Abstract
Rh C glycoprotein (Rhcg) is an NH3-specific transporter expressed in both intercalated cells (IC) and principal cells (PC) in the renal collecting duct. Recent studies show that deletion of Rhcg from both intercalated and principal cells inhibits both basal and acidosis-stimulated renal ammonia excretion. The purpose of the current studies was to better understand the specific role of Rhcg expression in intercalated cells in basal and metabolic acidosis-stimulated renal ammonia excretion. We generated mice with intercalated cell-specific Rhcg deletion (IC-Rhcg-KO) using Cre-loxP techniques; control (C) mice were floxed Rhcg but Cre negative. Under basal conditions, IC-Rhcg-KO and C mice excreted urine with similar ammonia content and pH. Mice were then acid loaded by adding HCl to their diet. Ammonia excretion after acid loading increased similarly in IC-Rhcg-KO and C mice during the first 2 days of acid loading but on day 3 was significantly less in IC-Rhcg-KO than in C mice. During the first 2 days of acid loading, urine was significantly more acidic in IC-Rhcg-KO mice than in C mice; there was no difference on day 3. In IC-Rhcg-KO mice, acid loading increased principal cell Rhcg expression in both the cortex and outer medulla as well as expression of another ammonia transporter, Rh glycoprotein B (Rhbg), in principal cells in the outer medulla. We conclude that 1) Rhcg expression in intercalated cells is necessary for the normal renal response to metabolic acidosis; 2) principal cell Rhcg contributes to both basal and acidosis-stimulated ammonia excretion; and 3) adaptations in Rhbg expression occur in response to acid-loading.
Keywords: collecting duct
the rhesus (rh)-related proteins are increasingly recognized as mediating a central role in mammalian ammonia transport. In the kidney, Rh C glycoprotein (Rhcg), a member of this family, is expressed in the distal nephron and collecting duct (5, 8, 29, 30, 32). Metabolic acidosis, which increases renal ammonia1 excretion, increases renal Rhcg expression (30, 31), and Rhcg deletion impairs both basal and acidosis-stimulated renal ammonia excretion (1, 16). Importantly, collecting duct-specific Rhcg deletion alone reduces renal ammonia excretion in both basal conditions and metabolic acidosis (16), indicating that collecting duct Rhcg expression is necessary for both basal and acidosis-stimulated ammonia excretion.
The collecting duct contains two morphologically and physiologically distinct cell types, intercalated and principal cells. Intercalated cells are generally believed to primarily participate in acid-base homeostasis, with a lesser role in maintenance of NaCl balance, whereas principal cells are generally thought important in the maintenance of sodium, potassium, and water balance, but not acid-base homeostasis. Intercalated cells express abundant Rhcg in both the apical and basolateral plasma membrane as well as in apical cytoplasmic vesicles (31). Furthermore, Rhcg expression is increased in the intercalated cell apical and basolateral plasma membranes in response to metabolic acidosis, due to both increased protein expression and subcellular redistribution (30, 31). These observations suggest that intercalated cell Rhcg-mediated NH3 secretion contributes to increased renal ammonia excretion in metabolic acidosis.
However, Rhcg is also expressed in principal cells (5, 30–32). Principal cells are approximately twice as numerous as intercalated cells, and principal cell Rhcg expression increases in conditions of increased collecting duct ammonia secretion, including metabolic acidosis and reduced renal mass (15, 31). Thus principal cells may contribute to transcellular ammonia secretion and thereby to acid-base homeostasis.
The purpose of this study was to better understand the specific role of intercalated cell-specific Rhcg-mediated ammonia secretion in renal ammonia excretion. We generated mice with intercalated cell-specific Rhcg deletion using mice with loxP sites flanking critical exons in the Rhcg gene (floxed Rhcg), which we have described previously (14, 16). We enabled intercalated cell-specific Rhcg knockout (IC-Rhcg-KO) using transgenic mice expressing Cre-recombinase under control of the H+-ATPase B1 subunit promoter (B1-Cre), an intercalated cell-specific promoter (25). We then determined the effect of intercalated cell-specific Rhcg deletion on basal and acidosis-stimulated renal ammonia excretion. Because IC-Rhcg-KO mice express Rhcg only in connecting segment (CNT) cells and principal cells, we also determined the effect of acid-loading on principal cell-specific Rhcg expression. Our results show that intercalated cell Rhcg is necessary for the normal increase in ammonia excretion during metabolic acidosis and suggest that at least when intercalated cell Rhcg is absent, principal cells contribute to renal ammonia excretion during acidosis via increases in both Rhcg and Rhbg.
METHODS
Animals.
Transgenic mice with loxP sites flanking exons 5–9 of the Rhcg gene have been reported previously (14). Animals transgenic for Cre-recombinase under control of the B1 subunit of the H+-ATPase promoter (B1-Cre) have also been reported previously (25). Animal breeding was performed in the University of Florida College of Medicine Cancer and Genetics Transgenic Animal Core Facility by trained personnel. Mice used in this project were the result of mating mice homozygous for floxed Rhcg alleles and also expressing B1-Cre (floxed Rhcg, B1-Cre-positive) with mice homozygous for floxed Rhcg alleles but not expressing B1-Cre (floxed Rhcg, B1-Cre-negative). Genotyping was performed using tail-clip DNA obtained at ∼2 wk of age as described previously (14, 16, 25). All animal studies were approved by the University of Florida College of Medicine and the North Florida/South Georgia Veterans Health System Institutional Animal Care and Use Committees.
Antibodies.
Affinity-purified antibodies to Rhcg and Rhbg generated in our laboratory have been characterized previously (21, 32). Antibodies to phosphate-dependent glutaminase (PDG) were provided by Dr. Norman Curthoys (Colorado State University), and antibodies to the a4 subunit of H+-ATPase were provided by Dr. Fiona Karet (Cambridge Institute for Medical Research, Cambridge, UK). Antibodies to phosphoenolpyruvate carboxykinase (PEPCK) were obtained from Cayman Chemical (Ann Arbor, MI), and antibodies to glutamine synthetase and aquaporin-2 (AQP2; AB3274) were obtained from Chemicon (Temecula, CA).
Acid loading.
An acid diet was prepared as we described previously (16). Briefly, we added 0.4 M HCl to powdered standard rodent chow in a ratio of 1 ml/g chow. The control diet was identical, except that deionized water was substituted for HCl. Adult male animals, >6 mo of age, were placed into metabolic cages (Tecniplast diuresis metabolic cage, Fisher Scientific). Animals were allowed to acclimate for 1 day while receiving the normal diet and then either continued the normal diet or were changed to the HCl diet; daily food intake was measured. Urine was collected under mineral oil, and daily urine volume and pH were recorded. Urine samples were stored at −20°C until analyzed for ammonia concentration.
Electrolyte measurements.
Urine ammonia was measured using a modification of a commercially available kit (A7553, Pointe Scientific, Canton, MI) as described previously (16). Urine pH was measured using a micro-pH electrode (ROSS semimicro-pH, Orion 8115BN, Thermo Scientific). Serum bicarbonate was measured as total CO2 using a commercially available kit (C750–120, Pointe Scientific) as described previously (16). Plasma Na+ and K+ were measured using a flame photometer (Instrumentation Laboratory, Lexington, MA). Urinary titratable acid was measured using methods we described previously (16).
Tissue preparation for immunolocalization.
Mice were anesthetized with inhalant isoflurane. The kidneys were preserved by in vivo cardiac perfusion with PBS (pH 7.4) followed by periodate-lysine-2% paraformaldehyde (PLP) and then cut transversely into several 2- to 4-mm-thick slices and immersed for 24–48 h at 4°C in the same fixative. Kidney samples from each animal were embedded in polyester wax made using polyethylene glycol 400 distearate (Polysciences, Warrington, PA) with 10% 1-hexadecanol, and 2- or 3-μm-thick sections were cut and mounted on gelatin-coated glass slides.
Immunohistochemistry.
Immunolocalization was accomplished using immunoperoxidase procedures. Sections were dewaxed in ethanol, rehydrated, and then rinsed in PBS. Endogenous peroxidase activity was blocked by incubating the sections in Peroxidase Blocking Reagent (DakoCytomation, Carpinteria, CA) for 45 min. The sections were blocked for 15 min with Serum-Free Protein Block (DakoCytomation) and were then incubated at 4°C overnight with anti-Rhcg antibody. The sections were washed in PBS and incubated for 30 min with polymer-linked, peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical, Concord, CA), again washed with PBS, and then exposed to diaminobenzidine (DAB) for 5 min. The sections were washed in distilled water, dehydrated with xylene, mounted, and observed by light microscopy. Comparisons of labeling were made only between sections of the same thickness from the same immunohistochemistry experiment. Sections were examined on a Nikon E600 microscope equipped with DIC optics and photographed using a DXM1200F digital camera and ACT-1 software (Nikon). Color correction was performed using Adobe Photoshop CS2 software (Adobe Systems, San Jose, CA).
Double-immunolabeling procedure.
Double immunolabeling was accomplished using sequential immunoperoxidase procedures described in detail previously (16). Briefly, tissue sections were labeled with the first primary antibody using Vector SG (Vector Laboratories) as the chromogen to produce a blue label, as described above. The above procedure was repeated with the substitution of a second primary antibody (Rhcg) and the substitution of DAB for Vector SG. This label was easily distinguishable from the blue label produced by the Vector SG. The sections were then washed with glass-distilled water, dehydrated with xylene, mounted with Permount, and observed by light microscopy.
Protein preparation.
Animals were anesthetized with inhalant isoflurane, and the kidneys were rinsed by in vivo cardiac perfusion with PBS (pH 7.4), rapidly removed, frozen in liquid nitrogen, and then stored frozen at −70°C until used. In some experiments, the right renal vasculature was clamped after in vivo cardiac perfusion with PBS, the right kidney was removed, and then the left kidney was perfused with PLP fixative for immunohistochemistry. Tissues were homogenized in T-PER Tissue Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL) using microtube pestles (USA Scientific, Ocala, FL), and protein was extracted according to the manufacturer's recommended procedures. An aliquot was obtained for protein determination using a BCA assay, and the remainder was stored frozen at −70°C until used.
Immunoblotting procedure.
Ten micrograms of renal protein were electrophoresed on 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). Gels were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk, and incubated at 4°C overnight with primary antibody diluted in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween 20, pH 7.65) with 5 g/dl nonfat dry milk. Loading and transfer equivalence were assessed with Ponceau S staining. After washing, membranes were exposed to secondary antibody, goat anti-rabbit IgG (Promega, Madison, WI) or goat anti-mouse IgG (Upstate, Temecula, CA), conjugated to horseradish peroxidase at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized by using enhanced chemiluminescence (SuperSignal West Pico Substrate, Pierce) and the Kodak Image Station 440CF digital imaging system. Band density was quantified using Kodak 1D, version 5.0, software (Kodak Scientific Imaging, New Haven, CT). Band density was normalized such that mean density in the same region (cortex or outer medulla) in control kidneys was 100. Absence of saturation was confirmed by examining pixel intensity distribution in all immunoblots.
Immunodot analysis.
Extracted protein was diluted with T-Per containing protease inhibitors to a final concentration of 2 μg/μl. A piece of nitrocellulose was aligned beneath a multichannel slotted gasket, and 1.0 μg of each sample was placed at regular intervals. The nitrocellulose was allowed to air dry and stained with a Ponceau S solution containing 5% concentrated acetic acid. Otherwise, protein detection was performed using similar techniques as used for the immunoblot analysis with the exception that the blocked nitrocellulose was placed in a multichannel plexiglass holder (TMR Engineering, Micanopy, FL) to analyze several antibodies at one time. In preliminary studies, we confirmed that immunodot and immunoblot analysis gave quantitatively similar results.
Statistics.
Results are presented as means ± SE. Statistical analyses were performed using Student's t-test, and P < 0.05 was taken as statistically significant; n refers to the numbers of animal studied.
RESULTS
Characterization of intercalated cell-specific Rhcg deletion mice.
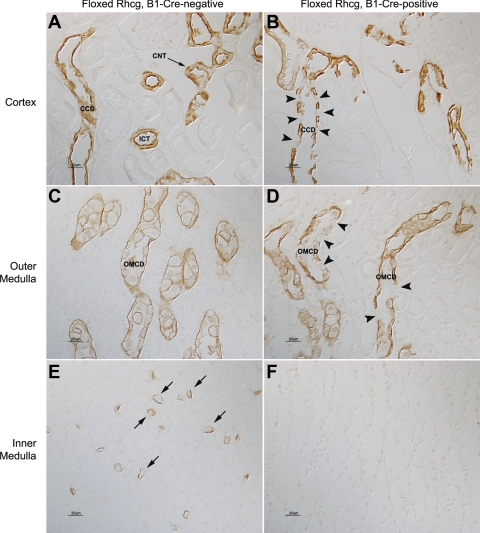
In wild-type mice, Rhcg is expressed in essentially all cells in the CCD and OMCD except for B-type intercalated cells, with significantly greater expression in intercalated cells than in principal cells (14, 16, 29, 32). Kidneys from floxed Rhcg, B1-Cre-positive mice, in contrast, had a substantial number of collecting duct cells in which no detectable Rhcg immunolabel was present (Fig. 1). The number of collecting duct cells lacking Rhcg immunolabel was substantially greater than the number of B-type intercalated cells, a cell population which normally does not express detectable Rhcg immunolabel (11). Kidneys from mice that were floxed Rhcg but were B1-Cre negative, exhibited a normal pattern of Rhcg immunolabel.
Fig. 1.
Rh glycoprotein C (Rhcg) immunolabel in floxed Rhcg, B1-Cre-negative, and floxed Rhcg, B1-Cre-positive mice. A: Rhcg immunolabel in cortex of floxed Rhcg, B1-Cre-negative mouse kidney. The normal apical and basolateral distribution of Rhcg immunolabel in cortical collecting duct (CCD) and in connecting tubule (CNT) and initial collecting tubule (ICT) is present. B: Rhcg immunolabel in floxed Rhcg, B1-Cre-positive mouse kidney. A large subpopulation of cells (arrowheads) has no detectable Rhcg in the CCD. The number of Rhcg-negative cells is greater than the expected number of B-type intercalated cells, a cell population which does not express detectable Rhcg immunolabel. C: Rhcg immunolabel in the outer medulla and demonstration of normal Rhcg expression in outer medullary collecting duct (OMCD) of B1-Cre-negative mouse kidney. D: outer medulla from floxed Rhcg, B1-Cre-positive mouse kidney. A subpopulation of cells in the OMCD (arrowheads) has no detectable Rhcg. E: Rhcg immunolabel in the inner medulla and demonstration of normal Rhcg expression in inner medullary collecting duct intercalated cells (arrows) in B1-Cre-negative mouse kidney. F: inner medulla from floxed Rhcg, B1-Cre-positive mouse kidney. No Rhcg immunolabel is detectable.
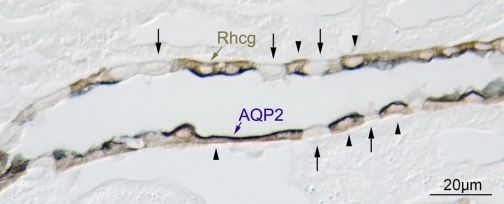
To confirm that the cells lacking Rhcg immunolabel were intercalated cells, we performed double-immunolabel of Rhcg with the principal cell-specific marker AQP2. These studies demonstrated that AQP2-positive cells, i.e., principal cells, exhibited intact Rhcg immunolabel, whereas AQP2-negative cells, i.e., intercalated cells, did not express detectable Rhcg immunolabel (Fig. 2). Intercalated cell number and distribution, identified from the H+-ATPase immunolabel, appeared normal, and general renal anatomy, assessed from hematoxylin- and eosin-stained kidney sections, was normal (data not shown). Thus floxed Rhcg, B1-Cre-positive mice have intercalated cell-specific Rhcg deletion. In the remainder of this report, we refer to floxed Rhcg, B1-Cre-positive mice as IC-Rhcg-KO mice and floxed Rhcg, B1-Cre-negative mice as control (C) mice.
Fig. 2.
Double-immunolabeling of CCD with aquaporin-2 (AQP2) and Rhcg in floxed Rhcg, B1-Cre positive mice. Double-immunolabel for AQP2 (blue) and Rhcg (brown) was performed to identify the Rhcg-negative cells in floxed Rhcg, B1-Cre-positive mice. Principal cells, identified by apical AQP2 immunolabel, expressed Rhcg immunolabel (arrowheads). Intercalated cells, identified as AQP2-negative cells, did not express detectable Rhcg immunolabel (arrows).
Urine ammonia excretion and pH under basal conditions.
We next examined the effect of intercalated cell-specific Rhcg deletion on basal renal ammonia metabolism. Table 1 shows these results. Intercalated cell-specific Rhcg deletion did not significantly alter either basal urine ammonia excretion or urine volume or pH. This contrasts with findings with both global and collecting duct-specific Rhcg deletion, where basal urine ammonia excretion was decreased significantly (1, 16). Similar to findings with collecting duct-specific Rhcg (16) or with global Rhcg deletion (1), serum Na+, K+, and HCO3− concentrations did not differ significantly between C and IC-Rhcg-KO mice under basal conditions.
Table 1.
Physiological parameters under basal conditions
| Parameter | IC-Rhcg-KO | C | P Value |
|---|---|---|---|
| Urine ammonia, μmol/day | 78.4 ± 7.8 (12) | 76.2 ± 7.5 (12) | NS |
| Urine pH | 6.10 ± 0.05 (12) | 6.06 ± 0.05 (12) | NS |
| Urine volume, ml/day | 1.81 ± 0.24 (12) | 1.66 ± 0.21 (12) | NS |
| Body weight, g | 34.3 ± 2.2 (12) | 39.4 ± 2.3 (12) | NS |
Values are means ± SE. Numbers in parentheses are numbers of animals in each group. IC-Rhcg-KO, intercalated cell-specific Rh glycoprotein (Rhcg) deletion; C, control; NS, not significant.
Urinary ammonia excretion in response to HCl-induced metabolic acidosis.
Because the ability to increase urinary ammonia excretion is the primary component of the renal response to metabolic acidosis, we examined the effect of intercalated cell-specific Rhcg deletion on the renal response to acid loading. Mice were acid loaded using HCl addition to their chow for 3 days, a time period that we have shown previously is sufficient for the maximal increase in urinary ammonia excretion in this model (16). Figure 3A summarizes urinary ammonia excretion in C and IC-Rhcg-KO mice. Acid loading increased urinary ammonia excretion in both IC-Rhcg-KO and C mice. There was no significant difference in urinary ammonia excretion between IC-Rhcg-KO and C mice either before acid loading or during the first 2 days of acid loading. However, on day 3, total urine ammonia was significantly less in IC-Rhcg-KO mice than in C mice. Thus intercalated cell-specific Rhcg expression is necessary for the delayed, day 3, increase in urinary ammonia excretion.
Fig. 3.
Urinary ammonia excretion and urine pH in response to HCl-induced metabolic acidosis. A: urinary ammonia excretion. Acid loading increased urine ammonia excretion in both control (C) and intercalated cell (IC)-Rhcg-knockout (KO) mice. There was no significant difference in urinary ammonia excretion before acid loading, or on days 1 or 2 of acid loading between C and IC-Rhcg-KO mice. On day 3, total urine ammonia was significantly less in IC-Rhcg-KO mice than in C mice. B: urine pH in response to acid loading. After acid loading, IC-Rhcg-KO mice excreted significantly more acid urine than did in C mice on days 1 and 2. *P < 0.01 vs. C. **P < 0.05 vs. C.
Urine pH in response to metabolic acidosis.
Luminal pH is an important regulator of collecting duct ammonia excretion, with increased acidification stimulating ammonia secretion. To assess whether intact urinary ammonia excretion in the initial response to metabolic acidosis in IC-Rhcg-KO was due to increased luminal acidification, we examined urine pH. Figure 3B summarizes these results. Acid-loaded IC-Rhcg-KO mice excreted significantly more acidic urine on the first 2 days of acid loading than did C mice; on day 3, urine pH did not differ significantly. Increased urine acidification may compensate, at least partially, for decreased Rhcg-dependent NH3 secretion in acid-loaded IC-Rhcg-KO mice during the first 2 days of acid loading.
Effect of IC-Rhcg-KO on titratable acid and net acid excretion.
A second major component of net acid excretion is titratable acid excretion. Under basal conditions, titratable acid excretion averaged 90.3 ± 13.9 μmol/day in C mice and 118.4 ± 10.6 μmol/day in IC-Rhcg-KO mice [P = not significant (NS); n = 8/group]. Thus intercalated cell-specific Rhcg deletion does not alter either ammonia or titratable acid excretion under basal conditions. In response to acid loading, there remained no significant difference between C and IC-Rhcg-KO mice (Fig. 4A).
Fig. 4.
Other effects of IC-Rhcg-KO. A: titratable acid excretion. There was no significant difference in titratable acid excretion between C and IC-Rhcg-KO mice, either under baseline conditions or in response to acid loading. B: net acid excretion. Net acid excretion did not differ significantly between C and IC-Rhcg-KO mice before acid loading or on days 1 or 2 of acid loading and was significantly less in IC-Rhcg-KO mice on day 3 of acid loading. C: dietary food intake. Food intake, and thus acid ingestion, did not differ significantly between C and IC-Rhcg-KO mice, either under baseline conditions or with acid loading. D: changes in body weight. Metabolic acidosis induces weight loss, but there were no significant differences in weight loss between C and IC-Rhcg-KO mice. E: urine volume. Urine volume did not differ significantly between C and IC-Rhcg-KO mice either before or during acid-loading; n = 8 at each time point in all graphs. P = not significant (NS).
Net acid excretion did not differ significantly between C and IC-Rhcg-KO mice under basal conditions or during the first 2 days of acid loading. On day 3 of acid loading, net acid excretion was significantly less in IC-Rhcg-KO mice than in C mice; this effect was solely due to differences in ammonia excretion (Fig. 4B). Thus the effects of IC-Rhcg-KO were specific to urine pH and ammonia excretion.
Electrolyte response to metabolic acidosis.
Serum Na+ and K+ concentrations did not differ significantly between C and IC-Rhcg-KO mice (Na+ concentration: C, 152.7 ± 1.3 vs. IC-Rhcg-KO, 156 ± 2, 155.9 ± 1.7 meq/l; K+ concentration: C, 4.1 ± 0.2 vs. IC-Rhcg-KO, 3.9 ± 0.1 meq/l; n = 8 in all groups, P = NS for both Na+ and K+ concentration). Serum HCO3− also did not differ significantly (C, 15.6 ± 1.5 vs. IC-Rhcg-KO, 14.8 ± 0.7 meq/l, P = NS, n = 8 in each group).
Extent of acid loading in C and IC-Rhcg-KO mice.
Because animals were acid loaded by adding HCl to their food, we could assess the acid loading by quantifying food intake. Food intake, and therefore acid ingestion, did not differ significantly between C and IC-Rhcg-KO mice (Fig. 4C).
Other systemic effects of acid loading in C and IC-Rhcg-KO mice.
Metabolic acidosis causes body weight reduction, due to catabolism of skeletal muscle to increase glutamine availability. Although there was a tendency for greater weight loss in IC-Rhcg-KO mice in response to metabolic acidosis, this difference was not statistically significant (Fig. 4D). Urine volume also did not differ significantly between C and IC-Rhcg-KO mice (Fig. 4E).
Principal cell Rhcg expression in response to metabolic acidosis.
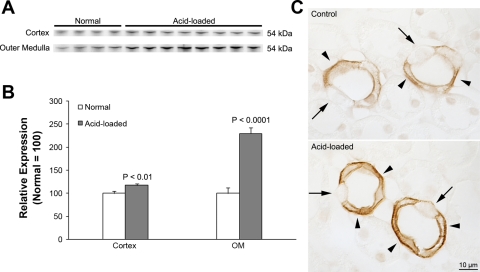
The increased urinary NH3 concentration on day 3 of acid loading in IC-Rhcg-KO mice suggests there may have been an increase at that time in NH3 transport mechanisms that do not involve intercalated cell Rhcg. Because IC-Rhcg-KO mice have intact Rhcg expression in principal cells (Fig. 2), one possibility is increased principal cell-specific Rhcg expression. To test this possibility, we examined Rhcg expression in IC-Rhcg-KO mice that were either acid loaded or received a normal diet for 3 days. Immunoblot analysis showed that Rhcg expression in both the cortex and outer medulla of IC-Rhcg-KO mice increased significantly in response to acid loading (Fig. 5). Immunohistochemistry confirmed increased Rhcg immunolabel in principal cells in both the CCD and the OMCD (Fig. 5). Double-immunolabel studies of kidneys from acid-loaded IC-Rhcg-KO mice using antibodies to Rhcg and to H+-ATPase confirmed that the increase in Rhcg expression occurred in principal cells and that it did not reflect induction of intercalated cell-specific Rhcg expression (Fig. 6). Thus principal cell Rhcg expression increases in IC-Rhcg-KO mice in response to metabolic acidosis and may thereby contribute to acidosis-stimulated ammonia excretion.
Fig. 5.
Rhcg expression in IC-Rhcg-KO mice fed a normal and acid diet. A: immunoblot analysis of Rhcg expression in the cortex and outer medulla. Rhcg protein expression in IC-Rhbg-KO mice is increased by acid loading. B: quantification of Rhcg protein expression. Cortical and outer medullary Rhcg expression is significantly greater in acid-loaded than in normal diet IC-Rhcg-KO mice. C: Rhcg immunolabel in the OMCD of normal and acid-loaded diet IC-Rhcg-KO mice. Acid loading increases Rhcg immunolabel in OMCD principal cells (arrowheads) compared with IC-Rhcg-KO mice fed a normal diet. Intercalated cells (arrows) do not express significant Rhcg immunolabel. Values are means ± SE; n = 4, normal diet and n = 8, acid-loaded diet.
Fig. 6.
Double-immunolabel for Rhcg and H+-ATPase in acid-loaded mice. A and B: Rhcg immunolabel (brown) and H+-ATPase immunolabel (blue) in CCD of acid-loaded C and IC-Rhcg-KO mice, respectively. In C mice, abundant Rhcg immunolabel is present in both principal cells and type A intercalated cells (arrowheads). Type B intercalated cells, identified by basolateral H+-ATPase immunolabel (arrow), do not express detectable Rhcg immunolabel. In IC-Rhcg-KO mice, neither type A intercalated cells (arrowheads) nor type B intercalated cells (arrow) express Rhcg immunolabel. C and D: Rhcg immunolabel and H+-ATPase immunolabel in OMCD of acid-loaded C and IC-Rhcg-KO mice, respectively. In C mice, abundant Rhcg immunolabel is present in both principal cells and IC (arrowheads). IC-Rhcg-KO mice do not express Rhcg immunolabel in intercalated cells; principal cell Rhcg immunolabel is intact. E and F: Rhcg immunolabel and H+-ATPase immunolabel in IMCD of acid-loaded control and IC-Rhcg-KO mice, respectively. In C mice, abundant Rhcg immunolabel is present only in intercalated cells (arrowheads). IC-Rhcg-KO do not express Rhcg immunolabel in either intercalated cells (arrowheads) or in nonintercalated cells. G, glomerulus; P, proximal tubule.
Rhbg expression in response to acid loading.
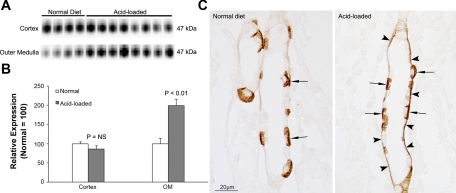
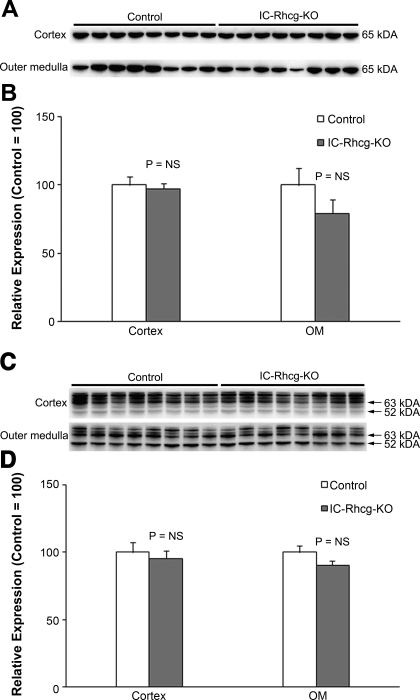
Rhbg is an ammonia-specific transporter (20, 21, 27) that is a homolog of Rhcg (19, 35, 38) and is expressed in the collecting duct in the same cells as Rhcg (29, 32). Under basal conditions, Rhbg expression did not differ between C and IC-Rhcg-KO mice (data not shown). However, acid loading increased Rhbg expression significantly in the outer medulla, but not the cortex, of IC-Rhcg-KO mice (Fig. 7). Immunohistochemistry identified increased Rhbg expression in OMCD principal cells (Fig. 7). Thus adaptations in Rhbg expression in principal cells occur in response to acid loading, suggesting Rhbg can contribute to renal ammonia metabolism.
Fig. 7.
Rhbg expression in IC-Rhcg-KO mice fed a normal and acid diet. A: immunoblot analysis of Rhbg expression in the cortex and outer medulla. B: quantification of Rhbg protein expression. Cortical Rhbg expression is not significantly different between IC-Rhcg-KO mice fed a normal and acid diet. In the outer medulla, Rhbg expression is significantly greater in acid-loaded than in normal-diet IC-Rhcg-KO mice. C: Rhbg immunolabel in the OMCD of normal-diet and acid-loaded IC-Rhcg-KO mice. Basolateral Rhbg immunolabel in OMCD principal cells (arrowheads) is increased in acid-loaded IC-Rhcg-KO mice relative to immunolabel in normal-diet IC-Rhcg-KO mice. Intercalated cell (arrows) basolateral Rhbg immunolabel appear unchanged.
Effect of intercalated cell-specific Rhcg deletion on enzymes involved in ammonia metabolism.
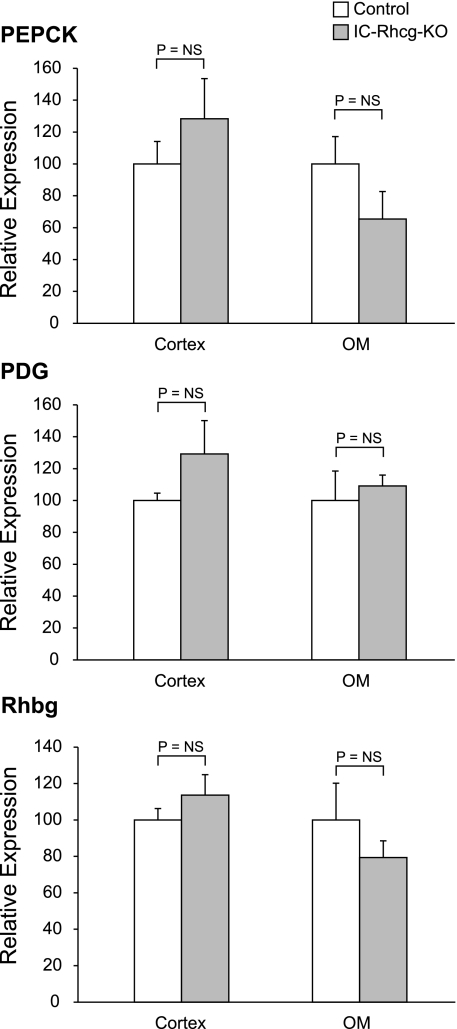
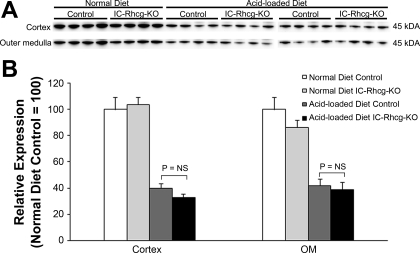
Because changes in ammonia-metabolizing enzymes in the proximal tubule are an important component of the regulation of renal ammonia excretion, we examined whether adaptive changes compensated for the lack of Rhcg in intercalated cells. With a normal diet, there was no significant difference in PEPCK or PDG expression between C or IC-Rhcg-KO mice in either the cortex or outer medulla (Fig. 8). Similarly, after acid loading, neither PEPCK nor PDG protein expression differed between C and IC-Rhcg-KO mice in either the cortex or outer medulla (Fig. 9).
Fig. 8.
Effect of IC-Rhcg-KO on phosphoenolpyruvate carboxykinase (PEPCK), phosphate-dependent glutaminase (PDG), and Rhbg expression in mice fed a normal diet. Rhbg, PEPCK, and PDG expression was quantified using immunodot analysis. A: PEPCK expression. IC-Rhcg-KO did not alter PEPCK expression in either the cortex or outer medulla (OM) in mice on a normal diet. B: PDG expression. IC-Rhcg-KO did not alter PDG expression in either the cortex or OM in mice on a normal diet. C: Rhbg expression. IC-Rhcg-KO did not alter Rhbg expression in either the cortex or OM in mice on a normal diet; n = 4/group.
Fig. 9.
PEPCK and PDG expression in acid-loaded IC-Rhcg-KO and C kidneys. A: immunoblot analysis of PEPCK expression in the cortex and OM of acid-loaded IC-Rhcg-KO and C mice. B: quantification of PEPCK protein expression. There was no significant difference in PEPCK expression between acid-loaded C and IC-Rhcg-KO mice in either the cortex or OM. C: immunoblot analysis of PDG expression in the cortex and OM of acid-loaded IC-Rhcg-KO and C mice. D: quantification of PDG protein expression. There was no significant difference in PDG expression between acid-loaded C and IC-Rhcg-KO mice in either the cortex or OM. Expression normalized to mean expression was equal to 100 in control mice in each region for each protein. Values are means ± SE; n = 8/group.
Glutamine synthetase is expressed in the late proximal tubule and may contribute to the regulation of net proximal tubule ammonia production by catalyzing the reaction of NH4+ with glutamate to form glutamine, thereby decreasing net ammonia production. With a normal diet, intercalated cell-specific Rhcg deletion did not significantly alter glutamine synthetase expression in either the cortex or the outer medulla of mice (Fig. 10). Acid loading decreased glutamine synthetase expression significantly in the cortex and outer medulla in both C and IC-Rhcg-KO mice, but the magnitude of the decrease did not differ between the genotypes. Thus intercalated cell-specific Rhcg deletion does not alter expression of PEPCK, PDG, or glutamine synthetase, either with a normal diet or after HCl-induced acid loading.
Fig. 10.
Glutamine synthetase (GS) expression in normal and acid-loaded IC-Rhcg-KO and C kidneys. A: immunoblot analysis of GS expression in the cortex and OM of IC-Rhcg-KO and C mice on a normal diet and when acid loaded. B: quantification of GS protein expression. GS expression did not differ significantly between C and IC-Rhcg-KO mice when on normal diet. Acid loading decreased GS expression compared with a normal diet, but there was no significant difference in GS expression in acid-loaded C and IC-Rhcg-KO mice in either the cortex or OM. Values are means ± SE; n = 4/group on a normal diet and n = 8/group on an acid-loaded diet. Results normalized to control mice on a normal diet was equal to 100.
DISCUSSION
The current studies examine the molecular mechanisms of intercalated cell-mediated ammonia secretion through the use of intercalated cell-specific Rhcg gene deletion. Several important observations result from these studies. First, intercalated cell Rhcg expression is necessary for the normal sustained increase in ammonia excretion in response to metabolic acidosis, but not for basal ammonia excretion. Second, principal cell Rhcg expression may contribute to both basal and acidosis-stimulated ammonia excretion. Third, increased Rhbg expression in principal cells in response to acid-loading suggests that Rhbg may contribute to renal ammonia excretion. These findings have important implications for our understanding of renal ammonia metabolism.
During the last decade, a novel, extended family of proteins has been recognized as having an important role in prokaryotic and eukaryotic ammonia metabolism. This family includes Mep proteins in yeast, Amt proteins in bacteria and plants, and Rh glycoproteins in higher organisms (22–24, 26, 28, 33, 35). Mammalian Rh glycoproteins are ammonia-specific transporters that are widely expressed in ammonia-transporting tissues, including kidneys, liver, gastrointestinal tract, lungs, skeletal muscle, and central nervous system (5, 9, 13, 18, 19, 29, 32, 39). Identification of these proteins has resulted in a fundamental change in our understanding of transcellular ammonia transport by leading to the recognition that NH3 transport involves specific proteins and is not solely mediated by diffusive movement. Thus NH3 is similar to other small, uncharged molecules, such as H2O and urea, in that it requires integral membrane proteins, in this case, Rh glycoproteins, for normal rates of transmembrane movement observed in the kidney.
A substantial body of evidence supports the essential role of Rhcg in intercalated cell-mediated renal ammonia excretion. The first evidence that intercalated cell-specific Rhcg-mediated ammonia transport was important in acid-base homeostasis were studies showing increased apical and basolateral plasma membrane Rhcg expression in intercalated cells in response to metabolic acidosis (30, 31). This increase involved at least two distinct mechanisms, increased total cellular Rhcg abundance and alterations in Rhcg's subcellular distribution, with redistribution from cytoplasmic sites to the apical plasma membrane (31). Other conditions in which intercalated cell Rhcg expression parallels ammonia excretion include reduced renal mass (15), ischemia-reperfusion injury (10), and cyclosporin nephropathy (17). Most recently, Rhcg gene-deletion studies confirmed a critical role of Rhcg in renal ammonia excretion (1), and studies using collecting duct-specific Rhcg deletion localized this role to the collecting duct, where intercalated cells reside (16). The current studies extend these previous studies by demonstrating that intercalated cell-specific Rhcg deletion is sufficient to impair the normal increase in renal ammonia excretion that occurs in response to metabolic acidosis, thereby confirming the importance of both Rhcg and intercalated cells in renal ammonia excretion and acid-base homeostasis.
It is important to recognize, however, that the current study likely underestimates the role of intercalated cell-specific Rhcg expression in acidosis-stimulated ammonia excretion. Acid-loaded IC-Rhcg-KO mice excreted more acidic urine than did control mice during the first 2 days of acid loading. Because luminal acidification independently increases collecting duct ammonia secretion (6, 7) and increases the diffusive component of collecting duct apical NH3 transport (12), the greater degree of urinary acidification in IC-Rhcg-KO mice likely causes an underestimation of the role of intercalated cell-specific Rhcg expression in acidosis-stimulated ammonia excretion. Indeed, it is likely that the inhibition of NH3 transport induced by intercalated cell-specific Rhcg deletion, by inhibiting transport of this basic compound into the luminal fluid, accounts, at least in part, for the greater degree of urinary acidification seen in acid-loaded mice with intercalated cell-specific Rhcg deletion.
In contrast to the necessary role of intercalated cell Rhcg in the renal response to metabolic acidosis, its expression does not appear necessary for normal rates of basal ammonia excretion. This contrasts with findings in studies in mice with either global Rhcg deletion (1) or combined intercalated and principal cell (i.e., collecting duct-specific) Rhcg deletion (16), where basal ammonia excretion was significantly decreased. Likely explanations for these differences between intercalated cell-specific and both global and collecting duct-specific Rhcg deletion are either that basal ammonia excretion involves principal cell-mediated ammonia secretion, at least when Rhcg is deleted from intercalated cells. Although it is possible that other adaptive mechanisms compensate when intercalated cell Rhcg is deleted, we identified no adaptive changes in PEPCK, PDG, glutamine synthetase, or Rhbg expression in response to intercalated cell-specific Rhcg deletion in mice on a normal diet.
The current study also adds additional evidence supporting the possibility that principal cell-specific Rhcg expression contributes significantly to both basal and acidosis-stimulated ammonia excretion. Both intercalated and principal cells express Rhcg (14, 30–32), and although principal cells express less Rhcg than intercalated cells, there are approximately twice as many principal cells as intercalated cells in both the CCD and OMCD. Direct measurements demonstrate that principal cell apical NH3 permeability, corrected for differences in apical plasma membrane folding, is ∼50% of that observed in intercalated cells (41). In addition to expressing Rhcg, principal cells express all of the critical transport activities necessary for H+ secretion and HCO3− reabsorption, including apical H+-ATPase and H+-K+-ATPase activities (36, 40) and basolateral Cl−/HCO3− exchange activity (37, 40). In the current study, normal basal ammonia excretion was maintained with only principal cell-specific Rhcg expression, whereas deletion of both intercalated cell and principal cell Rhcg in either a global Rhcg knockout model or a collecting duct-specific Rhcg knockout model decreases basal ammonia excretion significantly (1, 16). Although CNT cell Rhcg expression is preserved in both the current study and with collecting duct-specific Rhcg deletion, CNT cell Rhcg expression alone is not sufficient to maintain normal rates of basal ammonia excretion (16). Thus the finding of normal basal ammonia excretion in the current study suggests that principal cell-specific Rhcg expression is critical for basal ammonia excretion rates.
Principal cell Rhcg expression also is likely to contribute to adaptive changes in ammonia excretion. Increased principal cell Rhcg expression occurs both in chronic metabolic acidosis (31 and current study) and with reduced renal mass (15), suggesting that increased principal cell-specific Rhcg-mediated ammonia transport contributes to ammonia excretion in these conditions. The observation that urinary ammonia excretion does not increase on the first 2 days of acid loading in mice with combined intercalated and principal cell Rhcg deletion (16), but does increase in mice in which Rhcg is deleted only from intercalated cells (current study), also supports a role of principal cell Rhcg in the response to metabolic acidosis.
The results in the current report also provide additional evidence regarding the role of the Rhbg in renal physiology. Rhbg is an Rh glycoprotein that is closely related to Rhcg. It has ∼80% amino acid homology and a similar predicted tertiary structure to Rhcg, transports ammonia when expressed in heterologous expression systems (20, 21, 27), and is expressed in the basolateral plasma membrane of the same renal epithelial cells that express Rhcg (29, 30, 32). The role of Rhbg in renal ammonia excretion is incompletely defined at present. A previous study using genetic deletion of Rhbg and a different model of acidosis did not detect differences in either basal or acidosis-stimulated ammonia excretion (3), suggesting that Rhbg may not contribute to renal ammonia metabolism. Conversely, OMCD Rhbg expression increases in acid-loaded mice with either collecting duct-specific or intercalated cell-specific Rhcg deletion compared with acid-loaded mice with intact Rhcg expression (Ref. 16 and current study). These latter findings are consistent with adaptive changes in Rhbg expression contributing, under at least some conditions, to renal ammonia excretion. Whether RhBG is involved in human kidney ammonia metabolism is unclear; although high levels of RhBG mRNA are present (19), a recent study failed to detect RhBG protein expression in the human kidney (2).
The role of glutamine synthetase in the renal response to acidosis has been somewhat controversial. Previous studies in the rat did not find changes in glutamine synthetase expression (34), whereas studies in the mouse showed decreased glutamine synthetase expression and activity (4). The current findings of decreased glutamine synthetase protein expression in acid-loaded C and IC-Rhcg-KO mice provide further evidence that glutamine synthetase mediates an important role in acid-base homeostasis, at least in mice.
In summary, the current studies provide important new information regarding the roles of intercalated cells and principal cells in renal ammonia metabolism. These studies show that intercalated cell-specific Rhcg expression is not necessary for basal ammonia excretion or the early response to metabolic acidosis, but is necessary for normal delayed increases in ammonia excretion in response to metabolic acidosis, thereby demonstrating a central role of the intercalated cell in acid-base homeostasis. Principal cell Rhcg expression appears to contribute to both basal and metabolic acidosis-stimulated ammonia excretion. Third, by showing increased Rhbg expression in response to acid-loading in the OMCD of IC-Rhcg-KO mice, these studies support a role for Rhbg in renal ammonia metabolism.
GRANTS
This work was supported by funds from the National Institutes of Health (NIDDK R01–45788), the Gatorade Research Fund, and the Research Service of the Gainesville Veterans Affairs Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Gina Cowsert for secretarial assistance and Dr. Sharon W. Matthews for expert assistance in preparing tissues for immunohistochemistry.
Footnotes
Ammonia consists of two molecular species, NH3 and NH4+, which are in equilibrium with each other according to the reaction NH3 + H+ ↔ NH4+. We use the term “ammonia” to refer to the sum of these two molecular forms and refer to each molecular form as either “NH3” or “NH4+,” respectively.
REFERENCES
- 1.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Brown ACN, Hallouane D, Mawby WJ, Karet FE, Saleem MA, Howie AJ, Toye AM. RhCG is the major putative ammonia transporter expressed in human kidney and RhBG is not expressed at detectable levels. Am J Physiol Renal Physiol 296: F1279–F1290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol 289: F1281–F1290, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Conjard A, Komaty O, Delage H, Boghossian M, Martin M, Ferrier B, Baverel G. Inhibition of glutamine synthetase in the mouse kidney: a novel mechanism of adaptation to metabolic acidosis. J Biol Chem 278: 38159–38166, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Flessner MF, Wall SM, Knepper MA. Permeabilities of rat collecting duct segments to NH3 and NH4+. Am J Physiol Renal Fluid Electrolyte Physiol 260: F264–F272, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Hamm LL, Trigg D, Martin D, Gillespie C, Buerkert J. Transport of ammonia in the rabbit cortical collecting tubule. J Clin Invest 75: 478–485, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C Glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han KH, Mekala K, Babida V, Kim HY, Handlogten ME, Verlander JW, Weiner ID. Expression of the gas transporting proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the murine lung. Am J Physiol Lung Cell Mol Physiol 297: L153–L163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han KH, Kim HY, Croker BP, Reungjui S, Lee SY, Kim J, Handlogten ME, Adin CA, Weiner ID. Effects of ischemia-reperfusion injury on renal ammonia metabolism and the collecting duct. Am J Physiol Renal Physiol 293: F1342–F1354, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Han KH, Lee SY, Kim WY, Shin JA, Kim J, Weiner ID. Expression of ammonia transporter family members, Rh B glycoprotein and Rh C glycoprotein, in the developing rat kidney. Am J Physiol Renal Physiol (doi:10.1152/ajprenal.00607.2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289: F347–F358, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the intestinal tract. Am J Physiol Gastrointest Liver Physiol 288: G1036–G1047, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member, Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 296: F545–F555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C Glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim SW, Ahn KO, Kim WY, Han DH, Li C, Ghee JY, Han KH, Kim HY, Handlogten ME, Kim J, Yang CW, Weiner ID. Expression of ammonia transporters, Rhbg and Rhcg, in chronic cyclosporine nephropathy in rats. Nephron Exp Nephrol 110: e49–e58, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Chen Y, Mo R, Cc Hui Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem 275: 25641–25651, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Peng J, Mo R, Cc Hui, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem 276: 1424–1433, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Ludewig U. Electroneutral ammonium transport by basolateral Rhesus B glycoprotein. J Physiol 559: 751–759, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marini AM, Soussi-Boudekou S, Vissers S, Andre B. A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol 17: 4282–4293, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marini AM, Vissers S, Andre B. Ammonium transport in Saccharomyces cerevisiae. Yeast 11: 425, 1995. 7597846 [Google Scholar]
- 24.Marini AM, Vissers S, Urrestarazu A, Andre B. Cloning and expression of the MEP1 gene encoding an ammonium transporter in Saccharomyces cerevisiae. EMBO J 13: 3456–3463, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, Nelson RD. The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakhoul NL, Hamm LL. Non-erythroid Rh glycoproteins: a putative new family of mammalian ammonium transporters. Pflügers Arch 447: 807–812, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Nakhoul NL, DeJong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol 288: F170–F181, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Ninnemann O, Jauniaux JC, Frommer WB. Identification of a high affinity NH4+ transporter from plants. EMBO J 13: 3464–3471, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F1443–F1452, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003 [DOI] [PubMed] [Google Scholar]
- 33.von Wiren N, Gazzarrini S, Gojon A, Frommer WB. The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol 3: 254–261, 2000 [PubMed] [Google Scholar]
- 34.Watford M. Regulation of expression of the genes for glutaminase and glutamine synthetase in the acidotic rat. Contrib Nephrol 92: 211–217, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Weiner ID. The Rh gene family and renal ammonium transport. Curr Opin Nephrol Hyper 13: 533–540, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Weiner ID, Frank AE, Wingo CS. Apical proton secretion by the inner stripe of the outer medullary collecting duct. Am J Physiol Renal Physiol 276: F606–F613, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Weiner ID, Hamm LL. Regulation of intracellular pH in the rabbit cortical collecting tubule. J Clin Invest 85: 274–281, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B Glycoprotein and Rh C Glycoprotein in the mouse liver. Gastroenterology 124: 1432–1440, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Weiner ID, Wingo CS, Hamm LL. Regulation of intracellular pH in two cell populations of the inner stripe of the rabbit outer medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F406–F415, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Yip KP, Kurtz I. NH3 permeability of principal cells and intercalated cells measured by confocal fluorescence imaging. Am J Physiol Renal Fluid Electrolyte Physiol 269: F545–F550, 1995 [DOI] [PubMed] [Google Scholar]