Abstract
We have previously developed bioengineered three-dimensional internal anal sphincter (IAS) rings from circular smooth muscle cells isolated from rabbit and human IAS. We provide proof of concept that bioengineered mouse IAS rings are neovascularized upon implantation into mice of the same strain and maintain concentric smooth muscle alignment, phenotype, and IAS functionality. Rings were bioengineered by using smooth muscle cells from the IAS of C57BL/6J mice. Bioengineered mouse IAS rings were implanted subcutaneously on the dorsum of C57BL/6J mice along with a microosmotic pump delivering fibroblast growth factor-2. The mice remained healthy during the period of implantation, showing no external signs of rejection. Mice were killed 28 days postsurgery and implanted IAS rings were harvested. IAS rings showed muscle attachment, neovascularization, healthy color, and no external signs of infection or inflammation. Assessment of force generation on harvested IAS rings showed the following: 1) spontaneous basal tone was generated in the absence of external stimulation; 2) basal tone was relaxed by vasoactive intestinal peptide, nitric oxide donor, and nifedipine; 3) acetylcholine and phorbol dibutyrate elicited rapid-rising, dose-dependent, sustained contractions repeatedly over 30 min without signs of muscle fatigue; and 4) magnitudes of potassium chloride-induced contractions were 100% of peak maximal agonist-induced contractions. Our preliminary results confirm the proof of concept that bioengineered rings are neovascularized upon implantation. Harvested rings maintain smooth muscle alignment and phenotype. Our physiological studies confirm that implanted rings maintain 1) overall IAS physiology and develop basal tone, 2) integrity of membrane ionic characteristics, and 3) integrity of membrane associated intracellular signaling transduction pathways for contraction and relaxation by responding to cholinergic, nitrergic, and VIP-ergic stimulation. IAS smooth muscle tissue could thus be bioengineered for the purpose of implantation to serve as a potential graft therapy for dysfunctional internal anal sphincter in fecal incontinence.
Keywords: fecal incontinence, tissue engineering, smooth muscle contraction
fecal incontinence (FI) is an embarrassing disorder that can have overwhelming social and psychological impacts on an individual. The prevalence of FI has been estimated to fall within the range of 2.2–15%, with the numbers increasing with the age of the population. Although the etiology of FI can be multifactorial, it is well established that FI is secondary to obstetric trauma, idiopathic sphincteric degeneration and/or dysfunction, anorectal surgeries, and irritable bowel syndromes (1, 2).
The internal anal sphincter (IAS) contributes to 70% of the high anal canal pressure required to maintain rectoanal continence. This high pressure, dubbed as the basal sphincteric tone, arises from the state of contracture of the circular smooth muscle of IAS (29). The source of this high basal tone is thought to be primarily myogenic (25) and is modulated by neurohumoral components. Passage of colonic content through the anal canal during defecation is accompanied by relaxation of the IAS smooth muscle, with an associated giant migrating contraction propagating caudally in the colon. IAS dysfunction can largely be attributed to its weakened mechanical efficiency at the smooth muscle level or pudendal neuropathies (16, 24). Loss of basal sphincteric tone due to idiopathic degeneration of smooth muscle cells, stretching of elastic tissue and heightened collagen/fibril content as a result of aging, surgical trauma, or gut resections all lead to FI (19).
Conventional treatment options like dietary manipulations and antidiarrheal drugs can significantly reduce FI associated with chronic diarrhea. Other therapeutic efforts in FI focused on sacral nerve modulation, electrically stimulated gracilis muscle transposition, or biofeedback rehabilitation of the pelvic floor are not documented to be effective. Although these treatment options might provide relief in FI symptoms in individuals with structurally intact sphincters, sphincteric damage and dysfunction can only be corrected by invasive surgical options. Injection of bulking agents like glutaraldehyde cross-linked collagen, polytetrafluoroethylene, polydimethylsiloxane, etc., to improve sphincter function is accompanied by the danger of allergy and the possibility of migration of these particles into the lymphatic circulation, triggering a more severe allergic or foreign body response. Artificial anal sphincters could be implanted but present with the potential of device failure, infection of the electrodes, or severe foreign body reactions (11, 20, 29, 30).
The lack of an ideal therapeutic option for a disabling state such as FI has prompted our laboratory to develop a bioengineering solution focused on providing a positive remedy to FI due to degenerated IAS. We have previously bioengineered functional three-dimensional IAS constructs from mice, rabbits, and humans out of isolated circular smooth muscle cells from their respective internal anal sphincters (14, 27). Recently, we bioengineered mouse IAS rings for the purpose of implantation. We provide proof of concept that implantation of bioengineered IAS constructs into mice (of the same strain as the cell source) display healthy vascularization, physiological functionality before and after implantation, and no signs of rejection or inflammation.
Intestinal tissue engineering has shown exciting prospects in short bowel syndrome with and without the use of scaffolds (18). Skeletal muscle and cardiac pressure generating constructs have been developed before and tested in vitro for physiological function, but a successful implantation and comparisons of functionality have previously not been worked on (10, 15).
In this study, IAS rings were bioengineered in a parallel manner from circular smooth muscle cells from the mouse internal anal sphincter. One ring was tested for physiological functionality and another was implanted into an animal of the same strain. Harvested postimplant IAS displayed neovascularization. The functional studies on the postimplant harvested constructs focused on the following: 1) maintenance of overall IAS physiology; 2) maintenance of membrane ionic characteristics; 3) integrity of membrane receptors and intracellular signaling pathways for contraction and relaxation of IAS. Implanted rings showed similar functional behavior to bioengineered IAS rings preimplantation in their ability to 1) generate spontaneous basal tone; 2) contract in response to potassium chloride, acetylcholine, and phorbol dibutyrate; and 3) relax in response to vasoactive intestinal peptide (VIP), nifedipine, and nitric oxide donor sodium nitroprusside (SNP).
Our experiments provide proof of concept that bioengineered IAS constructs can be implanted in the animals without rejection, are neovascularized, and can remain physiologically functional. This implantable bioengineered IAS tissue could be used as autologous graft tissue for degenerated IAS. Combined with an increased knowledge of molecular mechanisms underlying smooth muscle function, this opens out doors to novel therapeutic options for severe FI attributed to IAS dysfunction.
METHODS
Animals.
C57BL/6J female, specific pathogen-free mice (8 wk old) were obtained from Jackson Laboratory (Bar Harbor, ME) and maintained under temperature-, humidity-, and light-controlled conditions. The study conformed to the Guidelines for the Care and Use of Laboratory Animals established by the University Committee on Use and Care of Animals at the University of Michigan, and protocols were approved by that committee (no. 09714).
Reagents.
All tissue culture reagents, including Dulbecco's modified Eagle's medium (DMEM), trypsin, media supplements like l-glutamine, and antibiotics, were purchased from Invitrogen (Carlsbad, CA). Sylgard, i.e., Poly(dimethylsiloxane), was purchased from World Precision Instruments (Sarasota, FL). Acetylcholine, VIP, phorbol dibutyrate, nifedipine, and SNP were obtained from Sigma (St. Louis, MO).
Isolation and culture of IAS cells.
IAS was identified and cultured as previously described (3, 5, 14, 27). Briefly, C57BL/6J mice were euthanized by carbon dioxide inhalation. Mouse sigmoid colon and rectum along with the anal canal was removed by sharp dissection. The tissue was kept moist in Hanks' balanced salt solution (HBSS; Hyclone, Logan, UT) containing antibiotics and antimycotics at 4°C. The fecal content was cleaned and it was slipped onto a moist Pasteur pipette. Adherent fat, connective tissue, striated muscle, blood vessels, and serosa were stripped off and cleaned with HBSS-wetted Kimwipe (Kimberly-Clark, Neenah, WI). The tissue was cut along its longitudinal axis and the mucosa was stripped off. The IAS was identified as a 2- to 3-mm strip of circular smooth muscle at the distal end of the rectum under a dissection microscope. IAS circular smooth muscle was removed by sharp dissection. Isolated IAS was then minced and digested twice with 0.1% type II collagenase (Worthington Biochemicals, Lakewood, NJ) and 0.1% 0.2 mg/ml DNAse (Roche Applied Sciences, Indianapolis, IN) at 32°C for 1 h each and filtered through a 500-μm Nytex (Tetko) mesh. The circular smooth muscle cells were collected by centrifuging and resuspended and washed three times to remove excess collagenase. Dispersed circular smooth muscle cells were then plated with growth media (GM: DMEM + 10% fetal bovine serum + 1.5% antibiotics/antimycotics + 1.5% l-glutamine) on to regular tissue culture T-75 flasks. Plates were incubated at 37°C with 5% CO2, with an accompanied medium change every 48 h until the cells were ready to be used.
Preparation of culture dishes.
The 35-mm tissue culture plates were coated with 0.8 ml Sylgard and allowed to cure for 2–3 days. Sylgard molds (5-mm diameter) were fixed to the center of the plate with insect pins to define luminal space. The plates were sterilized with ethanol and under UV light for 30–45 min. Thrombin (400 μl; 10.5 U/ml in GM) was mixed with 200 μl of fibrinogen (20 mg/ml) on the Sylgard-coated culture dish. In 30 min, the thrombin and fibrinogen polymerized to form a loose fibrin gel.
Bioengineering three-dimensional mouse IAS constructs.
Circular smooth muscle cells from the IAS were lifted off from their culture surface with 3 ml 0.25% trypsin-EDTA. The harvested cells were centrifuged and counted with a hemocytometer. Cell suspension concentration was adjusted to 1 × 105 cells/ml of GM; 2 ml of this cell suspension was plated on top of the loose fibrin gel in each prepared culture plate. As the cells proliferated in the fibrin gel, they started contracting the gel, toward the center of the plate where the post was affixed. GM was switched out to differentiation medium (DM: DMEM 365 ml, Media-199 100 ml, horse serum 35 ml, Pen/Strep 5 ml). This aided smooth muscle differentiation. The cells migrated in the fibrin gel, arranged themselves concentrically around the Sylgard post, and resulted in a thick three-dimensional IAS ring held together by cellular ECM in 4–6 days. The DM was replenished every 2 days. At this point, they were ready to be tested for physiological functionality and implantation.
Surgical procedure.
Surgical procedure for implantation of bioengineered IAS constructs has been described by Hashish et al. (13). Briefly, the Alzet model 1004 microosmotics pumps (Durect, Cupertino, CA) were primed per manufacturer's instructions. Sufficient volume of human recombinant fibroblast growth factor-2 (ProSpec-Tany TechnoGene) was loaded into pumps to allow for continuous infusion over a 21-day period. A sterile polyethylene catheter (0.76 mm, Durect) was attached. This pump-catheter complex was kept in sterile 0.9% saline at room temperature for 48 h prior to implantation.
The mice were anesthetized using an intraperitoneal injection of 100 mg/kg of ketamine and 5 mg/kg xylazine. After confirmation of adequate anesthesia, the surgical area on the back of the mouse was clipped of hair and prepped with chlorhexidine.
A 2-cm transverse skin incision was made in the upper back of the mouse (midscapular incision). A subcutaneous pocket was created caudally, which was made large enough to fit the pump and catheter comfortably. The bioengineered IAS ring was inserted near the tip of the catheter, ∼0.5 cm away from the skin incision. A 6-0 Prolene suture was placed loosely around the ring to mark its position. The catheter tip was then cut to length so that the tip was directed toward the implanted ring. The catheter was then secured to the underlying tissue by a single 4-0 Prolene suture.
The skin incision was then closed with 4-0 Vicryl sutures. The animals were returned to an approved facility for recovery in standard fashion.
Ring harvest.
Mice were euthanized 25–28 days postimplantation. The area of the previous incision was reentered and the rings were smoothly removed from the surrounding tissue after localization of the Prolene suture. The harvested construct was placed in PBS and was assessed immediately for physiological functionality as per protocols outlined below.
Histology.
Following measurement of physiological functionality (below), half of the harvested ring tissue was placed in zinc-formalin for fixation, and the other half was placed in Tissue-Tek OCT compound (Ted Pella) and frozen in liquid nitrogen. Slides were then prepared and sectioned by the Tissue Core at the University of Michigan Comprehensive Cancer Center. Hematoxylin and eosin (H&E) and Masson's trichrome staining were used for morphological assessment. Immunofluorescence assays were carried out for α-smooth muscle actin (F3777, Sigma), smooth muscle-specific heavy caldesmon (c-4562, Sigma), and endothelial-specific antigen von Willebrand's factor (factor VIII; sc-14014, Santa Cruz Biotechnology). Slides were visualized by an Olympus FV500 fluorescence microscope in the University of Michigan Microscope and Image Analysis Laboratory.
Western blot analysis.
A small portion of the postimplant bioengineered IAS ring and intact mouse IAS tissue were minced, sonicated, and lysed in RIPA buffer. Equal amounts of protein were separated on SDS-PAGE and electrophoretically transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% nonfat dry milk for 1 h and incubated with the appropriate dilution of the following primary antibodies in 3% nonfat dry milk in PBS with Tween-20 (PBST): c-Kit (sc-168, Santa Cruz Biotechnology) and prolyl-4-hydroxylase (MAB2073 Millipore). β-Actin (A5441, Sigma) was used as a loading control. Unbound primary antibody was removed by washing with PBST. The membrane was then incubated for 1 h with the appropriate dilution of horseradish peroxidase-conjugated secondary antibody. PBST washes were used to remove unbound secondary antibody. Blots were visualized with chemiluminescence.
Measurement of physiological functionality.
Protocols for measurement of force generation were adapted from previous work on three-dimensional IAS constructs (14, 27). The tissue bath in the force transducer set up consisted of a Sylgard coated 35-mm culture plate with a stainless steel pin fixed to the center of the plate. The plate held 4 ml of basal serum-free DMEM and was placed on a heated aluminum platform that maintained the temperature at 37°C ± 1°C. Before any measurements were made, noise was measured by recording tension with air and warm bath fluid without hooking the bioengineered tissue. The mean noise due to bath fluid was subtracted from total tension recordings with the tissue. Passive tension was obtained by hooking on the tissue with no stretch in zero calcium + 50 mM EGTA at the end of the day. This passive tension value was subtracted from total tension recordings with the tissue to obtain active tension. Values of force reported are only active tension values.
The IAS ring was separated from the Sylgard mold using forceps, and one end was anchored to the fixed pin in the tissue bath. The other end of the ring was hooked on to the movable measuring arm of an isometric force transducer (Harvard Apparatus, Holliston, MA). Stock solutions of the contractile and relaxant agonists were 40 times more concentrated than the required final concentration so that a uniform application of 100 μl resulted in the desired final concentrations in a 4-ml static bath. Bath diffusion times were assessed by use of methylene blue and averaged up to 44.3 ± 2.8 s for complete uniform diffusion of 100 μl in 4 ml. Basal DMEM soaking the ring was changed every 30 min, or at the end of a set of experiments, whichever was earlier.
Testing protocol.
Bioengineered rings or harvested postimplant rings were hooked on to the force transducer with no additional stretch. Basal tone was established spontaneously with no external stimulation or stretch over time as shown in Fig. 7. A 20% stretch was applied on the constructs by a micromanipulator and this remained the length at which all tests were carried out. The baseline of force established by the rings at this length was arbitrarily set to zero, to observe effects of drugs on the constructs. The rings were allowed to develop stable baseline before any contractile agonist/relaxant peptide was added to the tissue bath. Maintenance of membrane characteristics were studied by treatment with 30 mM potassium chloride. Receptor integrity and intracellular signaling mechanism preservation was studied by observing 1) relaxation of the basal tone of the IAS ring by addition of 1 μM VIP, 10 μM nifedipine, and 10 μM SNP; 2) cholinergic stimulation with 1 μM acetylcholine; 3) and direct stimulation of signal pathway intermediary PKC with 1 μM phorbol dibutyrate.
Fig. 7.
Spontaneous tone. The harvested postimplant IAS (B) as well as the bioengineered ring (A) displayed the ability to generate spontaneous basal tone, in the absence of any external stimulation. Active tone in the postimplant harvested IAS was 114 ± 28.4 μN, whereas the active tone in the preimplant rings was 94.7 ± 19.9 μN. Values are means ± SE of 6–10 individual animal experiments. Graphs are representative tracings to display the trend of basal tone establishment.
Data analysis.
Data was acquired by LabScribe2 (iWorx, Dover, NH) at a frequency of 0.02 Hz. GraphPad Prism 5.01 for Windows (GraphPad Software, San Diego, CA; www.graphpad.com) was used for all further data analysis. Second-order Savitsky-Golay smoothing was applied on raw data. Values were expressed as means and SE of 4–10 experiments. Contraction and relaxation were quantified as a percentage of each individual construct's basal tone. KCl-induced contraction was expressed as a percentage of peak maximal contraction induced by acetylcholine.
RESULTS
Bioengineering IAS rings.
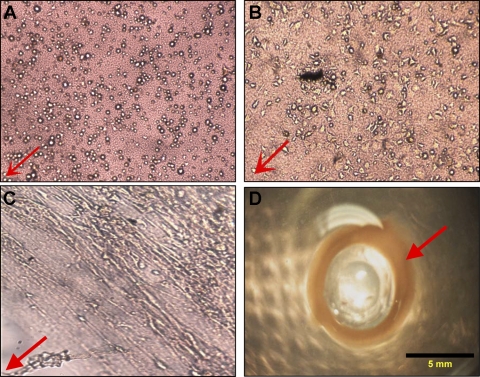
Mouse IAS cells isolated from primary cultures were expanded separately on T-75 culture flasks, and 200,000 cells were seeded onto loose thrombin polymerized fibrin gels. The cells in the fibrin gel started extending, 4 h postseeding (Fig. 1B). Over the next 24 h, the cells proliferated and oriented themselves concentrically around the Sylgard post (Fig. 1C). In 3 days, the fibrin gel shrank and contracted toward the center of the 35-mm culture dish, resulting in the formation of a three-dimensional mouse IAS ring (Fig. 1D). The different time points in the formation of the bioengineered ring are depicted in Fig. 1. The resulting rings had an inner diameter of 5 mm and an approximate thickness and width of 1–2 mm. They were stable in culture for over 35 days. IAS rings were bioengineered in a parallel manner. One ring was implanted, whereas the other was assessed for physiological functionality between 7–14 days.
Fig. 1.
Red arrow indicates the direction of the Sylgard post, toward the center of the 35-mm dish. A: ×10 optical image of mouse internal anal sphincter (IAS) cells in the fibrin gels, 15 min after they were seeded. B: ×10 optical image of mouse IAS cells extending in smooth muscle morphology, 4 h postplating on a fibrin gel. C: ×10 optical image showing concentric alignment of cells, 2 days after initial cell seeding, and compaction of the loose fibrin gel with cells and endogenous ECM. D: fully formed bioengineered mouse IAS ring (red arrow), thickened around the Sylgard post. The ring is 2 mm wide and thick, with an inner luminal diameter of 5 mm.
Implantation of bioengineered mouse IAS rings.
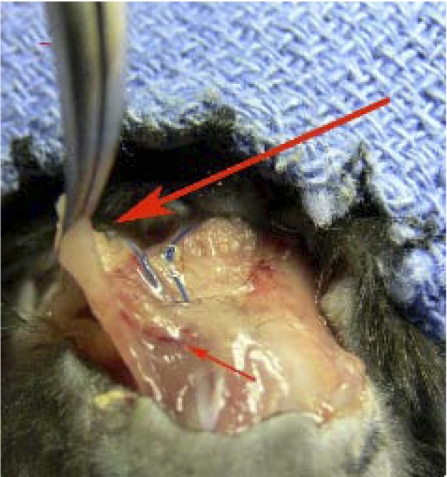
Parallel IAS constructs in culture were implanted into C57BL/6J mice. The period of implantation lasted between 25–28 days, after which the animals were euthanized and the IAS constructs were harvested and reassessed for physiological functionality. On the day of harvesting the IAS ring, visual inspection of the implanted ring in situ revealed significant muscle attachment to the back of the mouse. Blood vessels were visible on the base of the ring. Figure 2 shows the ring still attached to the animal, with signs of neovascularization around the ring. There were no signs of inflammation or abscesses and no visual indicators of tissue involution. The growth factor pump supplies FGF only for 21 days, and the rings survived at least a week over the 21 days, without a continuous supply of FGF.
Fig. 2.
Harvesting the ring from the back of the mouse. Large arrow indicates the implanted bioengineered mouse IAS ring, with its location marked by the blue Prolene sutures. The ring showed healthy color and no visible signs of inflammation. There were no abscess formations observed. Small red arrow points to blood vessels on the base of the ring, suggesting vascularization.
Histology.
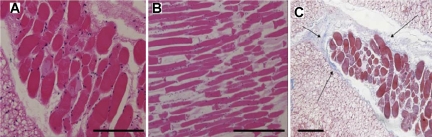
The implanted mouse IAS constructs were fixed and sectioned after physiological assessment. H&E staining showed that we were able to recover intact smooth muscle and confirm that the alignment of the smooth muscle cells was maintained in vivo postimplantation (Fig. 3, A and B). The sections were stained with Masson's trichrome to clearly identify collagen in the implanted constructs. Very little collagen deposition was observed around the outer ridge of the smooth muscle construct, as indicated by the thin blue lines in Fig. 3C. Any other collagen visualized was associated with neovascularization.
Fig. 3.
A and B: hematoxylin and eosin-stained section of the harvested postimplant mouse IAS construct shows alignment of smooth muscle cells were maintained on implantation. C: Masson's trichrome-stained section of the implanted mouse IAS: the smooth muscle was stained red, whereas the surrounding collagen was stained blue. Slide shows the maintenance of the muscle during the 4 wk of implantation and very little collagen deposition (small arrows) on the outline of the ring, with virtually no infiltration. Scale bar= 100 μm.
Immunofluorescence.
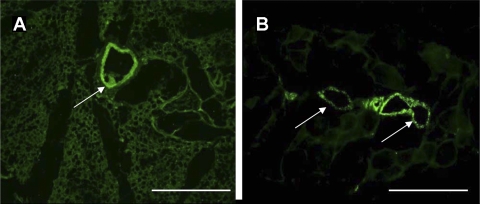
Visual vascularization was confirmed with endothelial specific antigen von Willebrand's factor (factor VIII) staining. Figure 4, A and B, shows bright green positively stained blood vessels in and around the implanted IAS ring. Moreover, the implanted smooth muscle also maintained its phenotype and stained positive for α-smooth muscle actin and smooth muscle specific h-caldesmon (Fig. 5), indicating the maintenance of the smooth muscle phenotype over 4 wk of implantation.
Fig. 4.
A and B: FITC-conjugated von Willebrand's factor (factor VIII), an endothelial specific antigen, staining sections of harvested postimplant mouse IAS. The positive stains (white arrows) are cross sections of blood vessels, revealing neovascularization in and around the implanted smooth muscle area. Scale bar 100 μm.
Fig. 5.
Immunofluorescence. Adjacent sections of harvested IAS were assayed with α-smooth muscle actin stained positive (left) and smooth muscle specific heavy caldesmon stained positive (right); this indicated the maintenance of the smooth muscle phenotype. Scale bar 100 μm.
Western blot analysis.
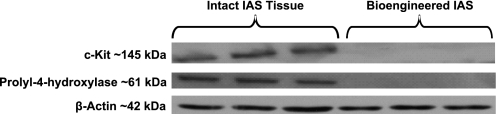
Lysates from intact native mouse IAS and implanted bioengineered IAS were probed for the presence of interstitial cells of Cajal by using c-Kit and fibroblasts by using prolyl-4-hydroxylase (Fig. 6). The native intact mouse IAS displayed bands at ∼145 kDa for c-Kit and ∼61 kDa for prolyl-4-hydroxylase, whereas the bioengineered IAS did not show any bands, indicating the absence of fibroblasts or Kit-positive ICCs in the implanted construct.
Fig. 6.
Western blot analysis. Western blot of bioengineered implanted IAS and native intact IAS lysates probed for c-Kit and prolyl-4-hydroxylase. Only the intact IAS is positive for c-Kit and prolyl-4-hydroxylase, indicating the absence of Kit-positive cells and fibroblasts in the bioengineered and implanted IAS construct. β-Actin was used as loading control.
Establishment of spontaneous basal tone.
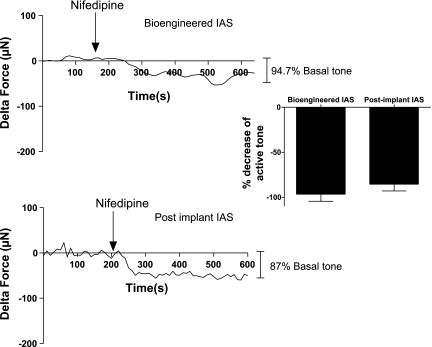
Mouse IAS rings generated tone spontaneously, pre- and postimplantation when attached to the measuring arm of the force transducer with no external stimulation whatsoever. The pattern of basal tone establishment in IAS constructs pre- and postimplantation were similar (Fig. 7). The active basal tone observed in the preimplant rings was 94.7 ± 19.9 μN (n = 5) and the active basal tone in the harvested postimplant IAS rings was 114 ± 28.4 μN (n = 8). Spontaneous basal tone generation is a characteristic property of IAS physiology and it was preserved over the course of implantation.
Relaxation of basal tone by nifedipine.
Nifedipine (10 μM) resulted in a drop in the basal tone of the IAS both pre- and postimplantation as shown in Fig. 8. Nifedipine blocked basal tone by 96.5 ± 7.75% in bioengineered IAS and 85.3 ± 7.5% in postimplant harvested IAS. The bioengineered IAS as well as the postimplant IAS reached their nadirs within 100 s of treatment of nifedipine. The drop in basal tone by the dihydropyridine blocker suggests the preservation of functional L-type calcium channels in the smooth muscle, maintained during bioengineering and even postimplantation.
Fig. 8.
Relaxation of basal tone by nifedipine. Treatment with 10 μM nifedipine, a dihydropyridine calcium channel blocker, resulted in an average relaxation of 85.3 ± 7.5% (postimplant IAS) and 96.5 ± 7.75% (bioengineered IAS) of active basal tone. Reported values are means ± SE of 3–5 individual animal experiments.
VIP-induced relaxation of basal tone.
Both IAS constructs pre- and postimplantation relax from their basal tone in response to 1 μM VIP (Fig. 9). The implanted IAS construct maintained the same extent of relaxation of basal tone. The harvested IAS ring relaxed 84 ± 9.7% of basal tone, whereas the preimplant counterpart relaxed 74.8 ± 19% of its basal tone. Both constructs reached their respective nadirs between 90 s (postimplant) and 120 s (bioengineered IAS) after the addition of VIP. The relaxation in response to VIP treatment indicated the preservation of VIP receptors on the smooth muscle, and the intracellular signaling mechanisms mediating VIP-induced relaxation.
Fig. 9.
Relaxation of basal tone induced by VIP. Two representative graphs were chosen from each experimental set, to elucidate the response of each construct to VIP. Reduction of basal force in response to 1 μM VIP is quantified as the change of force with respect to a stable baseline, expressed as a percentage of each individual construct's active tone. Reported values are means ± SE of 6–10 experiments. Bioengineered rings show a mean relaxation of basal tone by 68.08 ± 36%, whereas postimplant rings display a reduction of 84 ± 9.7%.
Relaxation of basal tone by nitric oxide donor SNP.
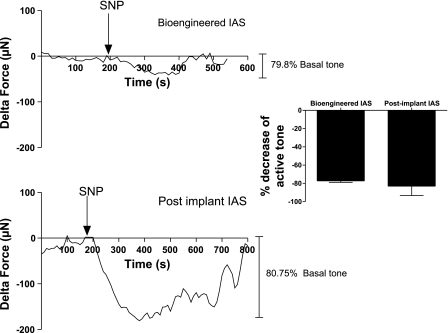
Treatment of the IAS constructs with 10 μM SNP resulted in a rapid relaxation of basal tone in bioengineered IAS and postimplantation IAS as shown in Fig. 10. The average relaxation in the bioengineered IAS before implantation was 77.17 ± 1.714%. The drop in basal tone was 83 ± 10% in harvested postimplant IAS constructs. Relaxation of basal tone by SNP suggests the intact maintenance of intracellular pathways mediated by nitrergic signaling in the implanted IAS smooth muscle.
Fig. 10.
Relaxation of basal tone by NO donor sodium nitroprusside (SNP). SNP (10 μM) resulted in an average relaxation of 83 ± 10% (postimplant) and 77.17 ± 1.714% (bioengineered IAS) of active basal tone. Values are means ± SE of 4 experiments.
Contractility of implanted mouse IAS constructs.
IAS rings pre- and postimplantation responded in a dose-dependent manner to acetylcholine (Fig. 11) and PKC activator, phorbol dibutyrate (PdBU) (Fig. 12). The doses tested were serial from 1 nM to 1 μM, allowing up to 70 s for each individual dose to diffuse completely in the tissue bath. Both constructs responded repeatedly to increasing doses, resulting in a rapid rising contraction, which was sustained over a total of 30 min.
Fig. 11.
Acetylcholine dose response in bioengineered and postimplant constructs. Serial doses starting from 1 nM were injected into the tissue bath containing the construct. It took up to 44 s for the injected compound to diffuse uniformly into the tissue bath. The rings responded to each dose, in a cumulative manner eliciting stronger rapid-rising contractions with increasing dose. Both constructs responded in a similar dose-dependent manner and sustained contractions over 30 min, with no visible signs of muscle fatigue. Dose-response experiments were carried out individually in 7 different animal experiments.
Fig. 12.
Dose response to phorbol dibutyrate (PdBU). Mouse IAS constructs responded in a dose-dependent manner to PdBU, with rapid rising contractions that were sustained over 30 min. Doses were serially administered starting 1 nM and went up to a maximum of 1 μM. Dose-response experiments were carried out individually in 7 different animal experiments.
Figure 13 shows the response of the individual constructs to a dose of 1 μM acetylcholine, and the initial slopes of rise of contraction are almost identical between an implanted and a preimplant ring. The harvested IAS maintained the magnitude of contraction and also the sustainability of contraction for up to 300 s postcholinergic stimulation. The postimplant IAS ring maintained the membrane receptors mediating cholinergic contraction and the intracellular mechanisms involving PKC activation resulting in smooth muscle contraction.
Fig. 13.
Response to 1 μM acetylcholine. Bioengineered IAS pre- and postimplant developed rapid rising contractions in response to cholinergic stimulation with 1 μM acetylcholine with almost identical initial contraction rates. Contraction was maintained over 5 min poststimulation. Values were mean ± SE of 6–11 experiments.
Response to potassium chloride.
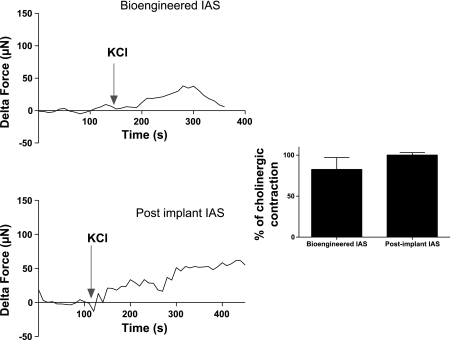
Bioengineered preimplant IAS and harvested postimplant IAS both produce rapid rising contractions in response to 30 mM potassium chloride (Fig. 14). Peak maximal KCl-induced contractions were almost equal to 1 μM acetylcholine-induced contractions. In the harvest postimplant IAS, KCl contractions were 100.2 ± 2.9% (n = 6) of acetylcholine-induced contractions. In bioengineered IAS, KCl contractions were 82.6 ± 14% (n = 4) of peak maximal agonist-induced contractions. These suggest that the integrity of the membrane's ionic characteristics as well as the mechanisms involved in electromechanical coupling in smooth muscle contraction were preserved over the course of implantation of IAS.
Fig. 14.
Response to potassium chloride. Bioengineered IAS and harvested postimplant IAS both contracted rapidly in response to treatment with 30 mM potassium chloride. Graphs show representative tracings to portray the trend of KCl-induced contraction. The magnitude of contraction was normalized and expressed as a percentage of peak maximal contraction induced by 1 μM acetylcholine treatment for each individual ring, and 30 mM KCl induced 100.2 ± 2.9% (postimplant) and 82.6 ± 14% (bioengineered IAS) of agonist induced contraction. Values are means ± SE of 4–6 experiments.
DISCUSSION
FI has several associated primary risk factors, with aging and sphincteric degeneration contributing in a major way to lower continence pressures (4, 7, 19). The IAS is vital in the maintenance of rectoanal continence, the restoration of which is the key to FI therapeutics. Currently available therapies like injection of bulking agents have limited results in providing a positive remedy to partially degenerated sphincters (29).
We have previously bioengineered a three-dimensional model of the IAS from a highly enriched population of sphincteric circular smooth muscle cells from rabbits and humans. It was established that physiological functionality and alignment of the constituent smooth muscle cells of bioengineered IAS rings matched closely to the IAS smooth muscle in vivo (14, 27). In the present study, IAS rings were bioengineered from a highly enriched population of circular smooth muscle cells isolated from the IAS of C57BL6 mice for the purpose of implantation.
As an extension of our previous work, we implanted bioengineered IAS rings under the skin on the backs of C57BL/6 mice. A microosmotic pump was implanted to deliver fibroblast growth factor in balanced solution to the IAS ring for 21 days. This promoted smooth muscle proliferation and angiogenesis as reported before by others (18). Moreover, the balanced solution also avoided any ischemic shocks the ring may have experienced, thus promoting a healthy neovascularization.
The animals tolerated the initial implantation surgery well, without any external signs of rejection, possibly because the cells constituting the bioengineered construct were derived from mice of the same strain. Upon harvesting the IAS ring after 28 days, neovascularization was visually confirmed by the rich pink color of the ring and the formation of blood vessels in the vicinity of the ring. Visual vascularization was also confirmed by von Willebrand's factor (factor VIII) staining (Fig. 4). Morphological assessment with routine H&E staining of cross sections of the harvested ring revealed that the smooth muscle alignment achieved during the bioengineering process was maintained during the course of the implantation (Fig. 3).
Previously, Dunn and colleagues (18) implanted tissue engineered intestinal smooth muscle constructs but failed to evoke significant contractions once these constructs were retrieved. Moreover, they seemed to have dedifferentiated into synthetic muscle upon implantation. Other work involving the injection of muscle-derived stem cells did not unequivocally address the issue of whether the cells just acted as a bulking agent or whether they were improving the inherent functionality of the smooth muscle itself (17).
Physiological functions of the IAS rings were assessed on the harvested postimplant IAS construct. These were focused on assessing the maintenance of 1) generation of spontaneous basal tone and blockade of tone by L-type calcium channel blocker nifedipine, 2) membrane receptors and intracellular signaling mechanisms for relaxation of basal tone by VIP-ergic and nitrergic stimulation, 3) contraction by cholinergic stimulation, and 4) integrity of the constituent smooth muscle cell membrane ionic characteristics in its response to potassium chloride. These properties were compared with physiological functionality of the bioengineered IAS rings before implantation.
Harvested postimplant IAS rings maintained the property of bioengineered IAS rings of exhibiting spontaneous basal tone in the absence of any external stimulation. Both sets of constructs generated similar magnitudes of active basal tone. This indicated that the implanted IAS preserved the specificity of sphincteric circular smooth muscle cell type. Basal tone was insensitive to the addition of nerve blocker tetrodotoxin. These data strongly suggested that the tone generated in our constructs was of myogenic nature, in line with previously documented work (25).
To further our physiological examination on the implanted IAS, we probed the blockade of basal tone by an L-type calcium channel blocker. It has been shown before that blocking of the L-type calcium channel using dihydropyridine blocker nifedipine lowers or blocks the generation of the spontaneous basal tone of the mouse and human IAS and the lower esophageal sphincter (9, 21). In our study, we find that 10 μM nifedipine lowers the basal tone of the IAS even postimplantation by up to 85.3 ± 7.5% (Fig. 8). Implantation thus preserves the membrane characteristics and the integrity of the major physiologically relevant L-type calcium channel responsible for the generation and maintenance of IAS basal tone.
VIP, an important component in nonadrenergic/noncholinergic relaxation of IAS and gastrointestinal smooth muscle, has been shown to act directly on receptors on smooth muscle cells causing relaxation with associated activation of PKA and PKG (6, 12, 22, 23, 26). VIP induced >90% relaxation of basal tone in the postimplant IAS as well as the bioengineered IAS rings. The extents of relaxation of basal tone were similar in harvested as well as preimplant IAS rings (Fig. 9). This indicated the preservation of the integrity of VIP-mediated relaxation of tone during implantation. The intracellular mechanisms by which VIP acts, by downstream activation of PKA, were confirmed by a direct relaxation in response to cyclic AMP in the postimplant IAS and an inhibition of VIP-induced relaxation in the presence of PKA inhibitor, H-89 (data not shown).
Nitric oxide is known to play a major role in inhibitory neurotransmission in the gut and the IAS. The rectoanal inhibitory reflex resulting in a transient relaxation of the IAS is one of the most important physiological functions of the IAS. Nitric oxide donor SNP is known to cause relaxation of basal tone in smooth muscle strips in vitro (8, 21). SNP spontaneously releases cell permeable nitric oxide in solution, resulting in a rapid relaxation. The extent of relaxation was 83 ± 10% of basal tone in postimplant constructs (Fig. 10), suggesting the preservation of NO-mediated relaxation of basal tone. The data thus suggest the preservation of both VIP- and NO-mediated relaxation of basal tone in the IAS after implantation.
The postimplant IAS ring contracts in response to acetylcholine treatment in a dose-dependent manner. Contraction of smooth muscle in response to acetylcholine is well documented and involves acetylcholine binding to the muscarinic receptor. In a G protein receptor-coupled signaling pathway, PKC is activated along with an increase in intracellular calcium concentration. Contraction pathways converge downstream to increase phosphorylation of myosin light chain to enable cross-bridge cycling and subsequent contraction (22, 28). PdBU is a membrane-permeable diacylglycerol analog that directly activates PKC bypassing the receptor-ligand interaction level, resulting in smooth muscle contraction. Our results indicate the preservation of the integrity of the membrane muscarinic receptors and the maintenance of intracellular contractile pathways; 30 mM KCl is a depolarizing concentration, leading to contraction, which is close to 100% of acetylcholine-induced peak maximal contraction, suggesting sound maintenance of basic electromechanical coupling in the implanted IAS.
Bioengineered IAS smooth muscle tissue that is already physiologically functional could be implanted to either supplement the function or replace an ailing degenerated IAS. Bioengineering is a scalable process in which the dimensions of the bioengineered ring as well as the number of cells the rings constitute could be scaled up to the species of interest. Studies have been initiated to intrinsically innervate the bioengineered tissues with enteric neurons, as a means of augmenting the physiology and bringing it closer to in vivo sphincteric function. These are important steps to be implemented before the bioengineered IAS can be used in FI therapeutics.
In conclusion, these studies provide proof of concept of a successful implantation of a three-dimensional bioengineered IAS construct that could be used to potentially treat FI associated with IAS dysfunction. Postimplant IAS rings maintain 1) the specificity of the constituent sphincteric circular smooth muscle phenotype, 2) overall IAS physiology and generation of spontaneous basal tone, 3) ionic integrity of the smooth muscle membranes, and 4) integrity of the membrane associated intracellular signal transduction pathways that mediate contraction and relaxation to physiologically relevant stimulants in a similar, if not better, manner. The implanted smooth muscle was neovascularized. The animals seemed to tolerate the implantation well for a month, without external signs of rejection. This concept could be extended toward bioengineering homologous IAS to restore inherent IAS smooth muscle contractility in FI.
GRANTS
This work was supported by National Institutes of Health Grants NIH/NIDDK DK071614, 1RC1DK087151 and the Training Program for Organogenesis T32HD007505.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Bharucha AE. Fecal incontinence. Gastroenterology 124: 1672–1685, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE, Zinsmeister AR, Locke RG, Seide BM, Mckeon K, Schleck CD, Melton JL., III Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology 129: 42–49, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Biancani P, Walsh J, Behar J. Vasoactive intestinal peptide: a neurotransmitter for relaxation of the rabbit internal anal sphincter. Gastroenterology 89: 867–874, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Bitar KN. Aging and Gi smooth muscle fecal incontinence: Is bioengineering an option. Exp Gerontol 40: 643–649, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Bitar KN, Hillemeier C, Biancani P, Balazovich KJ. Regulation of smooth muscle contraction in rabbit internal anal sphincter by protein kinase C and Ins(1,4,5)P3. Am J Physiol Gastrointest Liver Physiol 260: G537–G542, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Bitar KN, Makhlouf GM. Relaxation of isolated gastric smooth muscle cells by vasoactive intestinal peptide. Science 216: 531–533, 1982 [DOI] [PubMed] [Google Scholar]
- 7.Bitar KN, Patil SB. Aging and gastrointestinal smooth muscle. Mech Ageing Dev 125: 907–910, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cobine CA, Fong M, Hamilton R, Keef KD. Species dependent differences in the actions of sympathetic nerves and noradrenaline in the internal anal sphincter. Neurogastroenterol Motil 19: 937–945, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cook TA, Brading AF, Mortensen NJ. Effects of nifedipine on anorectal smooth muscle in vitro. Dis Colon Rectum 42: 782–787, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Dennis RG, Kosnik PE., 2nd Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim 36: 327–335, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Ganio E, Marino F, Giani I, Luc AR, Clerico G, Novelli E, Trompetto M. Injectable synthetic calcium hydroxylapatite ceramic microspheres (Coaptite) for passive fecal incontinence. Tech Coloproctol 12: 99–102, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Gilmont RR, Somara S, Bitar KN. VIP induces PKA-mediated rapid and sustained phosphorylation of HSP20. Biochem Biophys Res Commun 375: 452–456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashish M, Raghavan S, Somara S, Gilmont RR, Miyasaka EA, Bitar KN, Teitelbaum DH. Surgical implantation of a bioengineered internal anal sphincter. J Pediatr Surg 45: 52–58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hecker L, Baar K, Dennis RG, Bitar KN. Development of a three-dimensional physiological model of the internal anal sphincter bioengineered in vitro from isolated smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 289: G188–G196, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Huang YC, Khait L, Birla RK. Contractile three-dimensional bioengineered heart muscle for myocardial regeneration. J Biomed Mater Res A 80: 719–731, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Jones OM, Brading AF, Mortensen NJ. The physiology, pharmacology and therapeutic manipulation of the internal anal sphincter. Can J Physiol Pharmacol 16: 249–257, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Kang SB, Lee HN, Lee JY, Park JS, Lee HS, Lee JY. Sphincter contractility after muscle-derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis Colon Rectum 51: 1367–1373, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M, Wu BM, Stelzner M, Reichardt HM, Dunn JC. Intestinal smooth muscle cell maintenance by basic fibroblast growth factor. Tissue Eng Part A 14: 1395–1402, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Lewicky-Gaupp C, Hamilton Q, Ashton-Miller J, Huebner M, DeLancey JO, Fenner DE. Anal sphincter structure and function relationships in aging and fecal incontinence. Am J Obstet Gynecol 200: 559e551–e555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Luo Y, Higa M, Zhang X, Saijo Y, Shiraishi Y, Sekine K, Yambe T. Biochemical evaluation of an artificial anal sphincter made from shape memory alloys. J Artif Organs 10: 223–227, 2007 [DOI] [PubMed] [Google Scholar]
- 21.McDonnell B, Hamilton R, Fong M, Ward SM, Keef KD. Functional evidence for purinergic inhibitory neuromuscular transmission in the mouse internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 294: G1041–G1051, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Nurko S, Rattan S. Role of vasoactive intestinal polypeptide in the internal anal sphincter relaxation of the opossum. J Clin Invest 81: 1146–1153, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 126: S14–S22, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Rattan S. The internal anal sphincter: regulation of smooth muscle tone and relaxation. Neurogastroenterol Motil 17, Suppl 1: 50–59, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Said SI, Rattan S. The multiple mediators of neurogenic smooth muscle relaxation. Trends Endocrinol Metab 15: 189–191, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Somara S, Gilmont RR, Dennis RG, Bitar KN. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology 137: 53–61, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Somara S, Pang H, Bitar KN. Agonist-induced association of tropomyosin with protein kinase Cα in colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol 288: G268–G276, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Tjandra JJ, Lim JF, Hiscock R, Rajendra P. Injectable silicone biomaterial for fecal incontinence caused by internal anal sphincter dysfunction is effective. Dis Colon Rectum 47: 2138–2146, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Tjandra JJ, Lim JF, Matzel K. Sacral nerve stimulation: an emerging treatment for faecal incontinence. ANZ J Surg 74: 1098–1106, 2004 [DOI] [PubMed] [Google Scholar]