Abstract
Urothelium that lines almost the entire urinary tract performs important functions and is prone to assaults by urinary microbials, metabolites, and carcinogens. To improve our understanding of urothelial physiology and disease pathogenesis, we sought to develop two novel transgenic systems, one that would allow inducible and urothelium-specific gene expression, and another that would allow inducible and urothelium-specific knockout. Toward this end, we combined the ability of the mouse uroplakin II promoter (mUPII) to drive urothelium-specific gene expression with a versatile tetracycline-mediated inducible system. We found that, when constructed under the control of mUPII, only a modified, reverse tetracycline trans-activator (rtTA-M2), but not its original version (rtTA), could efficiently trans-activate reporter gene expression in mouse urothelium on doxycycline (Dox) induction. The mUPII/rtTA-M2-inducible system retained its strict urothelial specificity, had no background activity in the absence of Dox, and responded rapidly to Dox administration. Using a reporter gene whose expression was secondarily controlled by histone remodeling, we were able to identify, colocalize with 5-bromo-2-deoxyuridine incorporation, and semiquantify newly divided urothelial cells. Finally, we established that, when combined with a Cre recombinase under the control of the tetracycline operon, the mUPII-driven rtTA-M2 could inducibly inactivate any gene of interest in mouse urothelium. The establishment of these two new transgenic mouse systems enables the manipulation of gene expression and/or inactivation in adult mouse urothelium at any given time, thus minimizing potential compensatory effects due to gene overexpression or loss and allowing more accurate modeling of urothelial diseases than previously reported constitutive systems.
Keywords: uroplakin, transgenic, conditional, tetracycline
mammalian urothelium is a stratified, terminally differentiated epithelium that covers the mucosal surfaces of the bulk of the urinary tract including renal pelvis, ureters, bladder, and prostatic urethra. Previously portrayed as an inert, dispensable tissue, urothelium actually carries out crucially important functions under physiological conditions and is intimately involved in the pathogenesis of major urinary diseases (21, 29, 43, 49). First and foremost, the epithelium forms a formidable barrier that insulates the blood and tissues underlying the epithelium from exposure to urinary metabolites (27). Normally there is little, if any, water or electrolyte exchange across the urothelium, and in fact, few epithelial tissues including the epidermis can rival the urothelium in its effectiveness as a permeability barrier (36). Maintenance of this barrier function depends on the integrity of tight junction complexes, specialized membrane lipids, and a group of urothelium-specific proteins called uroplakins (UPs) that constitute the apical surface membrane (1, 27, 49). Deficiency in some of these components, particularly in the UPs, leads to an ineffective barrier and increased permeability to urea and water (12, 13, 25). Indeed, a compromised urothelial barrier has been associated with inflammatory diseases of the bladder such as interstitial cystitis (10, 14, 24, 26, 29, 37), although whether it is a cause or a consequence of this disease remains unclear. A reduced urothelial barrier may also allow urinary growth factors and carcinogens to gain access to the proliferative (basal) compartment of the urothelium, setting the stage for cellular transformation and tumorigenesis (5, 31). Second, urothelium possesses the remarkable capacity to vastly expand its surface area during bladder filling and completely return to its preexpansion state after bladder emptying during each micturition cycle (8, 27, 33). Mounting evidence suggests that such a reversible surface area adjustment is made possible by highly specialized membrane trafficking machineries uniquely designed for the urothelial superficial umbrella cells (7, 11, 22, 44, 49, 51). Interestingly, uropathogenic Escherichia coli seem to hijack some of these vesicular movement processes as a way to enter and/or exit the urothelium during urinary tract infections (4). Last but not the least is the fact that urothelium expresses a range of receptors and ion channels that are usually found in the neurons; these allow the urothelial cells to not only sense mechanical and chemical signals, but also emit specific transmitters to communicate with the neighboring tissues (2, 3). It has been suggested that alterations in these signaling steps might contribute to and be involved in the pathogenesis of several lower urinary tract syndromes that affect bladder contractibility and/or compliance such as overactive bladder and incontinence (17).
Despite its critical physiological functions and involvement in important diseases, urothelium somewhat lags behind other epithelia in the arsenal of investigative tools. New opportunities have been presented, however, due to the discovery of UPs whose promoters have allowed urothelium-specific targeting in transgenic and knockout mice (30, 34, 35, 53). Notwithstanding the progress, the existing targeting systems are constitutive in nature, i.e., gene expression or inactivation begins in embryogenesis (around day 15 gestation when UPs are first expressed) (9, 48). It is well-known that embryonic cells are more plastic and consequently more capable than adult cells to respond to genetic alterations by switching on compensatory mechanisms (15, 47). For instance, the loss of a molecule during embryogenesis can be functionally compensated for by a similar molecule in the same gene family or in the same signaling pathway or by another molecule with a similar function in a different gene family or pathway. Conversely, the overexpression of a molecule can be functionally inhibited by other molecule(s) with opposite functions. In both instances, the phenotypes that would have occurred, had gene expression or inactivation taken place in adult cells, are often masked. In this regard, gene expression and/or knockout acutely in adult cells would more faithfully model diseases that have an adulthood onset than the constitutive systems, although these inducible systems have not been reported for urothelium.
In this report, we address the pressing need for both temporally and spatially controlled gene expression and knockout in urothelium, by integrating the urothelium specificity of the mouse UPII promoter (mUPII) with the versatility of a tetracycline-induced gene expression system (40, 41, 49). We found that, when placed under the control of mUPII, a modified, reverse tetracycline trans-activator (rtTA-M2) (46) was dramatically more sensitive to doxycycline (Dox) induction in transcribing urothelial expression of a reporter gene than the conventional rtTA. With this system, rtTA-M2-induced gene expression was restricted to urothelium that lines the bladder as well as other parts of the urinary tract (28). Therefore, this urothelium-specific and inducible gene expression system will be broadly applicable for studying the role of activation of any gene of interest in urothelium. Additionally, we found that the reporter gene we used, whose expression relies on DNA synthesis and histone remodeling, can be used to measure and track the rate, location, and fate of urothelial proliferation and renewal, and to allow for the isolation of urothelial cells in different differentiation stages. Finally, by combining the UPII-driven rtTA-M2 with a tetracycline-operon-driven Cre transgenic line and a floxP-controlled reporter line, we established the feasibility of inactivating any gene of interest in urothelium in a tissue-specific and -inducible manner. The two novel transgenic systems that we generated and characterized should help accelerate the investigation on gene functions and urothelial physiology and improve our understanding of the pathogenesis of urothelial diseases.
MATERIALS AND METHODS
Generation of transgenic mice expressing Dox-controlled trans-activators.
Two different chimeric genes were constructed. The first consisted of from 5′- to 3′-end, a 3.6-kb mUPII, a 0.9-kb conventional rtTA, and a 0.6-kb mouse protamine 1 (MP1) gene fragment, the latter of which provided a poly A signal (Fig. 1). Briefly, a BamHI/SpeI double-digested rtTA fragment [a kind gift of Dr. J. Gordon of Washington University, St. Louis (42)] was inserted into pBS-mUPII (53) between the UPII promoter and the MP1 fragment. After the correct orientation of the chimeric gene was confirmed by restriction digestion and DNA sequencing, the 5.1-kb chimeric gene fragment was excised en bloc by KpnI/SpeI double digestion, purified by agarose gel and column chromatography. The second chimeric gene was constructed using a modified rtTA-M2 [a kind gift from Dr. W. Hillen of Friedrich-Alexander-Universität, Germany (46)] to replace the rtTA in the first construct. rtTA-M2 differed from rtTA in several amino acids (Fig. 1) (46). Briefly, a 0.9-kb rtTA-M2 fragment was extracted from pUHrT62–1 (46) using SacII and HindIII and inserted into pBS-mUPII between the mUPII promoter and the MP1 fragment to yield a pBS-mUPII-rtTA-M2/MP1 (Fig. 1). After verification with restriction digestion and sequencing, the 5.1-kb chimeric gene fragment was excised en bloc by KpnI/SpeI double digestion, purified by agarose gel and column chromatography.
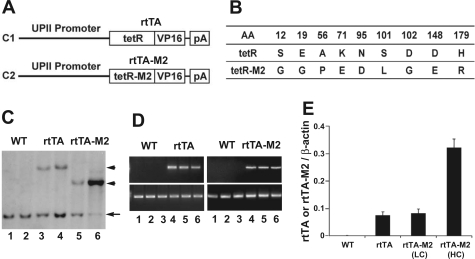
Fig. 1.
Construction of chimeric genes and generation and initial characterization of transgenic lines. A: schematic drawing of the chimeric genes. C1: construct 1 that consisted of an upstream mouse uroplakin II (UPII) promoter (3.6 kb) that controls the urothelial expression of a 0.9-kb conventional, reverse tetracycline trans-activator (rtTA). rtTA further consisted of a tetracycline-operon-binding domain (tetR) and a VP16 trans-activating domain. C2: construct 2 that consisted of the same UPII promoter driving a modified rtTA (rtTA-M2). pA stands for poly A signal provided by a 0.6-kb mouse protamine 1 intron fragment. B: comparison of amino acid sequences between rtTA and rtTA-M2. Numbers mark the amino acid (AA) positions where sequences differ, whereas single letters denote the residues. C: identification of transgenic founder mice by Southern blotting. Restriction-digested mouse tail genomic DNAs were reacted with a DNA probe located at the 3′-end of the UPII promoter. While wild-type (WT) mice (lanes 1 and 2) only had a 1.4-kb endogenous UPII fragment (arrow), the UPII-rtTA (rtTA) mice (lanes 3 and 4) harbored an additional 4.5-kb band (top arrowhead) and the UPII-rtTA-M2 (rtTA-M2) mice (lanes 5 and 6; bottom arrowhead) harbored an additional 3.0-kb band (size difference was due to different restriction sites in rtTA vs. rtTA-M2). Note the generation of 2 similar copy-numbered UPII-rtTA lines (one of which was chosen for further analysis), as well as a low- and a high-transgene copy line for the UPII-rtTA-M2. D: RT-PCR analysis of the transgene expression in mouse urothelia. Left, top: primers specific for rtTA detected a 500-bp band in 3 UPII-rtTA transgenic mice (lanes 4–6), but not in 3 WT littermates (lanes 1–3). Right, top: primers specific for rtTA-M2 detected a 500-bp band in 3 UPII-rtTA-M2 transgenic mice (lanes 4–6), but not in 3 WT littermates (lanes 1–3). RT-PCR using primers specific for β-actin was performed in parallel as internal controls. E: real-time PCR quantification of transgene expression in mouse urothelia. The relative abundance of rtTA or rtTA-M2 was expressed as a ratio to simultaneously amplified β-actin. Note that not only the transgene copy number (C) but also the expression level was similar between the rtTA line and the low copy (LC) line of rtTA-M2. N = 6 per group.
The two different chimeric genes (UPII-rtTA and UPII-rtTA-M2) were microinjected in separate experiments into the pronuclei of fertilized eggs derived from the FVB/N inbred strain. Identification of positive founders was carried out using genomic Southern blotting of tail DNA biopsies. Briefly, genomic DNA was digested with NcoI, resolved by agarose gel electrophoresis, transferred to a nylon membrane, and hybridized with a 550-bp DNA probe located at the 3′-end of the 3.6-kb mouse UPII promoter (Fig. 1). The probe allowed the simultaneous detection of a 1.4-kb endogenous mouse UPII fragment and different sized transgene fragments (Fig. 1). All animal experiments were carried out in accordance with the regulations and policies set forth by federal and local authorities and under an active protocol approved by the Institutional Animal Care and Use Committee (IACUC).
Reporter mice and other mouse strains.
The reporter mouse strain for urothelium-specific and inducible gene expression system harbored a transgene consisting of tetracycline-responsive elements (TRE) driving the expression of a downstream histone 2B gene further fused at 3′-end to an enhanced green fluorescent protein gene [kindly provided by Dr. E. Fuchs of the Rockefeller University (45)]. Thus, the reporter enhanced green fluorescent protein (EGFP) expression would rely not only on the binding of Dox-activated rtTA or rtTA-M2 to TRE, but also on histone remodeling during which cyclin D1-dependent factors bind to the H2B DNA sequence, thereby altering the conformational structure of the H2B sequence and allowing for the transcription of EGFP to proceed (18, 45). H2B also supplied a nuclear localization signal that allowed EGFP to be translocated into the nucleus for improved histological detection.
The reporter system for the urothelium-specific and inducible knockout consisted of two mouse lines. The TRE-Cre line in which the TRE drive the expression of Cre recombinase was obtained from the Jackson Laboratories. The ROSA26-LacZ reporter line, also obtained from the Jackson Laboratories, harbored a bacterial β-galactosidase reporter gene under the control of ROSA26 promoter and the expression of the reporter relies on Cre-mediated deletion of loxP-flanked “STOP” sequence between the promoter and the reporter gene.
Cross-breeding and genotyping.
For the evaluation of the inducible gene expression system, UPII-rtTA and UPII-rtTA-M2 mice were crossed, in separate experiments, with TRE-EGFP mice to generate double transgenic mice UPII-rtTA/TRE-EGFP or UPII-rtTA-M2/TRE-EGFP. For the evaluation of the inducible knockout system, the UPII-rtTA-M2 mice were chosen to cross with TRE-Cre mice and then with ROSA26-LacZ reporter mice to generate UPII-rtTA-M2/TRE-Cre/ROSA-LacZ triple transgenic mice.
Transgene alleles (UPII-rtTA, UPII-rtTA-M2, TRE-Cre, and ROSA26-LacZ) were identified by genomic PCR of tail DNAs. The primers were rtTA (forward: 5′-TGCAGAGCCAGCCTTCTTAT-3′; reverse: 5′-AGGGCATCGGTAACATCG-3′), rtTA-M2 (forward: 5′-AAGTCATTCCGCTGTGCTCT-3′; reverse: 5′-CAAAATCGTCAAGAGCGTCA-3′), EGFP (forward: 5′-CAAGACACGTGCTGAAGTCAA-3′; reverse: 5′-AAAGGGCAGATTGTGTGGAC-3′), Cre (forward: 5′-CGAACGCACTGATTTCGAC-3′; reverse: 5′-GGCAACACCATTTTTTCTGAC-3′), and LacZ (forward: 5′-CTGGCGTAATAGCGAAGAGG-3′; reverse: 5′-AAATTCAGACGGCAAACGAC-3′). The PCR conditions were 94°C for 4 min for the first cycle; 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s for 35 cycles.
Real-time quantitative PCR.
The expression level of rtTA and rtTA-M2 in urothelia was compared by real-time PCR using a QuantiTect SYBR Green PCR Kit (Qiagen). Double-strand urothelial cDNA was used for PCR using primers described above and at 95°C for 10 min for the first cycle, 95°C for 20 s, 58°C for 30 s, and 72°C for 30 s for 50 cycles. The PCR products were quantified by direct incorporation of SYBR Green I into newly synthesized DNA. The abundance of PCR products was expressed as a ratio to simultaneously amplified β-actin using specific primers (forward: 5′-TGTTACCAACTGGGACGACA-3′; reverse: 5′-TCTCAGCTGTGGTGGTGAAG-3′).
Induction of gene expression and knockout with Dox and treatment of mice with 5-bromo-2-deoxyuridine.
For the induction of urothelial gene expression or knockout, mice were fed ad libitum with a grain-based regular diet supplemented with Dox (20 mg/kg diet; Bio-Serv). This diet was well-tolerated by mice, and the amount of consumption was not significantly different between Dox-absent diet and Dox-containing diet.
For the identification of urothelial cells undergoing proliferation, mice were injected intraperitoneally with 50 mg/kg body wt of 5-bromo-2-deoxyuridine (BrdU), twice a day for 10 consecutive days until they were killed.
In situ hybridization, fluorescent microscopy, and immunohistochemical staining.
For in situ hybridization, sense and anti-sense cRNA probes for mouse UPII, rtTA-M2, or EGFP were prepared by in vitro transcription of linearized plasmids containing the respective cDNAs in the presence of digoxigenin-UTP (Roche). The quality of the probes was verified by overlaying agarose gel-resolved and nitrocellulose-adherent probes with anti-digoxigenin antibody followed by a Western blotting-like procedure. Mouse bladders were fixed in PBS-buffered 4% paraformaldehyde and paraffin-embedded. Sections (4-μm-thick) were digested with proteinase K and then incubated with digoxigenin-tagged cRNA probes and the hybridization was visualized by anti-digoxigenin immunohistochemical staining (Biochain).
The expression of EGFP was assessed by directly examining the frozen sections of 4% paraformaldehyde-fixed mouse bladder and other tissues with a Zeiss fluorescent microscope. Alternatively, paraffin-embedded bladder sections were used for double immunofluorescent staining and DAPI counterstaining. For paraffin sections, antigen unmasking was employed by microwaving deparaffinized sections in a citrate buffer (pH 6.0) for 15 min at the maximal microwave power output. The primary antibodies used in this study and their dilutions were rabbit anti-pan-uroplakin antibody (1:5,000) (50), goat anti-EFGP (1:1,000; Abcam), rat anti-BrdU (1:200; Abcam), rabbit anti-type IV collagen (1:200; Abcam), and rabbit anti-keratin 5 (1:400; Covance). Fluorescence-labeled secondary antibodies were purchased from Invitrogen.
Histochemical detection of bacterial β-galactosidase reporter activity.
Freshly dissected mouse tissues were prefixed in 4% paraformaldehyde (pH 7.4) and embedded in OCT medium in a liquid nitrogen bath. Frozen sections (10-μm-thick) were incubated at 37°C for 2 h with a solution containing 0.1% X-gal (5-bromo-4-chloro-3-indolyl-d-galactopyranoside), 0.2 mM potassium ferricyanide, 0.2 mM potassium ferrocyanide, 1.3 mM MgCl2, 15 mM NaCl, and 44 mM HEPES (pH 7.4). After a brief wash in the HEPES buffer without the substrata, the sections were counterstained with 1% neutral red.
RESULTS AND DISCUSSION
Development of transgenic lines expressing a conventional rtTA or a rtTA-M2 in urothelium.
As the first step to achieve inducible gene expression in urothelium, we constructed a chimeric gene so that a 3.6-kb mUPII gene promoter could drive the urothelium-specific expression of a conventional rtTA (Fig. 1A, C1). When combined with another transgenic line that harbored a gene of interest under the control of TRE, alternatively named tetracycline “operon,” expression of that gene would take place on tetracycline treatment, which would change the structure of rtTA from a non-TRE-binding conformation to a TRE-binding one (40, 41, 49). As a safeguard, we constructed a second chimeric gene by similarly fusing the 3.6-kb mUPII promoter with a modified rtTA (rtTA-M2; Fig. 1A, C2), the latter of which has been shown in cell culture and a handful of in vivo tissues to bind to TRE more stringently in the absence of tetracycline (less background/leakage, hence higher specificity), and to bind to TRE more avidly in the presence of tetracycline (hence, higher sensitivity) (19, 20, 32, 39). rtTA-M2 differs from rtTA in nine amino acid residues located in the TRE-binding domain (Fig. 1B). Pronuclear microinjection of the two chimeric genes (UPII-rtTA and UPII-rtTA-M2) in separate experiments into fertilized eggs from inbred FVB/N mice resulted in two transgenic lines for each chimeric gene (Fig. 1C). Because the two UPII-rtTA transgenic lines harbored a similar number of transgene copies, as evidenced by their relative ratio to the endogenous UPII gene (Fig. 1C, lanes 3 and 4; compare top and bottom band intensity), and expressed almost the same amounts of rtTA mRNA in the urothelium (data not shown), we chose one line for subsequent cross-breeding experiments. The UPII-rtTA-M2 construct yielded a low-copy and a high-copy line (Fig. 1C, lanes 5 and 6), both of which were retained for further analyses. Semiquantitative PCR (Fig. 1D) and real-time quantitative PCR (Fig. 1E) using urothelial total RNAs showed that the low-copied UPII-rtTA-M2 line expressed very similar amounts of rtTA-M2 mRNA in urothelium to those of the rtTA mRNA of the UPII-rtTA line (Fig. 1E), indicating that the level of mRNA expression of the two versions of tetracycline trans-activators in urothelium in these two transgenic lines was not significantly different. In comparison, the high-copied UPII-rtTA-M2 line expressed a significantly greater amount of mRNA than the low-copy UPII-rtTA-M2 line, consistent with our previous observation that the UPII promoter-driven transgene expression is copy number dependent (35, 52, 53).
Ability of rtTA-M2, but not rtTA, to trans-activate urothelial expression of a reporter gene on Dox administration.
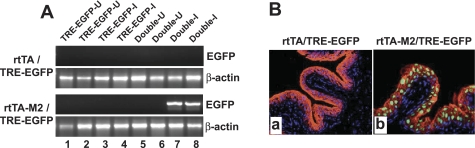
To assess the functionality of the urothelially expressed tetracycline trans-activators, we crossed one of the UPII-rtTA lines and the low-copied UPII-rtTA-M2 line with a reporter line in which the TRE drives the expression of an EGFP gene fused in frame, at the 5′-end, to the histone 2B (H2B) coding sequence. H2B serves two purposes: 1) the protein supplies a nuclear localization signal that confined EGFP to the nuclei for easier histological detection and 2) the DNA sequence forms a transcription-incompatible loop that is released by cyclin D1-dependent factors when host cells undergo proliferation (6, 16). Single and double transgenic mice [UPII-rtTA/TRE-EGFP or UPII-rtTA/TRE-EGFP-M2 (low-copy line)] were fed with either a regular diet or the same diet supplemented with Dox (20 mg/kg diet) for 1 mo. Upon RT-PCR analysis of the urothelial total RNAs, single transgenic reporter mice (TRE-EGFP) did not express EGFP, in the absence or presence of Dox (Fig. 2A, lanes 1–4). Double transgenic mice (UPII-rtTA/TRE-EGFP or UPII-rtTA-M2/TRE-EGFP) also did not express EGFP in the absence of Dox (Fig. 2A, lanes 5 and 6), indicating an extremely low background expression in the absence of the Dox inducer. Surprisingly, however, even after 1 mo of Dox treatment, the UPII-driven rtTA failed to trans-activate the EGFP reporter gene (Fig. 2A, top, lanes 7 and 8). In striking contrast, the UPII-driven rtTA-M2 efficiently activated the EGFP reporter (Fig. 2A, 3rd panel, lanes 7 and 8), despite the fact that the trans-activators (rtTA and rtTA-M2) of the two selected lines were expressed at an almost identical level (Fig. 1, D and E). Fluorescent microscopy confirmed the RT-PCR results, as the urothelial cells of the Dox-treated UPII-rtTA/TRE-EGFP double transgenic mice had no green fluorescence (Fig. 2Ba), whereas those of the Dox-treated UPII-rtTA-M2/TRE-EGFP double transgenic mice exhibited strong green fluorescence in the nuclei (Fig. 2Bb). The inability of the UPII-driven rtTA to trans-activate the EGFP reporter gene persisted even after a 7-mo treatment with Dox (data not shown). These results strongly suggest that the modified rtTA-M2 is considerably superior to the conventional rtTA in trans-activating Dox-mediated gene expression in the urothelial context and that rtTA-M2 is a preferred tetracycline trans-activator for inducible gene expression in urothelium.
Fig. 2.
Inducibility of reporter expression by doxycycline (Dox) in 2 different types of double transgenic mice. The UPII-rtTA line and the low-copied UPII-rtTA-M2 line were intercrossed separately with a transgenic reporter line in which tetracycline-responsive elements (TRE) drive the expression of an enhanced green fluorescent protein (EGFP; see materials and methods for details). A: RT-PCR analysis of EGFP expression. Single (TRE-EGFP) and double transgenic (rtTA/TRE-EGFP vs. rtTA-M2/TRE-EGFP) mice either received regular diet (uninduced or U) or the same diet supplemented with Dox (induced or I). Note the total absence of EGFP induction by Dox in the rtTA/TRE-EGFP double transgenic mice (top; lanes 7 and 8), and in stark contrast, the strong induction of EGFP (400-bp band) in the rtTA-M2/TRE-EGFP double transgenic mice (3rd panel; lanes 7 and 8). B: fluorescent microscopy of frozen bladder section from a Dox-treated (for 1 mo) rtTA/TRE-EGFP double transgenic mouse showing the lack of green fluorescence in urothelium (a), whereas bladder section from a rtTA-M2/TRE-EGFP mouse showed strong nuclear green fluorescence (b). The sections were counterstained with a pan-uroplakin antibody (red) and DAPI (blue).
Retention of urothelial specificity in the inducible gene expression system.
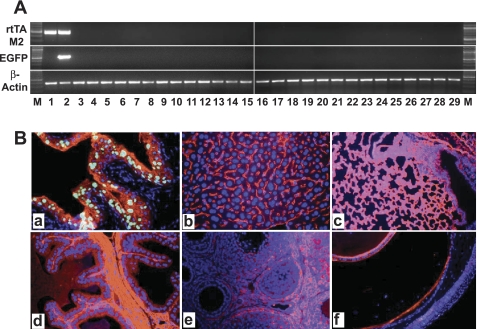
We next examined whether the introduction of the inducible mechanism in UPII-controlled urothelial gene expression compromised the urothelial specificity. By extracting total RNAs from a range of epithelial and nonepithelial tissues of Dox-treated UPII-rtTA-M2/TRE-EGFP double transgenic mice and then by RT-PCR analyses, we found the EGFP reporter expression to be restricted to the urothelium (Fig. 3A, middle, lane 2). Fluorescent microcopy (Fig. 3B) provided additional proof on a protein level that the urothelium specificity of the inducible system was retained.
Fig. 3.
Assessment of urothelial specificity of the inducible gene expression system. A: RT-PCR analyses of the expression of rtTA-M2 and EGFP. M, DNA ladder. Lane 1, pooled urothelia from rtTA-M2/TRE-EGFP double transgenic mice that did not receive Dox treatment. Lanes 2–29, different tissues from rtTA-M2/TRE-EGFP double transgenic mice that had been treated with Dox for 1 mo (from 2–29: urothelium, tail, renal cortex, prostate, testis, penile urethra, seminal vesicle, liver, spleen, forestomach, stomach, esophagus, small intestine, large intestine, trachea, pancreas, lung, heart, thymus, thyroid gland, cornea and conjunctiva, forebrain, hindbrain, skeletal muscle, trunk skin, breast, ovary, and uterus). RT-PCR of β-actin was performed as an RNA quality control. Note the detection of the 500-bp rtTA-M2 band specifically in the urothelia of the double transgenic mice without (lane 1) or with (lane 2) Dox treatment. Also note the 400-bp EGFP band only in the urothelia, but not in any other tissues, of the double transgenic mice that had been treated with Dox. B: fluorescent microcopy of frozen sections of representative tissues from an rtTA-M2/TRE-EGFP double transgenic mouse treated with Dox for 1 mo. Urothelium was counterstained with a pan-uroplakin antibody, while all other tissues were counterstained with an antibody against type IV collagen. Note that green fluorescence was only visible in urothelial cells (a; nuclei), but not in liver (b), lung (c), testis (d), ovary (e), or eye (f). All panels are of the same magnification: ×200.
Histone H2B-controlled reporter gene expression as a tracer of urothelial proliferation.
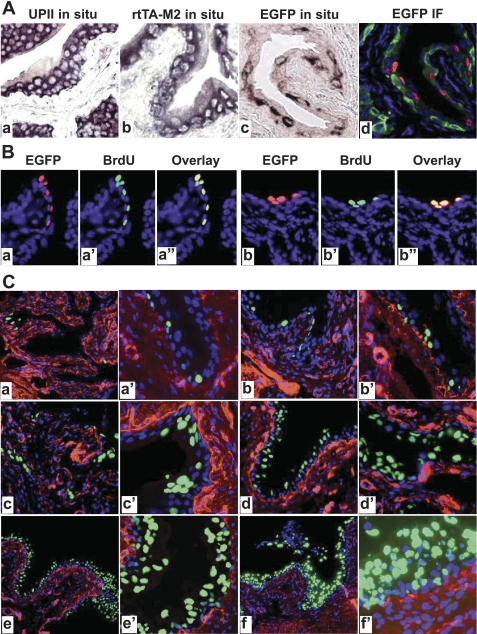
A byproduct of using the H2B as a secondary control of EGFP reporter expression in our inducible system was that it allowed us to identify and trace, in a time-dependent manner, the location and number of newly divided urothelial cells. By in situ hybridization of the Dox-treated UPII-rtTA-M2/TRE-EGFP mice, we found that the rtTA-M2 mRNA was expressed strongly and uniformly in almost all urothelial cells including the basal cells (Fig. 4Ab), much like the endogenous UPII mRNA (Fig. 4Aa). This was in agreement with our previous observations that the onset of UP expression was earlier in rodent urothelia than those of the higher mammals such as cattle and humans (34, 49). The expression of EGFP mRNA (Fig. 4Ac) and protein (Fig. 4Ad), however, was detected in a relatively smaller proportion of the urothelial cells (in this case, after a 10-day Dox treatment), and the positive cells often existed as clusters (red nuclei in Fig. 4Ad). Given the fact that only cells undergoing proliferation synthesize cyclin D1-associated factors that can bind to the H2B DNA sequence, thus unfolding it and allowing EGFP transcription (18, 45), there is reason to believe that these EGFP-expressing urothelial cells may represent the newly divided urothelial cells. This indeed turned out to be the case. When we injected the UPII-rtTA-M2/TRE-EGFP mice intraperitoneally with BrdU for 10 days while placing them on the Dox diet, we found by double immunofluorescent staining that the EGFP-expressing cells overlapped precisely with the BrdU-incorporating cells (Fig. 4, Ba″ and Bb″). Interestingly, the EGFP-positive, and for that matter, BrdU-containing cells were sometimes found in intermediate and superficial layers, suggesting that the newly divided cells had left their proliferative (basal) compartment and moved into differentiated compartments. Therefore, the present inducible system may provide a useful tool to study urothelial cell heterogeneity, growth, proliferation, renewal, and potentially the origin and properties of urothelial stem cells. By pulse-treating the double transgenic mice with the Dox and then chase the EGFP-positive cells, one might also be able to isolate urothelial cells from different compartments (basal, intermediate, and superficial) for gene and protein profiling studies.
Fig. 4.
Correlation of EGFP expression with urothelial proliferation. A: paraffin sections from WT (a) and rtTA-M2/TRE-EGFP double transgenic mice treated with Dox for 10 days (b–d) were subject to in situ hybridization (a–c) or anti-EGFP immunofluorescent staining (d). While anti-sense probes for endogenous mouse UPII (a) and for rtTA-M2 (b) labeled urothelial cells almost uniformly, anti-sense probe for EGFP labeled urothelial cells selectively (c). An adjacent section to (c) stained with anti-EGFP (red) and counterstained with anti-keratin 5 (green) and DAPI (blue) confirmed the spotty expression of EGFP in the urothelial cells. B: colocalization of EGFP-expressing and 5-bromo-2-deoxyuridine (BrdU)-incorporating cells. Double transgenic mice harboring UPII-rtTA-M2 and TRE-EGFP transgenes were treated with Dox and BrdU simultaneously for 10 days and their bladders were paraffin-sectioned and stained with anti-EGFP (a and b; red), anti-BrdU (a′ and b′; green), and counterstained with DAPI (blue). The images of anti-EGFP and anti-BrdU staining were merged (a″ and b″; overlay). Note that EGFP-expressing cells overlapped precisely with BrdU-incorporating cells (yellow), suggesting that these cells were undergoing proliferation and represented newly divided cells. C: time course of Dox-induced EGFP expression in the UPII-rtTA-M2/TRE-EGFP transgenic mice. The double transgenic mice were treated with Dox for 3 days (a and a′), 10 days (b and b′), 20 days (c and c′), 40 days (d and d′), 90 days (e and e′), and 150 days (f and f′). The bladders from the Dox-treated mice were processed for frozen sections and antibody staining (anti-type IV collagen; red) and counterstaining (DAPI; blue). Note the increased EGFP expression with increased time of Dox induction. Magnifications: all panels in A and B: ×200; and in C: a, b, c, d, e, and f, ×50; a′, b′, c′, d′, e′, and f′, ×200.
When exposed to the Dox inducer continuously for increasing periods of time, the expression of EGFP in the UPII-rtTA-M2/TRE-EGFP double transgenic mice also increased as expected (Fig. 4C). By 5 mo of Dox treatment, the majority of urothelial cells expressed EGFP.
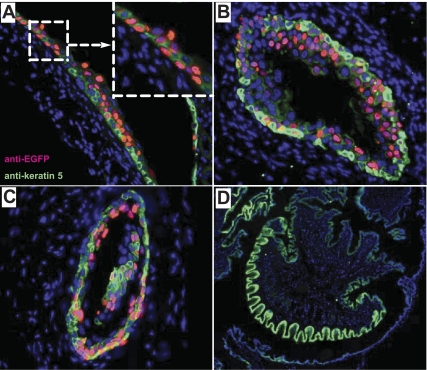
Because urothelium lines not only the bladder, but also other parts of the urinary tract, we examined whether the EGFP reporter gene could also be induced by Dox in nonbladder urothelial cells. We found that the renal pelvic (Fig. 5A), ureteral (Fig. 5B), and prostatic urethral (Fig. 5C) urothelia, but not that of the penile urethral epithelium (Fig. 5D), of the UPII-rtTA-M2/TRE-EGFP mice expressed EGFP on Dox induction. This pattern of EGFP expression corresponded well with urothelial distribution morphologically and with the uroplakin expression immunologically in the wild-type controls (28) (and data not shown).
Fig. 5.
Dox-induced EGFP expression in other parts of the urinary tract. Double transgenic UPII-rtTA-M2/TRE-EGFP mice were treated with Dox for 10 days and their renal pelvis (A), ureter (B), prostatic urethra (C), and penile urethra (D) were paraffin-sectioned and stained with anti-EGFP (red), anti-keratin 5 (green; as indicated in A), and DAPI (blue). Note that, with the exception of penile urethra which is lined by squamous epithelium, EGFP expression could be induced in other parts of the urinary tract lined by the urothelium. A, inset (top right dashed box) was an enlarged view of renal pelvic urothelium showing both basal and suprabasal EGFP expression. Magnification of all panels: ×200.
Temporally and spatially controlled gene knockout in urothelium.
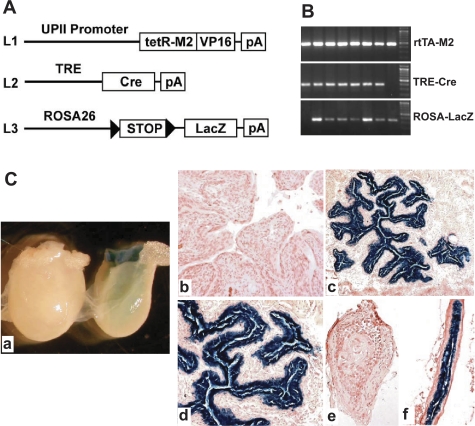
To examine whether the UPII-driven rtTA-M2 was capable of inducibly knocking out genes of interest within the urothelium, we crossed this mouse line (Fig. 6A, L1) with another transgenic line in which TRE drives the expression a Cre recombinase (Fig. 6A, L2). Double transgenic offspring from these crosses were further crossed with a reporter line in which the ROSA26 viral promoter drives the expression of a bacterial LacZ gene whose expression replies on the removal of a loxP-flanked STOP sequence (Fig. 6A, L3). Triple transgenic mice containing all three transgene alleles (L1–3) were obtained (Fig. 6B) and were fed with a regular diet or the same diet containing 20 mg/kg Dox. With the regular diet, no bacterial β-galactosidase activity was detected by histochemical staining of the whole bladder (Fig. 6Ca, left) or of the frozen section of the urothelium (Fig. 6Cb). In contrast, bladder (Fig. 6Ca, right) and frozen sections of the urothelia of Dox-treated triple transgenic mice exhibited exceedingly strong bacterial β-galactosidase activity (Fig. 6, Cc and Cd; dark blue color). In addition, while the ureter from a triple transgenic mouse on a regular diet lacked β-galactosidase activity (Fig. 6Ce), the ureter from a Dox-treated triple transgenic mouse had strong β-galactosidase activity in its urothelium (Fig. 6Cf). These results firmly established the feasibility of inducibly knocking out any gene of interest in the urothelium.
Fig. 6.
Generation and characterization of a urothelium-specific and inducible knockout system. A: schematic drawing showing the knockout strategy consisting of 3 different lines. Line 1 (L1) harboring the UPII-driven rtTA-M2; line 2 (L2) harboring a Cre recombinase gene under the control of TRE; line 3 (L3) harboring a ROSA26 promoter driving a bacterial LacZ reporter gene whose expression depends on the excision of a loxP-flanked STOP sequence on Cre expression (arrowheads denote loxP sites). B: PCR genotyping of the offspring derived from multiple intercrosses among the 3 mouse lines in A. Primers specific for rtTA-M2, TRE-Cre, and ROSA-LacZ transgene alleles amplified a 500-, a 380-, and a 350-bp PCR product, respectively, in triple transgenic mice (all lanes except the first and last lane that represented double transgenic mice). C: induction of bacterial LacZ reporter gene expression in UPII-rtTA-M2/TRE-Cre/ROSA26-LacZ triple transgenic mice. The bladders (a-d) and ureters (e and f) of the triple transgenic mice receiving no Dox treatment (a, left, and b) or treated with Dox for 10 days were histochemically stained for bacterial β-galactosidase activity (see materials and methods for details) and photographed either with a dissecting microscope (a) or a conventional transmission light microscope (b-e). Note the complete absence of urothelial staining in untreated triple transgenic mice (a, left, b, and e) and the extremely strong staining of β-galactosidase activity in Dox-treated triple transgenic mice (a, right, c, d, and f).
Key characteristics and broad applicability of the new conditional systems for studying urothelial biology and diseases.
An unexpected finding we made in this study has to do with the failure of the conventional version of the rtTA to trans-activate, in the presence of Dox, urothelial expression of the EGFP reporter (Figs. 1 and 2). This is contrary to the successful use of rtTA for gene induction in several nonurothelial tissues (40, 41, 49). We believe that the lack of rtTA activity cannot be entirely attributed to the stringent reporter system we employed which is secondarily dependent on urothelial histone remodeling. This is because 1) even after a 7-mo Dox treatment, during which at least some urothelial cells should have undergone histone remodeling, no EGFP expression was detected by RT-PCR in UPII-rtTA/TRE-EGFP double transgenic mice and 2) a modified version of rtTA, e.g., rtTA-M2, efficiently trans-activated urothelial expression of the EGFP reporter in UPII-rtTA/TRE-EGFP mice treated with Dox for only 3 days (Fig. 4). It is more probable, therefore, that urothelium is less accessible to the Dox inducer than other organ systems, thus requiring a more sensitive trans-activator (e.g., rtTA-M2) that functions even at a low (available) level of Dox. As mentioned, normal urothelium is designed to be impermeable to water and small molecules (21, 27), and as such, urothelial cells are unlikely to be exposed to any urinary Dox despite the potentially high concentration of Dox in the urine. Similarly, urothelium may be underlined by a less porous basement membrane than other epithelia, reducing its chance to be exposed to Dox from the circulation. Regardless of the exact cause(s), it is clear from our present study that rtTA-M2 is far more sensitive than rtTA in urothelium-based gene induction and should be the trans-activator of choice, as has recently been demonstrated in several other tissues as well (19, 20, 32).
The UPII-driven rtTA-M2 system we described here has several important features that make it a powerful and versatile tool for studying urothelial biology and diseases. The new system retains the strict urothelial specificity (Fig. 3) as with the UPII-based constitutive expression system (30, 34, 53), it has no background in the absence of Dox (Figs. 2 and 3), it responds rapidly to Dox in transcribing the reporter gene, and like the constitutive gene expression system, the new system induces gene expression in almost all urothelial layers (Fig. 4). The last feature is highly advantageous to the tumorigenesis studies, because introduction of genes into less differentiated cells is desirable for transforming the urothelium. Based on these features, it is expected that the UPII-rtTA-M2 transgenic mice can be used broadly to induce the urothelial expression of any gene placed under the control of TRE. Additionally, the UPII-rtTA-M2/TRE-Cre double transgenic mice we reported can be used widely to inducibly inactivate any gene flanked by loxP in urothelium (Fig. 6). Together, these two systems offer exciting new opportunities for studying the roles of even ubiquitously expressed genes in urothelial physiology and disease pathogenesis.
It should be noted that alternative approaches have been tested to express or inactivate genes in adult mouse bladder urothelium. A notable example is the adenoviral-driven gene expression via intravesicle delivery, a method that has been used successfully to study bladder tumorigenesis (38) and vesicular trafficking in the superficial umbrella cells (22). Due to technical difficulties with trans-urethral catherization in male mice, only female mice are amenable to trans-urethral intravesicle inoculation, whereas male mice require a surgical procedure. This would be cumbersome if a large number of animals are involved. Interanimal heterogeneity, due to varied degree of viral infection of urothelium, could also occur. Adenovirus per se and its associated solvent used to expose the urothelial surface are also known to cause urothelial damage and induce proliferation, which could confound data interpretation as to whether the result is the singular effect of the gene expression or a combinatorial effect of gene expression and the adenoviral infection. It is our belief that the availability of different approaches including the ones described here will prove to be highly complementary, and together, they can be tailored to different needs, and will be significantly more powerful and provide more accurate information than a single approach alone.
GRANTS
This work was supported in part by research grants from the National Institutes of Health (DK-69688) and Veterans Affairs’ Research and Development Service (Merit Review) and a grant-in-aid from Arnold Goldstein Fund for Urological Research at New York University School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Apodaca G. The uroepithelium: not just a passive barrier. Traffic 5: 117–128, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Birder LA. Urothelial signaling. Auton Neurosci 153: 33–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med 13: 625–630, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Boireau S, Buchert M, Samuel MS, Pannequin J, Ryan JL, Choquet A, Chapuis H, Rebillard X, Avances C, Ernst M, Joubert D, Mottet N, Hollande F. DNA-methylation-dependent alterations of claudin-4 expression in human bladder carcinoma. Carcinogenesis 28: 246–258, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bowman TL, Hurt MM. The coding sequences of mouse H2A and H3 histone genes contains a conserved seven nucleotide element that interacts with nuclear factors and is necessary for normal expression. Nucleic Acids Res 23: 3083–3092, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Guo X, Deng FM, Liang FX, Sun W, Ren M, Izumi T, Sabatini DD, Sun TT, Kreibich G. Rab27b is associated with fusiform vesicles and may be involved in targeting uroplakins to urothelial apical membranes. Proc Natl Acad Sci USA 100: 14012–14017, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chlapowski FJ, Bonneville MA, Staehelin LA. Lumenal plasma membrane of the urinary bladder. II. Isolation and structure of membrane components. J Cell Biol 53: 92–104, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erman A, Veranic P, Psenicnik M, Jezernik K. Superficial cell differentiation during embryonic and postnatal development of mouse urothelium. Tissue Cell 38: 293–301, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Graham E, Chai TC. Dysfunction of bladder urothelium and bladder urothelial cells in interstitial cystitis. Curr Urol Rep 7: 440–446, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Guo X, Tu L, Gumper I, Plesken H, Novak EK, Chintala S, Swank RT, Pastores G, Torres P, Izumi T, Sun TT, Sabatini DD, Kreibich G. Involvement of vps33a in the fusion of uroplakin-degrading multivesicular bodies with lysosomes. Traffic 10: 1350–1361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu P, Deng FM, Liang FX, Hu CM, Auerbach AB, Shapiro E, Wu XR, Kachar B, Sun TT. Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol 151: 961–972, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283: F1200–F1207, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Hurst RE, Moldwin RM, Mulholland SG. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology 69: 17–23, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Jonkers J, Berns A. Conditional mouse models of sporadic cancer. Nat Rev Cancer 2: 251–265, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Kaludov NK, Bowman TL, Sikorski EM, Hurt MM. Cell cycle-regulated binding of nuclear proteins to elements within a mouse H3.2 histone gene. Proc Natl Acad Sci USA 93: 4465–4470, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanai A, de Groat W, Birder L, Chai T, Hultgren S, Fowler C, Fry C. Symposium report on urothelial dysfunction: pathophysiology and novel therapies. J Urol 175: 1624–1629, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kanda T, Sullivan KF, Wahl GM. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr Biol 8: 377–385, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Katsantoni EZ, Anghelescu NE, Rottier R, Moerland M, Antoniou M, de Crom R, Grosveld F, Strouboulis J. Ubiquitous expression of the rtTA2S-M2 inducible system in transgenic mice driven by the human hnRNPA2B1/CBX3 CpG island. BMC Dev Biol 7: 108, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerrison JB, Duh EJ, Yu Y, Otteson DC, Zack DJ. A system for inducible gene expression in retinal ganglion cells. Invest Ophthalmol Vis Sci 46: 2932–2939, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297: F1477–F1501, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khandelwal P, Ruiz WG, Balestreire-Hawryluk E, Weisz OA, Goldenring JR, Apodaca G. Rab11a-dependent exocytosis of discoidal/fusiform vesicles in bladder umbrella cells. Proc Natl Acad Sci USA 105: 15773–15778, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Keay SK, Freeman MR. Heparin-binding epidermal growth factor-like growth factor functionally antagonizes interstitial cystitis antiproliferative factor via mitogen-activated protein kinase pathway activation. BJU Int 103: 541–546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong XT, Deng FM, Hu P, Liang FX, Zhou G, Auerbach AB, Genieser N, Nelson PK, Robbins ES, Shapiro E, Kachar B, Sun TT. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol 167: 1195–1204, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol 278: F540–F553, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol 278: F867–F874, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Liang FX, Bosland MC, Huang H, Romih R, Baptiste S, Deng FM, Wu XR, Shapiro E, Sun TT. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol 171: 835–844, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebert M. Basic science research on the urinary bladder and interstitial cystitis: new genetic approaches. Urology 57: 7–8, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Lin JH, Zhao H, Sun TT. A tissue-specific promoter that can drive a foreign gene to express in the suprabasal urothelial cells of transgenic mice. Proc Natl Acad Sci USA 92: 679–683, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messing EM. Growth factors and bladder cancer: clinical implications of the interactions between growth factors and their urothelial receptors. Semin Surg Oncol 8: 285–292, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Michalon A, Koshibu K, Baumgartel K, Spirig DH, Mansuy IM. Inducible and neuron-specific gene expression in the adult mouse brain with the rtTA2S-M2 system. Genesis 43: 205–212, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Minsky BD, Chlapowski FJ. Morphometric analysis of the translocation of lumenal membrane between cytoplasm and cell surface of transitional epithelial cells during the expansion-contraction cycles of mammalian urinary bladder. J Cell Biol 77: 685–697, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mo L, Cheng J, Lee EY, Sun TT, Wu XR. Gene deletion in urothelium by specific expression of Cre recombinase. Am J Physiol Renal Physiol 289: F562–F568, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Mo L, Zheng X, Huang HY, Shapiro E, Lepor H, Cordon-Cardo C, Sun TT, Wu XR. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J Clin Invest 117: 314–325, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol Renal Fluid Electrolyte Physiol 271: F886–F894, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 69: 9–16, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, Wang X, Shen TH, Matos T, Shen MM, Cordon-Cardo C, Abate-Shen C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev 23: 675–680, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu Z, Thottassery JV, Van Ginkel S, Manuvakhova M, Westbrook L, Roland-Lazenby C, Hays S, Kern FG. Homogeneity and long-term stability of tetracycline-regulated gene expression with low basal activity by using the rtTA2S-M2 transactivator and insulator-flanked reporter vectors. Gene 327: 61–73, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Rossi FM, Blau HM. Recent advances in inducible gene expression systems. Curr Opin Biotechnol 9: 451–456, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Ryding AD, Sharp MG, Mullins JJ. Conditional transgenic technologies. J Endocrinol 171: 1–14, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. J Biol Chem 274: 38071–38082, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Sun TT. Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing. Am J Physiol Renal Physiol 291: F9–F21, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13: 830–846, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science 303: 359–363, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci USA 97: 7963–7968, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell 108: 135–144, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Viana R, Batourina E, Huang H, Dressler GR, Kobayashi A, Behringer RR, Shapiro E, Hensle T, Lambert S, Mendelsohn C. The development of the bladder trigone, the center of the anti-reflux mechanism. Development 134: 3763–3769, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. Uroplakins in urothelial biology, function, and disease. Kidney Int 75: 1153–1165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu XR, Lin JH, Walz T, Haner M, Yu J, Aebi U, Sun TT. Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J Biol Chem 269: 13716–13724, 1994 [PubMed] [Google Scholar]
- 51.Yu W, Khandelwal P, Apodaca G. Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells. Mol Biol Cell 20: 282–295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang ZT, Pak J, Huang HY, Shapiro E, Sun TT, Pellicer A, Wu XR. Role of Ha-ras activation in superficial papillary pathway of urothelial tumor formation. Oncogene 20: 1973–1980, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Zhang ZT, Pak J, Shapiro E, Sun TT, Wu XR. Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer Res 59: 3512–3517, 1999 [PubMed] [Google Scholar]