Abstract
A better understanding of the central control of the physiology of deglutition is necessary for devising interventions aimed at correcting pathophysiological conditions of swallowing. Positive modulation of the cortical swallowing network can have clinical ramifications in dysphagia due to central nervous system deficits. Our aim was to determine the effect of nutritive sensory input on the cortical swallowing network. In 14 healthy right-handed volunteers, we utilized a paradigm-driven protocol to quantify the number of activated voxels and their signal intensity within the left hemispheric cortical swallowing network by high-resolution functional MRI (fMRI) during five different swallowing conditions. Swallowing conditions included a dry swallow (saliva) and natural water-, lemon-, popcorn-, and chocolate-flavored liquid swallows. Each flavored liquid was presented simultaneously by its image, scent, and taste in random order and tested over three runs. fMRIs were analyzed in a blinded fashion. Average fMRI blood oxygenation level-dependent signal intensity and number of activated voxels during swallowing concurrent with nutritive gustatory, olfactory, and visual stimulations were significantly increased compared with dry/natural water swallows throughout the cortical swallowing network (P < 0.001 and P < 0.05, respectively). Subregion analysis showed the increased activity for flavored liquids in prefrontal, cingulate gyrus, and sensory/motor cortex, but not in precuneus and insula. Concurrent gustatory, olfactory, and visual nutritive stimulation enhances the activity of the cortical swallowing network. This finding may have clinical implications in management of swallowing disorders due to cortical lesions.
Keywords: flavor, deglutition, olfaction, gustation
the main physiological function of the digestive tract and ingestion-related behavior in all multicellular life forms in the animal kingdom is to acquire food and water to meet the nutritional needs of the organism (30, 39). Deglutition, the primary ingestive behavior that develops in utero in mammalian species (42), contributes to several critical fetal developmental processes (39). The physical act of swallowing requires extensive sensory/motor coordination of oral, pharyngeal, laryngeal, esophageal, and diaphragmatic muscles (1, 2) that are primarily mediated by the “swallowing center” of the brain stem (19, 20). However, a large body of physiological evidence indicates that the cerebral cortex plays a fundamental role, not only in initiation (5, 36), but also in regulation and modulation, of deglutition (18, 29, 35). Furthermore, clinical observations have long documented the development of swallowing disorders due to cerebrovascular accident (13, 37) or traumatic brain injury (7, 12) in the absence of brain stem involvement (9, 14). In humans, recent cortical mapping studies using functional brain imaging (4, 10, 15, 16, 24, 28, 33, 45, 47) show that volitional swallowing bilaterally activates a number of cerebral cortical areas, including cingulate gyrus, prefrontal and sensory/motor cortices, insula, and precuneus, collectively considered to represent the “cortical swallowing network.”
However, the physiology of the cortical swallowing network is incompletely understood. Behavioral studies quantifying deglutitive biomechanical parameters following various gustatory and olfactory stimuli (27, 32, 48, 49), brain magnetic stimulation studies of the effects of gustatory stimulation on the cortical swallowing motor pathways (31), and brain imaging studies evaluating the effect of acid-induced esophageal afferent signals on the swallowing network (21) indicate significant modulatory input to this distributed network from a variety of peripheral sensory fields.
A better understanding of the influence of physiological stimuli on cortical swallowing network activity may have ramifications for the management of dysphagic patients. The aim of the present study, therefore, was to test the hypothesis that cortical swallowing network activity can be augmented by food-related sensory stimuli. Using functional MRI (fMRI), we determined and compared the effect of several common flavors, such as milk chocolate, lemonade, and popcorn, on a priori-defined components of the cortical swallowing network.
METHODS
Study protocol.
We studied 14 (7 female) right-handed, healthy subjects (28 ± 10 yr old). The Human Research Review Committee of the Medical College of Wisconsin approved the study protocol, and all volunteers signed a written informed consent. All participants completed a detailed health-related questionnaire. After a detailed interview with a physician, subjects were confirmed to have no gastrointestinal disease and no gross deficit in their sense of taste or smell for common flavors. The subjects were asked to fast for 6 h before the study, but they were allowed to drink water. They were placed supine in a 3.0-T scanner (Signa System, General Electric Medical Systems, Waukesha, WI) equipped with a custom-designed three-axis eight-channel head coil for rapid gradient field switching and a shielded transmit/receive birdcage radiofrequency coil. A rear projection screen at the head of the scanner bed displayed visual cues and different visual stimuli. All anatomic scans were acquired using the high-resolution spoiled gradient recalled acquisition technique, consisting of 120 sagittal whole brain 1.2-mm-thick slices over a 240-mm field of view and 256 × 256 within-slice pixel resolution. These high-resolution anatomic images were used for subsequent superposition of cortical activity regions derived from the lower-resolution echo planar blood oxygenation level-dependent (BOLD) contrast image data in each subject.
Data acquisition.
Paradigm-driven fMRI data were acquired during 120-s runs while subjects performed seven random single-trial swallows. Each run was repeated three times. During each MRI scan, subjects had either no external sensory stimulant other than visual cues to swallow their saliva (dry swallow) or an additional sensory stimulant as follows: water, lemonade, milk chocolate, or popcorn. Dry swallow runs were always performed at the beginning (3 runs) and at the end (3 runs) of each MRI scan session, and four flavor swallow runs were randomly performed between dry swallow runs. Each flavor was represented by an image (visual representation), standard scent (olfactory representation), and taste (gustatory representation) of the respective flavor. Each tastant was delivered by a Harvard infusion pumping system through individual polyvinyl catheters to the mouth at a controlled flow rate of 2 ml/min (∼0.6 ml per swallow) during each sensory stimulant run. Popcorn taste was simulated by a mixture of commercial dry popcorn and powdered popcorn butter in water. Bottled water, lemonade, and chocolate milk were used for the other tastes. Nutritive contents for a total of 12 ml of each flavor delivered to subjects during a study session, on the basis of information provided on their respective “nutritional fact” labels, were as follows: 9 Cal, 1,000 mg fat, 200 mg sodium, 100 mg carbohydrate, and 100 mg protein for popcorn; 6 Cal, 0 mg fat, 0 mg sodium, 1,500 mg carbohydrate, and 0 mg protein for lemonade; 9 Cal, 100 mg fat, 11 mg sodium, 20 mg potassium, 1,400 mg carbohydrate, and 400 mg protein for chocolate milk. All tastants were prepared at room temperature to reduce the effect of temperature variability. Scent was delivered orthonasally by a custom-made apparatus. Prior to each scan, a cotton-tipped applicator was immersed in the standard scented liquid extract of each flavor, and then excess liquid was removed from the tip. The applicator was secured to a delivery system and guided through a fixed 6-ft-long plastic tube to position the applicator tip 1 cm from the nares. To reduce cross-contamination of different flavors after each run, we asked the volunteers to perform multiple wet swallows and waited 1 min for aroma and taste to clear. Before initiation of the next flavor, we verified with every subject that the prior flavor had completely resolved.
fMRI analysis.
Fifteen contiguous, sagittal gradient high-resolution echo planar slices of the left hemisphere were obtained. MRIs were acquired at a repetition time (TR) of 1.2 s and an echo time of 20 ms. We studied only the left hemisphere to facilitate using high-resolution echo planar imaging techniques (21). The slice thickness for our functional image was 4.5 mm, and a slice-wise pixel resolution of 96 × 96 pixels over a 240-mm field of view yielded a within-slice resolution of 2.5 × 2.5 mm. The reason for our unilateral brain imaging is that, at the time of our study, these high-resolution echo planar image scanning specifications and short TR (<1.5 s) needed for studying swallowing, with scanner and computer processing limitations in our institution, made whole brain scanning untenable. For example, we were able to accommodate only 25 slices (which is not enough for both hemispheres) in TR = 1,200 ms used for current study. Furthermore, there has been no evidence showing that fMRI activity associated with volitional swallowing was restricted to one hemisphere (16, 24, 28, 34). Studies from our laboratory and others showed bilateral activation of the cortical swallowing network, with greater registration reported in the left hemisphere for all brain regions except the insula, in which right hemispheric dominance has been reported in some studies and left hemispheric dominance has been reported in other studies (16, 24, 28, 33, 45). Since the limitation of the scanner technology constrains imaging of both hemispheres and all historical data point to either hemisphere as a viable target for measuring fMRI signal changes, the left hemisphere was arbitrarily chosen for our study scans (21).
All imaging data were mapped stereotaxically to the Talairach-Tournoux coordinate system for comparison and display purposes. The regions of interest (ROI) were defined anatomically a priori and included the cingulate gyrus, insula, prefrontal region, sensory/motor cortex, and precuneus. The cingulate region was defined as the portion of the cortex confined by the cingulate sulcus and consisted of Brodmann areas (BA) 23, 24, 25, 29, 30, 31, 32, and 33. The prefrontal region was defined as predominantly the dorsolateral prefrontal cortex (BA 9 and 46), anterior prefrontal cortex (BA 10), and ventromedial prefrontal cortex (BA 11 and 47). The insula was defined as the region deep within the lateral fissure consisting of several long gyri parallel to the lateral fissure, as well as several short gyri more rostrally (BA 13). The sensory region consisted of BA 1, 2, and 3. The motor region was defined as the precentral gyrus on the lateral surface of the cortex extending into the longitudinal cerebral fissure medially (BA 4), as well as the supplementary motor cortex and supplementary motor area (BA 6 and 8). The precuneus region was defined as the medial surface of the parietal cortex between the cuneus and the paracentral lobule (BA 7).
Data analysis.
All fMRI signal analysis was carried out using analysis of functional neuroimaging (AFNI) (8) software. Subtle changes in head position during MRI scanning sessions were corrected using three-dimensional volume registration that realigns motions of a few millimeters and rotations of a few degrees using first-order Taylor series expansions at each point in the six motion parameters (3 shifts, 3 angles) and Fourier interpolation. All fMRI signal intensity data were normalized, voxel by voxel, to the mean signal intensity over the entire time series. General linear modeling (GLM) techniques that compute the voxel-wise hemodynamic response function from the magnetic signal time series were used to detect cortical regions that exhibit significant BOLD changes compared with random Gaussian variation of the signal. As a conservative estimate, we used an uncorrected P < 10−5 as the cutoff threshold when identifying regional cortical activation using AFNI. We considered statistical output of the GLM to be significant if the probability of making a type I error was <0.05 after correction for multiple comparisons.
We identified the number of activated voxels based on the GLM test in each ROI and over the whole cortical swallowing network. The average and peak signal intensity change for all activated voxels in each ROI was also calculated. Average BOLD signal waveforms during the interstimulus period of each sensory stimulant were generated across all significantly activated voxels within each ROI of all subjects and during all trials (n = 294). This was done by calculating the mean percent signal intensity change for each of 12 TRs (14.4 s) after a swallow among all activated voxels within an ROI of every subject. Group activity maps representative of each stimulant were also created. Additional statistical analysis further compared dry and water swallows with swallowing activity during flavor swallows and relevant modulation of the cortical swallowing network.
We determined whether a distinct area in each ROI of the cortical swallowing network was activated by flavored sensory stimuli compared with either water or dry swallow. Signal intensity values were compared using two statistical techniques: 1) all nonparametric statistical values are presented as median with range and compared using Kruskal-Wallis test and multiple comparisons, and 2) all parametric statistical values are reported as means ± SE and compared using ANOVA with repeated measures and Tukey's post test for multiple pair-wise comparisons. Significance level for all hypothesis testing was set at P < 0.05 corrected for multiple comparisons when appropriate.
RESULTS
All subjects showed regional cerebrocortical fMRI BOLD response to swallowing with or without a flavor. fMRI activity was observed in all known regions of the cortical swallowing network, including the cingulate gyrus, insula, precuneus, prefrontal, sensory, and motor regions. Table 1 illustrates the median number and range of activated voxels within each ROI. The number of activated voxels was smaller and more variable in the insula and precuneus than in the other cortical regions. The average percent BOLD signal increase and number of activated voxels in the cortical swallowing network were slightly higher during a dry swallow prior to the flavored swallows (pre runs) than during a dry swallow after the flavored swallows (post runs); however, the difference did not reach statistical significance.
Table 1.
Number of activated voxels in cortical swallowing network
| Region | Dry Swallow (Pre) | Chocolate | Lemon | Popcorn | Water | Dry Swallow (Post) |
|---|---|---|---|---|---|---|
| Cingulate | 16 (4–35) | 31 (25–82) | 27 (4–41) | 26 (6–49) | 13 (5–39) | 10 (3–24) |
| Insula | 0 (0–11) | 6 (0–73) | 1 (0–31) | 0 (0–71) | 4 (0–22) | 0 (0–12) |
| Prefrontal | 17 (7–36) | 107 (71–150)* | 79 (43–115)* | 68 (38–177)* | 35 (14–56) | 17 (5–31) |
| Precuneus | 4 (0–32) | 2 (0–100) | 0 (0–96) | 9 (0–87) | 7 (0–43) | 0 (0–33) |
| Sensory/motor | 16 (9–23) | 41 (30–115)* | 57 (27–95)* | 43 (26–101)* | 32 (17–40) | 15 (9–30) |
| Swallowing network | 58 (46–128) | 238 (151–455)* | 190 (127–296)* | 169 (102–290)* | 117 (57–167) | 50 (41–84) |
Values are medians (range).
Significantly more activated voxels with flavored stimuli than dry swallow in prefrontal and sensory/motor regions and swallowing network (P < 0.05).
Cingulate gyrus.
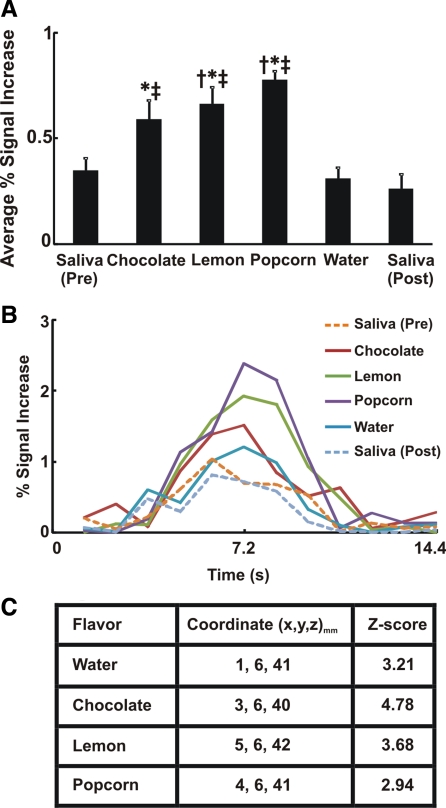
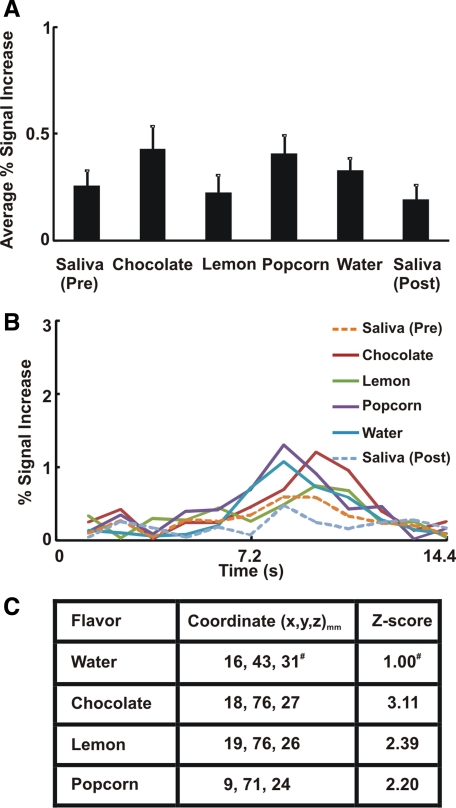
Swallow-related average percent signal intensity change was significantly greater with flavored stimuli than with dry and water swallows (Fig. 1A). The average swallow-related BOLD signal waveform across all subjects and trials (n = 294 for each flavor) in the cingulate gyrus is presented in Fig. 1B. The average BOLD response peaked at 7.2 s after flavored liquid stimuli compared with 6 s after dry swallows (Fig. 1B). We analyzed the anterior (BA 23, 24, 25, 32, and 33) and posterior (BA 29, 30, and 31) cingulate regions separately and observed a similar increased activity with flavored stimuli compared with dry and water swallows in both subregions. Talairach-Tournoux coordinates of peak fMRI signal intensity change in the cingulate gyrus are located in the midcingulate (or dorsal anterior cingulate) region (Fig. 1C).
Fig. 1.
Swallow-related cortical activity in cingulate gyrus. A: average percent signal increase in cingulate gyrus. Flavored swallows showed significantly more functional MRI (fMRI) activity than dry/water swallow. We performed ANOVA and Tukey's test for multiple pair-wise comparisons. †P < 0.01 vs. dry swallow (pre). *P < 0.05 vs. water. ‡P < 0.01 vs. dry swallow (post). Values are means ± SE. B: average swallow-related blood oxygenation level-dependent (BOLD) response waveforms across all trials in cingulate gyrus (n = 294). Note considerable differences in magnitude of fMRI activity waveforms for flavor-stimulated swallows compared with dry and water swallows. C: coordinates of peak signal intensity with each flavor within the cingulate gyrus based on the Talairach-Tournoux system. x, y, and z (in mm) represent left, posterior, and superior directions, respectively. Corresponding Z score of peak activity voxel with each flavor is also presented.
Insula.
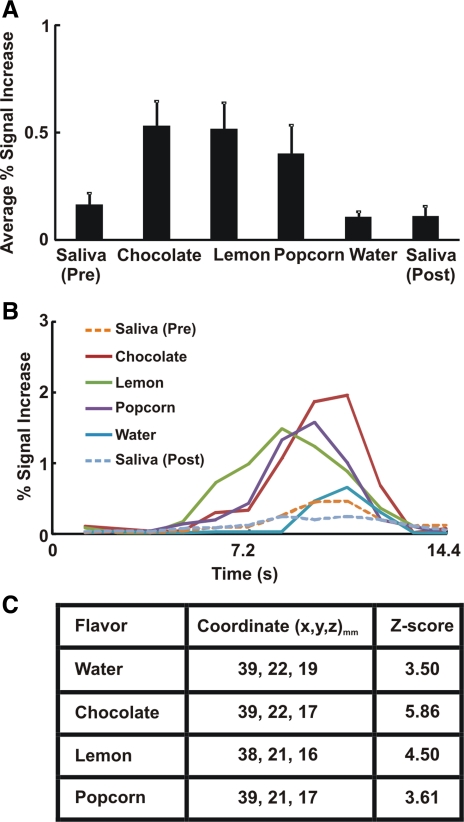
The average signal intensity increase associated with the swallow paradigm in the insula was highly variable and did not reach statistical significance when flavored swallows were compared with unflavored swallows (Fig. 2A). The average BOLD signal waveform related to swallowing across all subjects and trials (n = 294 for each flavor) in the insula is shown in Fig. 2B. The insular average BOLD response peaked at 8.4–10.8 s after swallowing with or without flavor (Fig. 2B). We analyzed anterior and posterior insular swallow-related BOLD activity separately and found no statistical difference when we compared signal intensity for dry or water swallows with that for other flavored stimuli due to high variability of response between subjects. Talairach-Tournoux coordinates of peak fMRI signal intensity change within the insula are located in the dorsal anterior insula (Fig. 2C).
Fig. 2.
Swallow-related cortical activity in insula. A: average percent signal increase in insula. Difference between flavored stimuli and dry swallow failed to reach statistical significance. We performed nonparametric Kruskal-Wallis test because of unequal variances among groups. Values are means ± SE. B: average swallow-related BOLD response waveforms across all trials in insula (n = 294). Note differences in the magnitude of fMRI waveforms for the flavor-stimulated swallows; however, these differences did not reach statistical significance. C: coordinates of peak signal intensity with each flavor within insula based on the Talairach-Tournoux system, with x, y, and z as described in Fig. 1 legend. Corresponding Z score of peak activity voxel with each flavor is also presented.
Prefrontal cortex.
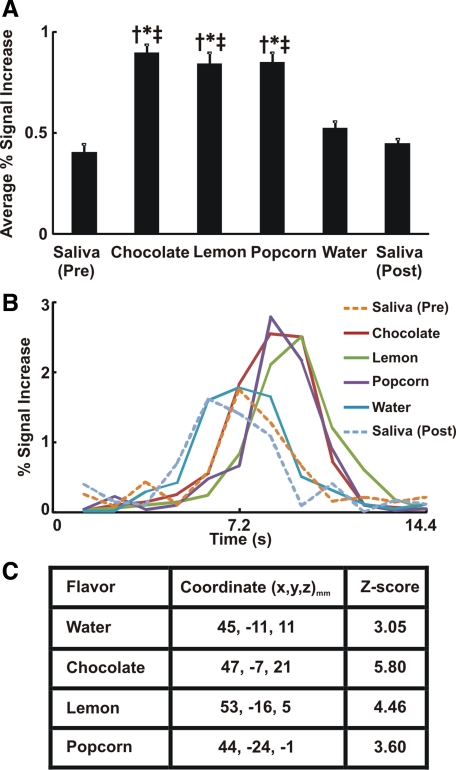
The prefrontal cortex is significantly activated during a swallow. Swallow-related average signal intensity increased significantly with flavored stimuli compared with dry and water swallows (Fig. 3A). The average swallow-related BOLD signal waveform across all subjects and trials (n = 294) is presented in Fig. 3B. The average BOLD response related to flavored stimuli in the prefrontal cortex peaked at 8.4–9.6 s compared with 6–7.2 s after dry and water swallows (Fig. 3B). Talairach-Tournoux coordinates of peak fMRI signal intensity within the prefrontal cortex are located dorsolaterally (Fig. 3C).
Fig. 3.
Swallow-related cortical activity in prefrontal cortex. A: average percent signal increase in prefrontal cortex. Average fMRI signal intensity for all flavored swallows was significantly different from that for dry/water swallow. We performed ANOVA and Tukey's post test for multiple pair-wise comparisons. †P < 0.001 vs. dry swallow (pre). *P < 0.01 vs. water. ‡P < 0.001 vs. dry swallow (post). Values are means ± SE. B: average swallow-related BOLD response waveform across all trials in prefrontal cortex (n = 294). Note considerable differences in magnitude of fMRI activity waveforms for the flavor-stimulated swallows compared with dry swallow and water. C: coordinates of peak signal intensity with each flavor within prefrontal cortex based on the Talairach-Tournoux system, with x, y, and z as described in Fig. 1 legend. Corresponding Z score of peak activity voxel with each flavor is also presented.
Sensory/motor cortex.
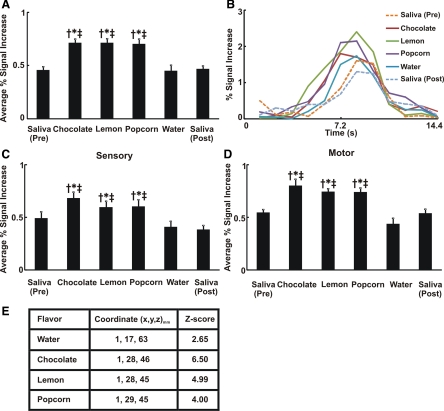
The swallow-related average percent signal intensity change was significantly increased with flavored stimuli compared with dry and water swallows (Fig. 4A). The average swallow-related BOLD response across all subjects and trials (n = 294) is presented in Fig. 4B. The average BOLD signal in the sensory/motor cortex peaked at 7.2–8.4 s after swallowing of flavored or nonflavored liquids (Fig. 4B). We analyzed swallow-related BOLD activity separately in motor and supplementary motor cortices (precentral gyrus, BA 4 and 6) and the sensory cortex (postcentral gyrus, BA 1, 2, 3, and 43). Both subregions showed significantly increased activity with flavored stimuli compared with dry and water swallows; flavored stimuli increased the average signal intensity more robustly within the motor and supplementary motor cortex (Fig. 4D; 2-way ANOVA with repeated measures, P < 0.01) than in the swallow-related sensory cortex (Fig. 4C). Talairach-Tournoux coordinates of the peak fMRI signal intensity increase are located in the rolandic opercular region within the sensory/motor cortex (Fig. 4E).
Fig. 4.
Swallow-related cortical activity in sensory/motor cortex. A: average percent signal increase in sensory/motor cortices. Average fMRI signal intensity change for all flavored swallows was significantly different from that for dry/water swallow. We performed ANOVA and Tukey's test for multiple pair-wise comparisons. †P < 0.001 vs. dry swallow (pre). *P < 0.001 vs. water. ‡P < 0.001 vs. dry swallow (post). Values are means ± SE. B: average swallow-related BOLD response waveform across all trials in sensory/motor cortices (n = 294). Note considerable differences in magnitude of fMRI activity waveforms for flavor-stimulated swallows compared with dry swallow and water. C: average percent signal increase in sensory cortex. Average fMRI signal intensity change for all flavored swallows was significantly different from that for dry/water swallow. We performed ANOVA and Tukey's test for multiple pair-wise comparisons. †P < 0.001 vs. dry swallow (pre). *P < 0.001 vs. water. ‡P < 0.001 vs. dry swallow (post). Values are means ± SE. D: average percent signal increase in motor cortex. Average fMRI signal intensity change for all flavored swallows was significantly different from that for dry/water swallow. We performed ANOVA and Tukey's test for multiple pair-wise comparisons. †P < 0.001 vs. dry swallow (pre). *P < 0.001 vs. water. ‡P < 0.001 vs. dry swallow (post). Values are means ± SE. Note more robust swallow-related BOLD response to flavored liquids in motor cortex than sensory cortex (P < 0.01). E: coordinates of peak signal intensity with each flavor within sensory/motor cortex based on Talairach-Tournoux system, with x, y, and z as described in Fig. 1 legend. Corresponding Z score of peak activity voxel with each flavor is also presented.
Precuneus.
The average percent signal intensity change associated with the swallowing paradigm in the precuneus region with flavored swallows was variable and did not reach statistical significance compared with dry swallowing (Fig. 5A). The average BOLD signal waveform related to swallowing in the precuneus region across all subjects and trials (n = 294) is shown in Fig. 5B. The average BOLD response peaked at 8.4–9.6 s after swallowing of nonflavored and flavored liquids. Talairach-Tournoux coordinates of the peak fMRI signal intensity change in the precuneus are presented in Fig. 5C.
Fig. 5.
Swallow-related cortical activity in precuneus region. A: average percent signal increase in precuneus. Difference between flavored stimuli and dry swallow failed to reach statistical significance. We performed nonparametric Kruskal-Wallis test because of unequal variances among groups. Values are means ± SE. B: average swallow-related BOLD response waveforms across all trials in precuneus (n = 294). Magnitude of fMRI waveforms for flavor-stimulated swallows did not differ significantly from that for dry swallow. C: coordinates of peak signal intensity with each flavor within precuneus region based on Talairach-Tournoux system, with x, y, and z as described in Fig. 1 legend. Corresponding Z score of peak activity voxel with each flavor is also presented. #Group analysis of water swallows did not show any significant activity within precuneus. Z score within the region is presented only for comparative purposes.
Total cortical swallowing network.
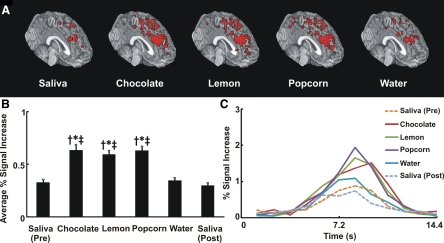
Activated cortical regions of the swallowing network from grouped analysis in the rendered anatomic display are shown in Fig. 6A. The swallow-related average percent signal intensity change throughout the entire cortical swallowing network subregions significantly increased with flavored stimuli compared with dry and water swallows (Fig. 6B). The average BOLD signal waveform related to swallowing in the cortical swallowing network across all subjects and trials and regions (n = 294) is shown in Fig. 6C. The average BOLD signal in the cortical swallowing network peaked at 8.4–9.6 s after swallowing of flavored and nonflavored liquids (Fig. 6C).
Fig. 6.
Total cortical swallowing network activity. A: activated cortical regions during swallow showing total cortical swallowing network. Significant activity associated with each stimulus throughout cortical swallowing network from medial anterior oblique view of a glass brain (all the activity is seen through the tissue). Note more robust activity with flavor-associated swallows than with saliva or water swallows. B: average percent signal increase in cortical swallowing network. Average fMRI signal intensity change for all flavored swallows was significantly different from that for dry/water swallow. We performed ANOVA and Tukey's test for multiple pair-wise comparisons. †P < 0.001 vs. dry swallow (pre). *P < 0.001 vs. water. ‡P < 0.001 vs. dry swallow (post). Values are means ± SE. C: average swallow-related BOLD response waveform across all trials in cortical swallowing network (n = 294). Magnitude of fMRI activity waveforms for the flavor-stimulated swallows was considerably different from that for dry swallow and water.
DISCUSSION
We have demonstrated the enhancing effect of concurrent olfactory, gustatory, and visual stimulations by the ingested material on the cortical swallowing network. Our findings indicate that, although the fMRI signal intensity changes of the total swallowing network show a significant increase during swallowing of liquids that stimulates all three ingestion-related senses compared with inert materials, such as pure water or saliva, this effect is not distinguishable in all components of the swallowing network. Notably, activity of the insula and precuneus regions, contrary to cingulate gyrus, sensory/motor, and prefrontal regions, exhibits significant variability and, overall, does not show a statistically significant augmentation due to flavor of the ingested materials.
Flavor of prepared food, one of the most complex and powerful human sensations (11), engages almost all human senses, particularly the retronasal olfactory system, as well as the gustatory system (41). The sense of taste influences evaluating the nutritious content of food (6), such as sweet for energy-rich carbohydrates, salty for proper dietary electrolyte balance, or umami (savory) for amino acids (50), and prevents the ingestion of possible toxic or noxious substances that may taste bitter and sour, respectively (6). Peripheral taste signals are modulated and relayed through the nucleus tractus solitarius and the taste nucleus of the thalamus (43) projecting bilaterally onto the anterior insula (40, 43, 45) and adjacent frontoparietal opercula (17, 40, 45), as the primary, and onto the orbitofrontal cortex (OFC) (3), as the secondary, gustatory cortex. Olfactory signals pass through the olfactory bulb to project bilaterally onto the frontotemporal pyriform cortex (51, 52), as the primary, and onto the OFC (46, 52), as the secondary, olfactory cortex. In fact, the OFC receives convergent somatosensory, visual, olfactory, and gustatory afferents (38, 43, 44) and combines these inputs with hedonic experience to form a sensory integration area, i.e., the “flavor center” (26, 41). The brain flavor perceptual system is directly linked to other brain systems, such as memory, language, motivation, and emotion circuitry, that are closely involved in the complex control of eating behavior (41). Various cortical drives, such as appetite, thirst, and hunger, are known to stimulate complex eating behaviors consummating in swallowing (39). It is known that the human sensory systems generate flavor as the main internal representation of food, and swallowing is the essential step in ingestion of the food. Therefore, investigation of the interaction between the respective cortical networks of sensory systems and swallowing is essential to our understanding of the brain-gut interaction in the context of eating behavior.
Activation of insula, precuneus, cingulate, sensory/motor, and prefrontal cortices has been reported in relation to swallowing and various other gastrointestinal events, such as subliminal esophageal acid exposure (25), heartburn (22), and external anal sphincter contraction (23). Activation of these same areas by gustatory and olfactory stimuli has also been reported. Despite the common areas of processing, the observed augmentation of these regions during swallowing of flavored fluid compared with inert water does not represent the summation of the deglutition-related activity and that induced by sensory stimulation of the flavor. This assertion is supported by the fact that the nutritive stimuli, i.e., flavored water, were delivered continuously into the mouth, as described in methods; therefore, any activity induced by them would have formed the baseline on which the intermittent swallowing activity was registered and processed. Since the measurement of percent fMRI signal intensity change is determined by signal increase from baseline, the comparison would only take into account signal change due to swallowing.
Because, in this study, we only investigated the effect of flavored nutritive stimuli on left hemispheric swallow-related cortical activity, the enhancing effect might have been underestimated. This may explain why the upward trend in insular activity in response to flavored stimuli did not reach statistical significance. Furthermore, if we consider the small volume of swallowed testants in the present study that potentially could have resulted in their reduced contact with the entire surface of the tongue, it is possible that we may have underestimated the magnitude of gustatory effects on enhancing the cortical swallowing network. Despite the shortcoming imposed by technical limitations, the findings of the present study indicate an accentuating effect of flavored nutritive stimuli on the cortical swallowing network. We did not compare taste prototypes, such as dextrose water, NaCl, quinine, or hydrochloric acid, as they are less representative of common nutritive and flavored liquids and lack the widely recognizable associated image or odor of flavor. Furthermore, it was not the aim of the present study to differentiate the individual contribution of each sensory stimulus to the observed enhancement of swallow-related cortical activity. Future studies delineating the degree of influence of flavor prototypes (sweet, sour, savory, salty, and bitter) and each of the basic senses (gustation, olfaction, and vision) in accentuating swallow-related cortical activity are awaited to achieve optimal modulatory outcome.
In the present study, we did not evaluate the functional consequences of the observed enhancement of swallowing network activity. However, a number of previous studies evaluated the effect of various tastants and odorants on swallowing function. Measuring swallowing intervals by recording submental electromyography showed facilitation of voluntary swallowing induced by chemical stimulation of the posterior tongue and pharyngeal region in humans (49). Studies of deglutition using concurrent gustatory and olfactory stimulation showed an increase in frequency and velocity of swallowing after retronasal olfactory stimulation compared with orthonasal stimulation (48). Sweet and bitter tastes have been shown to modulate excitability of the human cortical swallowing motor pathway (31). Furthermore, earlier studies showed different influences of various tastes, temperature, and carbonation of ingested substances on upper esophageal sphincter opening and deglutitive submental muscle contractions (32). Previous studies also demonstrated a differing modulatory effect of various tastes on biomechanical parameters of swallowing, such as the duration of oral bolus preparation and deglutitive submental muscle electromyogram amplitude and duration (27). The flavor-induced stimulation of the cortical swallowing network, as documented in the present study, provides a mechanistic explanation for those previously reported modulatory effects of gustatory and olfactory stimulation on the deglutitive biomechanical parameters. However, the central control mechanisms of swallowing associated with the above-mentioned modulations in response to gustatory and olfactory stimulation were not investigated in these studies. We recognize that these modulations can occur at the brain stem or the cerebral cortical level or both; therefore, cortical enhancement does not exclude the possibility of a contribution of the brain stem swallowing center to enhancement of cortical swallowing network activity. The findings of the present study suggest the potential for a new approach to dysphagic patients utilizing more robust gustatory, olfactory, and visual stimuli to enhance their deglutitive function. This assertion is also supported by earlier studies using flavored substances in healthy individuals (see above).
In summary, concurrent olfactory, gustatory, and visual stimulation of the ingested substances enhances the activity of the cerebral cortical swallowing network. This increased activity may have implications in the management of dysphagic conditions.
GRANTS
This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants 5R01 DK-025731-29 and 2T32 DK-061923-06.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Ardran GM, Kemp FH. Closure and opening of the larynx during swallowing. Br J Radiol 29: 205–208, 1956 [DOI] [PubMed] [Google Scholar]
- 2.Asoh R, Goyal RK. Manometry and electromyography of the upper esophageal sphincter in the opossum. Gastroenterology 74: 514–520, 1978 [PubMed] [Google Scholar]
- 3.Baylis LL, Rolls ET, Baylis GC. Afferent connections of the caudolateral orbitofrontal cortex taste area of the primate. Neuroscience 64: 801–812, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Birn RM, Bandettini PA, Cox RW, Jesmanowicz A, Shaker R. Magnetic field changes in the human brain due to swallowing or speaking. Magn Reson Med 40: 55–60, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Car A. [Macrophysiological and microphysiological study of the deglutition area of the frontal cortex.] (Abstract). J Physiol (Paris) 63: 183A, 1971 [PubMed] [Google Scholar]
- 6.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature 444: 288–294, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Cherney LR, Halper AS. Swallowing problems in adults with traumatic brain injury. Semin Neurol 16: 349–353, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia 12: 146–156, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Dziewas R, Soros P, Ishii R, Chau W, Henningsen H, Ringelstein EB, Knecht S, Pantev C. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage 20: 135–144, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Farb P, Armelagos G. Consuming Passions: The Anthropology of Eating. Boston: Houghton Mifflin, 1980 [Google Scholar]
- 12.Field LH, Weiss CJ. Dysphagia with head injury. Brain Inj 3: 19–26, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. Br Med J 295: 411–414, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamdy S, Aziz Q, Rothwell JC, Crone R, Hughes D, Tallis RC, Thompson DG. Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet 350: 686–692, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG, Tallis RC, Thompson DG. The cortical topography of human swallowing musculature in health and disease. Nat Med 2: 1217–1224, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol Gastrointest Liver Physiol 277: G219–G225, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Hausser-Hauw C, Bancaud J. Gustatory hallucinations in epileptic seizures. Electrophysiological, clinical and anatomical correlates. Brain 110: 339–359, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Hockman CH, Bieger D, Weerasuriya A. Supranuclear pathways of swallowing. Prog Neurobiol 12: 15–32, 1979 [DOI] [PubMed] [Google Scholar]
- 19.Jean A. Control of the central swallowing program by inputs from the peripheral receptors. J Auton Nerv Syst 10: 225–233, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Jean A. [Localization and activity of medullary swallowing neurones.] J Physiol (Paris) 64: 227–268, 1972 [PubMed] [Google Scholar]
- 21.Kern M, Chai C, Lawal A, Shaker R. Effect of esophageal acid exposure on the cortical swallowing network in healthy human subjects. Am J Physiol Gastrointest Liver Physiol 297: G152–G158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kern M, Hofmann C, Hyde J, Shaker R. Characterization of the cerebral cortical representation of heartburn in GERD patients. Am J Physiol Gastrointest Liver Physiol 286: G174–G181, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kern MK, Arndorfer RC, Hyde JS, Shaker R. Cerebral cortical representation of external anal sphincter contraction: effect of effort. Am J Physiol Gastrointest Liver Physiol 286: G304–G311, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol 280: G354–G360, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Kern MK, Shaker R. Cerebral cortical registration of subliminal visceral stimulation. Gastroenterology 122: 290–298, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6: 691–702, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Leow LP, Huckabee ML, Sharma S, Tooley TP. The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chem Senses 32: 119–128, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85: 938–950, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Martin RE, Kemppainen P, Masuda Y, Yao D, Murray GM, Sessle BJ. Features of cortically evoked swallowing in the awake primate (Macaca fascicularis). J Neurophysiol 82: 1529–1541, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Miller AJ. Deglutition. Physiol Rev 62: 129–184, 1982 [DOI] [PubMed] [Google Scholar]
- 31.Mistry S, Rothwell JC, Thompson DG, Hamdy S. Modulation of human cortical swallowing motor pathways after pleasant and aversive taste stimuli. Am J Physiol Gastrointest Liver Physiol 291: G666–G671, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Miura Y, Morita Y, Koizumi H, Shingai T. Effects of taste solutions, carbonation, and cold stimulus on the power frequency content of swallowing submental surface electromyography. Chem Senses 34: 325–331, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope 109: 1417–1423, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. AJNR Am J Neuroradiol 20: 1520–1526, 1999 [PMC free article] [PubMed] [Google Scholar]
- 35.Narita N, Yamamura K, Yao D, Martin RE, Sessle BJ. Effects of functional disruption of lateral pericentral cerebral cortex on primate swallowing. Brain Res 824: 140–145, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen WPT. The Cerebral Cortex of Man. New York: McMillan, 1950 [Google Scholar]
- 37.Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil 74: 1295–1300, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci 14: 5437–5452, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross MG, Nijland MJ. Development of ingestive behavior. Am J Physiol Regul Integr Comp Physiol 274: R879–R893, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Schoenfeld MA, Neuer G, Tempelmann C, Schussler K, Noesselt T, Hopf JM, Heinze HJ. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience 127: 347–353, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Shepherd GM. Smell images and the flavour system in the human brain. Nature 444: 316–321, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Sherman DJ, Ross MG, Day L, Ervin MG. Fetal swallowing: correlation of electromyography and esophageal fluid flow. Am J Physiol Regul Integr Comp Physiol 258: R1386–R1394, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci 7: 890–901, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Small DM, Bender G, Veldhuizen MG, Rudenga K, Nachtigal D, Felsted J. The role of the human orbitofrontal cortex in taste and flavor processing. Ann NY Acad Sci 1121: 136–151, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport 10: 7–14, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature 392: 282–286, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Toogood JA, Barr AM, Stevens TK, Gati JS, Menon RS, Martin RE. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: a functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp Brain Res 161: 81–90, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Welge-Lussen A, Ebnother M, Wolfensberger M, Hummel T. Swallowing is differentially influenced by retronasal compared with orthonasal stimulation in combination with gustatory stimuli. Chem Senses 34: 499–502, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Yahagi R, Okuda-Akabane K, Fukami H, Matsumoto N, Kitada Y. Facilitation of voluntary swallowing by chemical stimulation of the posterior tongue and pharyngeal region in humans. Neurosci Lett 448: 139–142, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi S. Basic properties of umami and effects on humans. Physiol Behav 49: 833–841, 1991 [DOI] [PubMed] [Google Scholar]
- 51.Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA 94: 4119–4124, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature 360: 339–340, 1992 [DOI] [PubMed] [Google Scholar]