Abstract
Adenosine can mediate the tubuloglomerular (TGF) response via activation of A1 receptors on the afferent arteriole, but both adenosine A1 and A2 receptors can regulate preglomerular resistance. We tested the hypothesis that adenosine A2 receptors offset the effect of A1 receptors and modulate the TGF. Maximal TGF responses were measured in male Sprague-Dawley rats as changes in proximal stop-flow pressure (ΔPSF) in response to increased perfusion of the loop of Henle (0 to 40 nl/min) with artificial tubular fluid (ATF). The maximal TGF response was studied after 5 min of intratubular perfusion (10 nl/min) with ATF alone, or with ATF plus the A2A receptor antagonist (ZM-241385; 10−7 or 10−5 mol/l), A1 receptor antagonist (PSB-36; 10−8 mol/l), or with a combination of A1 (PSB-36; 10−8 mol/l) and A2A (ZM-241385; 10−7 mol/l) antagonists. The maximal TGF response (ΔPSF) with ATF alone was 11.7 ± 1.0 mmHg. Specific A2 inhibition (low dose) enhanced the maximal TGF response (15.7 ± 0.8 mmHg; P < 0.01), whereas a high dose (unspecific inhibition) attenuated the response (5.0 ± 0.4 mmHg; P < 0.001). A1 inhibition alone led to a paradoxical TGF response, with an increase in PSF of 3.1 ± 0.5 mmHg (P < 0.05). Simultaneous application of A1 and A2 antagonists abolished the TGF response (ΔPSF: 0.4 ± 0.3 mmHg). In conclusion, adenosine A2 receptors modulate the TGF response by counteracting the effects of adenosine A1 receptors.
Keywords: adenosine receptors, kidney, micropuncture, renal microcirculation
adenosine is an endogenous purine nucleoside that modulates kidney function. Cellular signaling by adenosine occurs through G protein-coupled receptor subtypes (i.e., A1, A2, and A3). The adenosine A2 receptor family consists of two subtypes, A2A and the A2B, which possess a high and a low agonist affinity, respectively (33). Adenylate cyclase, thus cAMP production, is stimulated by the adenosine A2A and A2B receptors but inhibited by A1 and A3 receptors (33). Extracellular adenosine can derive from cellular adenosine release or by extracellular breakdown of ATP, AMP, or cAMP and is generated at enhanced rates when tubular NaCl reabsorption is increased (33).
The tubuloglomerular feedback (TGF) mechanism is a negative feedback loop that senses changes in luminal NaCl delivery at the macula densa in the juxtaglomerular apparatus and adjusts the vascular tone of the afferent arteriole accordingly (28). Osswald and coworkers (20) first proposed that local generation of adenosine, as a consequence of increased NaCl transport, may elicit TGF-induced afferent arteriole vasoconstriction. The TGF mechanism contributes to the kidney's ability to regulate renal microcirculation, fluid homeostasis, and, consequently, blood pressure. Adenosine, via activation of A1 receptors, has been demonstrated to mediate the TGF response in both rats (8, 29) and mice (4, 30), whereas angiotensin II, nitric oxide, and superoxide have important roles in modulating the response (32, 35). ATP has been shown to mediate vasoconstriction of preglomerular vessels via P2X1 receptor activation, thus contributing to autoregulatory behavior of the kidney (12). During the last years, it has been debated whether that released ATP acts directly through stimulation of P2 receptors or if it is hydrolyzed to adenosine, which evokes TGF responses through activation of A1 receptors (11, 27).
Both adenosine A1 and A2 receptors are expressed in renal afferent arterioles and can regulate preglomerular resistance (13, 33, 34). Adenosine A1 receptors mediate constriction, and A2 receptors mediate dilatation of the afferent arteriole (10, 17). However, the role of A2 receptors in modulating the TGF response has not been established. Therefore, we tested the hypothesis that activation of A2 receptors may counteract A1-mediated contraction and dampen the TGF response.
MATERIALS AND METHODS
Animals.
This study was approved by the Georgetown University Animal Care and Use Committee and performed according to the National Institutes of Health guidelines for the conduct of experiments in animals. Male Sprague-Dawley rats (Charles River Laboratories International) weighing 250–350 g were maintained on a standard rat chow (0.3 g sodium/100 g content) with free access to food and tap water until the day of the study.
Surgical preparation.
On the day of the experiment, the rats were anesthetized by an intraperitoneal injection of thiobarbital (Inactin, 120 mg/kg body wt; Research Biochemicals, Natick, MA). The animals were placed on a servo-regulated heating pad with a rectal probe to maintain their body temperature at 37°C. The trachea was cannulated to allow spontaneous breathing. Catheters were placed in the left jugular vein for infusion of maintenance fluid (0.9% NaCl; 5 ml·h−1·kg body wt−1) and in the right femoral artery for the recording of mean arterial pressure (MAP). The bladder and the left ureter were cannulated to ensure unrestricted urine flow. The left kidney was exposed by a flank incision, cleaned from surrounding tissue, stabilized in a Lucite cup without stretching the renal vessels, and bathed in 0.9% NaCl solution maintained at 37°C. Micropuncture experiments were initiated after a 30- to 40-min stabilization period.
TGF experiments.
TGF was determined by the stop-flow technique. Under a stereomicroscope, randomly chosen proximal tubular segments on the kidney surface were punctured with a sharpened micropipette (7–9 μm OD) containing artificial tubular fluid (ATF; in mM: 128 NaCl, 4 NaHCO3, 5 KCl, 2 CaCl2, 7 urea, and 2 MgCl2, pH 7.4) stained with Lissamine green dye (2 g/l) to identify the nephron and the direction of the tubular flow. Subsequently, grease (T grade; Apiezon, Manchester, UK) was inserted in the micropuncture site with a micropipette (7–9 μm OD) connected to a hydraulic drive (Trent Wells, La Jolla, CA) to halt tubular flow. A perfusion pipette containing ATF with testing compounds or vehicle was inserted in the late proximal tubule downstream from the grease block and connected to a nanoliter microperfusion pump (Vestavia Scientific, Birmingham, AL). A pressure pipette (3–5 μm OD) was inserted in the proximal tubule upstream from the grease block to measure proximal stop-flow pressure (PSF) or in unobstructed nephrons to determine proximal free-flow pressure (PFF). The micropressure system (model 900A; World Precision Instruments, Sarasota, FL) was connected to a Powerlab (AD Instruments, Colorado Springs, CO) to record MAP, PFF, and PSF.
Study design.
The maximal TGF response for each nephron was assessed by the difference in PSF at zero loop perfusion and during perfusion at 40 nl/min (for 2 min). The adenosine receptor antagonists were dissolved in dimethyl sulfoxide (DMSO) and added to ATF (1% DMSO). The stop-flow measurements were taken before and after 5 min of intratubular administration at a low, non-TGF-activating perfusion rate (i.e., 10 nl/min).
The following protocols were employed: protocol 1, A2A receptor antagonist (ZM-241385; 10−7 mol/l); protocol 2, A2A receptor antagonist (ZM-241385; 10−5 mol/l); protocol 3, A1 receptor antagonist (PSB-36; 10−8 mol/l); and protocol 4, A1 + A2A receptor antagonists (PSB-36; 10−8 mol/l + ZM-241385; 10−7 mol/l).
Drugs.
ZM-241385 (4-{2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a] [1,3,5]triazin-5-ylamino]ethyl}phenol), purchased from Tocris Bioscience (Ellisville, MO), is a potent and highly selective A2A adenosine antagonist and is active in vivo (9, 15, 18, 21). It has binding affinities of 540, 1.4, 31, and 270 nM for human (h) A1, hA2A, hA2B, hA3 receptors, respectively, and shows selectivities of 1,000, 91, and 500,000 over A1, A2B, and A3 sites (16). Thus the lower dose of ZM-241385 used in the present study (i.e., 10−7 mol/l) may inhibit both A2A and A2B receptors, but not A1 and A3 receptors. The high dose, however, is nonspecific and may affect all four adenosine receptors.
PSB-36 {1-butyl-8-[hexahydro-2,5-methanopentalen-3a(1H)-yl]-3,7-dihydro-3-(3-hydroxypropyl)-1H-purine-2,6-dione}, purchased from Tocris Bioscience, is a potent and selective A1 adenosine receptor antagonist and is active in vivo (1, 3). It has binding affinities of 0.12, 552, 187, and 6,500 nM for rat (r) A1, rA2A, hA2B, and rA3 receptors, respectively (1). Thus, at the concentration used for PSB-36 (i.e., 10−8 mol/l), it should only inhibit A1 receptors.
Statistics.
Values are presented as means ± SE. Single comparisons between normally distributed parameters were tested for significance with Student's paired or unpaired t-test. For the stop-flow pressure measurements, multiple groups were compared by one-way ANOVA. The Bonferroni posttest for paired multiple comparisons was used to allow for more than one comparison with the same variable. This states a significance level of P/M, where M is the number of comparisons to be made. Statistical significance was defined as P < 0.05.
RESULTS
All animals in this study were in good condition, and at the time of experiments there were no differences in body weights among the groups (Table 1). Blood pressure, heart rate, urine flow, PFF, and PSF (at 0 nl/min) were at a similar level.
Table 1.
Body weight, blood pressure, and proximal tubule pressures
| Control (ATF) | A2A Blockade (10−7 M ZM) | A2A Blockade (10−5 M ZM) | A1 Blockade (10−8 M PSB) | A1+A2A Blockade (10−8 PSB+10−7 M ZM) | |
|---|---|---|---|---|---|
| Body wt, g | 320 ± 21 | 328 ± 11 | 315 ± 35 | 290 ± 3 | 310 ± 8 |
| MAP, mmHg | 93 ± 3 | 94 ± 3 | 90 ± 2 | 93 ± 4 | 98 ± 7 |
| PFF, mmHg | 11.5 ± 0.4 | 10.0 ± 0.0 | 11.0 ± 1.0 | 10.5 ± 0.5 | 11.3 ± 0.5 |
| PSF, mmHg | |||||
| 0 nl/min | 38.1 ± 1.3 | 36.8 ± 1.4 | 34.6 ± 1.5 | 35.3 ± 1.0 | 38.7 ± 0.9 |
| 40 nl/min | 26.4 ± 1.4* | 21.1 ± 1.7* | 29.6 ± 1.5* | 38.4 ± 1.5* | 38.3 ± 0.9 |
| m/n | 6/9 | 4/12 | 3/5 | 3/9 | 3/9 |
Values are means ± SE. ATF, artificial tubular fluid; ZM, ZM-241385; PSB, PSB-36; MAP, mean arterial pressure; PFF, proximal free-flow pressure; PSF, proximal stop-flow pressure; m/n, animals and nephrons, respectively; M, mol/l.
P < 0.05 compared with PSF, at 0 nl/min, within each group (paired t-test).
Effect of adenosine A2A receptor antagonist (ZM-241385).
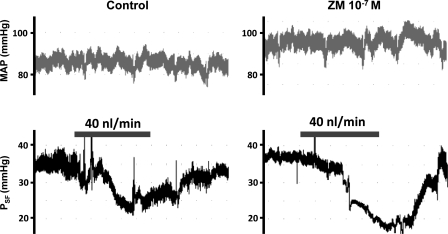
The maximal control TGF response decreased PSF by 11.7 ± 1.0 mmHg (P < 0.05) (Fig. 1A). After intratubular pretreatment with a low dose of ZM-241385 (10−7 mol/l), the maximal TGF response was clearly enhanced (15.7 ± 0.8 mmHg) compared with control (P < 0.01) (Fig. 1B). Pretreatment with a high dose of ZM-241385 (10−5 mol/l) attenuated the maximal response (5.0 ± 0.4 mmHg) compared with the control (P < 0.001) (Fig. 1C). Examples of original recordings of PSF are shown in Fig. 2.
Fig. 1.
Change in proximal stop-flow pressure in response to increased perfusion of loop of Henle from 0 to 40 nl/min. Broken lines represent individual nephrons. The maximal tubuloglomerular feedback (TGF) response was studied after 5 min of intratubular pretreatment (10 nl/min) with artificial tubular fluid (ATF) alone (control) (A) or with A2A receptor antagonist (ZM-241385) at 10−7 mol/l (B) or at 10−5 mol/l (C). M, mol/l. *P < 0.05 compared with proximal stop-flow pressure, at 0 nl/min, within each group (paired t-test).
Fig. 2.
Sample recordings of mean arterial pressure (MAP) and proximal stop-flow pressure (PSF) during loop of Henle perfusion with ATF alone (control) or with A2A receptor antagonist (ZM-241385). Bars represent a loop of Henle perfusion of 40 nl/min for 2 min.
Effect of adenosine A1 receptor antagonist (PSB-36).
After intratubular pretreatment with PSB-36 (10−8 mol/l), an anomalous TGF response was observed with a paradoxical increase in PSF (−3.1 ± 0.5 mmHg; P < 0.05) (Fig. 3A). Examples of original recordings of PSF are shown in Fig. 4.
Fig. 3.
Change in proximal PSF in response to increased perfusion of loop of Henle from 0 to 40 nl/min. Broken lines represent individual nephrons. The maximal TGF response was studied after 5 min of intratubular pretreatment (10 nl/min) with A1 receptor antagonist [PSB-36 (PSB); 10−8 mol/l] (A) or with the combination of A1 (PSB-36; 10−8 mol/l) and A2A [ZM-241385 (ZM); 10−7 mol/l] receptor antagonists (B). *P < 0.05 compared with proximal PSF, at 0 nl/min, within each group (paired t-test).
Fig. 4.
Sample recordings of MAP and proximal PSF during loop of Henle perfusion with A1 receptor antagonist (PSB-36) or with the combination of A1 (PSB-36) and A2A (ZM-241385) receptor antagonists. Bars represent a loop of Henle perfusion flow of 40 nl/min for 2 min.
Effect of adenosine A1 + A2A receptor antagonists (PSB-36 + ZM-241385).
After intratubular pretreatment with the combination of PSB-36 (10−8 mol/l) and the lower concentration of ZM-241385 (10−7 mol/l), the TGF response was abolished (0.4 ± 0.3 mmHg) (Fig. 3B). The maximal TGF response in the presence of both A1 and A2A receptor antagonists was significantly different from the response with A1 receptor antagonist alone (P < 0.05). Examples of original recordings of PSF are shown in Fig. 4.
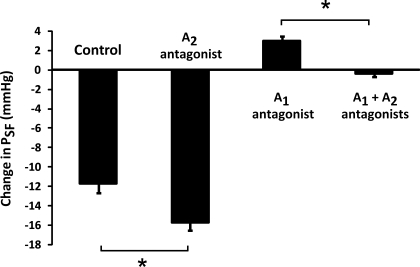
A summary of the maximal TGF responses for the different adenosine receptor antagonists is shown in Fig. 5. Inhibition of A2 receptors enhanced the TGF response, and A1 inhibition caused an abnormal TGF response, whereas A1 + A2 inhibition abolished the TGF response. A2 inhibition affected the TGF similarly, with or without inhibition of A1 receptors.
Fig. 5.
Effects of adenosine receptor antagonists on maximal TGF responses in response to an increase in perfusion of loop of Henle from 0 to 40 nl/min. The A2A receptor antagonist (ZM-241385; 10−7 mol/l) enhanced the TGF response compared with control (ATF alone). The A1 receptor antagonist (PSB-36; 10−8 mol/l) was associated with a significant inverse TGF response (P < 0.05). Simultaneous treatment with A1 (PSB-36; 10−8 mol/l) and A2A (ZM-241385; 10−7 mol/l) receptor antagonists abolished the TGF response. Values are means ± SE. *P < 0.05.
DISCUSSION
In the present study, using the stop-flow technique to determine TGF, we show adenosine A2 receptors modulate the responsiveness of TGF by counteracting the positive effect mediated by adenosine A1 receptors.
Adenosine is an important paracrine agent regulating microvascular tone of the kidney via activation of A1 and A2 receptors. In contrast to the vasculature of most other organs, both A1 and A2 receptors are widely expressed in the preglomerular microcirculation (13, 34). Studies in isolated and perfused renal afferent arterioles have demonstrated a biphasic response to adenosine, with contraction in the low concentration range and vasorelaxation at high concentration (5, 10, 17). Vasoconstriction induced by adenosine is mediated via A1 receptors, whereas vasodilatation by A2 receptor modulates the response (5, 7, 10, 17). The TGF mechanism is a negative feedback loop that enhances the vascular tone of the afferent arteriole when tubular NaCl delivery at the macula densa is increased (28). Increased tubular flow rates are associated with increased adenosine formation in the juxtaglomerular apparatus (20, 23). Studies in gene-deficient mice, or with receptor antagonists, have suggested that TGF is mediated via activation of adenosine A1 receptors (4, 8, 29, 30). However, studies have suggested that TGF signals are coupled to autoregulatory preglomerular vasoconstriction through ATP-mediated activation of P2X1 receptors (12). A direct role of ATP in mediating the TGF response may be possible, but the evidence for this is currently not compelling. Mice lacking P2X1 receptors, which are present in vascular smooth muscle cells of afferent arteriole (6), display impaired preglomerular autoregulation (12), but have largely normal TGF responses (27). Furthermore, pharmacological inhibition of P2 receptors with suramin did not significantly inhibit TGF of microperfused afferent arterioles with attached macula densas (25). The controversies regarding the role of adenosine or ATP as the signaling molecule may be because of regional differences of the kidney, since autoregulation studies were performed in juxtamedullary arterioles, whereas the TGF evidence comes from superficial nephrons.
Today, there is less information regarding the functional significance of A2 receptors in TGF. Given the dilatory function of A2 receptors in the afferent arteriole, we hypothesized that they might modulate TGF. In the present study, the role of A2 receptors in regulation of TGF was studied by intratubular administration of potent and highly selective adenosine receptor antagonists. As previously shown by micropuncture experiments, an intraluminal delivery of adenosine analogs targets the vascular effector cells in the juxtaglomerular apparatus (8, 14, 26, 29). The mechanisms for this are not clear, but access to the vascular receptor sites through permeable junctional complexes and intracellular pathways has been suggested (26). In the experimental approach used in this and similar studies, the concentration of adenosine antagonists at the receptor site is unknown. However, it is reasonable to assume that concentrations at the receptor sites will be considerably lower than perfusate concentrations. To avoid substantial dilution and to allow time for transportation to the effector site, intratubular pretreatment was performed before the maximal TGF response was assessed. Pretreatment alone had no effect on PSF in any group.
Our findings demonstrate that A2 receptors modulate TGF by counteracting the A1 receptor-mediated contraction. Even though a potent and highly selective A2A receptor antagonist was used (ZM-241385), the narrow window for receptor inhibition makes it difficult to rule out the possibility that also A2B receptors contributed to our findings. Studies in isolated afferent arterioles showed that pretreatment with A2A antagonist (ZM-241385; 10−7 mol/l) caused a monophasic constrictor response to adenosine, which did not differ from combined A2A and A2B (MRS-1706; 10−6 mol/l) receptor inhibition (17). The authors therefore suggested a predominant role for A2A receptors in adenosine-mediated dilatation of the arterioles.
Pretreatment with ZM-241385, at concentrations compatible with activation of A2 receptors, enhanced the maximal TGF response, whereas at a high concentration the TGF response was attenuated compared with controls. This discrepancy is most likely because of nonspecific inhibition of adenosine receptors, including A1, at the high concentration (i.e., 10,000-fold higher than needed for A2A activation). Studies have shown that preconstricted efferent arterioles dilate in response to increased macula densa NaCl, and in response to A2 receptor activation (2, 24). A possible efferent arteriole TGF may work in the opposite direction from that in afferent arterioles, thus modulating the glomerular filtration. Our results, however, indicate that the site of action is predominantly the afferent arterioles, since activation of adenosine postglomerular receptors would have the opposite result. However, it is possible that the effect observed with a much higher dose of ZM-241385 not only is mediated by inhibition of afferent A1 receptors, but also inhibition of efferent A2 receptors.
Interestingly, inhibition of adenosine A1 receptor, with a potent and selective antagonist (PSB-36; 10−8 mol/l), was associated with an anomalous TGF response, where increased loop of Henle perfusion from 0 to 40 nl/min caused a negative change in PSF. The observed effect suggests A2 receptor activation alone and is supported by the finding that cumulative application of adenosine in A1 receptor-deficient mice is associated with a monophasic dilatory response (17). In previous studies, A1 receptor inhibition was not associated with a reversed TGF response (14, 29). This discrepancy could be explained by the fact that the intratubular pretreatment used in the present study was more effective or by differences in the antagonists used.
Intraluminal administration of adenosine A1 receptor analogs enhances TGF-mediated changes in PSF, an effect that is not dependent on the presence of a luminal NaCl signal (8, 26). These findings are consistent with the concept that activation of A1 receptors on vascular cells of the afferent arterioles participates in the mediation of the TGF responses, and has been confirmed by an abolished TGF in A1 receptor-deficient mice (4, 30). Given that specific A1 receptor inhibition, in our study, was associated with a reversed TGF response, one would expect this to occur also in A1 receptor-deficient mice. This was not systematically studied in A1-deficient mice. However, perfusion with the A2 receptor agonist 5′-ethylcarboxamidoadenosine reduced or even reversed the TGF response at higher concentrations (26), supporting our finding that A2 receptor activation may modulate the TGF response.
Although we interpret the effects of luminal perfusion of adenosine receptor antagonists as acting on the afferent arterioles, it is possible that intraluminal administration of these antagonists activated adenosine receptors located in thick ascending limb of Henle's loop and possibly on the macula densa cells (34) and, consequently, modified intracellular cAMP levels. In this regard, the A2 receptor antagonist would decrease cAMP levels in the macula densa cells, thus enhancing the maximal TGF response. The A1 receptor antagonist would increase cAMP levels in the macula densa cells, thus dilating afferent arterioles and attenuating the TGF response. However, if adenosine receptors located on macula densa cells would be the only site of action during intratubular administration, a revered TGF response during A1 inhibition is not likely to occur. Considering that pretreatment was used and that adenosine analogs may access vascular cells in the juxtaglomerular apparatus, we find it unlikely that adenosine receptors located on macula densa cells would be the only target.
In conclusion, our findings demonstrate an important role of adenosine A2 receptors in modulating the TGF response by counteracting adenosine A1 receptor-mediated vasoconstriction. The mechanism responsible for A2 receptor-mediated vasodilatation of afferent arterioles and its modulation of TGF is not clear. However, stimulation of A2 receptors has been linked to release of nitric oxide (5, 10, 19, 22, 31). Future studies may investigate the possible role of nitric oxide.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-072183 (W. J. Welch), The Wenner-Gren Foundation, The Swedish Society of Medicine, and the Magnus Bergvall Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Muller CE. Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther 308: 358–366, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Al-Mashhadi RH, Skott O, Vanhoutte PM, Hansen PB. Activation of A(2) adenosine receptors dilates cortical efferent arterioles in mouse. Kidney Int 75: 793–799, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bilkei-Gorzo A, Abo-Salem OM, Hayallah AM, Michel K, Muller CE, Zimmer A. Adenosine receptor subtype-selective antagonists in inflammation and hyperalgesia. Naunyn Schmiedebergs Arch Pharmacol 377: 65–76, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 281: R1362–R1367, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Carlstrom M, Lai EY, Ma Z, Patzak A, Brown RD, Persson AE. Role of NOX2 in the regulation of afferent arteriole responsiveness. Am J Physiol Regul Integr Comp Physiol 296: R72–R79, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Chan CM, Unwin RJ, Bardini M, Oglesby IB, Ford AP, Townsend-Nicholson A, Burnstock G. Localization of P2X1 purinoceptors by autoradiography and immunohistochemistry in rat kidneys. Am J Physiol Renal Physiol 274: F799–F804, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Feng MG, Navar LG. Adenosine A2 receptor activation attenuates afferent arteriolar autoregulation during adenosine receptor saturation in rats. Hypertension 50: 744–749, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Franco M, Bell PD, Navar LG. Effect of adenosine A1 analog on tubuloglomerular feedback mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 257: F231–F236, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Golembiowska K, Dziubina A, Kowalska M, Kaminska K. Effect of adenosine A(2A) receptor antagonists on l-DOPA-induced hydroxyl radical formation in rat striatum. Neurotox Res 15: 155–166, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Hansen PB, Hashimoto S, Oppermann M, Huang Y, Briggs JP, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the mouse kidney due to preferential activation of A1 or A2 adenosine receptors. J Pharmacol Exp Ther 315: 1150–1157, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Inscho EW. ATP, P2 receptors and the renal microcirculation. Purinergic Signal 5: 447–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol 283: F41–F51, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Kawabata M, Ogawa T, Takabatake T. Control of rat glomerular microcirculation by juxtaglomerular adenosine A1 receptors. Kidney Int Suppl 67: S228–S230, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Keddie JR, Poucher SM, Shaw GR, Brooks R, Collis MG. In vivo characterisation of ZM 241385, a selective adenosine A2A receptor antagonist. Eur J Pharmacol 301: 107–113, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Klotz KN. Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch Pharmacol 362: 382–391, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, Persson AE. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int 70: 690–698, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Lasley RD, Kristo G, Keith BJ, Mentzer RM., Jr The A2A/A2B receptor antagonist ZM-241385 blocks the cardioprotective effect of adenosine agonist pretreatment in in vivo rat myocardium. Am J Physiol Heart Circ Physiol 292: H426–H431, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Li JM, Fenton RA, Cutler BS, Dobson JG., Jr Adenosine enhances nitric oxide production by vascular endothelial cells. Am J Physiol Cell Physiol 269: C519–C523, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Osswald H, Hermes HH, Nabakowski G. Role of adenosine in signal transmission of tubuloglomerular feedback. Kidney Int Suppl 12: S136–S142, 1982 [PubMed] [Google Scholar]
- 21.Poucher SM, Keddie JR, Brooks R, Shaw GR, McKillop D. Pharmacodynamics of ZM 241385, a potent A2a adenosine receptor antagonist, after enteric administration in rat, cat and dog. J Pharm Pharmacol 48: 601–606, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Ray CJ, Marshall JM. The cellular mechanisms by which adenosine evokes release of nitric oxide from rat aortic endothelium. J Physiol 570: 85–96, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Y, Arima S, Carretero OA, Ito S. Possible role of adenosine in macula densa control of glomerular hemodynamics. Kidney Int 61: 169–176, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Ren Y, Garvin JL, Carretero OA. Efferent arteriole tubuloglomerular feedback in the renal nephron. Kidney Int 59: 222–229, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int 66: 1479–1485, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Schnermann J. Effect of adenosine analogs on tubuloglomerular feedback responses. Am J Physiol Renal Fluid Electrolyte Physiol 255: F33–F42, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Schnermann J, Briggs JP. Tubuloglomerular feedback: mechanistic insights from gene-manipulated mice. Kidney Int 74: 418–426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnermann J, Persson AE, Agerup B. Tubuloglomerular feedback. Nonlinear relation between glomerular hydrostatic pressure and loop of henle perfusion rate. J Clin Invest 52: 862–869, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnermann J, Weihprecht H, Briggs JP. Inhibition of tubuloglomerular feedback during adenosine1 receptor blockade. Am J Physiol Renal Fluid Electrolyte Physiol 258: F553–F561, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng B, Ledent C, Mustafa SJ. Up-regulation of A 2B adenosine receptor in A 2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol 44: 905–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorup C, Persson AE. Inhibition of locally produced nitric oxide resets tubuloglomerular feedback mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 267: F606–F611, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Vitzthum H, Weiss B, Bachleitner W, Kramer BK, Kurtz A. Gene expression of adenosine receptors along the nephron. Kidney Int 65: 1180–1190, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Welch WJ, Tojo A, Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol 278: F769–F776, 2000 [DOI] [PubMed] [Google Scholar]