METHODS
Materials.
Standard α-MEM that contains ascorbic acid (Asc-α-MEM), α-MEM without ascorbic acid (heretofore referred to as α-MEM), and DMEM-nutrient mixture F-12 (DMEM-F-12) were obtained from Invitrogen (Carlsbad, CA), as were reverse transcriptase PCR reagents, Taq DNA polymerase, and custom primers. Holotransferrin and sodium selenite were purchased from Sigma (St. Louis, MO). Purified porcine insulin was obtained from Elanco Products (Indianapolis, IN). l-Ascorbic acid was obtained from Wako Pure Chemical Industries (Osaka, Japan). 3,4-Dehydro-l-proline (DHP) was purchased from Sigma. Electrophoresis reagents and PVDF membrane were obtained from Bio-Rad (Hercules, CA). Tri-Reagent was obtained from Molecular Research Center (Cincinnati, OH). Western immunoblotting was performed using enhanced chemiluminescence (ECL) Plus reagents from Amersham (Piscataway, NJ). Antibodies directed toward the β-subunit of the insulin receptor (antibody C-19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), anti-phospho ERK1/2 was obtained from Cell Signaling Technology (Beverly, MA), and anti-ERK1/2 was obtained from Upstate Biotechnology (Lake Placid, NY). U-0126 was from Calbiochem (La Jolla, CA).
Cell culture conditions and biochemical analyses.
The ATDC5 cell line was obtained from the Riken Cell Bank (Tsukuba, Japan). Cells were cultured as described previously (4, 29). To study hypertrophic cells, chondrogenesis was first induced by plating the cells at 3.4 × 103 cells/cm2 and allowing them to proliferate for 3 days until they reached confluence. At confluence, cells were induced to differentiate with Asc-α-MEM or DMEM-F-12 supplemented with 1,600 nM porcine insulin. Both media (pH 7.45) were supplemented with 5% FBS, 10 μg/ml human transferrin, 30 nM sodium selenite, and 2% antibiotic-antimycotic solution. Media were replaced every other day for the duration of the experiment.
To assess cell proliferation, cells were grown to confluence in DMEM-F-12 using six-well plates. Cells were induced to differentiate in Asc-α-MEM or DMEM-F-12 containing 1,600 nM insulin. At indicated times, cells were released from the plate by trypsinization and counted.
Histochemical quantitation of chondrocyte differentiation was assessed as proteoglycan accumulation, as measured by staining of cell monolayers with Alcian blue and neutral red (29). Cells were first rinsed with PBS three times and then fixed with 100% methanol for 10 min at −20°C. Staining was accomplished by applying a solution of 0.1% Alcian blue 8 GX in 0.1 M HCl to the cells for 2 h at room temperature. To quantify the intensity of the staining, the stained culture plates were rinsed with PBS three times, and each well was extracted with 1 ml of 6 M guanidine-HCl overnight at room temperature. The optical density of extracted dye was measured at 650 nm. Total cellular material was quantified in parallel by staining with neutral red, which is a weak base that is taken up by viable cells. The detection of cellular neutral red content was measured as optical density at 550 nm of the same extracts used for Alcian blue quantification. This method was used because it allowed us to combine analyses for an indicator of cell growth (proliferation and/or hypertrophy) with quantification of an indicator of chondrocyte differentiation.
Western immunoblotting of cell lysates was carried out using previously described methods (29). Where indicated, blots were stripped according to the ECL Plus protocol prior to reprobing (29). Immunoblotting results were quantified using Gel-Pro Analyzer software (Media Cybernetics, Silver Spring, MD).
Determination of gene expression by PCR.
To determine the expression levels of collagen II, bone morphogenic protein 2 (BMP2), collagen X, Indian hedgehog (Ihh), p21, Sox9, β-actin, and Runx2 in ATDC5 cells, total RNA was prepared from triplicate plates using TRIzol reagent. Semiquantitative PCR was performed as described previously (29). Primer sequences used for detection of collagen II, collagen X, BMP2, Ihh, and β-actin were those used by Phornphutkul et al. (27). Primers used for Sox9 were those used by Kojima et al. (19), and primers used for detection of p21 and Runx2 were those used by Chen et al. (7). After reverse transcription, PCR was performed as described previously (28). The optimal cycle number was determined empirically for each primer set to maximize the sensitivity of quantification. PCR products were electrophoresed in 1.5% agarose gels containing ethidium bromide. Resulting photographs were quantified as described above for Western blots.
Preconditioning of tissue culture plates.
ATDC5 cells were plated in DMEM-F-12 medium using six-well, tissue culture-treated polystyrene plates (Fisher Scientific, Pittsburgh, PA). At confluence, cells were allowed to differentiate in α-MEM or Asc-α-MEM. Cells were maintained in this medium for 5 days, after which they were removed from the plates by incubation for 1 h at 37°C with hypotonic buffer (water with 0.5% Triton X-100). The plates were rinsed six times with Hanks' buffered salt solution (HBSS) and maintained in HBSS at 4°C until use.
Statistical analyses.
Except where noted, the significance of differences between groups was determined by one-way ANOVA, followed by a Tukey post hoc test using GraphPad Prism software (GraphPad Software, San Diego, CA). Differences were considered significant at P < 0.05.
RESULTS
A comparison of ATDC5 cell differentiation induced by ascorbic acid vs. high-dose insulin.
We observed previously that ATDC5 cells can differentiate in Asc-α-MEM without the addition of insulin or IGF-I (16). This was in contrast to DMEM-F-12, which is used routinely to differentiate these cells (4). When the components of Asc-α-MEM are compared with DMEM-F-12, Asc-α-MEM contains 50 μg/ml of ascorbic acid, approximately twice the concentration of calcium, and higher concentrations of several nonessential amino acids.
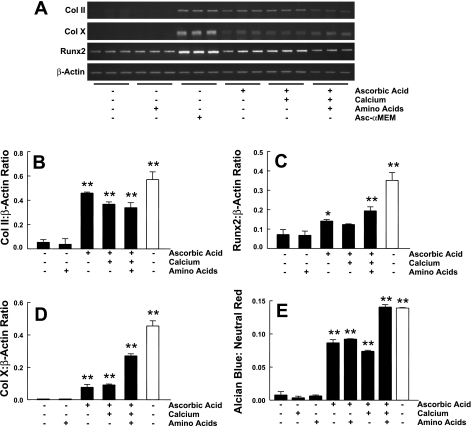
To determine which components in Asc-α-MEM could support ATDC5 cell differentiation, we tested eight different conditions in which calcium, nonessential amino acids, and ascorbic acid were added alone or in combination to DMEM-F-12 at concentrations equal to those found in Asc-α-MEM. We studied a duration of incubation of 14 days. At the end of this time, total RNA was isolated and analyzed by semiquantitative RT-PCR for three markers of chondrocyte differentiation: collagen II, Runx2, and collagen X. ATDC5 cells grown in Asc-α-MEM were used for comparison. Expression levels were normalized to the expression of β-actin, which remained stable throughout the experiment. Results (Fig. 1, A–D) showed that the expression of all three differentiation markers was increased only in DMEM-F-12 medium, to which ascorbic acid was added. A parallel experiment was done in which cells were stained for proteoglycan accumulation using Alcian blue. Similar results were obtained (Fig. 1E). We also noted that cells cultured in the presence of ascorbic acid maintained a round shape characteristic of chondrocytes and formed cell clusters during the culture period. Controlling for calcium and essential amino acid concentrations did not suffice to induce differentiation, although these factors may have enhanced the effect of ascorbic acid. We concluded that ascorbic acid is the key component in the standard α-MEM formulation that allows for differentiation of ATDC5 cells in the absence of insulin or IGF-I.
Fig. 1.
ATDC5 cell differentiation in various medium conditions. A: ATDC5 cells were incubated with DMEM-F-12 supplemented with 90 mg/ml calcium, nonessential amino acids, or 50 μg/ml ascorbic acid alone or in combination. Cells were also maintained in standard α-MEM that contains ascorbic acid (Asc-α-MEM). Three markers of differentiation, collagen (Col) II, Runx2, and Col X, and a control gene, β-actin, were assessed using semiquantitative RT-PCR. Triplicate samples were analyzed for each condition. Quantification of these results, calculated as the ratio of Col II (B), Runx2 (C), and Col X (D) to β-actin expression, is shown as means ± 1 SD for triplicate determinations. E: proteoglycan accumulation was assessed using Alcian blue and normalized for cell content using neutral red staining. The dyes were extracted, and absorbance was determined. The ratio of Alcian blue to neutral red was calculated (n = 3 for each condition). Filled bars, DMEM-F-12; open bars, Asc-α-MEM. *P < 0.05 vs. DMEM-F-12 alone; **P < 0.01 vs. DMEM-F-12 alone, as determined by ANOVA. A 2nd replicate experiment gave similar results.
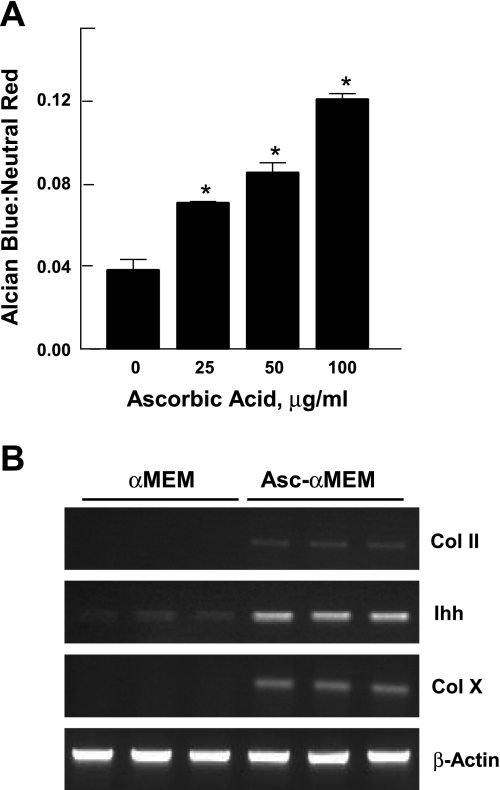
We next examined the concentration dependence of the differentiation process on ascorbic acid. Ascorbic acid, 0–100 μg/ml, was added to DMEM-F-12, and differentiation was assessed by measuring proteoglycan accumulation. Results (Fig. 2A) showed a dose-dependent effect ≤100 μg/ml, twice the concentration in the Asc-α-MEM medium.
Fig. 2.
The effect of ascorbic acid on the induction of differentiation markers in ATDC5 cells. A: ATDC5 cells cultured in DMEM-F-12 were incubated with various concentrations of ascorbic acid (0–100 μg/ml) for 14 days. Proteoglycan accumulation was analyzed by Alcian blue staining and normalized for cell content using neutral red staining. Results, expressed as the ratio of Alcian blue to neutral red, are presented as the mean ± 1 SD for triplicate determinations. *P < 0.01 vs. control, as determined by ANOVA. An additional experiment gave similar results. B: cells were grown in α-MEM or Asc-α-MEM for 10 days. The expression of Col II, Indian hedgehog (Ihh), and Col X was assessed in triplicate samples by semiquantitative RT-PCR. β-Actin was used as an internal control.
The effect of ascorbic acid on ATDC5 cell differentiation was further supported by a direct comparison of ATDC5 cells maintained in α-MEM vs. Asc-α-MEM. Only Asc-α-MEM medium was able to induce ATDC5 cell differentiation, as measured by collagen II, Ihh, and collagen X mRNA expression (Fig. 2B).
We went on to assess the temporal aspects of ATDC5 cell proliferation and differentiation in Asc-α-MEM relative to the process induced by high dose-insulin (1,600 nM) in DMEM-F-12. ATDC5 cells were grown to confluence and then cultured under these two conditions for ≤14 days. At various time points, cells were collected by trypsinization and counted. Results (Fig. 3A) showed that cells maintained in DMEM-F-12 containing high-dose insulin proliferate at a significantly higher rate than cells maintained in Asc-α-MEM. In a parallel experiment, mRNA levels of chondrogenic differentiation markers collagen II, Sox9, Ihh, p21, and BMP2 were measured at intervals throughout the experiment. Results (Fig. 3, B–F) showed that all of these markers were induced in cells cultured in both Asc-α-MEM and DMEM-F-12 with high-concentration insulin. The pattern and level of induction were similar with the exception of collagen II, which was more potently induced by Asc-α-MEM.
Fig. 3.
The time course of the effect of ascorbic acid on cell proliferation and chondrogenic-related gene expression. ATDC5 cells were grown to confluence in DMEM-F-12. At confluence (day 0), cells were induced to differentiate for 14 days in the presence of Asc-α-MEM (●) or DMEM-F-12 with 1,600 nM insulin (○). A: On days 0, 1, 2, 4, 7, and 10, cells were counted. Data represent the mean (n = 3 for each condition). A similar experiment was performed in which cells were lysed and RNA was prepared. The expression of Col II, Sox9, Ihh, p21, BMP2, and β-actin was assessed using semiquantitative RT-PCR. The ratio of Col II (B), Sox9 (C), Ihh (D), p21 (E), and bone morphogenetic protein 2 (BMP2; F) to β-actin expression is shown as means ± 1 SD for triplicate analyses. *P < 0.05 vs. the corresponding time point; **P < 0.001 vs. the corresponding time point.
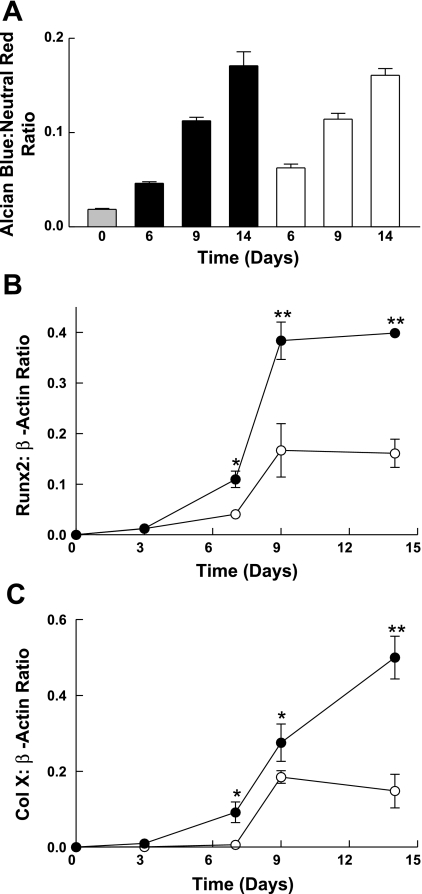
A parallel experiment in which differentiation was assessed as proteoglycan accumulation was done (Fig. 4A). Again, induction of differentiation was similar for the two culture conditions. Additional analyses for collagen X and Runx2 mRNA expression (Fig. 4, B and C), both markers of chondrocyte hypertrophy, showed induction that occurred more rapidly and was greater in magnitude in Asc-α-MEM than in DMEM-F-12 with high-concentration insulin. We concluded that ascorbic acid initiates a differentiation program in ATDC5 cells that mimics the pattern observed using the standard method of exposure to high-concentration insulin.
Fig. 4.
The effect of ascorbic acid on the induction of markers of chondrocyte hypertrophy. ATDC5 cells were grown to confluence and induced to differentiate as for Fig. 3 (filled bars or ●, Asc-α-MEM; open bars or ○, 1,600 nM insulin in DMEM-F-12) for ≤14 days. A: On days 0, 6, 9, and 14, cells were stained with Alcian blue and neutral red. Dye was extracted and absorbance measured. The ratio of Alcian blue to neutral red is shown as the mean + 1 SD for triplicate analyses. Differences between groups were not significant, as determined by ANOVA. A parallel experiment was done to assess the expression of Runx2 (B) and Col X (C) using semiquantitative RT-PCR. Data are shown as the mean ± 1 SD for triplicate analyses. Additional experiments gave similar results. *P < 0.05; **P < 0.001 vs. corresponding time point by ANOVA.
Growth factor signaling in ATDC5 cells induced to differentiate by ascorbic acid vs. high-dose insulin.
The use of insulin at high concentration would be expected to induce downregulation of insulin and IGF-I receptors through ligand-induced endocytosis (28). Therefore, we took advantage of the ability of ascorbic acid to induce differentiation to study growth factor and insulin signaling during the differentiation process. Cells were cultured in Asc-α-MEM or 1,600 nM insulin in DMEM-F-12 for ≤14 days. Insulin and IGF-I receptor content were analyzed by direct Western immunoblotting. Results (Fig. 5A) showed a rapid and persistent downregulation of insulin and IGF-I receptor β-subunits in the presence of insulin. Levels persisted at predifferentiation levels in the presence of ascorbic acid. A modest upregulation of the content of both receptors was seen on day 6 in cells cultured in Asc-α-MEM. Total ERK content was constant throughout the differentiation period.
Fig. 5.
The effect of ascorbic acid on insulin and IGF-I receptor content and ERK activation. ATDC5 cells were maintained for the indicated times in Asc-α-MEM or DMEM-F-12 containing 1,600 nM insulin. A: at the times indicated, duplicate cell lysates were prepared and analyzed by Western immunoblotting for the IGF-I receptor-β (IGF-Iβ) subunit and the insulin receptor-β (IRβ) subunit. Samples were also analyzed for total ERK to assess sample loading and transfer. B: a similar experiment was performed in which duplicate samples were prepared from cells maintained in DMEM-F-12 (0) or Asc-α-MEM for a duration of 14 days. At indicated times, these samples were analyzed by Western immunoblotting for the IGF-I receptor β-subunit, insulin receptor β-subunit, phosphorylated ERK (p-ERK), and total ERK.
To examine the role of the ERK pathway in Asc-α-MEM-induced ATDC5 cell differentiation, we assessed ERK activity at various stages of the differentiation process (Fig. 5B). ERK phosphorylation, an indicator of ERK activity, was increased in the presence of Asc-α-MEM relative to DMEM-F-12 maintenance medium. A sharp increase in ERK phosphorylation occurred on days 2 and 6, coincident with the changes in IGF-I receptor content.
The role of collagen synthesis in ascorbic acid-induced ATDC5 cell differentiation.
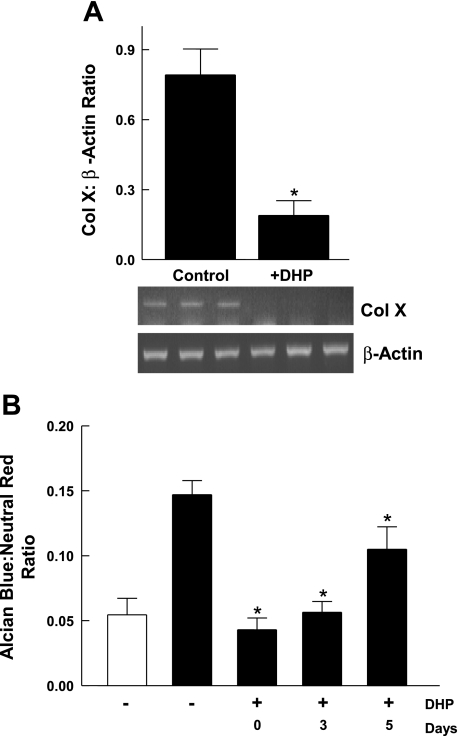
Given that ascorbic acid is required for the formation of collagen triple helices, we hypothesized that the ability of ascorbic acid to induce ATDC5 cell differentiation depends on its ability to promote synthesis of collagen matrix. To test this hypothesis, we analyzed the effect of an inhibitor of collagen triple helix formation, DHP, on Asc-α-MEM-induced ATDC5 cell differentiation. Cells were maintained in Asc-α-MEM in the presence or absence of DHP for 10 days. Total RNA was collected and analyzed for collagen X expression. Results (Fig. 6A) showed that DHP at 0.4 mM completely inhibited the induction of collagen X. The effect of DHP on proteoglycan accumulation was assessed using Alcian blue staining with normalization to the intensity of neutral red staining, a measurement of cell content (Fig. 6B). In this experiment, DHP was added at different stages of the differentiation process (day 0, 3, or 5).
Fig. 6.
The effect of a collagen synthesis inhibitor on ascorbic acid-induced ATDC5 cell differentiation. A: cells were grown to confluence in DMEM-F-12 and then switched to Asc-α-MEM. 3,4-Dehydro-l-proline (DHP; 0.4 mM) or vehicle (control) was added to the medium. Cells were lysed on day 10. Total RNA was extracted and analyzed for the expression of Col X and β-actin using semiquantitative RT-PCR. Data representing the ratio of Col X to β-actin expression are shown as the mean + 1 SD for triplicate samples. *P < 0.001, control vs. DHP by unpaired t-test. A, bottom: Col X and β-actin expression. Triplicate samples were analyzed for each condition. A replicate experiment gave similar results. B: cells were grown to confluence in DMEM-F-12 (open bar) and then switched to Asc-α-MEM (filled bars). DHP (0.4 mM) was added starting on day 0, 3, or 5. Cells were maintained until day 14. At that time, cells were stained for proteoglycan accumulation using Alcian blue and neutral red. The dye was extracted and absorbance determined. Data representing the ratio of Alcian blue to neutral red are shown as the mean + 1 SD for triplicate determinations (n = 3 for each condition). *P < 0.01 vs. Asc-α-MEM/DHP.
Cells were cultured for ≤14 days. Proteoglycan accumulation was completely inhibited relative to the level observed with Asc-α-MEM when DHP was added on day 0 or day 3. Only partial inhibition of proteoglycan accumulation was observed when DHP was added on day 5. As noted above (see Fig. 3A), when ATDC5 cells at confluence are induced to differentiate in the presence of ascorbic acid, they go through several cycles of cell proliferation prior to undergoing hypertrophy. We observed 20% inhibition of cell proliferation in the presence of DHP (data not shown), indicating that collagenous matrix can modulate both chondrocyte proliferation and differentiation.
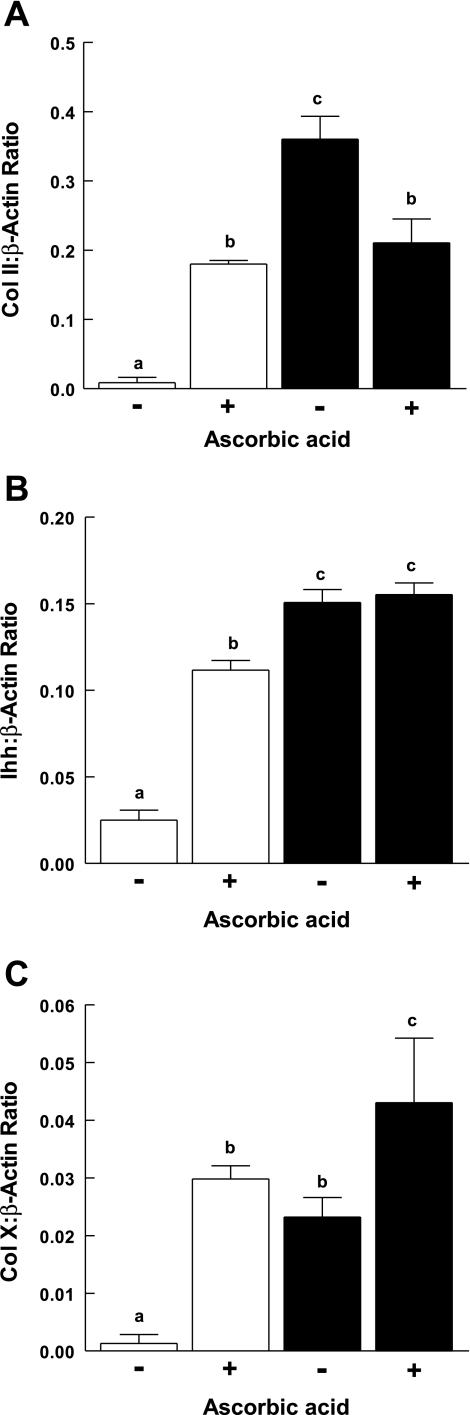
These results were interpreted as consistent with cell-matrix interactions that are stimulatory for chondrocyte differentiation. To further assess this, culture plates were conditioned by culturing ATDC5 cells for 5 days in the presence of Asc-α-MEM. Cells were then removed using hypotonic buffer. ATDC5 cells were cultured on the conditioned plates for 7 days in α-MEM or Asc-α-MEM. Results (Fig. 7) showed that, on conditioned plates, the preformed matrix was sufficient to induce differentiation of ATDC5 cells as assessed by collagen II, Ihh, and collagen X expression. Conditioning of the tissue culture plate induced collagen X expression in the absence of ascorbic acid to a level similar to that seen in cells cultured in Asc-α-MEM without tissue culture plate conditioning. The addition of ascorbic acid to cultures on conditioned plates enhanced collagen X expression. There was no effect on Ihh. At 7 days, cells cultured in Asc-α-MEM showed a lower level of collagen II induction relative to cells cultured in preconditioned plates. We interpreted this as consistent with an acceleration of the differentiation process with a concomitant, late decrease in collagen II expression.
Fig. 7.
The effect of extracellular matrix produced by ascorbic acid-induced ATDC5 cells on chondrocyte differentiation. Plates were conditioned by culturing ATDC5 cells in α-MEM (open bars) or Asc-α-MEM (filled bars) for 5 days. Cells were removed, and fresh ATDC5 cells were plated and maintained in α-MEM with (+) or without (−) ascorbic acid for 7 days. The expression of Col II (A), Ihh (B), and Col X (C) was determined using RT-PCR. Data normalized to β-actin expression are shown as the mean + 1 SD for triplicate samples. Letters above the bars indicate groups that differ significantly from one another based on ANOVA. An additional experiment gave similar results.
Role of ERK signaling in matrix-induced ATDC5 cell differentiation.
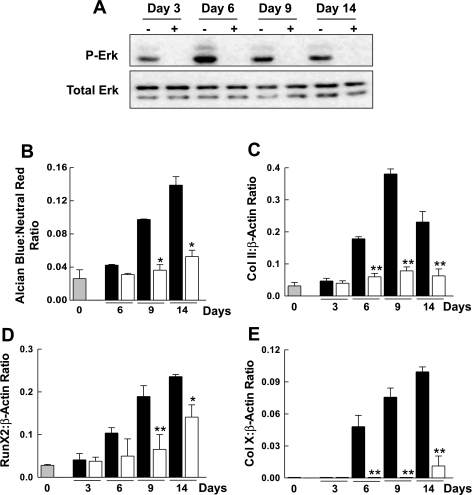
It is well established in a number of cell systems that ERK activity is stimulated by cell-matrix interactions (32, 37, 39). As noted above, the ERK pathway has an important role in the chondrocyte differentiation process. To examine the role of ERK signaling in ascorbic acid-induced ATDC5 cell differentiation, we studied the effect of U-0126 on ERK phosphorylation and chondrocyte differentiation. ATDC5 cells grown in Asc-α-MEM were incubated with U-0126 or DMSO for ≤14 days. Cultures were assessed for effect of the inhibitor on ERK phosphorylation and markers of chondrogenic differentiation and hypertrophy. Results (Fig. 8) confirmed that U-0126 at a concentration of 10 μM abolished ERK phosphorylation at all experimental time points used to assess chondrocyte differentiation. The presence of U-0126 was associated with a marked reduction in the accumulation of sulfated glycosaminoglycans (Alcian blue staining) relative to control at 6, 9, and 14 days. U-0126 also inhibited the induction of collagen II, Runx2, and collagen X. A different pharmacological inhibitor of MEK1, PD-98059, was able to inhibit both ascorbic acid-induced ERK phosphorylation and ATDC5 cell differentiation. However, PD-98059 was unable to suppress ERK activation and ATDC5 cell differentiation as effectively as U-0126 (data not shown).
Fig. 8.
The effect of the ERK pathway inhibitor U-0126 on chondrocyte hypertrophy. A: ATDC5 cells at confluence were incubated continuously for 14 days with Asc-α-MEM containing either 0.1% DMSO vehicle (−) or 10 μM U-0126 (+). Levels of p-ERK and total ERK were determined by Western blot analysis. B: ATDC5 cells at confluence (gray bar) were switched to Asc-α-MEM in the absence (black bars) or presence (open bars) of U-0126 (10 μM) for ≤14 days. At the times indicated, cells were stained with Alcian blue and neutral red. Data representing the ratio of Alcian blue to neutral red are shown as the mean ± 1 SD for triplicate determinations. C–E: a similar experiment was performed in which the expression of Col II (C), Runx2 (D), and Col X (E) was analyzed by semiquantitative RT-PCR. Data represent the ratio of the expression of each gene to β-actin. Gene expression data are shown as the mean + 1 SD for triplicate determinations. *P < 0.01 vs. corresponding control; **P < 0.001 vs. corresponding control. Additional experiments gave similar results.
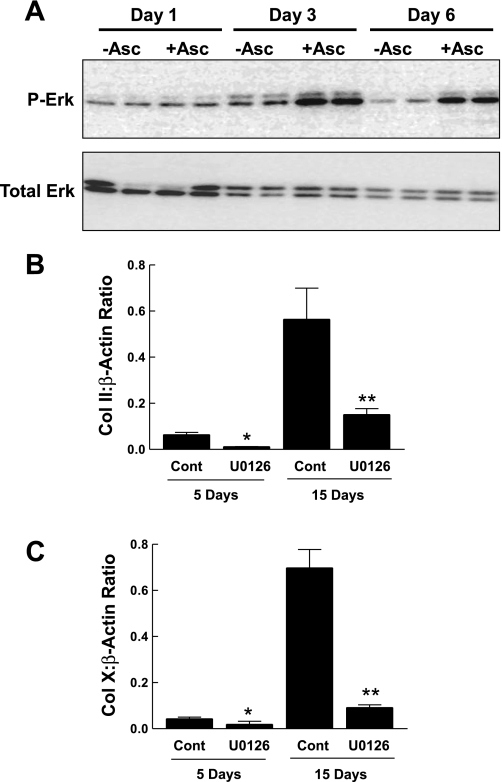
To further test our hypothesis that the activation of ERK is likely mediated by cell-matrix interaction, we assessed ERK phosphorylation in cells that were induced to differentiate by preconditioning of the tissue culture plates. On days 3 and 6, basal ERK phosphorylation was markedly higher in cells grown on plates preconditioned by cells grown in Asc-α-MEM relative to plates conditioned by cells maintained in α-MEM without ascorbic acid (Fig. 9A). We examined the induction of several markers of differentiation by preconditioning of the tissue culture plates, with or without ERK pathway inhibition by U-0126. Results (Fig. 9, B and C) demonstrated that the differentiation of ATDC5 cell culture on preformed matrix is markedly attenuated when ERK is inhibited by U-0126.
Fig. 9.
The effect of preconditioned plates and ERK signaling. A: preconditioned plates were prepared as in Fig. 7, using α-MEM without (−Asc) or with (+Asc) ascorbic acid. Fresh ATDC5 cells were seeded on the preconditioned plates and cultured for ≤15 days in α-MEM. At the time points indicated, cell lysates were prepared and analyzed for p-ERK and total ERK by Western immunoblotting. B: ATDC5 cells seeded on plates preconditioned by ATDC5 cells grown in the presence of ascorbic acid were maintained in the absence or presence of 10 μM U-0126. At the times indicated, total RNA was prepared and analyzed by RT-PCR for Col II. C: the samples from the experiment in B were analyzed for Col X expression. Data representing the ratio to β-actin expression are shown as the mean + 1 SD of triplicate analyses. *P <0.05 or **P <0.001 vs. corresponding control. An additional experiment gave similar results.
DISCUSSION
It is well established that ascorbic acid is necessary for the differentiation of mesenchymal cell types, including adipocytes, osteoblasts, myoblasts, and chondrocytes (21, 43–45). Although insulin at high concentrations has been used routinely to induce differentiation of ATDC5 cells, we found previously that ascorbic acid-containing α-MEM alone was sufficient to induce differentiation in this cell line (16). The present studies were aimed at characterizing ascorbic acid-induced ATDC5 cell differentiation and investigating the mechanisms of ascorbic acid action. Our results show that ascorbic acid-treated cells produce a pattern of gene expression similar to that seen in insulin-induced ATDC5 cell differentiation. This observation provided the basis for further studies to characterize growth factor signaling during ATDC5 cell differentiation in the absence of tonic growth factor exposure.
We previously demonstrated the effect of high-dose insulin on insulin and IGF-I receptors (28). Those studies showed a rapid, marked, and persistent downregulation of receptor abundance. In contrast, such changes were not seen during ascorbic acid-induced ATDC5 cell differentiation. This result indicates that the differentiating effect of ascorbic acid probably does not depend on induction of IGF-I. In addition, it appears that insulin and IGF-I receptors are persistently expressed during ATDC5 cell differentiation and that their downregulation in the presence of high-concentration insulin is a consequence of ligand-induced receptor endocytosis and degradation. Thus, ascorbic acid may be a useful agent for studies on the modulation of growth factor signaling during ATDC5 cell differentiation.
Our results also indicate that ascorbic acid induction of ATDC5 cell differentiation is dependent on the synthesis of collagenous extracellular matrix and that this effect requires ERK signaling. Ascorbic acid stimulates procollagen hydroxylation and processing and is required for collagen fibril assembly and collagen secretion (33–35). The rate-limiting step in this overall process is the hydroxylation and secretion of unprocessed procollagen chains that accumulate in the endoplasmic reticulum of ascorbic acid-deficient cells (22).
The requirement of ATDC5 cell differentiation for intact collagen production and secretion is supported by the observation that chondrocyte hypertrophy was inhibited by a collagen synthesis inhibitor. Maximal inhibition occurred only when collagen synthesis was inhibited at the start of the differentiation process, suggesting that collagen matrix formed before day 5 was sufficient to induce an intermediate degree of differentiation. Similar observations have been reported for osteoblasts, in which inhibition of collagen I synthesis inhibited ascorbic acid induction of osteoblast proliferation (13) and differentiation (9). The reduction of proliferation in the presence of DHP is unlikely to be the factor accounting for a 90% decrease in chondrocyte differentiation measured by collagen X expression. In fact, we (29) demonstrated that inhibition of ATDC5 cell proliferation by the addition of ERK inhibitor PD-98059 resulted in enhanced differentiation.
In an extension of our studies showing that collagen synthesis was critical to the induction of ATDC5 cell differentiation in response to ascorbic acid, we were able to demonstrate that extracellular matrix synthesized by cells exposed to ascorbic acid was sufficient to promote differentiation in the absence of ascorbic acid. Given that the collagenous matrix was effective when produced by cells early in their differentiation program, and given that the inhibitor of collagen synthesis could only partially inhibit differentiation when added several days after initiation of chondrocyte differentiation, it is likely that collagens expressed early in the differentiation process, such as collagen types II, IX, and IV, are playing a major role in the induction process. The important role of early collagen in chondrocyte differentiation is supported by observations made by several groups. Bosnakovski et al. (5) showed that collagen II hydrogels could stimulate the differentiation of bone marrow mesenchymal stem cells in the absence of growth factors. Qi and Scully (31) observed an increase in bovine articular chondrocyte proteoglycan synthesis in a dose-dependent fashion in the presence of collagen II in the extracellular matrix.
Growth plate cartilage is an avascular tissue that consists of chondrocytes and extracellular matrix. The importance of interactions between extracellular matrix and growth factors is supported by a number of studies. Extracellular signaling involves the interaction of cell surface receptors with soluble cytokines and growth factors, with extracellular matrix constituents such as collagen and proteoglycans, or with the cell surface proteins of neighboring cells. Extracellular matrix has other physiological functions, such as promoting the availability of nutrients and mediating cellular functions through direct interaction with cell surface receptors (1, 10, 11). Integrin family members are a family of receptors that transmit information from the matrix to the cells. In the growth plate, the interaction of integrins with matrix protein has various effects on cell proliferation and differentiation of cells via the activation of a variety of signal transduction pathways (14). The ERK pathway has a central role in transducing integrin signals, in particular those that regulate gene expression through activation of transcription factors (8, 11, 23). We previously made the observation that collagen X expression and glycosaminoglycan accumulation were enhanced in ATDC5 cells by inhibition of the ERK pathway when IGF-I was used to promote differentiation (29). More recent work using metatarsal explants from E15.5 mouse embryos (30) resulted in the converse observation. Inhibition of ERK signaling using U-0126 attenuated the differentiation process, with no subsequent effect on chondrocyte proliferation. In the latter model, chondrocyte proliferation and differentiation are occurring simultaneously in separate zones within the metatarsal growth plate. The results of the present studies support a prodifferentiation role for the ERK pathway, one that is downstream from extracellular matrix biosynthesis and possibly integrin signaling.
In summary, we have shown that we can induce ATDC5 chondrocyte to differentiation in ascorbic acid-containing medium and that the resulting pattern of gene expression closely mimics that seen with the standard insulin-mediated induction of ATDC5 cell differentiation. This activity of ascorbic acid is dependent on collagen synthesis and ERK activation. Furthermore, the extracellular matrix produced by ATDC5 cells exposed to ascorbic acid is sufficient to promote differentiation in an ERK-dependent manner. Our findings support a critical role for matrix proteins in the early signaling events that mediate chondrogenesis. Furthermore, our results indicate that ascorbic acid-containing growth medium can be used to study chondrogenic signaling events in the absence of the confounding effects of tonic pharmacological growth factor stimulation.
GRANTS
C. Phornphutkul was funded by the Department of Pediatrics, Rhode Island Hospital, Research Fund and National Center for Research Resources Grant No. P20-RR-024484 (to C. Phornphutkul; Q. Chen, principal investigator). This work was also supported by National Institute of Child Health and Human Development Grant R01-HD-24455 (to P. A. Gruppuso).
DISCLOSURES
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
REFERENCES
- 1.Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development 117: 1183–1198, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Altaf FM, Hering TM, Kazmi NH, Yoo JU, Johnstone B. Ascorbate-enhanced chondrogenesis of ATDC5 cells. Eur Cell Mater 12: 64–69, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol 143: 213–221, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Atsumi T, Miwa Y, Kimata K, Ikawa Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev 30: 109–116, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng 93: 1152–1163, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cancedda R, Descalzi Cancedda F, Castagnola P. Chondrocyte differentiation. Int Rev Cytol 159: 265–358, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Fink T, Zhang XY, Ebbesen P, Zachar V. Quantitative transcriptional profiling of ATDC5 mouse progenitor cells during chondrogenesis. Differentiation 73: 350–363, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Clark EA, Hynes RO. 1997 keystone symposium on signal transduction by cell adhesion receptors. Biochim Biophys Acta 1333: R9–R16, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res 7: 235–246, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol 9: 701–706, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol 9: 691–700, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Habuchi H, Conrad HE, Glaser JH. Coordinate regulation of collagen and alkaline phosphatase levels in chick embryo chondrocytes. J Biol Chem 260: 13029–13034, 1985 [PubMed] [Google Scholar]
- 13.Harada S, Matsumoto T, Ogata E. Role of ascorbic acid in the regulation of proliferation in osteoblast-like MC3T3-E1 cells. J Bone Miner Res 6: 903–908, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Hirsch MS, Lunsford LE, Trinkaus-Randall V, Svoboda KK. Chondrocyte survival and differentiation in situ are integrin mediated. Dev Dyn 210: 249–263, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Horovitz O, Knaack D, Podleski TR, Salpeter MM. Acetylcholine receptor alpha-subunit mRNA is increased by ascorbic acid in cloned L5 muscle cells: Northern blot analysis and in situ hybridization. J Cell Biol 108: 1823–1832, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MS, Wu KY, Auyeung V, Chen Q, Gruppuso PA, Phornphutkul C. Leucine restriction inhibits chondrocyte proliferation and differentiation through mechanisms both dependent and independent of mTOR signaling. Am J Physiol Endocrinol Metab 296: E1374–E1382, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone 18: 281–288, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Knaack D, Podleski T. Ascorbic acid mediates acetylcholine receptor increase induced by brain extract on myogenic cells. Proc Natl Acad Sci USA 82: 575–579, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima Y, Hayashi Y, Mizuno K, Sasaki S, Fukui Y, Koopman P, Morohashi K, Kohri K. Up-regulation of SOX9 in human sex-determining region on the Y chromosome (SRY)-negative XX males. Clin Endocrinol (Oxf) 68: 791–799, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Kronenberg HM. Developmental regulation of the growth plate. Nature 423: 332–336, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem 264: 17281–17286, 1989 [PubMed] [Google Scholar]
- 22.Meier S, Solursh M. Ultrastructural analysis of the effect of ascorbic acid on secretion and assembly of extracellular matrix by cultured chick embryo chondrocytes. J Ultrastruct Res 65: 48–59, 1978 [DOI] [PubMed] [Google Scholar]
- 23.Meredith JE, Jr, Winitz S, Lewis JM, Hess S, Ren XD, Renshaw MW, Schwartz MA. The regulation of growth and intracellular signaling by integrins. Endocr Rev 17: 207–220, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Nakajima M, Negishi Y, Tanaka H, Kawashima K. p21(Cip-1/SDI-1/WAF-1) expression via the mitogen-activated protein kinase signaling pathway in insulin-induced chondrogenic differentiation of ATDC5 cells. Biochem Biophys Res Commun 320: 1069–1075, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Nandan D, Clarke EP, Ball EH, Sanwal BD. Ethyl-3,4-dihydroxybenzoate inhibits myoblast differentiation: evidence for an essential role of collagen. J Cell Biol 110: 1673–1679, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143: 420–430, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Phornphutkul C, Wu KY, Auyeung V, Chen Q, Gruppuso PA. mTOR signaling contributes to chondrocyte differentiation. Dev Dyn 237: 702–712, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phornphutkul C, Wu KY, Gruppuso PA. The role of insulin in chondrogenesis. Mol Cell Endocrinol 249: 107–115, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Phornphutkul C, Wu KY, Yang X, Chen Q, Gruppuso PA. Insulin-like growth factor-I signaling is modified during chondrocyte differentiation. J Endocrinol 183: 477–486, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Provot S, Nachtrab G, Paruch J, Chen AP, Silva A, Kronenberg HM. A-raf and B-raf are dispensable for normal endochondral bone development, and parathyroid hormone-related peptide suppresses extracellular signal-regulated kinase activation in hypertrophic chondrocytes. Mol Cell Biol 28: 344–357, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi WN, Scully SP. Effect of type II collagen in chondrocyte response to TGF-beta 1 regulation. Exp Cell Res 241: 142–150, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Salasznyk RM, Klees RF, Hughlock MK, Plopper GE. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun Adhes 11: 137–153, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Schwarz RI. Procollagen secretion meets the minimum requirements for the rate-controlling step in the ascorbate induction of procollagen synthesis. J Biol Chem 260: 3045–3049, 1985 [PubMed] [Google Scholar]
- 34.Schwarz RI. Ascorbate stabilizes the differentiated state and reduces the ability of Rous sarcoma virus to replicate and to uniformly transform cell cultures. Am J Clin Nutr 54: 1247S–1251S, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Schwarz RI, Kleinman P, Owens N. Ascorbate can act as an inducer of the collagen pathway because most steps are tightly coupled. Ann NY Acad Sci 498: 172–185, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Schwarz RI, Mandell RB, Bissell MJ. Ascorbate induction of collagen synthesis as a means for elucidating a mechanism of quantitative control of tissue-specific function. Mol Cell Biol 1: 843–853, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shakibaei M, John T, de Souza P, Rahmanzadeh R, Merker HJ. Signal transduction by beta1 integrin receptors in human chondrocytes in vitro: collaboration with the insulin-like growth factor-I receptor. Biochem J 342: 615–623, 1999 [PMC free article] [PubMed] [Google Scholar]
- 38.Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol 133: 457–468, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res 91: 769–775, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Spindler KP, Shapiro DB, Gross SB, Brighton CT, Clark CC. The effect of ascorbic acid on the metabolism of rat calvarial bone cells in vitro. J Orthop Res 7: 696–701, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Stevens DA, Williams GR. Hormone regulation of chondrocyte differentiation and endochondral bone formation. Mol Cell Endocrinol 151: 195–204, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Sugimoto T, Nakada M, Fukase M, Imai Y, Kinoshita Y, Fujita T. Effects of ascorbic acid on alkaline phosphatase activity and hormone responsiveness in the osteoblastic osteosarcoma cell line UMR-106. Calcif Tissue Int 39: 171–174, 1986 [DOI] [PubMed] [Google Scholar]
- 43.Taylor SM, Jones PA. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell 17: 771–779, 1979 [DOI] [PubMed] [Google Scholar]
- 44.Torii Y, Hitomi K, Tsukagoshi N. l-ascorbic acid 2-phosphate promotes osteoblastic differentiation of MC3T3-E1 mediated by accumulation of type I collagen. J Nutr Sci Vitaminol (Tokyo) 40: 229–238, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Vogel Z, Daniels MP, Chen T, Xi ZY, Bachar E, Ben-David L, Rosenberg N, Krause M, Duksin D, Kalcheim C. Ascorbate-like factor from embryonic brain. Role in collagen formation, basement membrane deposition, and acetylcholine receptor aggregation by muscle cells. Ann NY Acad Sci 498: 13–27, 1987 [DOI] [PubMed] [Google Scholar]
- 46.Wallis GA. Bone growth: coordinating chondrocyte differentiation. Curr Biol 6: 1577–1580, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Wegger I, Palludan B. Vitamin C deficiency causes hematological and skeletal abnormalities during fetal development in swine. J Nutr 124: 241–248, 1994 [DOI] [PubMed] [Google Scholar]