Abstract
TLR4 ligation by pathogen-associated molecular patterns, such as Gram-negative bacteria-derived LPS, triggers a nonhematopoietic cell-mediated ileus during early endotoxemia. Our objective was to investigate the quantitative contributions of the two downstream signaling pathways of TLR4, namely the adapter proteins myeloid differentiation primary response gene 88 (MyD88) and Toll-IL-1-resistance (TIR) domain-containing adaptor-inducing IFN-β (TRIF). Six hours after intraperitoneal injection of highly purified LPS (UP-LPS, 5 mg/kg), in vivo gastrointestinal transit and intestinal muscularis gene transcripts of inflammatory mediators chemokine (C-X-C motif) ligand 10, synonymous IP-10 (CXCL10), granulomonocyte colony stimulating factor (GM-CSF, synonymous CSF-2), IL-1β, IL-6, IL-10, and inducible NO synthase (iNOS) were assessed in mice with transgenic loss-of-function for MyD88 or TRIF. LPS-induced MyD88 and TRIF mRNA upregulation was quantified within the intestinal muscularis of TLR4-competent and TLR4-mutant mice, and MyD88 mRNA levels were additionally measured in TLR4 bone marrow chimeras. MyD88 deficiency completely protected mice from early endotoxin-induced ileus, while TRIF deficiency partially ameliorated ileus severity. LPS induction of the primary downstream signaling element MyD88 was TLR4 dependent and was derived in equal amounts from both the hematopoietic and the nonhematopoietic cells. Conversely, no induction of TRIF mRNA was detectable. Significant gene induction of all inflammatory mediators was dependent on intracellular signal transduction by MyD88, while the TRIF MyD88-independent pathway predominantly regulated the molecular levels of CXCL10. In summary, MyD88 and TRIF are nonredundant signaling pathways in early endotoxin-induced rodent ileus, but MyD88 is the essential adaptor molecule for transduction of early TLR4-induced ileus and inflammatory signaling. The dependency of ileus on individual adaptor protein pathways is also reflected in the manifestation of specific molecular inflammatory events within the intestinal muscularis externa.
Keywords: inflammation, Toll-like receptor 4, intestine, motility, Toll-interleukin-1-resistance domain-containing adaptor-inducing interferon-β, lipopolysaccharide
mammals are constantly challenged by exposure to environmental threats at specific vulnerable large body surface interfaces, such as the skin, lung, and intestine. The intestinal mucosa must master the dual bidirectional tasks of nutrient absorption and maintenance of a competent tightly regulated barrier to prevent harmful bacterial invasion (2). Toll-like receptors (TLR) recognize specific motifs of microbial and noninfectious elements termed pathogen-associated molecular patterns and danger-associated molecular patterns, respectively. These TLR-associated signaling mechanisms are essential for launching innate and adaptive immune responses (3). Mutualism between the host and the 500 families of normally innocuous intralumenal bacteria provides a vital nutritional function and is permitted by local tolerance. The noninflamed homeostatic steady-state of the gut is maintained by the sophisticated regulation of low TLR expression and hyporesponsiveness to conserved bacterial motifs of the gut epithelium and classical immune cells like dendritic cells, lymphocytes, and macrophages, which are distributed throughout the gut wall (1). However, first line defense mechanisms like the secretion of antimicrobial peptides, the generation of an inflammatory cytokine storm, and cellular immunity must be readily inducible on demand to defeat dangerous microbial intruders or systemic microbial spread, as a breach in the mucosal barrier can transform commensal bacteria into harmful pathogens.
Gram-negative endotoxemia is ever increasingly prevalent in humans and is frequently accompanied by severe ileus, which furthers a self-sustained circle of bacterial overgrowth, mucosal barrier failure, and gastrointestinal dysmotility (6). The lipid A moiety of LPS is an integral bacterial membrane component that is well known to cause septic ileus via its exclusive and potent action as a TLR4 ligand (8). Soluble LPS binding protein chaperones LPS to CD14, which then transfers LPS to myeloid differentiation factor-2 causing TLR4 to homodimerize and activate its signaling cascades. Critical downstream signaling components of the TLR4 pathway consist of the activation of two Toll/IL-1 receptor (TIR) domain containing intracellular adapter protein pairs: 1) myeloid differentiation primary response gene 88 (MyD88)/Toll-IL-1 receptor (TIR) domain containing adaptor protein (TIRAP), and 2) Toll-IL-1-resistance (TIR) domain-containing adaptor-inducing IFN-β (TRIF, formerly Toll-receptor-associated activator of interferon)/TRIF-related adaptor molecule (TRAM, synonymous TICAM2), namely the MyD88-dependent and MyD88-independent pathways, respectively (36).
The MyD88-dependent signaling pathway is initiated as activated TLR4 binds TIRAP, which then recruits MyD88 resulting in the activation of interleukin-1R-associated kinase (IRAK)-1 and -4. The IRAKs in turn phosphorylate TNF-receptor associated receptor 6 (TRAF6) causing the activation of the transcriptional regulators NF-κB, p38 MAPK, ERK1/2, and JNK via TAK1 (TGF-β-activated kinase 1, also called mitogen-activated protein kinase 7; Refs. 9, 24).
Translocation of cell membrane-bound TLR4 to the endosome by dynamin-controlled endocytosis characterizes the MyD88-independent TRIF pathway for LPS (20). Bridged by the adaptor protein TRAM, endosomal TLR4 binds TRIF, which then causes the activation of the transcriptional regulators interferon regulatory factors (IRF) 3 and 7, which induce three genes containing IFN-stimulated regulatory elements: 1) type I interferon-β (IFN-β), 2) IFN-inducible protein 10 [IP-10/chemokine (C-X-C motif) ligand 10 (CXCL10)], and 3) chemokine (C-C motif) ligand 5 [CCL5 or regulated upon activation, normal T cell expressed and secreted (RANTES)], a potent chemoattractant for monocytes and T cells (22).
Recent studies in transgenic loss-of-function animals have further addressed the importance of the distinct inflammatory effects of the individual TLR4/MyD88 and TLR4/TRIF pathways. It has been shown that MyD88 knockout mice are refractory to endotoxin-induced shock over a time period of 96 h, but interestingly the activation of NF-κB and the mitogen-activated protein (MAP) kinase family was not abolished (21). Mice deficient in both adaptors (MyD88 and TRIF) showed complete loss of NF-κB activation in response to TLR4 stimulation, as indicated by the absence of macrophage TNF-α production (7, 19). However, NF-κB activation by the MyD88-independent pathway occurs in a time-delayed manner, as it requires de novo IRF 3-dependent TNF-α synthesis to subsequently activate NF-κB (14).
Recently, Buchholz et al. (8) clearly demonstrated that importantly nonhematopoietic cells are the primary responding cells mediating early ileus after a single dose of endotoxin exposure. At a molecular level, this study also indicated that both hematopoietic and the nonhematopoietic derived cells interact synergistically to generate a fully competent inflammatory cytokine response (8). However, functionally, the endotoxin-triggered early disruption of propulsive gastrointestinal motility is solely effected by the nonhematopoietic lineage without the necessity of TLR4 bone marrow-derived cell signaling.
Unfortunately, detailed investigations into the distinct contributions of TLR4-MyD88 and TLR4-TRIF signaling pathways to the pathogenesis of endotoxin-induced ileus are lacking. Therefore, in this study we report that single exposure to a pure TLR4 agonist strictly requires downstream MyD88 signaling to trigger early gastrointestinal dysmotility, while the TRIF pathway apparently contributes as an ancillary pathway in the presence of functional MyD88 signaling. The dominant role of MyD88-mediated TLR4 signaling is reflected in the absence of molecular inflammatory hallmark events within the intestinal muscularis externa of LPS-treated MyD88-deficient mice. In contrast, TRIF-dependent CXCL10 gene expression was still present in MyD88-deficient mice but was not potent enough to induce early endotoxin-induced ileus by itself.
MATERIALS AND METHODS
Animals, genetic modification, and experimental groups.
Male inbred TLR4-competent (C3H/HeOuJ = TLR4WT), TLR4-mutant (C3H/HeJ-Pde6brd1 = TLR4Lps-d), and C57Bl/6 (TRIF+/+) mice aged-matched littermates of 8–12 wk were obtained from Jackson Laboratory (Bar Harbor, ME). Male TRIF-deficient (TRIFLPS2/LPS2, synonymous TRIF−/−), MyD88-competent (MyD88+/+), and MyD88-deficient (MyD88−/−) mice were bred at the University of Pittsburgh (T. R. Billiar; originally provided from R. Medhzitov, Howard Hughes Medical Institute). Mutant MyD88 and TRIF strains were backcrossed at least 10 times into a C57/Bl6 background. Animals were maintained on a 12-h light-dark cycle in a pathogen-free facility at the University of Pittsburgh (Pittsburgh, PA) and were fed a standard diet with water ad libitum.
MyD88 deficiency results in impaired early immune response based on its function as a convergent pathway for all TLRs (except TLR3), which are key sensors of infection, which leads to an increased susceptibility to nonlethal Gram-negative and Gram-positive sepsis as observed for TLR4 immunoneutralization (30). To prevent neonatal death of the adaptor protein loss-of-function mice, MyD88+/+ and MyD88−/− mice were provided water supplemented with trimethoprim (0.032 mg/ml) and sulfamethoxazole (0.04 mg/ml) until 6 wk of age. To allow systemic antibiotic clearance, oral prophylactic antibiotic treatment was stopped for at least 2 wk before utilization in experiments.
TLR4 chimera animals were generated by lethal irradiation and reciprocal bone marrow transplantation as described previously, and the reliability of the adoptive transfer model was positively confirmed by selective TLR4-dependent NO-release of the transplanted bone marrow (8). Briefly, lethally irradiated mice (single dose of 10 Gy total body irradiation by a dual Cesium137 source) were reconstituted with ∼5 × 105 cells constructing four groups of chimera mice: TLR4-competent bone marrow in TLR4-competent mice (bmTLR4WT/TLR4WT), TLR4 competent bone marrow in TLR4-mutant mice (bmTLR4WT/TLR4LPS-d), TLR4-mutant bone marrow in TLR4-competent mice (bmTLR4LPS-d/TLR4WT), and TLR4-mutant bone marrow in TLR4-mutant mice (bmTLR4LPS-d/TLR4LPS-d). Replacement of tissue immunocytes was allowed to take place during a repopulation period of 12 wk.
The experimental design was approved by the University of Pittsburgh Institutional Animal Care and Use Committee. The gastrointestinal tract was harvested 6 h after a single intraperitoneal injection of highly purified LPS from Escherichia coli (strain O111:B4, UP-LPS; List Laboratories), a pure TLR4 agonist, with a moderate dose of 5 mg/kg or sham treatment (0.9% saline). Animal numbers for the individual experiments are stated within the corresponding result section.
Functional studies.
Modulation of gastrointestinal motility of nonchimera mice by UP-LPS treatment was assessed by in vivo gastrointestinal transit as previously described (8). In short, animals were orally fed with a liquid, nonabsorbable fluorescent marker (10 μl FITC-dextran, 70 kDa; Molecular Probes) and aboral propulsion of dye was determined after a transit time of 75 min. The segmental distribution of the fluorescent marker was plotted, and a geometric center (GC) was calculated for statistical analysis (33).
Molecular studies.
Isolated muscularis externa extracts from nonchimera and chimera small intestines were snap-frozen on dry ice and stored at −80°C until further analysis. RNA was extracted using a standardized phenol-chloroform protocol. After DNAse treatment and cDNA synthesis (Invitrogen), quantitative real-time PCR was carried out. Peak mRNA expression of inflammatory mediators was quantified in duplicate by SYBR green two-step real-time RT-PCR and normalized to the murine housekeeping gene GAPDH. Gene of interest primer sequences are listed in Table 1.
Table 1.
Nucleotide sequences of oligonucleotide primers (RT-PCR)
| Target Gene | Abbreviation | Primer Sequences (5′ → 3′) |
|---|---|---|
| Chemokine (C-X-C motif) ligand 10 | CXCL10 (syn. IP-10) | (F) GCTGCCGTCATTTTCTGC |
| (R) TCTCACTGGCCCGTCATC | ||
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH | (F) TTCACCACCATGGAGAAGGC |
| (R) GGCATGGACTGTGGTCATGA | ||
| Granulo-monocyte colony stimulating factor | GM-CSF (syn. CSF-2) | (F) TAAGGTCCTGAGGAGGATGTG |
| (R) GAGGTTCAGGGCTTCTTTGA | ||
| Inducible nitric oxide synthase | iNOS | (F) GTGACGGCAAACATGACTTCAG |
| (R) GCCATCGGGCATCTGGTA | ||
| Interleukin-1β | IL-1β | (F) CAGGTCGCTCAGGGTCACA |
| (R) CAGAGGCAAGGAGGAAACACA | ||
| Interleukin-6 | IL-6 | (F) TCAATTCCAGAAACCGCTATGA |
| (R) CACCAGCATCAGTCCCAAGA | ||
| Interleukin-10 | IL-10 | (F) CACAAAGCAGCCTTGCAGAA |
| (R) AGAGCAGGCAGCATAGCAGTG | ||
| Myeloid differentiation primary response gene 88 | MyD88 | (F) TGCCGTCCTGTCTACATCTTTG |
| (R) GTTGCTCAGGCCAGTCATCA | ||
| Toll-IL-1-resistance (TIR) domain-containing adaptor-inducing | IFN-β (TRIF) | (F) GGTTCACGATCCTGCTCCTGAC |
| (R) CTTGAGTGTTCTCAGGCCCAGC |
F, forward; R, reverese.
Data analysis.
The results are expressed as means ± SE. Statistical analysis was performed by ANOVA with Tukey post hoc group comparisons using InerSTAT-a v1.3. Fold increase values for LPS effects in molecular studies were calculated over the corresponding sham-treated wild-type mice in a strain-specific fashion. A probability level of P < 0.05 was considered statistically significant.
RESULTS
Early delay in TLR-4-mediated endotoxin ileus is MyD88 dependent and partially TRIF dependent.
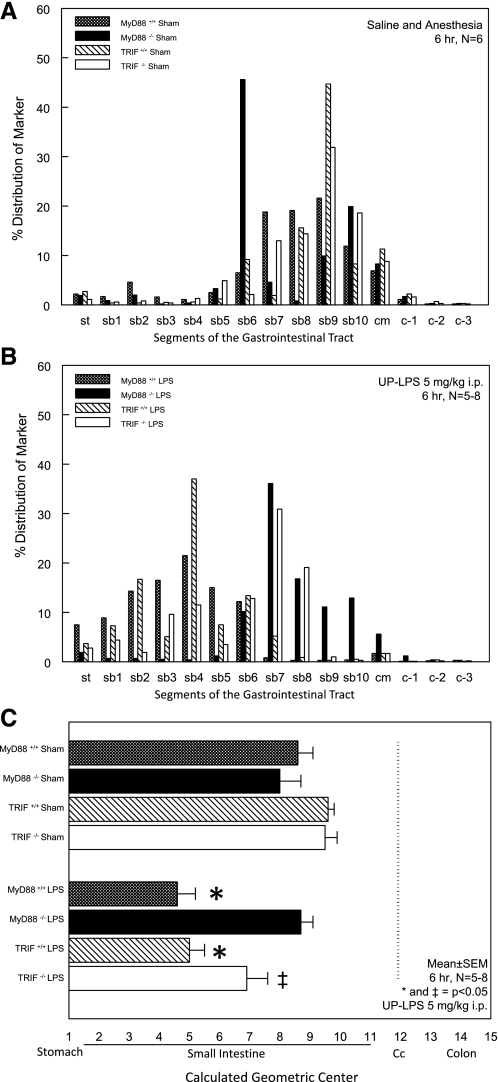
In vivo gastrointestinal motility was measured by calculating the GC of the segmental distribution of an orally fed, nondigestible fluorescent marker (n = 6). Seventy-five minutes after bolus dye ingestion, sham-treated MyD88+/+, MyD88−/−, TRIF+/+, and TRIF−/− mice displayed a normal aboral propulsion pattern with the majority of the fluorescent dye being located within the distal gut segments (MyD88+/+ sham GC: 8.2 ± 1.1, MyD88−/− sham GC: 8 ± 0.7, TRIF+/+ sham GC: 9.9 ± 0.3, and TRIF−/− sham GC: 9.9 ± 0.1; Fig. 1, A–C). Although an interesting observation for the sham-treated MyD88−/− mice, the detected biphasic profile is not unusual in individual distribution histograms and might just reflect gastric boluses of the FITC-dextran that are progressing through the small intestine after gastric emptying. However, this variation did not result in a significant difference in sham-treated MyD88+/+ mice utilizing the calculated GC. In MyD88+/+ and TRIF+/+ mice, LPS injection significantly decreased gastrointestinal transit with a GC of 4.6 ± 0.6 and 5.0 ± 0.3, respectively. In contrast, LPS treatment of MyD88−/− mice did not result in delayed gastrointestinal transit with a GC of 8.7 ± 0.4, indicating full protection from TLR4-mediated early endotoxin-induced ileus by deficiency of the MyD88-dependent pathway. In comparison, gastrointestinal motility in LPS-exposed TRIF−/− mice was still significantly diminished (GC: 6.9 ± 0.7), although the severity of ileus was partially ameliorated compared with TRIF+/+ mice (Fig. 1, A–C).
Fig. 1.
Myeloid differentiation primary response gene 88 (MyD88) deficiency fully protects from early endotoxin-induced ileus, whereas inhibition of the toll-IL-1-resistance (TIR) domain-containing adaptor-inducing IFN-β (TRIF) pathway only partially ameliorates severity of early onset ileus during endotoxemia. Six hours after intraperitoneal sham or UP-LPS treatment, the gastrointestinal distribution of an orally fed nonabsorbable dye was assessed with a transit time period of 75 min. A: individual segmental marker distribution in sham-treated animals. B: individual segmental marker distribution in LPS-treated mice. C: calculated geometric center was used for statistical analysis by ANOVA with Tukey post hoc group comparisons. Results are means ± SE; n = 5–8. *P < 0.05 for MyD88+/+ and TRIF+/+ LPS-treated mice compared with MyD88−/− LPS mice and all sham-treated mice. ‡P < 0.05 for TRIF−/− LPS-treated mice compared with sham-treated TRIF mice.
TLR4-dependent gene induction of downstream adaptor MyD88 occurs in both hematopoietic and nonhematopoietic cell lineages within the intestinal muscularis externa.
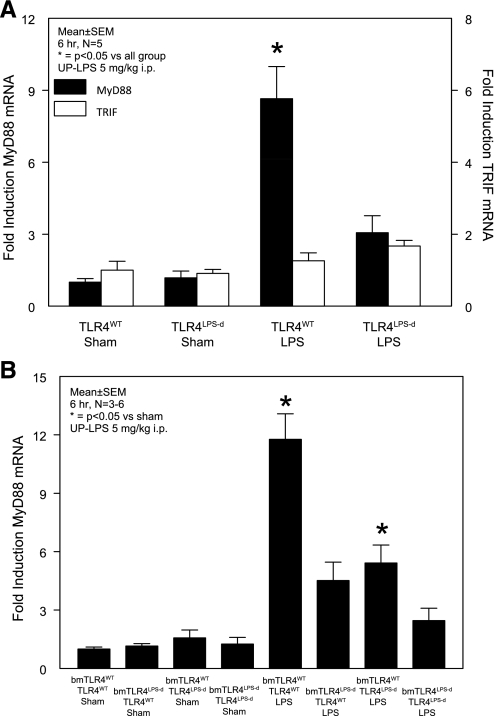
Downstream TLR4 molecular signaling events begin with the recruitment and activation of two discrete adapter protein pairs TIRAP/MyD88 and TRAM/TRIF. Alterations in the availability and magnitude of expression of these critical signaling elements are known to regulate and modify the effects of TLR4 ligands. Hence, we investigated whether changes of intestinal muscularis gene expression of MyD88 and TRIF occur during the course of early endotoxin-induced ileus. In LPS-treated TLR4WT mice, MyD88 mRNA expression showed a significant ninefold increase compared with sham TLR4WT and sham TLR4LPS−d mice (n = 5). A very minor induction of MyD88 gene transcription in LPS-treated TLR4LPS−d mice did not reach statistical significance (Fig. 2A).
Fig. 2.
A positive feedback loop with TLR4-dependent molecular self-regulation of the downstream signaling component MyD88 occurs in both hematopoietic and nonhematopoietic cell lineages within intestinal muscularis extracts. A: muscularis MyD88 and TRIF gene transcripts in TLR4-competent (TLR4WT) and TLR-mutant (TLR4LPS-d) mice. B: Contribution of the hematopoietic (bmTLR4WT/TLR4LPS-d) and nonhematopoietic cell lineages (bmTLR4LPS-d/TLR4WT) to TLR4-dependent muscularis MyD88 mRNA induction with 50% each of the maximal induction of fully competent TLR4 chimera mice (bmTLR4WT/TLR4WT). Results are means ± SE; n = 3–6; sham vs. UP-LPS 5 mg/kg ip at 6 h. *P < 0.05 for TLR4WT compared with sham-treated nonchimera and LPS-treated TRL4LPS-d. *P < 0.05 for bmTLR4WT/TLR4WT LPS mice compared with bmTLR4LPS-d/TLR4LPS-d LPS mice and all sham-treated chimera animals and bmTLR4WT/TLR4LPS-d LPS mice compared with all sham-treated chimera mice by ANOVA with Tukey post hoc group comparisons.
Next, the question arose whether cell-specific differences of TLR4-dependent MyD88 gene induction exists within the multicellular syncytium of the intestinal tunica muscularis. Principal cell types comprising the nonhematopoietic system, include smooth muscle cells, vascular endothelium, fibroblasts, and enteric neurons, while the hematopoietic system is mainly represented by the dense network of resident muscularis macrophages and intravascular leukocytes based on a lack of leukocytic extravasation in early endotoxin-induced ileus (8). Similar to TLR4-competent nonchimera mice, gene expression levels of intestinal muscularis MyD88 reached a significant 12-fold induction in fully competent TLR4 chimera mice (bmTLR4WT/TLR4WT) compared with all groups of sham-treated chimera mice. TLR4 competence of either the nonhematopoietic system (bmTLR4LPS-d/TLR4WT) or the hematopoietic system (bmTLR4WT/TLR4LPS-d) each demonstrated a fivefold increase in MyD88 gene expression induced by TLR4 ligation. Therefore, each cell lineage accounted for 50% of the maximal molecular signal in fully competent TLR4 chimera mice (n = 3–6; Fig. 2B).
In contrast to the MyD88-dependent pathway, no significant changes in TRIF gene expression were detected after LPS treatment of neither TLR4WT nor TLR4LPS-d (Fig. 2A), and therefore, TRIF mRNA levels were not further investigated in TLR4 chimera animals. Taken together, the data confirm the existence of a TLR4-dependent positive reinforcement of MyD88 signaling by positive self-regulation of this primary adaptor molecule. MyD88 gene transcription was equally induced in both the hematopoietic and nonhematopoietic cell lineages within the intestinal muscularis externa, which apparently required no cross talk between the cell types for the induction of the maximal molecular signal, as equally 50% of the signal was derived independently from each isolated system.
MyD88 plays the dominant role in transducing the LPS-triggered TLR4 molecular inflammatory response within the intestinal muscularis externa.
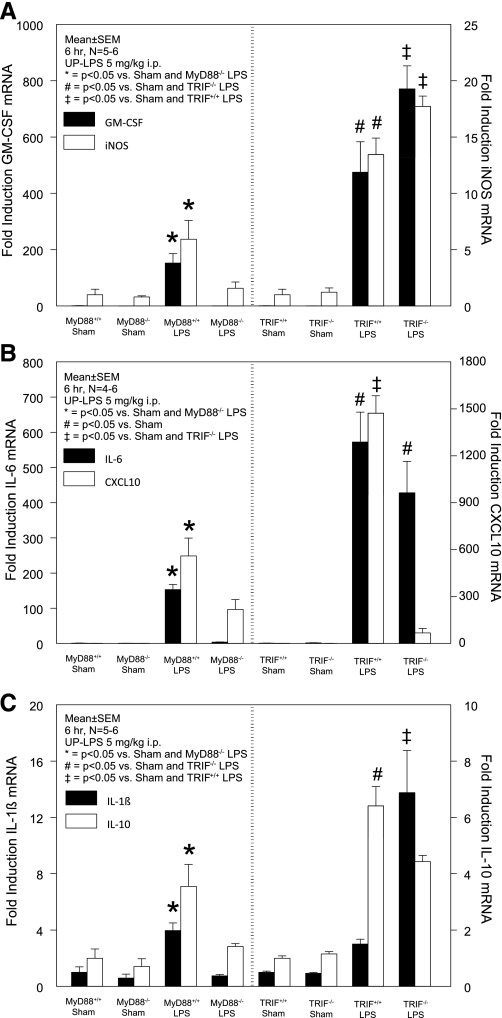
The induction of specific inflammatory genes plays a major role in orchestrating the multifaceted host defense response during inflammatory diseases. We have previously shown that both the hematopoietic and nonhematopoietic compartments contribute to TLR4-sensitive muscularis inflammatory signaling during early endotoxin-induced ileus (8). However, the individual quantitative contributions of MyD88 and TRIF signaling to the induction of specific inflammatory mediators during early endotoxin-induced ileus were not determined. Consequently, we analyzed LPS-induced changes in intestinal muscularis gene expression of the following inflammatory mediators: CXCL10, granulomonocyte colony stimulating factor (GM-CSF, syn. CSF-2), inducible NO synthase (iNOS), IL-1β, IL-6, and IL-10 in MyD88+/+, MyD88−/−, TRIF+/+, and TRIF−/− mice (n = 4–6) and calculated the fold increase in a strain-specific fashion. Technically, in general, MyD88 wild-type sham mice, which were treated with oral growth-inhibiting antibiotics for breeding purposes, consistently displayed a higher baseline of inflammatory gene expression compared with nonantibiotic-treated TRIF wild-type sham mice (C57Bl/6 mice), even though antibiotics were discontinued at least 2 wk before experimentation (ratio sham-treated MyD88+/+ to sham-treated TRIF+/+ mice: GM-CSF: 2.53 ± 1.1, iNOS: 3.2 ± 1.5, IL-6: 6.1 ± 3.2, CXCL10: 2.3 ± 0.7, IL-10: 1.7 ± 0.6, and Il-1β: 1.9 ± 0.8). Hence, the mRNA fold inductions observed in MyD88 mice by LPS relatively to sham MyD88 mice were comparatively less than the mRNA fold inductions by LPS in TRIF mice compared with sham-treated TRIF mice.
TLR4-dependent GM-CSF expression, known to be solely derived from nonhematopoietic cells, was significantly induced in LPS-treated MyD88+/+ (150-fold) mice, while no significant induction occurred in MyD88−/− mice. In comparison, GM-CSF was induced by LPS in both TRIF+/+ (470-fold) and TRIF−/− (770-fold) mice (Fig. 3A, left y-axis). A similar MyD88-dependent/TRIF-independent gene induction pattern was detected for both iNOS (Fig. 3A, right y-axis) and the major proinflammatory cytokine IL-6 (Fig. 3B, left y-axis). In contrast, the potent chemokine CXCL10 was only partially MyD88 dependent, as the 560-fold induction by LPS in MyD88+/+ was diminished to a 220-fold increase in MyD88−/− mice. However, CXCL10 gene transcription demonstrated a virtual complete dependency on the adaptor molecule TRIF. In TRIF+/+ mice, LPS caused an ∼1,500-fold induction of CXCL10, whereas only a 70-fold induction was observed in LPS-treated TRIF−/− mice compared with sham-treated TRIF+/+ mice (Fig. 3B, right y-axis). Interestingly, LPS-triggered gene induction of the inflammatory cytokine IL-1β showed a dependency on the MyD88-dependent pathway with a 4-fold and 0.8-fold increase in MyD88+/+ and MyD88−/− mice but was significantly higher induced in TRIF-deficient mice (14-fold) compared with a minor upregulation in TRIF+/+ mice (3-fold; Fig. 3C, left y-axis). Finally, we investigated molecular changes in IL-10, representing the anti-inflammatory gene target. IL-10 gene transcripts mirrored the gene induction pattern for IL-6, iNOS, and GM-CSF, mRNAs by demonstrating significantly increased transcripts in a MyD88-dependent manner with a threefold, sixfold, and fourfold induction in LPS-treated MyD88+/+, TRIF+/+, and TRIF−/− mice, respectively, but IL-10 gene levels in LPS-treated MyD88−/− mice remained at baseline levels of sham-treated mice (Fig. 3C, right y-axis).
Fig. 3.
MyD88 is the dominant pathway governing the TLR4-triggered molecular inflammatory responses within the intestinal muscularis externa during early endotoxemia. A–C: MyD88- and TRIF-dependent gene expression of muscularis inflammatory mediators. The remarkable differences in fold increase between the MyD88 strain and the TRIF strain are mainly a result of a nonsignificant higher baseline of inflammatory mediator expression in MyD88+/+ sham mice compared with TRIF+/+ sham mice. Results are means ± SE; n = 4–6; sham vs. UP-LPS 5 mg/kg ip at 6 h. iNOS, inducible nitric oxide synthase; GM-CSF, granulomonocyte colony stimulating factor; CXCL10, IP-10/chemokine (C-X-C motif) ligand 10. For P values, see individual panels, statistical analysis by ANOVA with Tukey post hoc group comparisons.
DISCUSSION
Recently, our group has published experimental evidence that nonhematopoietic cell lineages primarily mediate TLR4-triggered ileus during early endotoxemia. A unique feature of TLR4 signaling is that it distinctly utilizes both MyD88-dependent and TRIF-dependent pathways for signal transduction. The absolute requirement and quantitative contribution of each of the downstream signaling pathways during early endotoxemia have not been previously determined. In this study, we report that MyD88 and TRIF are nonredundant pathways of TLR4 signaling during early endotoxemia, although the presence of MyD88 is a prerequisite to elicit the functional consequence of early nonhematopoietic cell lineage mediated ileus. Furthermore, the MyD88-dependent pathway orchestrates the expression of the majority of the measured local counterbalanced pro- and anti-inflammatory mediators within the muscularis externa. Interestingly, a positive reinforcement of MyD88 signal transduction might result from TLR4-dependent induction of MyD88 mRNA, which occurred equally in both hematopoietic and nonhematopoietic cell lineages within the intestinal muscularis, while TRIF mRNA remained unchanged. Nonetheless, TLR4 also distinctly signals through the TRIF adaptor molecule and indeed this pathway is also activated within the muscularis externa, as evidenced by the TRIF-dependent induction of CXCL10 gene transcripts. Despite the reported lack of leukocytic extravasation in early TLR4-mediated ileus (8), the absence of CXCL10 induction in TRIF-deficient mice might participate in the amelioration of ileus severity in these mice. Taking the known time delay of TRIF-mediated NF-κB activation into account, the subordinate role of TRIF in endotoxin-induced ileus could possibly change over time.
Complementing the in vivo observed protection from endotoxin-induced ileus in MyD88-deficient mice, the MyD88-dependent pathway accounted for the majority of the measured LPS-triggered molecular inflammatory responses, such as IL-6, GM-CSF, and iNOS gene inductions. Consistent with these findings, adult MyD88-deficient mice were reported to be resistant to LPS treatment or polymicrobial sepsis produced by colon ascendens stent peritonitis (21, 35). Similarly, cecal ligation and puncture-induced acute kidney injury was diminished and serum TNF-α levels were significantly reduced in the absence of functional MyD88 signaling (16). Furthermore, cell-specific investigations showed that LPS or lipid A compound triggered release of cytokines from nonhematopoietic cells and peritoneal macrophage-derived IL-6, TNF-α, and NO secretion was abolished by MyD88 deficiency (21). Interestingly, although the LPS-triggered induction of the early inflammatory genes IL-1β, TNF-α, and IL-6 was abolished in MyD88-deficient macrophages, LPS still caused the delayed late-phase activation of NF-κB and JNK/p38 (22), which might be mediated by the time-delayed TRIF pathway. In addition, it was recently reported (17) that TRIF signaling regulates TNF-α biosynthesis in LPS-stimulated dendritic cells at the level of mRNA translation through activation of the protein kinase MK2. This phenomenon likely accounts as the underlying mechanism for the observation that dendritic cell-derived IL-12 synthesis is a MyD88-dependent process but TRIF signaling is critical for TLR synergy (23). However, although TLR4-mediated inflammatory gene induction does occur in bone marrow derived cells, it is fundamental to state that this immunostimulation is dispensable for the initiation of early onset ileus during endotoxemia and its role in regulating motility is currently unknown (8). Importantly, combining the three major points of our recent investigations in early endotoxin-triggered ileus that 1) MyD88 plays the dominant role in downstream TLR4 signaling while TRIF is subordinate (present study), 2) severe ileus does occur with TLR4 compentence of the nonhematopoietic cell lineage independent from hematopoietic TLR4 signaling (8), and 3) TLR4-mediated induction of inflammatory gene transcripts in hematopoietic cells alone does not cause early disruption of gastrointestinal motility (8), we conclude that TLR4/MyD88 signaling in nonhematopoietic cells comprises the cardinal pathway in causing early TLR4-triggered ileus. Taken together, the combined data would clearly predict that a hypothetical bmMyD88WT/TLR4LPS-d mouse would not be different from a bmTLR4WT/TLR4LPS-d mouse ileus in response to LPS. We considered the possibility of an interaction between MyD88 hematopoietic signaling and MyD88 nonhematopoietic signaling; however, at 6 h this appears unlikely, as functionally bmTLR4LPS-d/TLR4WT and bmTLR4WT/TLR4WT mice both have a similar degree of ileus. Therefore, in the following sections of the discussion, we will focus on TLR4/MyD88 signaling in nonhematopoietic cells.
Because TLR signaling comprises a vitally important function of immune host defense, the elements involved must be tightly controlled. First, the basic but sophisticated concept of TLR ligand specificity for essential microbial and noninfectious motifs enables the immune system to appropriately respond to a wide variety of pathogens. Also, the tight regulatory expression and subcellular distribution of TLRs serve to efficiently restrain the host from inappropriate immune activation (3). Additionally, exquisite regulation of the intracellular signaling machinery is achieved by both positive and negative regulators of key factors at multiple levels, such as adapter proteins, kinases, and transcription factors. This is exemplified by the downstream component modulation of MyD88-dependent NF-κB transcriptional activity with the positive regulators LRRFIP-2 and Flap-1 and the negative regulator Fliih (15), as well as NF-κB signal modulation by amplifier C/EBP6 and attenuator ATF-3 (25). However, the quantitative presence of these essential signaling elements itself will have major impact on the efficiency and adequacy of the microbe-elicited immune response. Therefore, we assessed the gene transcript levels of the TLR4 adapter proteins MyD88 and TRIF within the intestinal muscularis externa during early endotoxemia. Intriguingly, we confirmed the existence of a positive feedback loop for MyD88 expression that was TLR4 and MyD88 dependent, but no modulation of TRIF mRNA levels was detected. The positive self regulation of MyD88 could possibly lead to a reinforcement of the triggering inflammatory signal and/or prepare the host for intracellular replenishment of the dominant adapter protein for subsequent inflammatory signals. This scenario is not only true for the intestine during acute single injection of LPS-induced endotoxemia but seems to constitute a common feature of host defense. Indeed, LPS-induced MyD88 mRNA induction has been described in cultured fibroblast-like synoviocytes (18) and the murine macrophage cell line RAW 264.7 (27). Likewise, we have shown that both the hematopoietic and nonhematopoietic enteric cell lineages possess the pivotal capability to regulate their downstream signaling components upon TLR4 engagement. In theory, inflammatory cytokines via MyD88 signaling in hematopoietic and nonhematopoietic derived cells may both play a role in the systemic inflammatory response and distant organ injury (lungs and liver). However, likewise in hematopoietic inflammatory gene induction (8), both MyD88 and cytokine mRNA induced by TLR4/MyD88 signaling on hematopoietic-derived cells at the 6 h time point appear not to influence gastrointestinal motility because gastrointestinal transit is not altered in bmTLR4WT/TLR4LPS-d mice.
The intestinal contractile unit (smooth muscle cells and interstitial cells of Cajal) and the enteric nervous system are the major effectors of gastrointestinal motility, yet knowledge on cell-specific TLR4 downstream signaling in either of these nonhematopoietic cell types is sparse and functional cell-specific roles during sepsis have not been determined. However, LPS has been shown to cause a nuclear translocation of NF-κB in intestinal smooth muscle cells (31). Additionally, for the intestinal contractile cells some parallels might be drawn from investigations on vascular smooth muscle cells. It has been shown that ligation of TLR4 by either LPS or extracellular heat shock protein 90 converted cultured human aortic muscle cells into a proinflammatory phenotype in a MyD88-dependent fashion (12, 29). On the other hand, although in vivo E. coli-induced vascular dysfunction was TLR4 dependent, the functional change showed only an incomplete necessity of both MyD88 and TRIF signaling (10). In addition, studies by Choi et al. (11) employing a glycated serum albumin induced inflammatory response in vascular muscle demonstrated a requirement for TLR4 and signaling through both MyD88 and TRIF adaptor proteins. Therefore, the literature would suggest that intestinal smooth muscle cells could potentially participate as nonprofessional immunocytes in the early MyD88-dependent development of ileus and the generation of various inflammatory mediators but that, additionally, smooth muscle cell TRIF signaling might also contribute.
Coordinated inhibitory and excitatory enteric neuromuscular transmission is essential for propulsive peristalsis. Interestingly, TLRs have been anatomically localized to human and rodent enteric neurons, suggesting the potential for direct microbial TLR ligand modulation of their firing patterns (5, 31). However, a dearth of functional data exists regarding enteric neuronal function during endotoxemia. In cultures of murine myenteric neurons, commercial LPS caused the nuclear translocation of NF-κB (31) and importantly it exhibited direct neurotoxic properties on cultured porcine myenteric neurons (4). In central nervous system cultures, microglia can be indirectly activated by necrotic neurons in a MyD88-dependent fashion (28). Taken together, these studies indicate that neuronal cell loss and the secondary propagation of inflammation are both mediated through the MyD88-dependent pathway. Hence, the enteric nervous system might be a primary target during endotoxemia that could mediate the disruption of normal gastrointestinal motility and may participate in generating a subsequent molecular inflammatory response.
Although the small intestinal tract is relatively devoid of colonization with viable bacteria and first line defense mechanisms such as mucus layer and IgA secretion further reduce the microbial load at the intestinal surface, the small intestinal epithelium is constantly exposed to microbial stimuli. To restrain inappropriate continuous immune activation, intestinal epithelial cells and immune cells of the subepithelial layers follow the concept of low TLR expression (26), possibly mediated by the commensal flora itself as exemplified for LPS-dependent internalization/endocytosis of TLR4 (32). Therefore, alterations of commensal microflora by oral growth-inhibiting antibiotic treatment of MyD88-deficient mice may be accompanied by a disturbance in intestinal TLR4 homeostasis. If these changes underlie the observed marked differences between the fold increase yields in inflammatory gene upregulation between MyD88−/− strain and TRIF−/− strain is unclear, but MyD88+/+ mice constantly exhibited a two- to sixfold higher basal level of inflammatory gene expression compared with TRIF+/+ mice. However, prophylactic noneradicating antibiotic treatment of the MyD88-deficient strain was discontinued at least 2 wk before utilization for experiments to allow systemic antibiotic clearance and recovery of the intestinal microflora. Also, although we have not investigated the recovery of the intestinal microflora 2 wk after cessation of antibiotic treatment and commensal bacteria are reported to influence motility as outlined in a recent review (13), it can be deduced from data presented by Umenai et al. (34) that our results would only have been biased toward a diminished LPS-induced ileus response in MyD88+/+ mice. Because the antibiotic-treated MyD88 wild-type mice developed significant ileus that was similar to TRIF wild-type mice, the temporarily disturbed composition of the microflora did not appear to play a role in these mice.
In conclusion, four novel and important points can be stated based on our combined data (8): 1) LPS/TLR4-sensitive ileus at 6 h is regulated primarily through MyD88 signaling on nonhematopoietic cells, 2) TRIF signaling on nonhematopoietic cells partially contributes to the disruption in LPS-induced gastrointestinal motility dysfunction in the presence of functional MyD88, 3) distinct molecular inflammatory signaling can be attributed to each adaptor protein, and 4) unlike TRIF gene expression, adaptor protein MyD88 mRNA was induced on both hematopoietic and nonhematopoietic cell lineages within the intestinal muscularis externa, but both the regulation of this adaptor molecule and of inflammatory cytokines on hematopoietic cells do not appear to participate in altering motility during endotoxemia at 6 h.
GRANTS
This study was supported, in part, by grants from the National Institutes of Health (R01-GM-58241, R01-DK-068610, P50-GM-53789, and DK02488) and from the Deutsche Forschungsgemeinschaft (Bu2403/2-1; to B. M. Buchholz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the excellent technical help of Deborah Williams and Danielle Reiser (Department of Surgery, University of Pittsburgh). Data were partially previously presented in abstract form at the 2009 American College of Surgeons, Chicago, IL (J Am Coll Surg 209 Suppl 1: S11, 2009).
REFERENCES
- 1.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol 174: 4453–4460, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Abreu MT, Thomas LS, Arnold ET, Lukasek K, Michelsen KS, Arditi M. TLR signaling at the intestinal epithelial interface. J Endotoxin Res 9: 322–330, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Arciszewski M, Pierzynowski S, Ekblad E. Lipopolysaccharide induces cell death in cultured porcine myenteric neurons. Dig Dis Sci 50: 1661–1668, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem 57: 1013–1023, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer AJ, Schwarz NT, Moore BA, Turler A, Kalff JC. Ileus in critical illness: mechanisms and management. Curr Opin Crit Care 8: 152–157, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Beutler B, Hoebe K, Du X, Janssen E, Georgel P, Tabeta K. Lps2 and signal transduction in sepsis: at the intersection of host responses to bacteria and viruses. Scand J Infect Dis 35: 563–567, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Buchholz BM, Chanthaphavong RS, Bauer AJM. Nonhemopoietic cell TLR4 signaling is critical in causing early lipopolysaccharide-induced ileus. J Immunol 183: 6744–6753, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature 383: 443–446, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Cartwright N, McMaster SK, Sorrentino R, Paul-Clark M, Sriskandan S, Ryffel B, Quesniaux VF, Evans TW, Mitchell JA. Elucidation of toll-like receptor and adapter protein signaling in vascular dysfunction induced by gram-positive Staphylococcus aureus or gram-negative Escherichia coli. Shock 27: 40–47, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Choi KH, Park Jw, Kim HY, Kim YH, Kim SM, Son YH, Park YC, Eo SK, Kim K. Cellular factors involved in CXCL8 expression induced by glycated serum albumin in vascular smooth muscle cells. Atherosclerosis 209: 58–65, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Chung SW, Lee JH, Choi KH, Park YC, Eo SK, Rhim BY, Kim K. Extracellular heat shock protein 90 induces interleukin-8 in vascular smooth muscle cells. Biochem Biophys Res Commun 378: 444–449, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 136: 2003–2014, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science 309: 1854–1857, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Dai P, Jeong SY, Yu Y, Leng T, Wu W, Xie L, Chen X. Modulation of TLR signaling by multiple MyD88-interacting partners including leucine-rich repeat Fli-I-interacting proteins. J Immunol 182: 3450–3460, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Dear JW, Yasuda H, Hu X, Hieny S, Yuen PS, Hewitt SM, Sher A, Star RA. Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int 69: 832–836, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gais P, Tiedje C, Altmayr F, Gaestel M, Weighardt H, Holzmann B. TRIF signaling stimulates translation of TNF-α mRNA via prolonged activation of MK2. J Immunol 2010 [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez-Canas I, Juarranz Y, Santiago B, Arranz A, Martinez C, Galindo M, Paya M, Gomariz RP, Pablos JL. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology 45: 527–532, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signaling. Nature 424: 743–748, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9: 361–368, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11: 115–122, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Takeuchi O, Fujita T, Inoue Ji, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol 167: 5887–5894, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Krummen M, Balkow S, Shen L, Heinz S, Loquai C, Probst HC, Grabbe S. Release of IL-12 by dendritic cells activated by TLR ligation is dependent on MyD88 signaling, whereas TRIF signaling is indispensable for TLR synergy. J Leukoc Biol 2010April1 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Laird MHW, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, Vogel SN. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol 85: 966–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, Nykter M, Shmulevich I, Aderem A. Function of C/EBP[delta] in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol 10: 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med 203: 973–984, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ornatowska M, Azim AC, Wang X, Christman JW, Xiao L, Joo M, Sadikot RT. Functional genomics of silencing TREM-1 on TLR4 signaling in macrophages. Am J Physiol Lung Cell Mol Physiol 293: L1377–L1384, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pais TF, Figueiredo C, Peixoto R, Braz MH, Chatterjee S. Necrotic neurons enhance microglial neurotoxicity through induction of glutaminase by a MyD88-dependent pathway. J Neuroinflammation 5: 43, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel DN, Bailey SR, Gresham JK, Schuchman DB, Shelhamer JH, Goldstein BJ, Foxwell BM, Stemerman MB, Maranchie JK, Valente AJ, Mummidi S, Chandrasekar B. TLR4-NOX4-AP-1 signaling mediates lipopolysaccharide-induced CXCR6 expression in human aortic smooth muscle cells. Biochem Biophys Res Commun 347: 1113–1120, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T. Protection from lethal Gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci USA 106: 2348–2352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rumio C, Besusso D, Arnaboldi F, Palazzo M, Selleri S, Gariboldi S, Akira S, Uematsu S, Bignami P, Ceriani V, Menard S, Balsari A. Activation of smooth muscle and myenteric plexus cells of jejunum via Toll-like receptor 4. J Cell Physiol 208: 47–54, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Saitoh S, Miyake K. Regulatory molecules required for nucleotide-sensing Toll-like receptors. Immunol Rev 227: 32–43, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Schmidt J, Stoffels B, Moore BA, Chanthaphavong RS, Mazie AR, Buchholz BM, Bauer AJ. Proinflammatory role of leukocyte-derived Egr-1 in the development of murine postoperative ileus. Gastroenterology 135: 926–936, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umenai T, Hirai H, Shime N, Nakaya T, Asahara T, Nomoto K, Kita M, Tanaka Y, Imanishi J. Eradication of the commensal intestinal microflora by oral antimicrobials interferes with the host response to lipopolysaccharide. Eur J Clin Microbiol Infect Dis 29: 633–641, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Weighardt H, Kaiser-Moore S, Vabulas RM, Kirschning CJ, Wagner H, Holzmann B. Cutting edge: myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J Immunol 169: 2823–2827, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 4: 1144–1150, 2003 [DOI] [PubMed] [Google Scholar]