Abstract
Nedd4–2, a E3 ubiquitin ligase, regulates epithelial sodium channel-mediated transcellular Na+ transport in the collecting duct. We investigated the effect of Nedd4–2 on the junctional complex and paracellular conductance in mpkCCDc14 cells, a collecting duct cell line. We demonstrate that Nedd4–2 coimmunoprecipitated with and reduced the expression of transfected occludin in HEK293 cells. This interaction was mediated via a conserved PY motif in the COOH terminus of occludin and mutation of this PY motif increased the half-life of transfected occludin in HEK293 cells from 6.4 to 11.4 h. We demonstrate that Nedd4–2 ubiquitinates occludin, which was not seen when a catalytically inactive form of Nedd4–2 was used. Overexpression of Nedd4–2 in mpkCCDc14 cells reduced occludin at the tight junction and transiently increased paracellular conductance in a Ca2+ switch assay consistent with a delay in the formation of tight junctions. Conversely, siRNA-mediated knockdown of Nedd4–2 increased occludin levels and reduced paracellular conductance. In summary, we demonstrate that Nedd4–2 plays a role in tight junction assembly and the regulation of paracellular conductance in the collecting duct.
Keywords: epithelial cells, epithelial barrier function, ubiquitination
the final composition of urine is determined by the secretion and reabsorption of solutes across polarized epithelial cells that line the nephron. The tight junction (TJ) complex contributes to the development and maintenance of epithelial polarity by preventing the diffusion of cell membrane proteins between the apical and basolateral domains. TJs also contain pores that permit the selective paracellular diffusion of ions between cells, contributing to the transport characteristics of various nephron segments (8, 12). TJs include several integral membrane proteins like occludin, claudins, junctional adhesion molecules (JAM), tricellulin, cingulin, and the coxsackievirus and adenovirus receptor (4).
Occludin was discovered as a 65-kDa integral membrane protein at the TJs from chick liver (16). This protein contains four membrane-spanning segments in its NH2-terminal half with two extracellular loops, an intracellular loop and intracellular NH2+ and COO− termini (2, 16). Although occludin is not the primary molecule responsible for determination of the paracellular barrier, several studies indicate that occludin plays an important role in the regulation of TJ integrity (26, 44). For example, expression of a dominant negative mutant of occludin dramatically reduced transepithelial resistance and increased paracellular permeability (5). Similarly, stable expression of siRNAs against occludin resulted in an increase in the paracellular permeability of small organic cations and altered the cell response to cholesterol depletion (46).
When epithelial cells divide, TJs are remodeled by a process that involves the destruction and reassembly of multiple proteins into this complex. To maintain proper epithelial barrier function, the process of destruction and regeneration of TJs need to be very well-coordinated. Ubiquitination is one mechanism to internalize members of the TJ and other intercellular junction complexes and target them for degradation or for recycling. E-cadherin, an adhesion molecule of adherens junctions, is ubiquitinated by the RING type E3 ubiquitin ligase, Hakai, altering cell adhesion in Madin-Darby canine kidney (MDCK) cells (15). In these cells, Itch, a HECT type E3 ubiquitin ligase, was reported to associate with occludin and lead to its ubiquitination (43). Recently, another RING type E3 ubiquitin ligase, the ligand of Numb-protein X1 (LNX1p80), was identified as binding to claudin-1, a key component of TJs in MDCK cells (42). LNX1p80 leads to the ubiquitination, endocytosis, and lysosomal degradation of claudins.
In the current study, we examined the interaction between occludin and Nedd4–2, a HECT (homologous to E6-AP COOH terminus) domain containing E3 ubiquitin ligase naturally expressed in the connecting tubule and the collecting duct that regulates transepithelial Na+ transport (6, 13, 38). The occludin-Nedd4–2 interaction was demonstrated by coimmunoprecipitation and by overexpression of Nedd4–2 which accelerated ubiquitination of occludin in a heterologous system. Furthermore, we show that enhanced expression of Nedd4–2 reduced occludin in mpkCCDc14 cells, a collecting duct cell line, and delayed the formation of new TJs while siRNA-mediated knockdown of Nedd4–2 increased occludin and reduced paracellular conductance. Our experimental findings suggest that Nedd4–2, via ubiquitination of occludin, may be involved in the assembly and remodeling of TJ and the regulation of paracellular conductance in the collecting duct.
METHODS
Materials.
All cell culture media were obtained from Invitrogen Life Technologies (Gaithersburg, MD). Selenium, sorbitol, transferrin, triiodothyronine, N-acetyl-Leu-Leu-norleucinal (ALLN), benzamil, barium, 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), and poly l-lysine were purchased from Sigma (St. Louis, MO) and cycloheximide was purchased from EMD Chemicals (San Diego, CA). Anti-FLAG M2 antibody was purchased from Sigma; anti-HA antibody from US Biologicals (Swampscott, MA); anti-Xpress and anti-occludin antibody from Invitrogen; anti-α tubulin antibody, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and goat anti-mouse IgM from Santa Cruz Biotechnology (Santa Cruz, CA); and HRP-conjugated goat anti-rabbit IgG from Cell Signaling Technology (Danvers, MA). Anti-Nedd4–2 antibody was a gift from H. Pratt (Indiana Univ.).
Constructs.
FLAG-tagged occludin (Occludin_FLAG) was generated by cloning full-length human occludin into pFLAG-CMV-6c (Sigma) in frame with the NH2-terminal FLAG epitope. PCR primers used were hOcc_F: 5′-TCAAAGCTTCATCCAGGCCTCT TGAAAG and hOcc_R: 5′-TGACCCGGGCTATGTTTTCTGTCTATCATAGTCTCC, which includes flanking HindIII and SmaI sites. OccludinMut_FLAG was generated by mutating PPPY amino acids at position 9–12 to PAAY using the QuikChange Site Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Human Nedd4–2 that lacks the C2 domain was used in the studies reported here and the plasmid and adenoviral construct expressing this form of Nedd4–2 have been described before (20, 29). Plasmid Nedd4–2CS was created by changing cysteine to serine at position 821 in the HECT domain by QuikChange Site Directed Mutagenesis Kit. All constructs were verified by sequencing.
Transfections and coimmunoprecipitation.
Human embryonic kidney cell line, HEK293, was maintained in high-glucose DMEM containing 10% FBS and 1% penicillin streptomycin. Subconfluent HEK293 cells were transiently transfected with various plasmids using Lipofectamine 2000 (Invitrogen), the day after plating. Cycloheximide treatment, cell lysis, and Western blotting were performed as published (28). For immunoprecipitation experiments, 1 mg of total cell lysates was affinity-purified using the EZview Red ANTI-FLAG M2 Affinity Gel (Invitrogen) as recommended by the manufacturer.
For siRNA experiments, mpkCCDc14 cells were transfected by Nucleofection (Lonza, Walkersville, MD), using Cell Line Nucleofector Solution L according to the manufacturer's directions. Silencer siRNA (300 ng) or scrambled oligonucleotides purchased from Applied Biosystems (Foster City, CA) were used with 2 × 106 cells per Nucleofection. For quantitative measurements of Nedd4–2 and 18S RNA levels in mpkCCDc14 cells, we used Predeveloped TaqMan PCR assay reagents (Applied Biosystems) as previously described (28).
Transepithelial resistance and short-circuit measurement.
Mouse cortical collecting duct epithelia, mpkCCDc14 cells, were cultured as previously described (7, 29). mpkCCDc14 cells were seeded on collagen-coated transwell filters (12-mm-diameter Millicell-PCF, Millipore) and transepithelial electrical resistance (TER) and short-circuit currents (Isc) were measured in culture media at 37°C in Ussing chambers as described previously (7, 29). Short-term time course experiments were done in bicarbonate-free Ringer solution in traditional Ussing chambers to allow the rapid addition and removal of reagents. To test the effect of Nedd4–2 on paracellular conductance, 10 × 107 PFU of recombinant adenovirus expressing Nedd4–2 or empty virus in 100 μl serum-free media (SFM) was added to each transwell filter the day after being seeded. Alternatively, 60 × 107 PFU of recombinant adenovirus in SFM was added to mpkCCDc14 cells growing in 100-mm dishes and 5 h later, cells were washed, trypsinized, and seeded equally into six transwell filters. Both cells transduced with Nedd4–2 before being seeded on filters and those transduced after being seeded on filters were grown for another 24 to 48 h before measurements in Ussing chambers. Conductance was calculated as the reciprocal of the TER times 1,000 and presented as mΩ/cm2 or mS/cm2 where S is siemen.
Calcium switch assay.
Ca2+ switch assays were performed in mpkCCDc14 cells transduced with adenovirus and then seeded on filters. The day after being reseeded on filters, cell monolayers were rinsed three times with DPBS and then incubated with Ca2+- and Mg2+-free DMEM (Invitrogen) containing 2% dialyzed FBS for 4 h at 37°C. This 4-h time period was sufficient to abolish TER as measured in an Ussing chamber (Raikwar NS and Thomas CP, unpublished observation). Cells on filters were then incubated with Ca2+-sufficient SFM and then TER and Isc were measured at various time periods after.
In Nedd4–2 knockdown experiments, 2 × 106 mpkCCDc14 cells were mixed with siRNA and Nucleofector solution in a culture dish and the following day seeded onto collagen-coated transwell filters in complete media. Twenty-four hours later, cells were switched to Ca2+-free media for 4 h and then reincubated with Ca2+-sufficient SFM followed by TER measurements as described above.
Immunofluorescence microscopy.
MpkCCDc14 cells were first transduced with Nedd4–2 or empty adenovirus and then seeded on transwell filters. The following day, a Ca2+ switch was performed for 4 h, and 21 h later cells were washed three times in PBS and then fixed in 95% ethanol for 30 min. After three washes with PBS, cells on filters were blocked with 5% normal goat serum for 30 min before incubation with a 1:150 dilution of mouse anti-occludin antibody in blocking buffer for 1 h. Following multiple washes with PBS, AlexaFlour-488 goat anti-mouse IgG (Molecular Probes, Eugene, OR) was added at a dilution of 1:500 for 1 h. After several washes with PBS, filters were cut from inserts and then mounted with VectaShield (Vector Laboratories, Burlingame, CA) on slides. Fluorescent signals were captured in Bio-Rad Multi-Photon microscope (Hercules, CA).
Statistics.
Data are provided as arithmetic means ± SE. All data were tested for significance with a Student's t-test or one-way ANOVA where applicable, using SigmaStat (SPSS Chicago, IL). In all cases, P values <0.05 were considered statistically significant.
RESULTS
mpkCCDc14 cells expressing Nedd4–2 delayed the formation of TJ.
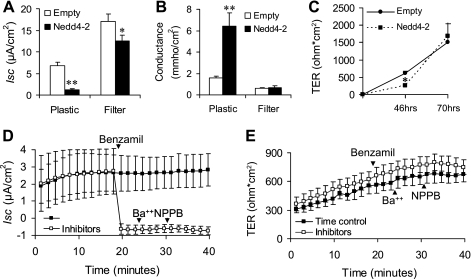
We previously reported that Nedd4–2 overexpressed via an adenoviral vector result in a ∼60% reduction in benzamil-sensitive Na+ transport in M1 cells, a mouse cortical collecting duct (CCD) cell line (29). To evaluate this further, we tested the effect of Nedd4–2 in mpkCCDc14 cells, an immortalized mouse CCD principal cell line that retained aldosterone-stimulable amiloride-sensitive Na+ transport (7). Our initial experiments performed by adenoviral overexpression of Nedd4–2 in cells seeded on transwell filters appeared to only modestly inhibit Na+ transport despite adjusting various parameters of viral transduction (data not shown). However, when cells were first transduced with Nedd4–2 before being seeded on transwell filters, we noticed an 82% inhibition of Na+ transport compared with a 27% reduction in cells transduced on filters, although both were statistically significant (Fig. 1A). The more striking observation, however, was a very dramatic fall in TER, corresponding to a large increase in conductance, in cells transduced on plastic with little to no change in cells transduced on filters (Fig. 1B). Time course experiments revealed that the decrease in TER in Nedd4–2-transduced cells was significantly different at 46 h, although the groups had equalized by 70 h (Fig. 1C). Together, these data suggested that Nedd4–2 overexpression in mpkCCDc14 cells delayed the formation of TJs. We confirmed that the short-circuit currents measured in mpkCCDc14 cells were almost entirely indicative of Na+ transport as benzamil, an ENaC-specific inhibitor, but not Ba2+, a K+ channel inhibitor or NPPB, a Cl− channel inhibitor, virtually abolished Isc (Fig. 1D). Similar results were seen even when Ba2+ and NPPB were added before benzamil (data not shown). Since substantially inhibiting Na+ transport with benzamil did not reduce TER, the effect of Nedd4–2 to inhibit TER is likely to be independent of its effect on transcellular Na+ transport and reflect changes in paracellular conductance (Fig. 1E).
Fig. 1.
Effects of Nedd4–2 overexepression on short-circuit current (Isc), transepithelial electrical resistance (TER), and conductance in mpkCCDc14 cells. A and B: mpkCCDc14 cells were transduced with Nedd4–2 or empty virus while subconfluent in culture dishes (plastic) or 24 h after being plated on transwell inserts (filters). Cells transduced on plastic were then plated into transwell filters. Forty-eight hours after being plated on filters, Isc and TER were measured in Ussing chambers at 37°C and conductance was calculated from TER. Nedd4–2 significantly reduced Isc in both groups compared with empty virus, although the effect was more robust in the first group. Nedd4–2 significantly increased conductance (∼3-fold) compared with empty virus but only in cells transduced on plastic; n = 12–14, means ± SE. C: time course of developing resistance in cells transduced on plastic and then plated on filters. Nedd4–2 significantly reduces TER at 46 h after being plated which is no longer evident at 70 h; n = 6, means ± SE. D and E: effect of benzamil, Ba2+, and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) on Isc and TER in mpkCCDc14 cells; n = 4, means ± SE. **P < 0.001; *P < 0.05 Tukey's pairwise multiple comparison.
Nedd4–2 reduces occludin protein levels.
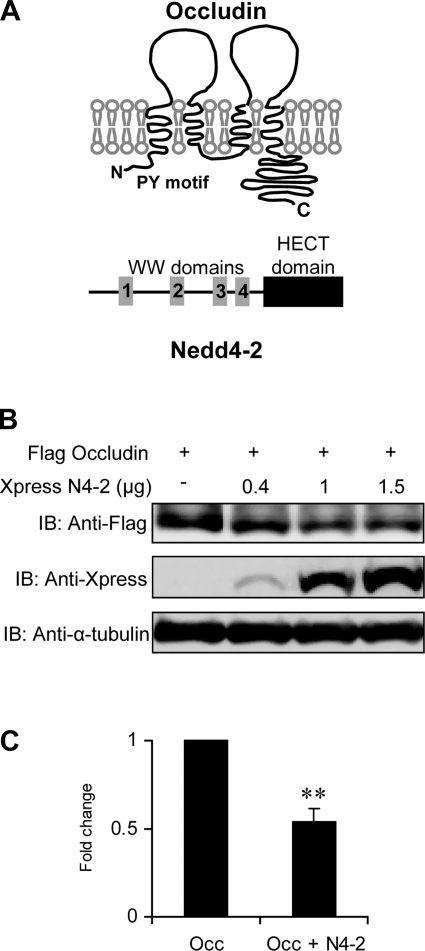
Nedd4–2 is a member of the Nedd4/Rsp5 family of E3 ubiquitin protein ligases with a COOH-terminal catalytic HECT domain, which carries the ubiquitin ligase function and multiple WW domains, which are protein-protein interaction domains (3, 31). The WW domains in Nedd4–2 and other members of this family typically bind to a PY motif on target proteins that is then subjected to polyubiquitination leading to the recycling or degradation of the target protein. Occludin is a transmembrane protein that contains a PY motif within the cytosolic NH2 terminus (Fig. 2A) (16). Given earlier reports that occludin is a functional target for another E3 ubiquitin ligase, itch (43), we postulated that the effect of Nedd4–2 on TER in the collecting duct was secondary to an interaction between Nedd4–2 and occludin. Upon cotransfection of Nedd4–2 and human occludin in HEK293 cells, we demonstrate a dose-dependent reduction in expressed occludin, consistent with a role for Nedd4–2 in enhancing its degradation (Fig. 2, B and C).
Fig. 2.
Identification of Nedd4–2 as an occludin-binding partner. A: schematic representation of occludin and Nedd4–2: occludin is a transmembrane protein with 4 transmembrane domains and cytosolic NH2- and COO-terminal domains. The location of the PY motif (PPPY9–12) is shown. Nedd4–2 isoforms are cytosolic proteins that contain 2–4 WW domains, a COO-terminal HECT domain and in some instances an NH2-terminal C2 domain. B: Nedd4–2 overexpression reduces occludin levels. HEK293 cells were transfected with Flag-tagged occludin and increasing amounts of Xpress-tagged Nedd4–2. Cell lysates were analyzed by Western blotting using anti-Xpress and anti-Flag and α-tubulin antibodies. The data show decreasing occludin protein levels that correlate with increasing Nedd4–2 expression. C: effect of 1.5 μg of Nedd4–2 (N4–2) from 3 different experiments was quantitated by densitometry and demonstrates a significant reduction in occludin (Occ) expression (n = 3; **P < 0.001).
NH2-terminal PY motif of occludin is involved in interaction with Nedd4–2.
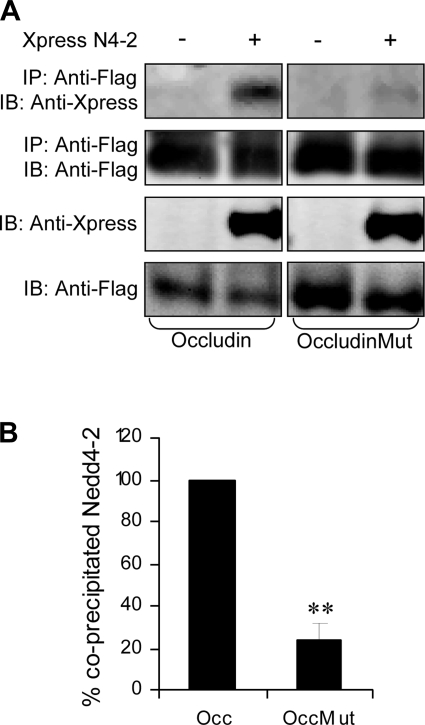
To examine the interaction more directly, we performed coimmunoprecipation experiments in HEK293 cells. When expressed together, Nedd4–2 could be pulled down with occludin using an anti-FLAG antibody. To determine whether the interaction of Nedd4–2 with occludin was mediated via its PY motif, we mutated the PY motif (PPPY9–12 to PAAY9–12). Under these conditions, the amount of immunoprecipitated Nedd4–2 was dramatically reduced (Fig. 3, A and B). We also noted that the PY mutation of occludin appeared to increase the abundance of expressed occludin, suggesting that the mutated form of occludin (occludinMut) was more stable.
Fig. 3.
Occludin interacts with Nedd4–2 via its conserved NH2-terminal PY motif. A and B: IP in HEK293 cells transfected with Flag-tagged occludin or occludin Mut with and without Xpress-tagged Nedd4–2. Protein lysates were subject to IP using an ANTI-FLAG Affinity Gel and then analyzed by immunoblotting using anti-Xpress and anti-Flag. Nedd4–2 coprecipitates with occludin and a PY mutation (PPPY9–12 to PAAY) significantly reduces coprecipitated Nedd4–2 proteins. Representative blot in A and pooled densitometric data from 3 experiments in B. Immunoprecipitated Nedd4–2 proteins were first expressed as a ratio of the immunoblottable Nedd4–2 and then normalized to the amount of immunoprecipitated occludin or OccMut in lysates used for the IP reaction and then compared with wild-type occludin that was set at 100%. Means ± SE, **P < 0.001, Tukey's pairwise multiple comparison.
PY domain of occludin regulates its stability.
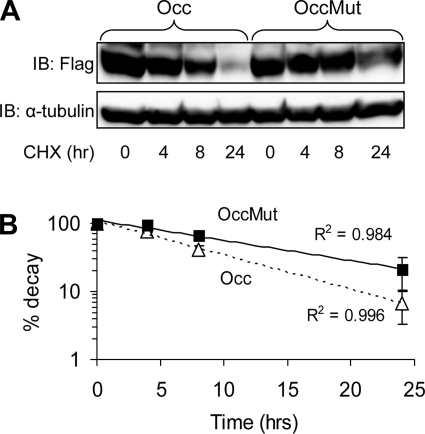
Since mutation of the PY domain of occludin appeared to increase its abundance in HEK293 cells compared with wild-type occludin, we next measured the half-life of these proteins. We adjusted the amount of transfected plasmids (3 μg occludin:1 μg occludin Mut) to keep expressed protein levels in HEK293 cells similar under basal conditions. Translation was then blocked by cycloheximide and occludin levels were determined by Western blotting in cell lysates obtained at timed intervals after cycloheximide exposure. Our data demonstrate that mutating the PY motif of occludin increases the half-life of occludin from 6.4 to 11.4 h (Fig. 4, A and B).
Fig. 4.
Mutation of PPPY9–12 enhances occludin half-life. A: HEK293 cells were transiently transfected with occludin or Occludin Mut (OccMut) and then treated with cycloheximide (CHX) in serum-free media for the indicated times. Proteins lysates were sequentially analyzed using anti-Flag (occludin) and α-tubulin antibody. Western blot is representative of 3 separate experiments. B: data from immunoblotting experiments were quantified by densitometry, normalized for α-tubulin, and used to derive exponential regression lines for both conditions. The t1/2 for OccMut is 11.4 h compared with 6.4 h for occludin. P < 0.05 by t-test, n = 3.
Nedd4–2 promotes ubiquitination of occludin that is mediated via its catalytic domain.
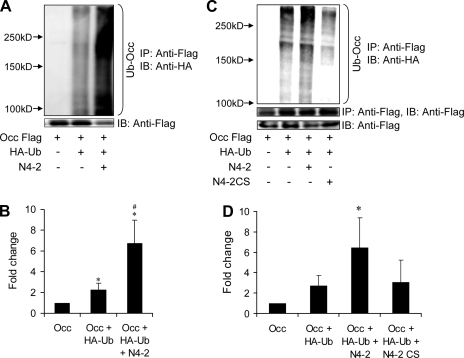
The association of occludin with Nedd4–2 together with the increased stability of mutated occludin indicated the possibility that occludin was being targeted for ubiquitination and degradation. We then studied the ubiquitination of FLAG-tagged occludin when cotransfected with HA-tagged ubiquitin in HEK293 cells. Immunoprecipitation was performed with anti-FLAG antibody and immunoprecipitated ubiquitinated occludin was detected by anti-HA antibody. Strong ubiquitination signals were detected, as evidenced by a high-molecular-weight smear (>100 kDa), increasing in intensity with increased amount of transfected occludin (data not shown). Furthermore, ubiquitination was significantly increased when Nedd4–2 was coexpressed (Fig. 5, A and B). These results are consistent with Nedd4–2-induced occludin polyubiquitination, although we cannot exclude the possibility that another ubiquitinated protein associates with occludin and is immunoprecipitated with it. We then tested a catalytically inactive form of Nedd4–2 where a cysteine residue at position 821 within the HECT domain is mutated to serine (Nedd4–2 CS). In contrast to wild-type Nedd4–2, the CS mutant had no effect on ubiquitination of occludin, nor did it reduce steady-state levels of occludin (Fig. 5, C and D).
Fig. 5.
Nedd4–2 enhances ubiquitination of occludin. A and B: HEK293 cells transfected with FLAG-occludin (Occ), HA-ubiquitin, and Nedd4–2 and subjected to sequential immunoprecipitation and immunoblotting with an anti-HA antibody. Ubiquitinated occludin is seen as a broad smear and is bracketed. Representative experiment in A and pooled data quantified by densitometry shown in B. *P < 0.05 against Occ; #P < 0.05 against Occ + HA-Ub; Tukey's pairwise multiple comparison, means ± SE, n = 4. C and D: experiments similar to A and B but examining the effect of a catalytically inactive form of Nedd4–2 (Nedd4–2CS). Representative experiment in A and pooled data quantified by densitometry shown in B. Catalytic activity of Nedd4–2 is required for ubiquitination of occludin. *P < 0.05 by ANOVA against each of the other conditions.
Nedd4–2 disrupts occludin assembly at TJs in mpkCCDc14 cells.
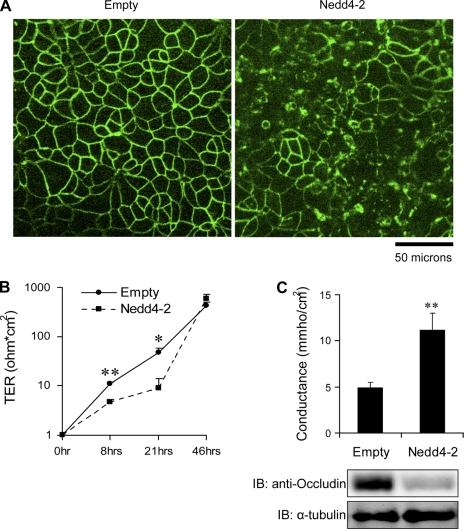
We then examined the effect of Nedd4–2 on cellular localization of endogenous occludin in mpkCCDc14 cells by indirect immunofluorescence microscopy. Cells were transduced with adenovirus expressing Nedd4–2 or empty virus before being plated on Millicell filters. Cells were then incubated in Ca2+- and Mg2+-free DMEM for 4 h, which was sufficient for complete loss of TER consistent with the disruption of TJs between cells (Raikwar NS and Thomas CP, unpublished observation). Cells were then switched back to Ca2+-containing growth media for ∼21 h to permit the regeneration of TJs and were then fixed and stained for endogenous occludin. As expected, occludin was localized as a TJ protein in the classic “chicken-wire” distribution at the cell-cell contact area in the control group (Fig. 6A). In Nedd4–2-transduced cells, on the other hand, most cells showed substantial disruption of the continuous occludin staining at the cell-cell border.
Fig. 6.
Nedd4–2 overexpression disrupts or delays occludin recruitment to tight junctions (TJs). A: mpkCCDc14 cells were transduced with Nedd4–2 or empty virus and then split and seeded into Millicell filters. Twenty-one hours after Ca2+ switch, cells were fixed and stained for occludin. Confocal micrographs from several random fields of each of 4 different filters in each group were analyzed and a representative figure is shown here. Linear staining of occludin at TJ observed in control group is substantially disrupted in Nedd4–2-transduced group. B: in parallel experiments, TER was measured at 8, 21, and 46 h following calcium switch in virus-transduced cells. Nedd4–2-treated cells had lower TER compared with control group at 8 and 21 h. **P < 0.001, *P < 0.05 by t-test; n = 6; means ± SE. C: mpkCCDc14 cells were transduced with Nedd4–2 or empty virus and then split and seeded into Millicell filters, subjected to Ca2+ switch, and 21–22 h later conductance was measured in Ussing chambers and occludin and α-tubulin by immunoblotting. Paracellular conductance increases with exogenous expression of Nedd4–2 which correlates with a decrease in endogenous occludin. **P < 0.001; t-test; n = 21 filters from 4 experiments; means ± SE.
Nedd4–2 reduces rate of induction of TER in mpkCCDc14 cells after Ca2+ switch.
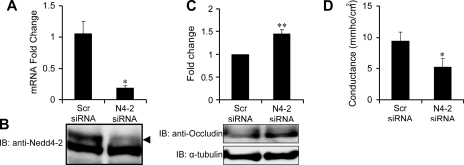
To further evaluate the role of Nedd4–2 and occludin in the induction of TER, a Ca2+ switch assay was preformed in cells plated on filters following the transduction of Nedd4–2. Cells were incubated in Ca2+-free media to abolish TER and then switched back to Ca2+-sufficient complete media and TER was measured at various time points. TER measurements of 8 and 21 h following Ca2+ switch were significantly different between the two groups but the difference was no longer evident at 46 h (Fig. 6B). These data demonstrate that the rate of recovery of TER after Ca2+ switch is slower when cells are overexpressing Nedd4–2. We correlated occludin expression with paracellular conductance after performing a Ca2+ switch in mpkCCDc14 cells. We demonstrate that the enhanced paracellular conductance seen with exogenous Nedd4–2 expression is associated with reduction in occludin levels (Fig. 6C). We then tested the role of endogenous Nedd4–2 in the development of paracellular conductance in mpkCCDc14 cells by knockdown of Nedd4–2. We confirmed knockdown of Nedd4–2 at the mRNA and protein level (Fig. 7, A and B). Nedd4–2 knockdown led to significantly enhanced occludin expression which correlated with a decline in paracellular conductance (Fig. 7, C and D). Together, these data indicate that in the mouse collecting duct, Nedd4–2 interaction with occludin occurs via its PY motif, leading to its ubiquitination and degradation. This results in diminished assembly of occludin at TJs and an increase in paracellular conductance.
Fig. 7.
Nedd4–2 overexpression results in enhanced paracellular conductance while suppression of Nedd4–2 decreases conductance after calcium switch. A: siRNA against Nedd4–2 (N4–2) reduces Nedd4–2 mRNA levels in mpkCCDc14 cells compared with scrambled RNA (Scr siRNA) sequence; n = 4; means ± SE. *P < 0.05. B: immunoblot demonstrating reduction in N4–2 (arrowhead) with N4–2 siRNA. C: knockdown of Nedd4–2 significantly increased occludin by immunoblotting. Pooled data from 3 experiments above and representatative immunoblot below (**P < 0.001). α-Tubulin was used as a loading control. D: knockdown of Nedd4–2 significantly reduces paracellular conductance compared with scrambled sequence. *P < 0.05; t-test; n = 12 filters from 3 experiments; means ± SE.
DISCUSSION
Nedd4–2 plays a central role in the regulation of aldosterone-stimulated amiloride-sensitive Na+ transport mediated in the collecting duct and connecting tubule (37, 40). In reconstituted systems, Nedd4–2 interacts with the PY domains at the COOH termini of ENaC subunits regulating endocytosis and degradation of cleaved ENaC subunits (21). Nedd4–2 is expressed in the native collecting duct and in collecting duct cell lines and, at least in mpkCCDc14 cells, its expression is reduced by aldosterone (20, 23). Aldosterone stimulation also leads to induction of the serine threonine kinase, sgk1, which phosphorylates Nedd4–2 and reduces the interaction between Nedd4–2 and ENaC (10, 39). The role of Nedd4–2 in the regulation of Na+ transport and volume homeostasis was confirmed in the Nedd4–2-null mouse, where hypertension and cardiac hypertrophy developed (35). Since its original discovery as an ENaC-interacting protein, Nedd4–2 has been shown to interact with a wide variety of ion channels and transporters including the Cl− channels, ClC-5, ClC-Ka, and Tweety, the cardiac and neuronal voltage-gated Na+ channels, Nav1.2, 1.5, 1.7, and 1.8 (37, 45).
In this manuscript, we describe a role for Nedd4–2 in regulating TJ assembly and paracellular conductance. We repeatedly observed a delay in forming TJs as evidenced by enhanced conductance in Nedd4–2-overexpressing mpkCCDc14 cells (Fig. 1). We demonstrate that Nedd4–2 coimmunoprecipitated with and reduced the expression of transfected occludin in HEK293 cells and endogenous occludin in mpkCCDc14 cells (Figs. 2B and 6C). We noted that immunoprecipitation with Nedd4–2 was severely reduced when the PY motif of occludin was mutated confirming that Nedd4–2 interacted with occludin via its PY motif. This PY motif is conserved in mammals and even in Xenopus laevis and zebrafish, although human occludin shares only ∼50% identity overall with its avian and marsupial orthologs (2). In keeping with a role for PY motif of occludin in regulating its turnover, we also noted that this mutation increased the stability of occludin (Fig. 4). The turnover rate of occludin is probably related to its interaction with ubiquitin ligases that target occludin for ubiquitination. Generally, polyubiquitinated proteins are targeted to the proteasome, while monoubiquitinated proteins may be targeted to the lysosome or be directed for recycling endosomes. We were unable to detect an increase in occludin levels with the proteasome inhibitors, MG132 or ALLN, or with a lysosome inhibitor, chloroquine (data not shown), although in other reports a significant increase in occludin-ubiquitin conjugates was noted after short-term treatment with MG132 (24, 27, 43). We confirmed that occludin is ubiquitinated by Nedd4–2 in a heterologous system, although our experiments do not differentiate mono and/or polyubiquitinated forms of occludin. In this regard, there are several conserved lysines within occludin that are possible ubiquitination sites.
We extended our studies to look at the effect of Nedd4–2 on localization of occludin in mpkCCDc14 cells during TJ formation. In low-Ca2+ media (1.6 μM Ca2+), occludin is localized in cytoplasmic granular structures and upon switching to normal Ca2+ (1.8 mM Ca2+) occludin is recruited to cell-cell contact borders as a continuous fine line consistent with its trafficking to the TJ (25, 34). Our studies in mpkCCDc14 cells demonstrate a reduction in occludin at intercellular junctions and an increase in occludin within the cytoplasm when Nedd4–2 was overexpressed, consistent with a delay in occludin trafficking from cytoplasm to plasma membrane, although our experiments do not distinguish between reduced delivery to the TJ and increased internalization from the TJ.
What is the effect of modulating occludin on TJ formation? The Ca2+ switch studies that we performed clearly demonstrate that a reduction in occludin associated with overexpression of Nedd4–2 in mpkCCDc14 cells results in a slower rate of TER development, although the final achieved TER was similar to controls. We confirmed a role for Nedd4–2 in TJ formation in mpkCCDc14 cells by demonstrating a reduction in conductance upon knockdown of endogenous Nedd4–2. In other studies, MAPK inhibition increased TER more than twofold in Ras-transformed MDCK cells by stabilizing occludin, and PKCη-induced threonine phosphorylation of occludin resulted in increased TER in Caco-2 cells (9, 41). Conversely, absence of occludin expression reduced the initial peak TER during TJ assembly but had little effect on steady-state TER in MDCK II cells (46). Yet, occludin-null mice survive beyond embryonic stage and do not appear to show abnormal structure or function of TJs (32). Polarized epithelial cells originated from occludin-deficient embryonic stem (ES) cells are capable of developing TJs that are indistinguishable from normal ES cells (33). These findings suggest that occludin is not the primary molecule responsible for determination of the paracellular barrier but rather that in certain cells and tissues, it functions as a regulator of paracellular permeability.
The role and function of TJ proteins in the distal nephron are just beginning to be understood. Compared with the proximal segments of the nephron, there is more occludin and ZO proteins in the distal nephron that correlates with its higher resistance (17). At least two genetic disorders of distal nephron ion transport arise from mutations in TJ proteins: loss-of-function mutations in claudin-16 or claudin-19 cause familial hypomagnesemia, hypercalciuria, and nephrocalcinosis secondary to reduced paracellular absorption of Mg2+ and Ca2+ in the thick ascending limb of Henle (19, 36). Gain-of-function mutations in WNK4 cause pseudohypoaldosteronism type II, an effect that may be mediated, in part, by an increase in paracellular Cl− permeability in the collecting duct (22). At this time, we can only speculate on the physiological relevance of the Nedd4–2-occludin interaction in the collecting duct. Nedd4–2 may specifically enhance paracellular chloride permeability increasing its backleak and diminishing NaCl absorption. The disruption of TJs may allow the diffusion of apical membrane proteins like ENaC to the basolateral domains, also contributing to diminishing Na+ transport. If itch is expressed in the collecting duct, then the relative role of itch vs. Nedd4–2 in occludin regulation also needs to be clarified in future studies. Perhaps Nedd4–2 plays an important role in the recovery of TJ integrity after ischemic or inflammatory injury to tubular epithelium. Corticosteroids are effective in a variety of inflammatory states and both glucocorticoids and aldosterone increase occludin expression in brain microvascular endothelial cells and in human colon, respectively (1, 14). Aldosterone is known to inhibit Nedd4–2 function by sgk1 activation in collecting duct epithelia and could secondarily increase occludin expression and enhance TER.
In summary, we demonstrate that Nedd4–2 plays a role in TJ assembly and the regulation of paracellular conductance, at least in collecting duct epithelial cells. This effect is mediated via its interaction with occludin, leading to its increased ubiquitination and a reduction in total occludin levels as well as a reduction in occludin at intercellular junctions during TJ assembly. The accelerated degradation of occludin could account for depletion of occludin from TJs. It is likely that insufficient occludin, which has been shown to interact with other TJ proteins such as ZO-1, ZO-3, or CLMP, perturbs TJ formation (11, 18, 30). Whether this effect of Nedd4–2 on occludin affects specific ion transport pathways in the collecting duct has not been determined.
GRANTS
This work was supported in part by a Veterans Affairs Merit Review Award and by the United States Public Health Service Grant HL-71664.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank D. Bohmann for the HA-tagged ubiquitin vector, H. Pratt for the anti-Nedd4–2 antibody, and E. A. Kelley for excellent support. The authors also acknowledge the University of Iowa DNA Core facility and Vector Core facility for services provided.
REFERENCES
- 1.Amasheh S, Milatz S, Krug SM, Bergs M, Amasheh M, Schulzke JD, Fromm M. Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem Biophys Res Commun 378: 45–50, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, Yonemura S, Furuse M, Tsukita S. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol 133: 43–47, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardley HC, Robinson PA. E3 ubiquitin ligases. Essays Biochem 41: 15–30, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Balda MS, Matter K. Tight junctions at a glance. J Cell Sci 121: 3677–3682, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci 112: 1879–1888, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Bens M, Chassin C, Vandewalle A. Regulation of NaCl transport in the renal collecting duct: lessons from cultured cells. Pflügers Arch 453: 133–146, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim Biophys Acta 1778: 770–793, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Lu Q, Schneeberger EE, Goodenough DA. Restoration of tight junction structure and barrier function by downregulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell 11: 849–862, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4–2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273: 29745–29753, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev 57: 883–917, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Flores SY, Loffing-Cueni D, Kamynina E, Daidie D, Gerbex C, Chabanel S, Dudler J, Loffing J, Staub O. Aldosterone-induced serum and glucocorticoid-induced kinase 1 expression is accompanied by Nedd4–2 phosphorylation and increased Na+ transport in cortical collecting duct cells. J Am Soc Nephrol 16: 2279–2287, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Förster C, Silwedel C, Golenhofen N, Burek M, Kietz S, Mankertz J, Drenckhahn D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J Physiol 565: 475–486, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 4: 222–231, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123: 1777–1788, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, Valencia L, Muriel P, Ponce L, Reyes JL. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int 57: 2386–2402, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol 141: 199–208, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci USA 106: 15350–15355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itani OA, Stokes JB, Thomas CP. Nedd4–2 isoforms differentially associate with ENaC and regulate its activity. Am J Physiol Renal Physiol 289: F334–F346, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Kabra R, Knight KK, Zhou R, Snyder PM. Nedd4–2 induces endocytosis and degradation of proteolytically cleaved epithelial Na+ channels. J Biol Chem 283: 6033–6039, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Kahle KT, Wilson FH, Lalioti M, Toka H, Qin H, Lifton RP. WNK kinases: molecular regulators of integrated epithelial ion transport. Curr Opin Nephrol Hypertens 13: 557–562, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Loffing-Cueni D, Flores SY, Sauter D, Daidie D, Siegrist N, Meneton P, Staub O, Loffing J. Dietary sodium intake regulates the ubiquitin-protein ligase Nedd4–2 in the renal collecting system. J Am Soc Nephrol 17: 1264–1274, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lui WY, Lee WM. cAMP perturbs inter-Sertoli tight junction permeability barrier in vitro via its effect on proteasome-sensitive ubiquitination of occludin. J Cell Physiol 203: 564–572, 2005 [DOI] [PubMed] [Google Scholar]
- 25.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci 109: 2287–2298, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Medina R, Rahner C, Mitic LL, Anderson JM, Van Itallie CM. Occludin localization at the tight junction requires the second extracellular loop. J Membr Biol 178: 235–247, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem 284: 21036–21046, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raikwar NS, Snyder PM, Thomas CP. An evolutionarily conserved N-terminal Sgk1 variant with enhanced stability and improved function. Am J Physiol Renal Physiol 295: F1440–F1448, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raikwar NS, Thomas CP. Nedd4–2 isoforms ubiquitinate individual epithelial sodium channel subunits and reduce surface expression and function of the epithelial sodium channel. Am J Physiol Renal Physiol 294: F1157–F1165, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raschperger E, Engstrom U, Pettersson RF, Fuxe J. CLMP, a novel member of the CTX family and a new component of epithelial tight junctions. J Biol Chem 279: 796–804, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol 10: 398–409, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Sachs LM, Shi YB. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc Natl Acad Sci USA 97: 13138–13143, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol 141: 397–408, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol 137: 1393–1401, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, Nair R, Weiss RM, Williamson RA, Sigmund CD, Snyder PM, Staub O, Stokes JB, Yang B. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4–2. Am J Physiol Renal Physiol 295: F462–F470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Snyder PM. Downregulating destruction: phosphorylation regulates the E3 ubiquitin ligase Nedd4–2. Sci Signal 2: pe41, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Snyder PM. Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology 146: 5079–5085, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Snyder PM, Olson DR, Thomas BC. SGK modulates Nedd4–2-mediated inhibition of ENaC. J Biol Chem 277: 5–8, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Staub O, Verrey F. Impact of Nedd4 proteins and serum and glucocorticoid-induced kinases on epithelial Na+ transport in the distal nephron. J Am Soc Nephrol 16: 3167–3174, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci USA 106: 61–66, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi S, Iwamoto N, Sasaki H, Ohashi M, Oda Y, Tsukita S, Furuse M. The E3 ubiquitin ligase LNX1p80 promotes the removal of claudins from tight junctions in MDCK cells. J Cell Sci 122: 985–994, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Traweger A, Fang D, Liu YC, Stelzhammer W, Krizbai IA, Fresser F, Bauer HC, Bauer H. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase itch. J Biol Chem 277: 10201–10208, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci 110: 1113–1121, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Yang B, Kumar S. Nedd4 and Nedd4–2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ 17: 68–77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol 288: C1231–C1241, 2005 [DOI] [PubMed] [Google Scholar]