Abstract
The sodium-chloride cotransporter (NCC) is the principal salt-absorptive pathway in the distal convoluted tubule. Recently, we described a novel pathway of NCC regulation in which phorbol esters (PE) stimulate Ras guanyl-releasing protein 1 (RasGRP1), triggering a cascade ultimately activating ERK1/2 MAPK and decreasing NCC cell surface expression (Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, Gamba G, Hoover RS. Proc Natl Acad Sci USA 104: 20120–20125, 2007). Little is known about the mechanisms which underlie these effects on NCC activity. Regulation of NCC via changes in NCC surface expression has been reported, but endocytosis of NCC has not been demonstrated. In this study, utilizing biotinylation, internalization assays, and a dynamin dominant-negative construct, we demonstrate that the regulation of NCC by PE occurs via an enhancement in internalization of NCC and is dynamin dependent. In addition, immunoprecipitation of NCC and subsequent immunoblotting for ubiquitin showed increased ubiquitination of NCC with phorbol ester treatment. MEK1/2 inhibitors and gene silencing of RasGRP1 indicated that this effect was dependent on RasGRP1 and ERK1/2 activation. Inhibition of ubiquitination prevents any PE-mediated decrease in NCC surface expression as measured by biotinylation or NCC activity as measured by radiotracer uptake. These findings confirmed that the PE effect on NCC is mediated by endocytosis of NCC. Furthermore, ubiquitination of NCC is essential for this process and this ubiquitination is dependent upon RasGRP1-mediated ERK1/2 activation.
Keywords: ubiquitin, ERK1/2, MAPK
the thiazide-sensitive sodium-chloride cotransporter (NCC), encoded by the SLC12A3 gene, in the distal convoluted tubule (DCT) of the kidney plays a key role in renal salt handling and blood pressure regulation. Residing in the DCT, NCC is responsible for the reabsorption of 5–10% of filtered sodium and chloride in the kidney (7). The cotransporter's importance in sodium balance, and therefore blood pressure regulation, is demonstrated not only by the efficacy of thiazide diuretics but also by the hypotension seen with loss of NCC function (Gitelman's syndrome) and the hypertension seen with increases in NCC activity (familial hyperkalemic hypertension or FHHt) (19, 27, 39). Recently, we identified a regulatory pathway for NCC, demonstrating that phorbol esters (PE) regulate NCC activity (12). Importantly, PE act as functional analogs of diacylglycerol (DAG) and do not act on NCC in this system via protein kinase C but instead mediate activation of Ras guanyl-releasing protein 1 (RasGRP1), ultimately leading to increased ERK1/2 MAPK activation. Following activation by DAG, RasGRP1 activates the small G protein Ras (specifically the isoform H-Ras in this system) by causing release of GDP from Ras. Ras then binds and is activated by GTP. Ras activates Raf, the first kinase in the ERK1/2 MAPK pathway. Raf then phosphorylates and activates MEK1/2, which in turn phosphorylates and activates ERK1/2. Activation of the Ras-MEK-ERK1/2 MAPK cascade by PE/RasGRP1 is necessary for the decreases in NCC activity seen with PE administration (12).
While the functional effect on NCC activity by PE correlates with a decrease in the amount of NCC expressed on the cell surface, the mechanisms by which this may occur are not known (12). Investigators exploring the modulation of NCC by other agents have found evidence that forward trafficking appears to be regulated (3, 9, 33). Whole animal studies have demonstrated the existence of a subapical pool of NCC and have shown a redistribution of NCC from the cell surface to this subapical pool under inhibition of the angiotensin-converting enzyme, but it is not clear whether this redistribution represents decreased forward trafficking or increased endocytosis (23, 24). Details of how these cell-trafficking events occur need to be further explored.
The role of ERK1/2 activation in the DAG/PE effect on NCC activity is of particular interest as ERK1/2 activation has been linked to ubiquitination of a number of proteins, including the epithelial sodium channel (ENaC) (1a, 13, 15, 18, 26, 28). Ubiquitin is an 8-kDa highly conserved protein that covalently attaches to lysine residues of target proteins either singly (monoubiquitination) or in polymerized chains (polyubiquitination). This attachment can tag a protein for endocytosis and/or degradation and is accomplished via the enzymes E1, E2, and E3, which act to transfer ubiquitin molecules to the target protein. In some systems, regulating the ubiquitination of a protein allows for modulation of that protein's endocytosis and/or degradation. ERK1/2 MAPK has clearly been shown to play a role in the regulation of ubiquitination and subsequent degradation in a variety of settings, from the regulation of ENaC to programmed cell death (1a, 13, 15, 18, 26, 28).
We therefore theorized a potential role for ERK1/2 MAPK activation in regulating ubiquitination and sought to assess the role of ubiquitination in cell-trafficking events that result in decreased NCC surface expression. In this study, using both a mammalian cell model with native NCC function and a heterologous mammalian expression system, we demonstrate that the regulation of NCC by DAG/PE occurs due to a decrease in NCC surface expression via enhanced, dynamin-dependent endocytosis. Furthermore, we show that ubiquitination of NCC is essential for this process and that this ubiquitination is dependent upon RasGRP1-mediated ERK1/2 activation.
MATERIALS AND METHODS
Materials.
Materials were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. The mouse DCT (mDCT) cells were a gift from Dr. Peter Friedman. The K44A dynamin construct was a gift from Dr. Eugene Chang. The Madin-Darby canine kidney (MDCK) cell lines expressing Flag-NCC were a gift from Dr. René Bindels. The NCC antibody was a gift from Dr. Mark Knepper.
Cell culture and treatments.
mDCT cells were plated on cell culture dishes and grown in growth medium containing a 50:50 mix of DMEM/F12, 5% heat-inactivated fetal bovine serum, and 1% penicillin/streptomycin/neomycin (P/S/N) at 37°C. Experiments were conducted when the cells reached 90–95% confluence. MDCK cell culture, immunocytochemistry, and the collection of images were performed as described elsewhere (5). Cells were treated with TPA (10−7 M) in culture medium where indicated and for the indicated time intervals.
Assessment of NCC function in mDCT cells.
mDCT cells were seeded in 12-well plates and prepared as described. The cells were then incubated in a serum-free, preuptake medium (130 mM Na gluconate, 2 mM K gluconate, 1.0 mM Ca gluconate, 1 mM Mg gluconate, 5 mM HEPES/Tris pH 7.4, 1 mM amiloride, 0.1 mM bumetanide) for 30 min. For studies examining forward trafficking, the cells were incubated with 10 μM befreldin A (BFA) for 1 h. During this time period, the cells were treated with the various agents being studied [100 nM TPA for 15 min and either 10 nM U0126 or vehicle (DMSO) for 15 min]. The medium was then changed to a 22Na+-containing medium (140 mM NaCl, 1 mM CaCl, 1 mM MgCl, 5 mM HEPES/Tris, pH 7.4, 1 mM amiloride, 0.1 mM bumetanide, 0.1 mM benzamil, 1 mM ouabain, and 1 μCi/ml of 22Na+) with or without thiazide (0.1 mM metolazone) and incubated for 20 min. Tracer uptake was then stopped via washes with ice-cold wash buffer. Cells were subsequently lysed with 0.1% SDS. Radioactivity was measured via liquid scintillation, and protein concentrations of the lysates were determined [bicinchoninic acid (BCA) protein assay, Pierce, Rockford, IL]. Uptakes were normalized to nanomoles per milligram. Thiazide-sensitive uptake was given by the difference of the uptakes with and without thiazide.
Cell surface biotinylation.
mDCT cells were incubated in a preuptake medium (detailed above) for 15 min. Then, 100 nM TPA or DMSO vehicle was added to the medium, and the cells were incubated for 15 more min. The preuptake medium was removed and uptake medium (as above, without 22Na+) was added, and the cells were incubated for 20 min. For studies examining forward trafficking, the cells were incubated with 10 μM BFA for 1 h. The cells were washed with PBS, and cell surface proteins were labeled with sulfo-NHS-SS- biotin (Pierce) in PBS for 30 min at 4°C. The reaction was quenched by adding 500 μl of the quenching solution (Pierce). The cells were harvested, lysed using lysis buffer containing protease inhibitor, and homogenized by sonication on ice. The cell lysates were centrifuged briefly, and the supernatant was collected. Eighty microliters of the supernatant from each group was stored separately at −80°C. Biotinylated proteins in the cell lysates were isolated by incubating with NeutrAvidin gel (Pierce) for 60 min at room temperature. The labeled proteins were eluted in SDS-PAGE sample buffer containing 50 mM DTT. Protein concentrations were determined by using a BCA protein assay kit (Pierce). The eluted proteins and the cell lysates were immunoblotted with polyclonal anti NCC antibody. For each group, 80 μg of total protein from the cell lysate was loaded along with 30 μl of biotinylated protein from the DMSO control group and the proportionate volume from the rest of the biotinylated protein groups. MDCK cells were grown to confluence on six-well filters, treated as wanted, placed on ice, and washed twice with ice-cold PBS, 1 mM CaCl2 and 0.1 mM MgCl2 (PBS-CM) as previously described (5). Surface proteins were biotinylated twice for 20 min each with freshly prepared ice-cold 1.5 mg/ml sulfo-NHS-SS-biotin (Pierce) in 10 mM tri-ethanolamine (pH = 8.9), 2 mM CaCl2, and 125 mM NaCl. Cells were subjected to gentle rocking to ensure uniform mixing during the biotinylation reactions. Then, excess biotin was quenched for 5 min with ice-cold 50 mM NH4Cl in PBS-CM, and the cells were washed three times with ice-cold PBS-CM. For biotinylation experiments, cells were lysed in 1 ml of lysis buffer [1% Triton X-100, 150 mM NaCl, 5 mM EDTA, 50 mM Tris (pH = 7.5)] for 30 min at room temperature.
Internalization experiments.
For internalization experiments, surface proteins were biotinylated as above before undergoing the internalization protocol (11). Cells were then warmed by reheating in a 37°C incubator for 5 min (to minimize thermal shock) before incubation with prewarmed culture medium. Cells were then treated with the agents of interest. Next, the cells were returned to ice, washed three times with ice-cold PBS-CM, treated three times for 20 min each with ice-cold 100 mM 2-sodium mercaptoethanesulfonic acid (MesNa) in 100 mM NaCl, 1 mM EDTA, 50 mM Tris (pH = 8.6), and 0.2% BSA. Excess MesNa was quenched for 10 min with ice-cold 120 mM iodoacetic acid in PBS-CM, and cells were lysed as for biotinylation. Subsequently, all lysates were precleared by centrifugation at 14,000 g and incubated with 30-μl equivalents of prewashed streptavidin-conjugated beads (Pierce) for 16 h. Finally, the beads were washed twice with high-salt buffer [500 mM NaCl, 5 mM EDTA, 50 mM Tris (pH = 7.5), 0.1% Triton X-100], twice with lysis buffer, and once with 10 mM Tris (pH = 7.5) and resuspended in 30 μl Laemmli buffer.
Immunoblotting.
For mDCT cells, a 7.5% polyacrylamide gel was used. Proteins were transferred electrophoretically to polyvinylidene difluoride membranes. After blocking with 3% BSA, the membranes were probed with corresponding primary antibodies [ubiquitin (Cell Signaling) 1:1,000, anti-RasGRP1 (Cell Signaling), dilution 1:200, or anti-NCC (Knepper) dilution 1:50,000] overnight at 4°C. The blots were washed in TBST. Signal detection for RasGRP1, NCC, and ubiquitin was done using IRDye800 goat anti-mouse IgG antibody, IRDye800 rabbit anti-goat IgG antibody, IRDye800 goat anti-rabbit, and IRDye680 goat anti-mouse (dilution 1:10,000, Rockland Immunochemicals) and subsequent scanning of the membrane by an Odyssey Infrared Imager. Intensity of the protein bands was analyzed by using Odyssey Infrared Imaging Software (Li-Cor Biosciences). For MDCK cells, PAGE and blotting, blocking, antibody incubation, and chemiluminescence of the membranes were performed as described (11). Ubiquitin (Zymed, San Francisco, CA) antibodies were diluted 1:250, followed by 1:10,000-diluted biotinylated anti-mouse IgG's (Jackson Laboratories) and by 1:8,000-diluted streptavidin-peroxidase. Mouse Flag antibodies (Zymed) were diluted 1:1,000.
Immunocytochemistry.
Essentially, immunocytochemistry and collection of images were performed as described previously (5), except that cells were not permeabilized with 1% Triton X-00 but with 1% saponin. Mouse Flag antibodies were diluted 1:100.
Immunoprecipitation.
MDCK cells were grown to confluence, treated as indicated, lysed [1% Triton X-100, 150 mM NaCl, 25 mM HEPES (pH = 7.4), supplemented with protease inhibitors and 20 mM NEM] and spun down to remove nonsolubilized material. Fifteen-microliter equivalents of protein A-agarose were preincubated with either rabbit FLAG antibodies in IPP500 [500 mM NaCl, 10 mM Tris (pH = 8.0), 0.1% NP-40, 0.1% Tween 20], rabbit antibodies to RasGRP1 (Santa Cruz Biotechnology), or rabbit antibodies to NCC and 0.1% BSA, and washed three times with IPP500. The precleared lysates were incubated for 16 h at 4°C with the antibody-bound protein A beads. The beads were washed three times with lysis buffer, and proteins were eluted using 15 μl Laemmli buffer.
Dominant-negative dynamin transfection.
mDCT cells were grown to 80% confluence in a medium containing a 50:50 mix of DMEM/F12 plus 5% heat-inactivated FBS, and 1% P/S/N. The heme agglutinin (HA)-K44A dynamin construct or wild-type dynamin was transiently transfected into these cells using GenePorter Transfection Reagent per the manufacturer's protocol (Genlantis). Confirmation of transfection was achieved by immunoblotting using a rabbit anti-dynamin antibody (1:1,000 dilution, Cell Signaling).
Gene silencing.
mDCT cells were grown to 80% confluence in a medium containing a 50:50 mix of DMEM/F12 plus 5% heat-inactivated FBS, and 1% P/S/N. Small interfering (si) RNA specific for RasGRP1 (Dharmacon) was transfected into these cells using GeneSilencer siRNA Transfection Reagent per the manufacturer's protocol (Genlantis). Nontargeting siRNA differing by a single base pair from the siRNA for RasGRP1 (Dharmacon) was used as a control (1).
ERK1/2 phosphorylation assay.
mDCT cells grown to 80% confluence after transfection with siRNA were incubated for 30 min in a preuptake medium. During this time period, the cells were treated with 100 nM TPA for 15 min. Cells were subsequently lysed in 1 ml of lysis buffer containing 50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1 tablet of Complete protease inhibitors (Roche). The lysates were homogenized by sonication on ice. Protein determination and immunoblotting were then performed as described above using antibodies for ohospho-ERK1/2 (1:1,000, Cell Signaling) and ERK1/2 (1:200, Santa Cruz Biotechnology). Densitometry was then performed, and relative densitometry was reported by expressing each group as a fraction of its phosphorylated form over total ERK1/2. Controls were then normalized to 1.
Statistical analysis.
Statistical analysis was performed using the SigmaStat software package (Systat, San Jose, CA). Data was analyzed for statistical significance using ANOVA (Holm-Sidak). A P value of <0.05 was taken as statistically significant.
RESULTS
PE enhance endocytosis of NCC.
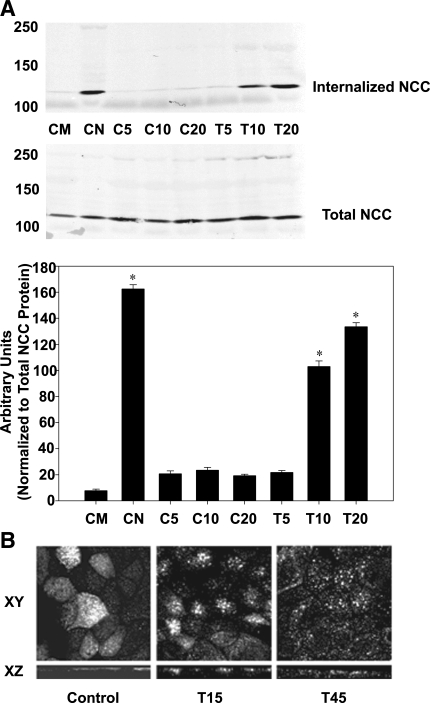
We previously demonstrated that the reduction of NCC activity with DAG/PE stimulation was tied to a decrease in NCC surface expression (12). To assess the mechanism by which this occurs, we performed internalization assays on mDCT cells. In this assay, cell surface proteins are first biotinylated at 4°C before the cells are rewarmed and treated with TPA. The cells are then treated with MesNa, a cell-impermeable acid which cleaves the biotin-protein attachments. Since MesNa is cell impermeable, any remaining biotinylated protein represents internalized proteins. This protocol is a well-established method for assessing internalization (5, 11, 21, 34). As shown in Fig. 1, treatment with MesNa virtually eliminated surface-expressed NCC (control with MesNa at time 0) compared with vehicle (control at time 0 without MesNa). Increased internalization of NCC with exposure to TPA (100 nM) vs. control (vehicle, DMSO) was evident as early as 10 min after exposure (P < 0.01). Over this time, total cellular NCC remained constant in both groups, suggesting that NCC degradation does not occur in response to PE in this time frame. These findings are consistent with images obtained using MDCK cells stably transfected with Flag-tagged NCC subjected to immunocytochemistry and confocal microscopy. These MDCK-Flag NCC cells were treated with 100 nM TPA for 15 and 45 min before fixation and immunocytochemistry. Subsequent confocal microscopy in both the XY and XZ planes show decreased apical NCC surface expression 15 and 45 min after treatment with TPA (Fig. 1B).
Fig. 1.
Internalization of sodium-chloride cotransporter (NCC) with TPA treatment. A: mouse distal convoluted tubule (mDCT) cells were grown to confluence, labeled with biotin at 4°C, then rewarmed at treated with 100 nM TPA or vehicle for the indicated times. Then, remaining surface biotin was stripped using mercaptoethanesulfonic acid (MesNa). Cells were lysed and biotinylated (internalized) proteins were recovered using streptavidin-agarose and immunoblotted for NCC. Densitometry is shown, normalized to total cellular NCC (n = 4). CM, time 0 control with MesNa; CN, time 0 control no MesNa; C5, DMSO control 5 min; C10, DMSO control 10 min; C20, DMSO control 20 min; T5, TPA 5 min; T10, TPA 10 min; T20, TPA 20 min. *P < 0.05 vs. control at similar time point. B: Madin-Darby canine kidney cells (MDCK) cells expressing Flag-NCC were grown to confluence on permeable supports, treated with TPA for 15 and 45 min (T15 and T45, respectively), fixed, and subjected to immunofluorescence using Flag antibodies. Confocal images were obtained in the XY and XZ planes.
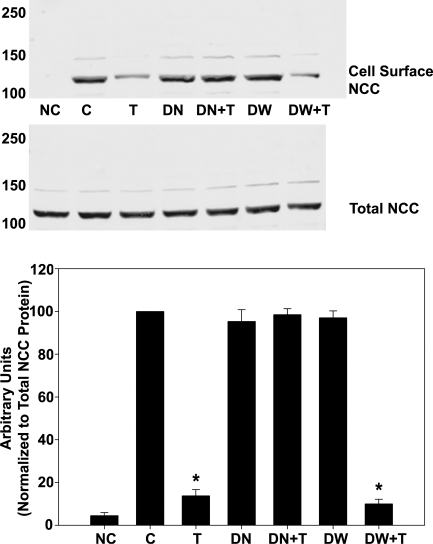
Dynamin is a GTPase critical for endocytosis, an effect inhibited by the dominant-negative dynamin mutant K44A (4). Therefore, to demonstrate that PE-induced NCC endocytosis is dynamin dependent, mDCT cells were transfected with HA-tagged dynamin dominant-negative construct to assess the dynamin dependence of the DAG/PE effect. Mock transfected cells behaved similarly to those observed in our previous experiments, resulting in a 76 ± 4% reduction in NCC surface expression with TPA compared with control, but mDCT cells expressing the dominant-negative dynamin showed no change in NCC surface expression with TPA administration (P < 0.05 vs. vehicle, Fig. 2). Cells transfected with wild-type dynamin behave as control. Taken together, these data indicate that the DAG/PE effect upon NCC occurs via enhancing dynamin-dependent endocytosis of surface NCC.
Fig. 2.
NCC surface expression after TPA treatment in cells expressing K44A dynamin. mDCT cells were grown to confluence, transfected with hemeagglutinin-tagged with either wild-type (DW) or K44A (DN) dynamin as described, then treated with 100 nM TPA (T) or vehicle (C) for 15 min before labeling with biotin at 4°C. Biotinylated (cell surface) proteins were recovered using streptavidin-agarose and immunoblotted for NCC. NC, no biotin control; DW+T, wild-type dynamin plus TPA; DN+T, DN plus TPA. Accompanying densitometry is shown (n = 4). *P < 0.05.
PE do not affect forward trafficking of NCC.
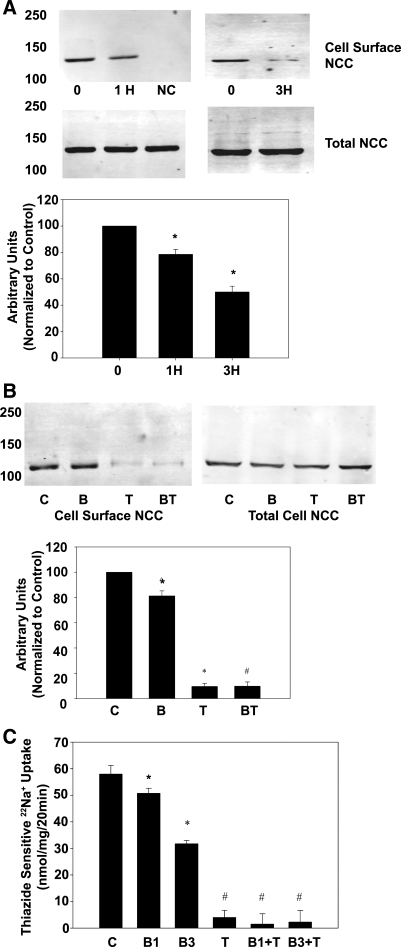
While these data show that TPA clearly enhances endocytosis, NCC has been shown in other systems to be regulated via affecting forward trafficking of NCC to the plasma membrane (3, 9, 33). Therefore, we examined the ability of TPA to affect forward trafficking of NCC. BFA (10 μM) was used to inhibit forward trafficking of NCC. BFA blocks transport of new proteins from the endoplasmic reticulum/Golgi to the plasma membrane. As shown in Fig. 3A, NCC surface expression decreased by 24 ± 3% over control after 1 h of BFA incubation, indicating continued endocytosis with inhibition of exocytosis (P < 0.05). At 3 h, NCC surface expression decreased by 52 ± 4% over control (Fig. 3A, P < 0.05). This is consistent with previously published reports of BFA inhibition of NCC forward trafficking, which demonstrated an NCC surface half-life of ∼3 h (33). NCC activity showed a similar decrease with BFA treatment, 12.4 ± 3.2% of control at 1 h and 45.3 ± 1.3% of control at 3 h (Fig. 3C, P < 0.05). In mDCT cells treated with a 1-h BFA incubation, TPA treatment essentially eliminated NCC surface expression (Fig. 3B). This was similar to TPA alone, indicating that TPA does not significantly act on NCC by affecting forward trafficking (33). This is in contrast to WNK4's effect on NCC, which acts via inhibition of forward trafficking (3, 9, 33). NCC activity was essentially eliminated by TPA compared with control by TPA alone or in groups treated with BFA for either 1 or 3 h (Fig. 3C, P < 0.05) There were no significant differences in surface expression between the TPA alone and the two BFA- and TPA-treated groups. This demonstrates that PE do not act on NCC via forward trafficking but instead via enhanced endocytosis.
Fig. 3.
Role of forward trafficking in TPA effect on surface expression and NCC activity. A: mDCT cells were grown to confluence, treated with 10 μM befreldin A (BFA) for 0, 1 (1H), or 3 h (3H) before labeling with biotin at 4°C. Biotinylated (cell surface) proteins were recovered using streptavidin-agarose and immunoblotted for NCC. NC, no biotin control. Accompanying densitometry is shown (n = 4). *P < 0.05 compared with time 0. B: mDCT cells prepared as above were treated with 10 μM BFA or vehicle for 1 h before treatment with 100 nM TPA. Cells were then labeled with biotin at 4°C. Biotinylated (cell surface) proteins were recovered using streptavidin-agarose and immunoblotted for NCC. C, vehicle; B, BFA alone; T, TPA alone; BT, BFA plus TPA. Accompanying densitometry is shown (n = 4). *P < 0.05 compared with control. #P < 0.05 compared with BFA group. C: mDCT cells were grown to confluence, treated with BFA or vehicle for either 1 or 3 h, and then treated with 100 nM TPA (T) or vehicle for 15 min. Cells were subsequently incubated in uptake medium with 22Na+ and either vehicle or 1 mM metolazone. Radioactive uptake was determined as in materials and methods. Thiazide-sensitive uptake was calculated as the difference in radiotracer uptake between the metolazone-containing and the metolazone-free groups. C, control, B1, BFA 1 h; B3, BFA 3 h; T, TPA; B1+T, BFA 1 h plus TPA; B3+T, BFA 3 h plus TPA (n = 4). *P < 0.05 compared with control. #P < 0.05 compared with control, B1, or B3 (no statistical difference among T, B1+T, and B3+T).
PE enhance ubiquitination of NCC.
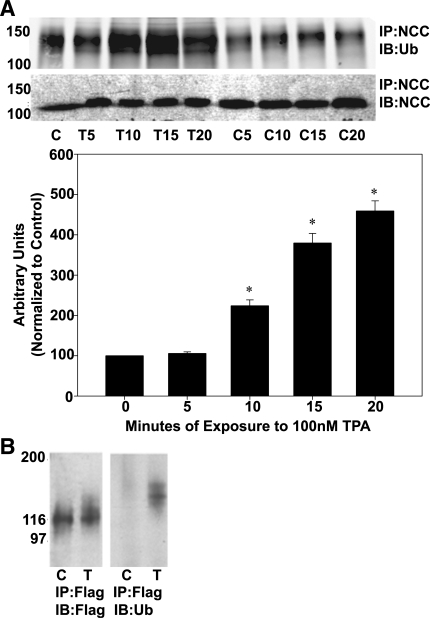
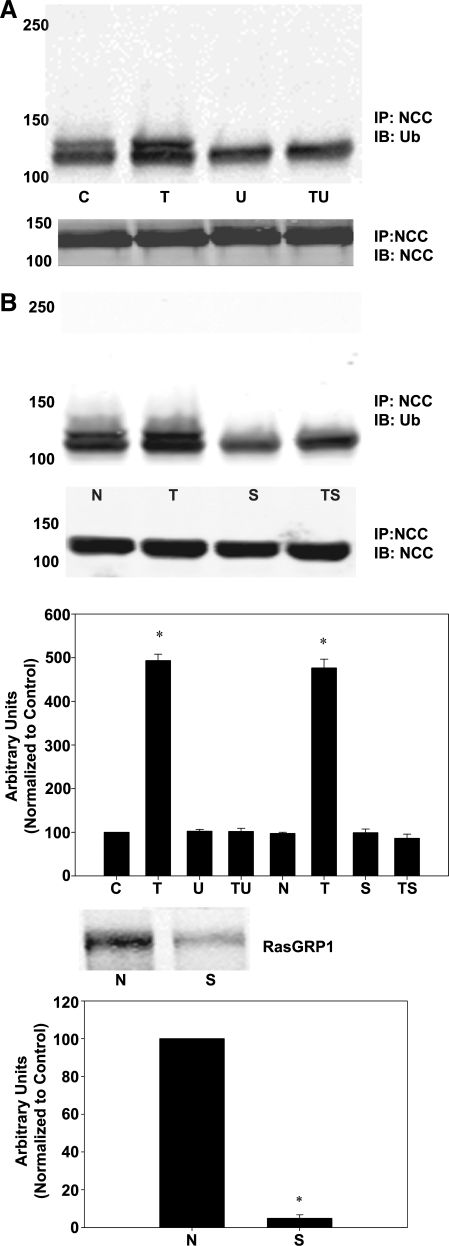
As previously discussed, we theorized that a potential signal for endocytosis of NCC was ubiquitination. To investigate a possible role for ubiquitin in mediating internalization of NCC in response to DAG/PE, we assessed the ability of TPA to augment ubiquitination of NCC. mDCT cells were treated with 100 nM TPA for varying time periods minutes before lysis and immunoprecipitation. Immunoblotting showed a significant increase in ubiquitinated NCC by 10 min of TPA treatment and a nearly fivefold increase in ubiquitinated NCC over control after 20 min of TPA treatment (P < 0.01 by ANOVA, n = 4, Fig. 4A). Ubiquitination of NCC was also assessed in the MDCK Flag-NCC cell line. These cells were treated with 100 nM TPA for 5 min, lysed, and immunoprecipitated with anti-FLAG antibody. Subsequent immunoblotting for ubiquitin clearly demonstrated a band centered around 140 kDa, representing ubiquitination of NCC (Fig. 4B). These data indicate that NCC is ubiquitinated after treatment with the PE TPA.
Fig. 4.
Ubiquitination of NCC after TPA treatment. A: mDCT cells were grown to confluence, treated with 100 nM TPA (T) or vehicle (DMSO, C) for the indicated times before lysis in RIPA buffer. NCC was then immunoprecipitated using NCC antibodies before immunoblotting with anti-ubiquitin or anti-NCC antibodies. Accompanying densitometry is shown (n = 4). *P < 0.01. B: MDCK cells stably expressing FLAG-tagged NCC were grown to confluence, treated for 5 min with (T) or without (C) TPA and lysed. The transporters were immunoprecipitated using rabbit FLAG antibodies (IP:Flag), and the immunoprecipitates were immunoblotted for ubiquitin (IB:Ub) or Flag (IB:Flag). Marker bands are indicated on the left by their molecular weights (in kDa).
Ubiquitination of NCC is dependent on RasGRP1-mediated ERK1/2 activation.
As we have previously demonstrated that PE regulate NCC via RasGRP1 activation of Ras, Raf, MEK1/2, and ultimately ERK1/2, presumably, DAG/PE act via this pathway to enhance NCC ubiquitination. To test this theory, we assessed the ability of TPA to enhance NCC ubiquitination while inhibiting portions of the pathway. mDCT cells were treated with 100 nM TPA for 15 min along with the MEK1/2 inhibitor U0126. Subsequent lysis, immunoprecipitation for NCC, and immunoblotting for ubiquitin demonstrated that while TPA alone results in a fivefold increase in ubiquitination (P < 0.01 by ANOVA, n = 4), the presence of a MEK1/2 inhibitor prevents TPA-induced enhancement in ubiquitination compared with either vehicle or U0126 alone (Fig. 5A).
Fig. 5.
NCC ubiquitination with TPA treatment with inhibition of the Ras guanyl-releasing protein 1 (RasGRP1) ERK1/2 pathway. A: mDCT cells were grown to confluence and treated with 10 nM U0126 (U) or vehicle (DMSO, C). Cells were then treated with 100 nM TPA (T) or vehicle (DMSO, C) for 15 min before lysis in RIPA buffer. NCC was then immunoprecipitated using NCC antibodies before immunoblotting with anti-ubiquitin (Ub) or anti-NCC antibodies (NCC). TU, TPA plus U0126 group. Accompanying densitometry is shown (n = 4). *P < 0.01. B: mDCT cells were grown to confluence and transfected with siRNA for RasGRP1 (S) or nontargeting small interfering (si) RNA (N) as described. Suppression of RasGRP1 expression is shown in immunoblot (P < 0.05, n = 4). Cells were then treated with 100 nM TPA (T) or vehicle (DMSO, C) for 15 min before lysis in RIPA buffer. NCC was then immunoprecipitated before immunoblotting with anti-ubiquitin or anti-NCC antibodies as above. TS, TPA+siRNA for RasGRP1. Accompanying densitometry is shown (n = 4). *P < 0.05.
To verify the involvement of RasGRP1 in this process, mDCT cells were transfected with siRNA specific for RasGRP1. Cells treated in this manner were again treated with TPA and assayed for NCC ubiquitination as above. Cells transfected with nonspecific siRNA continued to demonstrate a nearly fivefold increase in ubiquitinated NCC (P < 0.01). A 94 ± 5% reduction in RasGRP1 expression was achieved (Fig. 5B, P < 0.05 compared with transfection with nontargeting siRNA), and in these cells TPA did not have an effect on ubiquitination of NCC compared with cells transfected with nontargeting siRNA (Fig. 5A). These data indicate that TPA's ability to enhance NCC ubiquitination is dependent upon activation of RasGRP1 and ERK1/2.
Inhibition of ubiquitination by an E1 inhibitor does not affect ERK1/2 activation by TPA.
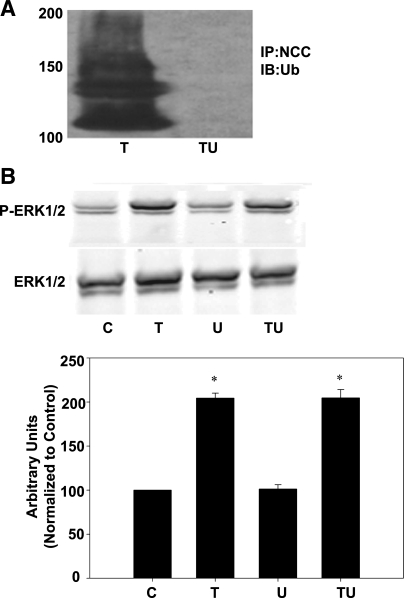
To directly assess the role of NCC ubiquitination in DAG/PE regulation of NCC, we utilized a chemical inhibitor of ubiquitination, 4[4-(5-Nitro-furan-2-ylmethylene)-3,4-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester (UBEI-41). UBEI-41 is a benzoic acid ethyl ester that inhibits the action of the E1 enzyme, inhibiting ubiquitination by preventing ubiquitin attachment (42). Incubation of mDCT cells with 50 μM UBEI-41 for 15 min eliminates ubiquitination of NCC in response to TPA (Fig. 6A). UBEI-41 had no effect on ERK1/2 activation by TPA, with the combination of TPA and UBEI-41 increasing ERK1/2 phosphorylation by 204 ± 9 compared with 205 ± 6% for TPA alone (P < 0.05 for either vs. vehicle by ANOVA, n = 4, Fig. 6B). Therefore, UBEI-41 effectively inhibits ubiquitination of NCC but has no effect on ERK1/2 activation by PE. Since RasGRP1 and ERK1/2 activation is necessary for TPA to enhance the ubiquitination of NCC and inhibition of ubiquitination has no effect on ERK1/2 activation, this demonstrates that ERK1/2 phosphorylation and activation lie upstream of ubiquitination of NCC.
Fig. 6.
Inhibition of ubiquitination does not affect TPA-mediated ERK1/2 phosphorylation A: mDCT cells were grown to confluence, treated with 100 nM TPA (T) in the presence or absence of UBEI-41 (U) for 15 min before lysis in RIPA buffer. TU, TPA+UBEI-41. NCC was then immunoprecipitated using NCC antibodies before immunoblotting with anti-ubiquitin or anti-NCC antibodies; n = 4. B: cells treated as described in A were lysed and immunoblotted for phospho-ERK1/2 and ERK1/2; n = 4. C, control. *P < 0.05.
Inhibition of ubiquitination prevents effect of TPA on NCC activity.
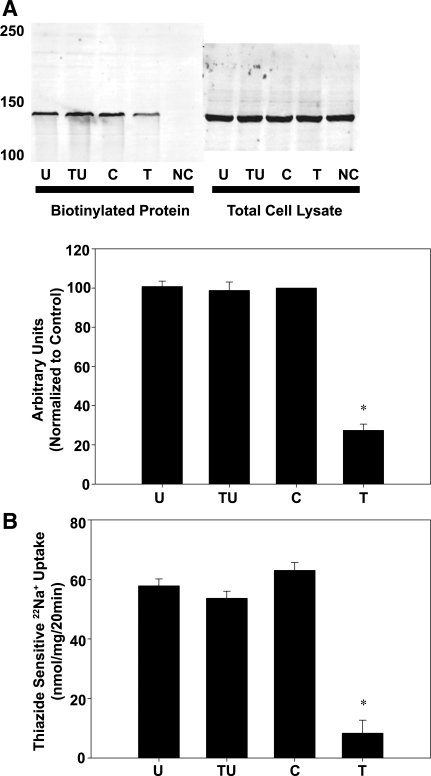
To determine whether ubiquitination of NCC was necessary for PE to modulate NCC surface expression and activity, the ubiquitin inhibitor UBEI-41 was used. In mDCT cells treated with TPA and biotinylated as described previously, the addition of UBEI-41 prevented the decrease in NCC surface expression seen with TPA alone (unchanged from baseline for TPA and UBEI-41 vs. 73 ± 4% decrease from baseline for TPA alone, Fig. 7A).
Fig. 7.
Inhibition of ubiquitination prevents a TPA-mediated decrease in NCC activity. mDCT cells were grown to confluence, treated with UBEI-41 (U) or vehicle for 15 min, and then treated with 100 nM TPA (T) or vehicle (DMSO, C) for 15 min. A: cells treated as described were labeled with biotin at 4°C. Biotinylated (cell surface) proteins were recovered using streptavidin-agarose and immunoblotted for NCC. NC, no biotin control. Accompanying densitometry is shown (n = 4). *P < 0.05. B: cells treated as above were subsequently incubated in uptake medium with 22Na+ and either vehicle or 1 mM metolazone. Radioactive uptake was determined as in materials and methods. Thiazide-sensitive uptake was calculated as the difference in radiotracer uptake between the metolazone-containing and the metolazone-free groups. TU, TPA+UBEI-41 (n = 6). *P < 0.01.
The impact of ubiquitination upon NCC function was then assessed by measuring NCC activity by a 22Na+ radiotracer uptake assay in the presence of 50 μM UBEI-41. Treatment with UBEI-41 for 15 min did not significantly alter the baseline activity of NCC (Fig. 7B). However, UBEI-41 did significantly alter the response to TPA, with TPA and UBEI-41 in combination causing no change in NCC activity while TPA alone resulted in a 87 ± 8% decrease, indicating that ubiquitination of NCC is essential in decreasing NCC activity (P < 0.01 vs. vehicle, n = 6). These data show that stimulation of NCC ubiquitination is essential for decreases in NCC surface expression and activity seen with TPA stimulation.
Taken together, these data demonstrate that PE regulate NCC activity by enhancing the ubiquitination of NCC, triggering its endocytosis, and thereby reducing its activity. Our data show that this is accomplished by PE-mediated activation of RasGRP1, triggering Ras, MEK1/2, and ERK1/2 MAPK activation. This ERK1/2 MAPK activation then leads to enhanced ubiquitination of NCC. The increased ubiquitinated NCC leads to increased endocytosis of NCC and decreased cell surface expression, ultimately leading to decreased NCC activity.
DISCUSSION
PE-mediated ERK1/2 activation almost completely suppresses NCC activity as measured by 22Na+ studies, accompanied by an ∼70% reduction in surface expression as measured by biotinylation (12). This finding was echoed by immunocytochemical staining and confocal microscopy of MDCK-FLAG NCC cells showing decreased apical cell surface expression with exposure to TPA (Fig. 1). In contrast to WNK4's reported effects on NCC, the PE do not appear to affecting forward trafficking of the cotransporter (3, 9, 33) Additionally, this work demonstrates that ERK1/2 MAPK activation by a PE/RasGRP1-mediated pathway triggers endocytosis of NCC. Tracking internalized NCC after PE treatment using biotinylation and subsequent treatment with MesNa showed a marked increase in internalized NCC in response to PE treatment, indicating endocytosis. The dynamin dependence of this finding was confirmed by introducing the dominant-negative dynamin mutant K44A into the system, which inhibited that change in NCC surface expression, indicating that PE's effect was via dynamin-dependent endocytosis of NCC. Using BFA to inhibit Golgi transport does not inhibit the effect of PE on surface expression, indicating that PE work primarily by stimulating endocytosis and not by affecting forward trafficking. To our knowledge, this is the first report of endocytosis of NCC.
In our mDCT cell model, NCC appears to be ubiquitinated and run as two ubiquitinated species (Fig. 4A). This is the first report of ubiquitination of NCC. Inhibition of ubiquitination with an E1 enzyme inhibitor eliminates the top band. This suggests that NCC may be constitutively ubiquitinated, with a second ubiquitination event mediated by PE stimulation. It is unclear whether this ubiquitination event represents short-chain polyubiquitination at the previous site or multiubiquitination at different sites, and this issue merits further study. Of note, while baseline ubiquitination is observed in the mDCT model, the transfected MDCK model does not appear to demonstrate significant baseline ubiquitination. This may be due to an alteration in baseline ubiquitination, due to a number of potential mechanisms. In ENaC, alterations in baseline ubiquitination were seen depending on the level of expression of the E3 ligase Nedd4–2 (44). These models may simply have varying expressions of the relevant E3 ligase, or for that matter a deubiquitinating enzyme (DUB), resulting in the differences in baseline ubiquitination. Determining the mechanism behind these differences merits future study.
In a series of whole animal studies conducted by Sandberg et al. (23, 24), NCC was shown by immunoelectron microscopy to reside in an apical (cell surface expressed) pool as well as in subapical vesicles. NCC appeared to redistribute within these pools in response to the administration of an angiotensin-converting enzyme inhibitor or angiotensin II infusion. These studies, however, could not distinguish between a decrease in forwarding trafficking and insertion of NCC in the plasma membrane or an increase in endocytosis of NCC from the plasma membrane (23, 24). Experiments using the Xenopus laevis expression system to coexpress WNK4 and enhanced green fluorescent protein-NCC have demonstrated reduced NCC cell surface expression in the presence of WNK4, a kinase associated with the form of genetic hypertension known as FHHt (40, 41). In this case, further studies demonstrated that the addition of a K44A dynamin-dominant negative did not affect WNK4 inhibition of NCC cell surface expression. This ultimately led to work using inhibitors of Golgi transport and lysosomal degradation to show that WNK4 likely impacted NCC surface expression by impeding forward trafficking to the cell surface and enhancing lysosomal degradation (3, 9, 33). While endocytosis of NCC has not been previously demonstrated, there are numerous examples of transport protein regulation via dynamin-dependent endocytosis, and ERK1/2 MAPK has also been shown to mediate changes in surface expression of ion transport proteins (10, 29, 35, 36, 43). We previously demonstrated that the RasGRP1-ERK1/2 MAPK pathway was essential for decreasing NCC surface expression and activity associated with DAG/PE (12). The PE TPA, a functional analog of DAG, activates RasGRP1, which in turn activates the small G protein Ras. Ras goes on to activate a cascade involving Raf and MEK1/2, which then phosphorylates ERK1/2 MAPK, activating it. This cascade represents a novel pathway for endocytosis-mediated regulation of NCC. Certain heterotrimeric G proteins activate phospholipase C, releasing DAG and inositol 1,4,5-trisphosphate from the cell membrane, making G-coupled protein receptors expressed in the DCT such as the calcium-sensing receptor a likely possibility. In addition, any hormones with receptors in the DCT and effects on ERK1/2 are likely candidate hormones. Possible hormonal targets therefore include epidermal growth factor and aldosterone.
Here, we investigated a potential role of ubiquitin in the regulation of NCC via the ERK1/2 MAPK pathway and demonstrated that in both mDCT and transfected MDCK cells expressing FLAG-NCC, TPA administration causes a marked increase in ubiquitination (Fig. 5). This increase in ubiquitination is of a similar time course to the increase in internalized NCC as measured by the biotinylation/MesNa assay, suggesting a possible direct relationship between these findings (Fig. 5). We further investigated the role of ERK1/2 MAPK in the ubiquitination of NCC and showed that inhibiting ERK1/2 activation by either blocking MEK1/2 or gene silencing RasGRP1 prevented an increase in ubiquitinated NCC by TPA (Fig. 6). This clearly demonstrates that ERK1/2 phosphorylation is required for ubiquitination of NCC in a pathway starting with DAG/PE activation of RasGRP1.
By inhibiting ubiquitination of NCC via an E1 inhibitor, we were able to directly assess the role of ubiquitination in triggering endocytosis and therefore decreasing NCC activity in this system. Prevention of NCC ubiquitination eliminated any change in NCC surface expression with PE treatment (Fig. 7). ERK1/2 activation by TPA, however, remained intact, indicating that ERK1/2 MAPK alone is not sufficient for the usual observed DAG/PE effect on NCC (Fig. 7).
Ubiquitination of NCC has not been previously described, but ubiquitination has been demonstrated to play a role in the regulation of other transport proteins (11, 14, 15, 37). It is a highly conserved 76-amino acid polypeptide with an ever-growing list of cellular functions (32). Ubiquitin is covalently linked to its substrate by an isopeptide bond between its C-terminal glycine and the ε-amino group of lysine residues. This occurs via the actions of three enzymes. First, an ubiquitin-activating enzyme (E1) binds to ubiquitin before transfer to an ubiquitin-conjugating enzyme (E2). Next, the ubiquitin is transferred to the substrate protein with the aid of an ubiquitin ligase (E3), either by directly binding to the substrate from E2 in a reaction catalyzed by E3 or by forming an intermediate complex with E3.
In this system, the increase in NCC ubiquitination appears tied to ERK1/2 MAPK activation, but the actual mechanism by which this occurs is unclear, with a number of plausible mechanisms existing. These include direct phosphorylation of NCC (as is the case with ERK1/2 and ENaC), stimulation of an E3 ligase (described in many systems, such as regulation of the tumor suppressor protein p53), or inhibition of a DUB (1a, 2, 15, 20, 26, 28, 30, 31, 38). In addition to these possibilities, ERK1/2 enhancement of ubiquitination could be a multiple-step process, with ERK1/2 activating a second mediator which then acts on NCC, a ubiquitin ligase, or other regulatory protein. Of these prospective pathways, the simplest would be direct phosphorylation of NCC by ERK1/2, thereby enhancing the action of an E3 ligase and thereby enhancing ubiquitination. NCC, however, contains no traditional consensus sites for ERK1/2 MAPK phosphorylation. While noncanonical phosphorylation sites certainly are possible and the other mechanisms have not been explicitly demonstrated in ERK1/2 MAPK, this raises the likelihood that ERK1/2 may act via a different mechanism (25). Potential pathways involving E3 ligases, deubiquitinating enzymes, or other mediators provide attractive possible targets, and further work is merited to single out the possible effectors from the hundreds of E3 ligases, dozens of DUBs, and countless other kinases.
The lysine(s) on NCC to which ubiquitin attaches are unknown, and ubiquitination at these sites can carry unique regulatory implications (16). Similarly, the pattern of ubiquitination, be it monoubiquitination, polyubiquitination, or multiubiquitination, can play a key determinant in regulation. For instance, monoubiquitination, ubiquitination at a single site by a single ubiquitin, can signal endocytosis and lysosomal degradation, with epidermal growth factor receptor and other tyrosine kinase receptors being good examples (17). Polyubiquitination, where a ubiquitin attached to a target protein is itself ubiquitinated at one of its own lysines, often forming long polymerized chains of ubiquitin molecules, is well studied. The sites at which ubiquitin polymerizes have been correlated with the degradation pathway for the ubiquitinated protein (6, 22). Multiubiquitination, or monoubiquitination at multiple sites on the target protein, has also been described in other systems and can serve as a signal for endocytosis (1a, 15, 15a, 20, 26, 28, 31, 38).
In this study, we have demonstrated that NCC is endocytosed in response to PE administration. Furthermore, we describe that NCC is ubiquitinated and that this ubiquitination is essential for the regulation of NCC by PE. Activation of ERK1/2 MAPK by a pathway involving RasGRP1, Ras, Raf, and MEK1/2 is essential for DAG/PE-mediated ubiquitination to occur. This work represents the first description of endocytosis of NCC. It is also the first report of ubiquitination of this cotransporter, and future study is warranted to further explore this means of NCC regulation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants K08 DK070668 (to R. S. Hoover) and K08 DK081728 (to B. Ko) and by grants from the Netherlands Organisation for Scientific Research NWO 916.36.122 (to E. J. Kamsteeg) and 865.07.002 (to P. M. T. Deen). P. M. T. Deen is a recipient of VICI grant 865.07.002 from the Netherlands Organization for Scientific Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Eugene Chang and Mark Musch for advice and discussions.
REFERENCES
- 1.Anonymous Whither RNAi? Nat Cell Biol 5: 489–490, 2003 [DOI] [PubMed] [Google Scholar]
- 1a.Booth RE, Stockand JD. Targeted degradation of ENaC in response to PKC activation of the ERK1/2 cascade. Am J Physiol Renal Physiol 284: F938–F947, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Butterworth MB, Edinger RS, Ovaa H, Burg D, Johnson JP, Frizzell RA. The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. J Biol Chem 282: 37885–37893, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int 69: 2162–2170, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 127: 915–934, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deen PMT, Van Balkom BWM, Savelkoul PJM, Kamsteeg EJ, Van Raak M, Jennings ML, Muth TR, Rajendran V, Caplan MJ. Aquaporin-2: COOH terminus is necessary but not sufficient for routing to the apical membrane. Am J Physiol Renal Physiol 282: F330–F340, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Galan JM, Haguenauer-Tsapis R. Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J 16: 5847–5854, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994 [PubMed] [Google Scholar]
- 9.Golbang AP, Cope G, Hamad A, Murthy M, Liu CH, Cuthbert AW, O'Shaughnessy KM. Regulation of the expression of the Na/Cl cotransporter by WNK4 and WNK1: evidence that accelerated dynamin-dependent endocytosis is not involved. Am J Physiol Renal Physiol 291: F1369–F1376, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Hasler U, Nunes P, Bouley R, Lu HAJ, Matsuzaki T, Brown D. Acute hypertonicity alters aquaporin-2 trafficking and induces a MAPK-dependent accumulation at the plasma membrane of renal epithelial cells. J Biol Chem 283: 26643–26661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, Klumperman J, Deen PM. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci USA 103: 18344–18349, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, Gamba G, Hoover RS. Phorbol ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci USA 104: 20120–20125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 Signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem 278: 18811–18816, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Lin DH, Sterling H, Wang Z, Babilonia E, Yang B, Dong K, Hebert SC, Giebisch G, Wang WH. ROMK1 channel activity is regulated by monoubiquitination. Proc Natl Acad Sci USA 102: 4306–4311, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik B, Yue Q, Yue G, Chen XJ, Price SR, Mitch WE, Eaton DC. Role of Nedd4–2 and polyubiquitination in epithelial sodium channel degradation in untransfected renal A6 cells expressing endogenous ENaC subunits. Am J Physiol Renal Physiol 289: F107–F116, 2005 [DOI] [PubMed] [Google Scholar]
- 15a.Monami G, Emiliozzi V, Morrione A. Grb10/Nedd4-mediated multiubiquitination of the insulin-like growth factor receptor regulates receptor internalization. J Cell Physiol 216: 426–437, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Moren A, Hellman U, Inada Y, Imamura T, Heldin CH, Moustakas A. Differential ubiquitination defines the functional status of the tumor suppressor Smad4. J Biol Chem 278: 33571–33582, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Mosesson Y, Shtiegman K, Katz M, Zwang Y, Vereb G, Szollosi J, Yarden Y. Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J Biol Chem 278: 21323–21326, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Nie L, Xu M, Vladimirova A, Sun XH. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO J 22: 5780–5792, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Shaughnessy KM, Karet FE. Salt handling and hypertension. J Clin Invest 113: 1075–1081, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem 277: 21843–21850, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Peng Y, Amemiya M, Yang X, Fan L, Moe OW, Yin H, Preisig PA, Yanagisawa M, Alpern RJ. ETB receptor activation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol Renal Physiol 280: F34–F42, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem 70: 503–533, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Sandberg MB, Maunsbach AB, McDonough AA. Redistribution of distal tubule Na+-Cl− cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol 291: F503–F508, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl cotransporter to apical membrane. Am J Physiol Renal Physiol 293: F662–F669, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Sheridan DL, Kong Y, Parker SA, Dalby KN, Turk BE. Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. J Biol Chem 283: 19511–19520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, Garty H. Interactions of beta and gamma ENaC with Nedd4 can be facilitated by an ERK-mediated phosphorylation. J Biol Chem 277: 13539–13547, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Simon DB, Nelson-Williams C, Johnson Bia M, Ellison D, Karet FE, Morey Molina A, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitelman HJ, Lifton RP. Gitelman's variant of Barter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Snyder PM, Steines JC, Olson DR. Relative contribution of Nedd4 and Nedd4–2 to ENaC regulation in epithelia determined by RNA interference. J Biol Chem 279: 5042–5046, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Sorribas V, Halaihel N, Puttaparthi K, Rogers T, Cronin RE, Alcalde AI, Aramayona J, Sarasa M, Wang H, Wilson P, Zajicek H, Levi M. Gentamicin causes endocytosis of Na/Pi cotransporter protein (NaPi-2). Kidney Int 59: 1024–1036, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J 15: 2371–2380, 1996 [PMC free article] [PubMed] [Google Scholar]
- 31.Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J 16: 6325–6336, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev 86: 669–707, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Subramanya AR, Liu J, Ellison DH, Wade JB, Welling PA. WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem 284: 18471–18480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Graaf SFJ, Rescher U, Hoenderop JG, Verkaart S, Bindels RJ, Gerke V. TRPV5 is internalized via clathrin-dependent endocytosis to enter a Ca2+-controlled recycling pathway. J Biol Chem 283: 4077–4086, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, Perry CJ, Kester L, Butterworth MB, Peters KW, Kleyman TR, Frizzell RA, Johnson JP. Clathrin-mediated endocytosis of the epithelial sodium channel: role of epsin. J Biol Chem 281: 14129–14135, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Wang WH, Lin DH, Sterling H. Regulation of ROMK channels by protein tyrosine kinase and tyrosine phosphatase. Trends Cardiovasc Med 12: 138–142, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83: 121–127, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson KD. Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin Cell Dev Biol 11: 141–148, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100: 680–684, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Kitagaki J, Dai RM, Tsai YC, Lorick KL, Ludwig RL, Pierre SA, Jensen JP, Davydov IV, Oberoi P, Li CCH, Kenten JH, Beutler JA, Vousden KH, Weissman AM. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res 67: 9472–9481, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Zeng WZ, Babich V, Ortega B, Quigley R, White SJ, Welling PA, Huang CL. Evidence for endocytosis of ROMK potassium channel via clathrin-coated vesicles. Am J Physiol Renal Physiol 283: F630–F639, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Zhou R, Patel SV, Snyder PM. Nedd4–2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem 282: 20207–20212, 2007 [DOI] [PubMed] [Google Scholar]