Abstract
Previous studies have shown that oral ingestion of nutrients stimulates secretion of the incretin hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1); however, it is unclear whether there is a dose-dependent response between the amount of nutrient ingested and the secretion of the hormones in vivo. Using our lymph fistula rat model, we previously demonstrated that both GIP and GLP-1 responded dose dependently to increasing amounts of infused dietary lipid and that the GLP-1-secreting cells were more sensitive to changes in intestinal lipid content. In the present study, we investigated the dose-dependent relationships between incretin secretion and the two remaining macronutrients, carbohydrate and protein. To accomplish this objective, the major mesenteric lymphatic duct of male Sprague-Dawley rats was cannulated. Each animal received a single bolus (3 ml) of saline, dextrin, whey protein, or casein hydrolysate (0.275, 0.55, 1.1, 2.2, 4.4 kcal) via a surgically inserted duodenal or ileal feeding tube. Lymph was continuously collected for 3 h and analyzed for GIP and GLP-1 content. Both GIP and GLP-1 outputs responded dose dependently to increasing amounts of dietary carbohydrate but not protein. Additionally, we found that the GIP-secreting cells were more sensitive than the GLP-1-secreting cells to changes in intestinal carbohydrate content.
Keywords: lymph, glucose-dependent insulinotropic polypeptide, glucagon-like peptide-1
glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are intestinal incretin hormones produced from enteroendocrine K cells and L cells, respectively. Although it has been reported that a small subset of duodenal endocrine cells produce both GIP and GLP-1 (25, 34), the majority of the GIP-secreting K cells are located in the proximal small intestine, whereas the GLP-1-secreting L cells are primarily located in the ileum. As incretin hormones, one of the primary roles for these molecules is to enhance postprandial insulin secretion. Beyond this, GIP and GLP-1 have several additional physiological functions. Both hormones upregulate insulin gene transcription and biosynthesis, stimulate pancreatic β-cell proliferation, and inhibit β-cell apoptosis (1, 19, 37). GLP-1 also maintains glucose homeostasis by regulating gastrointestinal motility and food intake (23, 26, 33). On the other hand, GIP enhances lipogenesis by stimulating lipoprotein lipase activity, enhancing fatty acid synthesis and incorporation into triglycerides, and downregulating glucagon-stimulated lipolysis, all of which promote fat deposition rather than mobilization (14). In fact, mice lacking the receptor for GIP are resistant to diet-induced obesity (24, 40), making GIP an attractive target for anti-obesity therapy.
GIP secretion is primarily stimulated by nutrients, whereas GLP-1 secretion is stimulated by a combination of nutrient, neural, and hormonal influences (1). Although we have an understanding of the factors that induce incretin secretion, the mechanisms underlying this process still remain to be elucidated. Whether or not all three macronutrients utilize the same cellular machinery to stimulate hormone release and whether the GIP-secreting K cells and the GLP-1-secreting L cells respond in a similar fashion to the same stimuli are questions yet to be answered. Currently, it is suggested that nutrient absorption is required for GIP release, whereas the presence of nutrients in the intestinal lumen is sufficient to stimulate GLP-1 secretion (12, 13).
Typically, the incretin hormones are measured in systemic blood; however, the concentration of both active GIP and GLP-1 in plasma is low because of portal dilution and rapid degradation by the enzyme dipeptidyl-peptidase IV (DPP-IV). The half-life of GLP-1 is less than 2 min, whereas the half-life for GIP is 7 min in humans but less than 2 min in rodents (1). Recently, we have demonstrated that intestinal lymph is an alternative fluid compartment for the collection and measurement of the incretin hormones. In our lymph fistula rodent model, a catheter is inserted into the major mesenteric lymphatic duct, allowing for continuous lymphatic drainage from the entire gastrointestinal tract (intestine, stomach, pancreas, and portions of the liver) of conscious animals. We found that the concentration of both incretin hormones is higher in intestinal lymph than in either peripheral or portal plasma (9, 21, 22). The higher incretin concentrations are likely due to less dilution by the circulating fluid and less degradation by DPP-IV. There is also evidence to suggest specific targeting of GLP-1 to the lymph compartment (9). The lymph fistula model is an excellent tool to study incretin biology because small changes in secretion can be detected over time. Additionally, the measured concentrations may more accurately mimic the concentration of incretin hormones sensed by the enteric neurons.
Using the lymph fistula model, we had previously shown that both GIP and GLP-1 responded dose dependently to increasing amounts of infused dietary lipid. Additionally, we found that the GLP-1-secreting L cells were more sensitive than the GIP-secreting K cells to changes in the intraluminal lipid content (39). In the present study, we investigated the dose-dependent relationships between incretin secretion and the two remaining macronutrients, carbohydrate and protein.
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats weighing 250–350 g (Harlan Laboratories, Indianapolis, IN) were acclimated to their environment for 2 wk prior to experimentation. During acclimatization, the animals were allowed free access to water and standard chow (Harlan Teklad LM-485 Mouse/Rat Sterilizable Diet, Harlan Laboratories). The animals were housed in a room with controlled humidity (50%) and temperature (21°C) and a 12-h light-dark cycle. The University of Cincinnati Institutional Animal Care and Use Committee approved all procedures.
Surgical procedure and recovery.
Prior to surgery, the animals were fasted overnight with free access to water. Under isoflurane anesthesia, a midline laparotomy was performed. The major mesenteric lymphatic duct was cannulated with polyvinyl chloride tubing (0.5 mm inner diameter, 0.8 mm outer diameter, Tyco Electronics, Castle Hill, Australia), with slight modifications to the procedure described by Bollman et al. (5). Instead of suture, the cannula was secured with a drop of cyanoacrylate glue (Krazy Glue, Columbus, OH). A silicone feeding tube (1.02 mm inner diameter, 2.16 mm outer diameter, VWR International, West Chester, PA) was introduced into the stomach via a small gastrotomy and advanced 1–2 cm beyond the pylorus into the duodenum. The feeding tube was secured with a purse-string ligature in the stomach. For a subset of animals, a smaller silicone feeding tube (0.64 mm inner diameter, 1.19 mm outer diameter, Braintree Scientific, Braintree, MA) was inserted directly into the small intestine, rather than the stomach, ∼25 cm proximal of the ileal-cecal junction, and secured with a purse-string ligature. Both the lymph cannula and the duodenal or ileal feeding tube were exteriorized through the right flank; the abdomen was then closed in two layers. After surgery, the animals were placed in Bollman restraint cages (4) and allowed to recover overnight (18–22 h); the animals were kept in a temperature-regulated chamber (24°C) to prevent hypothermia. To compensate for fluid and electrolyte loss due to lymphatic drainage, a 5% glucose-saline solution was infused into the duodenum at 3 ml/h for 6–7 h, followed by an overnight infusion at 3 ml/h of saline.
Nutrient doses and lymph collection.
To initially test the effect of dietary carbohydrate and protein on incretin secretion, a 3-ml bolus mixture of dextrin (Sigma-Aldrich, St. Louis, MO) plus 0.9% saline or whey protein (100% AnyWhey, Optimum Nutrition, Aurora, IL) plus 0.9% saline was provided as a single meal via the duodenal feeding tube. Because previous reports had documented incretin secretion following a whey-supplemented meal (11, 17, 35), we chose whey as our initial protein source. For each nutrient, five experimental doses were tested (0.275, 0.55, 1.1, 2.2, 4.4 kcal; Table 1, carbohydrate doses; Table 2, protein doses). Three additional groups of animals received a 3-ml bolus mixture of casein hydrolysate (N-Z-Case Plus, Sigma-Aldrich) plus 0.9% saline (0.275, 1.1, 4.4 kcal; Table 2). A group of control animals (n = 14) were given a 3-ml bolus of 0.9% saline. To test the effects of ileal carbohydrate exposure on incretin secretion, a 3-ml mixture of dextrin and 0.9% saline or saline alone was infused as a single bolus via the ileal feeding tube; three doses were tested (0.275, 0.55, 1.1 kcal; Table 1). In our previous study that investigated incretin secretion following increasing doses of dietary lipid (39), we also used lipid doses ranging from 0.275 to 4.4 kcal. The caloric amount of the highest dose (4.4 kcal) is equivalent to half the daily fat intake of the rat. To make comparisons to our previous data, we chose to use the same doses for the present study. The highest dose (4.4 kcal) is equivalent to a third of the daily protein intake and a tenth of the daily carbohydrate intake of the rat.
Table 1.
Carbohydrate doses
| Dose | Dextrin, g | Saline, ml | kcal | No. of Animals |
|---|---|---|---|---|
| Duodenal carbohydrate | ||||
| 1/16 | 0.0688 | 3.00 | 0.275 | 10 |
| 1/8 | 0.1375 | 3.00 | 0.55 | 8 |
| 1/4 | 0.275 | 3.00 | 1.1 | 8 |
| 1/2 | 0.55 | 3.00 | 2.2 | 8 |
| 1 | 1.1 | 3.00 | 4.4 | 10 |
| Ileal carbohydrate | ||||
| 1/16 | 0.0688 | 3.00 | 0.275 | 4 |
| 1/8 | 0.1375 | 3.00 | 0.55 | 4 |
| 1/4 | 0.275 | 3.00 | 1.1 | 4 |
Table 2.
Protein doses
| Dose | Protein, g | Saline, ml | kcal | No. of Animals |
|---|---|---|---|---|
| Whey protein | ||||
| 1/16 | 0.0806 | 3.00 | 0.275 | 8 |
| 1/8 | 0.1613 | 3.00 | 0.55 | 9 |
| 1/4 | 0.3225 | 3.00 | 1.1 | 7 |
| 1/2 | 0.645 | 3.00 | 2.2 | 8 |
| 1 | 1.29 | 3.00 | 4.4 | 8 |
| Casein hydrolysate | ||||
| 1/16 | 0.0688 | 3.00 | 0.275 | 5 |
| 1/4 | 0.275 | 3.00 | 1.1 | 5 |
| 1 | 1.1 | 3.00 | 4.4 | 5 |
The morning after surgery, lymph was collected in a conical centrifuge tube on ice for 1 h to establish fasting lymph, GIP, and GLP-1 outputs. The animals (n = 111, 4–10 animals per group, Tables 1 and 2) were then given the 3-ml bolus of carbohydrate-saline, protein-saline, protein hydrolysate-saline, or saline alone. Thirty minutes following the nutrient bolus, all animals were maintained on a saline infusion at 3 ml/h for the remainder of the collection period. Lymph samples were continuously collected on ice for 0.5, 1, 2, and 3 h following the nutrient bolus. Each sample contained 10% by volume of an antiproteolytic cocktail (0.25 M EDTA, 0.80 mg/ml aprotinin, 80 U/ml heparin).
Measurement of GIP and GLP-1 concentration.
GIP and GLP-1 concentrations were determined by using commercially available sandwich ELISA kits (LINCO Research, St. Charles, MO). The GIP ELISA is specific to active GIP(1-42) and nonactive GIP(3-42) and does not cross-react with glucagon, oxyntomodulin, GLP-1, or GLP-2. As reported by LINCO, both the intra-assay and interassay coefficients of variance (CV) are 3.5%. The final concentrations (pg/ml) were calculated using standards from LINCO; the GIP concentrations were then converted to picomolar (1 pM GIP = 4.4 pg/ml GIP). The GLP-1 ELISA measures biologically active GLP-1(7-37) and GLP-1(7-36)NH2 and will not detect glucagon, GLP-2, and inactive GLP-1(9-37) and GLP-1(9-36)NH2. As reported by LINCO, the intra-assay CV is 7.4% and the interassay CV is 8.0%. The final concentrations (pM) were calculated by use of standards provided by LINCO.
Data and statistical analysis.
Data are presented as means ± SE. Hourly outputs were calculated by multiplying together the hourly lymph volume and GIP or GLP-1 concentrations. Hourly lymph, GIP, and GLP-1 outputs were analyzed by a two-way ANOVA. The Bonferroni t-test was used as the posttest analysis. Differences were considered significant if P < 0.05. Cumulative 3-h secretion for each parameter was determined by summing the hourly outputs for GIP or GLP-1 above the fasting level. Total lymph, GIP, and GLP-1 outputs were examined by a one-way ANOVA with the Bonferroni t-test as the posttest analysis. Protein and protein hydrolysate total GIP and GLP-1 outputs were compared by a Student's t-test. Differences were considered significant if P < 0.05. Additionally, cumulative GIP and GLP-1 outputs were plotted as a function of infused nutrient dose after normalization of the data to saline levels. For each data set, best-fit lines were generated and subjected to linear regression analysis. Slopes were considered significantly different from zero if P < 0.05 (SigmaPlot, version 10.0).
RESULTS
Effect of dietary carbohydrate and whey protein on lymph flow.
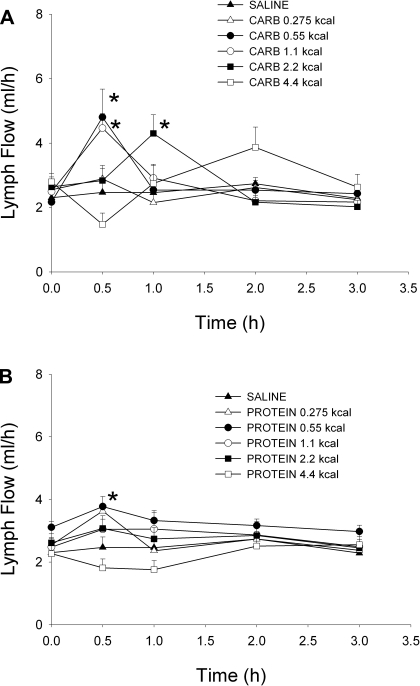
Lymph flow rates for the carbohydrate and protein doses are shown in Fig. 1, A and B, respectively. The fasting lymph flow rates did not differ among the five carbohydrate, five protein, or saline doses. The lymph flow rate for the 0.55 and 1.1-kcal carbohydrate doses (4.80 ± 0.87 and 4.46 ± 0.45 ml/h, respectively) and the 0.55-kcal protein dose (3.77 ± 0.32 ml/h) was significantly different from saline (2.47 ± 0.34 ml/h) 30 min following the nutrient bolus. Additionally, at 1 h, the lymph flow rate for the 2.2-kcal carbohydrate dose (4.30 ± 0.58 ml/h) was significantly different from saline (2.46 ± 0.26 ml/h). Cumulative 3-h lymph flow was similar for all 10 nutrient doses and was not significantly different from saline (data not shown).
Fig. 1.
Hourly lymph flow rate following a duodenal carbohydrate (A), whey protein (B), or saline bolus (0.275, 0.55, 1.1, 2.2, 4.4 kcal for each nutrient). Values are means + SE. *P < 0.05 vs. saline.
Effect of dietary carbohydrate and whey protein on GIP secretion.
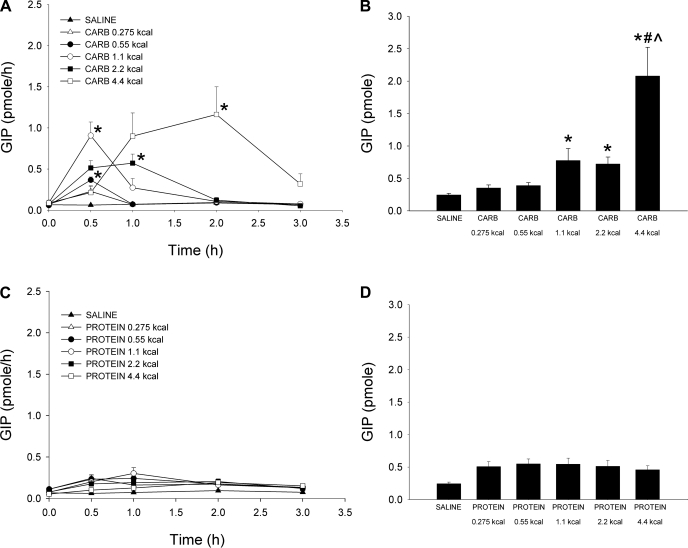
Hourly and cumulative 3-h lymphatic outputs were analyzed to determine the effect of dietary carbohydrate and protein on GIP secretion (Fig. 2). All five carbohydrate doses stimulated GIP secretion above the saline control, and by the end of the 3-h collection period GIP secretion had returned or was returning to baseline for each carbohydrate dose (Fig. 2A). GIP secretion peaked at 0.5 h for the 0.275-, 0.55-, and 1.1-kcal carbohydrate doses and was significantly greater than saline (0.06 ± 0.01 pmol/h) for the 0.55- and 1.1-kcal doses (0.37 ± 0.04 and 0.91 ± 0.17 pmol/h, respectively). For the largest two carbohydrate doses (2.2 and 4.4 kcal), GIP secretion peaked significantly at 1 and 2 h, respectively [1 h: 2.2 kcal carbohydrate (carb) 0.57 ± 0.11 pmol/h, saline 0.07 ± 0.01 pmol/h; 2 h: 4.4 kcal carb 1.16 ± 0.34 pmol/h, saline 0.09 ± 0.01 pmol/h]. Cumulative 3-h lymphatic GIP secretion ranged from 0.35 ± 0.05 (0.275 kcal carb) to 2.08 ± 0.44 pmol (4.4 kcal carb) (Fig. 2B). The total GIP output for the 4.4-kcal carbohydrate dose was significantly different from the 0.275 and 0.55-kcal carbohydrate doses and saline (0.55 kcal carb 0.39 ± 0.05 pmol; saline 0.24 ± 0.03 pmol). Total GIP secretion for carbohydrate doses 2.2 and 1.1 kcal (0.72 ± 0.11 and 0.77 ± 0.19 pmol, respectively) was also significantly different from saline.
Fig. 2.
Hourly (A and C) and cumulative (B and D) lymphatic glucose-dependent insulinotropic polypeptide (GIP) output following either a duodenal carbohydrate, whey protein, or saline bolus (0.275, 0.55, 1.1, 2.2, 4.4 kcal for each nutrient). Hourly output was determined by multiplying together the lymph flow rate by the hourly GIP concentration. Cumulative secretion was calculated by summing together the hourly GIP output values during the 3-h collection period. Values are means + SE. *P < 0.05 vs. saline at time of peak secretion (A). *P < 0.05 vs. saline, #P < 0.05 vs. carbohydrate 0.275 kcal, and ^P < 0.05 vs. carbohydrate 0.55 kcal (B).
In contrast to the hourly secretion pattern in response to carbohydrate, the experimental doses of whey protein did not significantly stimulate GIP secretion above the saline control (Fig. 2C). The cumulative lymphatic GIP secretion ranged from 0.46 ± 0.06 (4.4 kcal protein) to 0.55 ± 0.08 pmol (0.55 kcal protein) and paralleled the trends observed in the hourly output data (Fig. 2D).
Effect of dietary carbohydrate and whey protein on GLP-1 secretion.
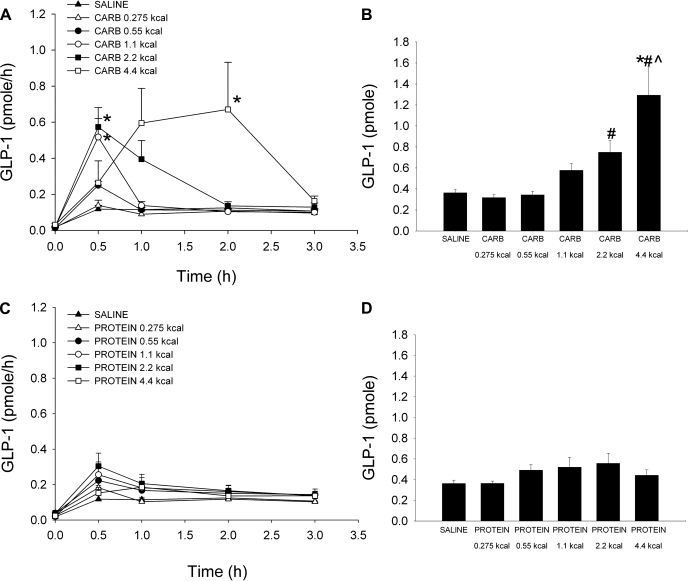
Hourly and 3-h cumulative lymphatic GLP-1 secretion was examined in response to increasing caloric doses of dietary carbohydrate and protein (Fig. 3). All carbohydrate doses stimulated GLP-1 secretion above baseline (Fig. 3A). GLP-1 secretion peaked at 0.5 h for the four lowest carbohydrate doses (0.275, 0.55, 1.1, and 2.2 kcal carb); the peak secretion values for the 1.1- and 2.2-kcal carbohydrate doses (0.52 ± 0.10 and 0.57 ± 0.11 pmol/h, respectively) were significantly greater than saline (0.12 ± 0.02 pmol/h). GLP-1 release peaked later at 2 h for the 4.4-kcal carbohydrate dose (0.67 ± 0.26 pmol/h) and was significantly different from saline (0.13 ± 0.01 pmol/h). By 3 h, GLP-1 levels had returned to baseline for all five doses. Cumulative lymphatic GLP-1 secretion was calculated by summing the hourly GLP-1 outputs over the 3-h collection period (Fig. 3B). The total GLP-1 secretion increased in response to larger amounts of dietary carbohydrate, ranging from 0.32 ± 0.03 pmol for the 0.275-kcal carbohydrate dose to 1.29 ± 0.27 pmol for the 4.4-kcal carbohydrate dose. The total GLP-1 secretion for the 4.4-kcal carbohydrate dose was significantly different from the 0.55 and 0.275-kcal carbohydrate doses and the saline control (0.55 kcal carb 0.34 ± 0.03 pmol; saline 0.36 ± 0.03 pmol); additionally the total GLP-1 secretion for the 2.2-kcal carbohydrate dose was significantly different from the 0.275-kcal carbohydrate dose (2.2 kcal carb 0.75 ± 0.11 pmol).
Fig. 3.
Hourly (A and C) and cumulative (B and D) lymphatic glucagon-like peptide-1 (GLP-1) output following either a duodenal carbohydrate, whey protein, or saline bolus (0.275, 0.55, 1.1, 2.2, 4.4 kcal for each nutrient). Hourly output was determined by multiplying together the lymph flow rate by the hourly GLP-1 concentration. Cumulative secretion was calculated by summing together the hourly GLP-1 output values during the 3-h collection period. Values are means + SE. *P < 0.05 vs. saline at time of peak secretion (A). *P < 0.05 vs. saline, #P < 0.05 vs. carbohydrate 0.275 kcal, and ^P < 0.05 vs. carbohydrate 0.55 kcal (B).
Similar to the hourly GIP response, none of the five whey protein doses significantly raised GLP-1 levels above the saline control (Fig. 3C). The cumulative lymphatic GLP-1 secretion ranged from 0.36 ± 0.02 (0.275 kcal protein) to 0.56 ± 0.10 pmol (2.2 kcal protein) (Fig. 3D); however, the changes were not significantly different from saline or each other.
Comparison of GIP and GLP-1 cumulative secretion in response to dietary carbohydrate and whey protein.
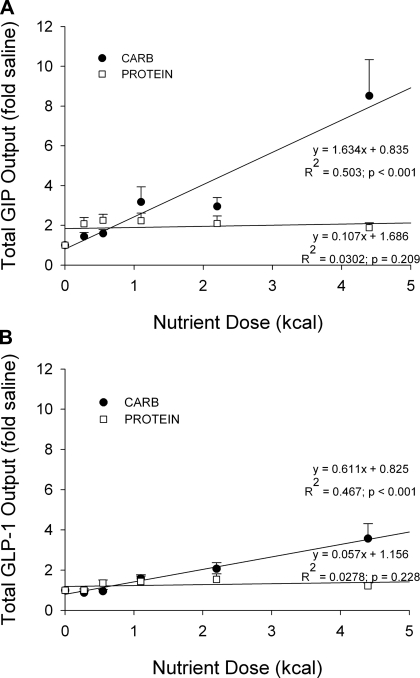
Cumulative GIP and GLP-1 outputs were plotted as a function of infused nutrient dose after first normalizing the data to saline levels to compare the secretory ability of GIP- and GLP-1-producing cells following increasing doses of carbohydrate or protein (Fig. 4). The equations for the best-fit lines generated for the GIP data are y = 1.634x + 0.835 (R2 = 0.503; P < 0.001) for carbohydrate and y = 0.107x + 1.686 (R2 = 0.0302; P = 0.209) for protein (Fig. 4A). The equations for the best-fit lines produced for the carbohydrate and protein GLP-1 data are y = 0.611x + 0.825 (R2 = 0.467; P < 0.001) and y = 0.057x + 1.156 (R2 = 0.0278; P = 0.228), respectively (Fig. 4B). The slopes for both the protein GIP and GLP-1 data are not significantly different from zero; on the other hand, the slopes for the carbohydrate GIP and GLP-1 data are significantly greater than zero, indicating that both GIP- and GLP-1-secreting cells responded dose dependently to increasing amounts of dietary carbohydrate, but not protein.
Fig. 4.
Cumulative lymphatic GIP (A) and GLP-1 (B) output plotted as a function of nutrient dose. Five carbohydrate (●) and 5 whey protein (□) doses were tested (0.275, 0.55, 1.1, 2.2, 4.4 kcal for each nutrient). Values (fold amounts above saline) are means + SE. Equations for best-fit lines generated for GIP and GLP-1 are shown. Only the slopes for the carbohydrate best-fit lines were significantly greater than zero (P < 0.001, carbohydrate GIP; P < 0.001, carbohydrate GLP-1; P = 0.209, protein GIP; P = 0.228, protein GLP-1).
Effect of ileal carbohydrate exposure on incretin secretion.
The steeper slope for the carbohydrate GIP data following the duodenal infusions suggests that the GIP-secreting K cells are more sensitive than the GLP-1-secreting L cells to caloric changes in dietary carbohydrate. Since less distal exposure to nutrients is one explanation for the difference in K cell and L cell responsiveness, the use of ileal infusions would allow exposure of the distal L cells to the same nutrient concentration as the proximal K cells during the duodenal infusions. Accordingly, incretin secretion was monitored after ileal infusions of the three lowest carbohydrate doses (3-ml bolus: 0.275, 0.55, and 1.1 kcal).
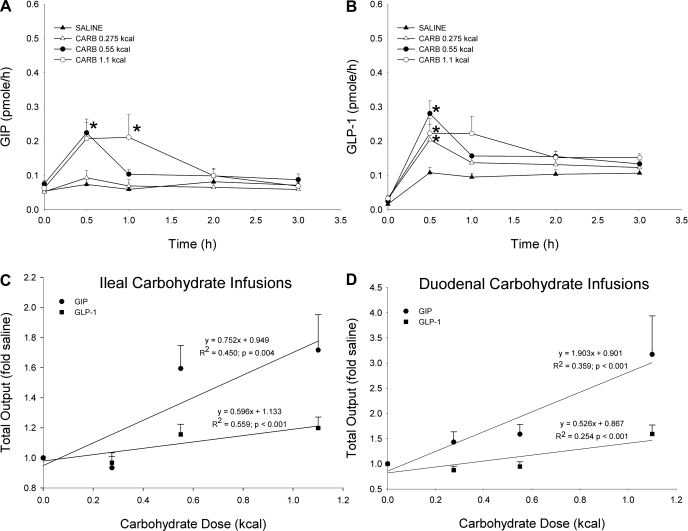
The hourly and 3-h cumulative lymphatic GIP and GLP-1 secretion was analyzed following increasing caloric doses of ileal-infused carbohydrate (Fig. 5). The two highest doses stimulated GIP secretion above the saline control (Fig. 5A). GIP secretion peaked significantly at 0.5 h for the 0.55-kcal dose and at 1 h for the 1.1-kcal dose (0.5 h: 0.55 kcal carb 0.22 ± 0.03 pmol/h, saline 0.07 ± 0.02 pmol/h; 1 h: 1.1 kcal carb 0.21 ± 0.06 pmol/h, saline 0.06 ± 0.01 pmol/h). Likely because of bypassing the large majority of the GIP-secreting K cells, GIP secretion following the ileal infusions is substantially lower than that following duodenal carbohydrate doses of equal caloric value. All three ileal carbohydrate doses elicited peaks in GLP-1 secretion (Fig. 5B) that were significantly greater than saline at 0.5 h (0.275 kcal carb 0.20 ± 0.04 pmol/h, 0.55 kcal carb 0.28 ± 0.04 pmol/h, 1.1 kcal carb 0.22 ± 0.04 pmol/h, saline 0.11 ± 0.02 pmol/h).
Fig. 5.
Hourly lymphatic GIP (A) and GLP-1 (B) output following either an ileal carbohydrate (carb; 0.275, 0.55, 1.1 kcal) or saline bolus. Hourly output was determined by multiplying together the lymph flow rate by the hourly GIP or GLP-1 concentration. Values are means + SE. *P < 0.05 vs. saline at time of peak secretion. Cumulative lymphatic GIP and GLP-1 output plotted a function of carbohydrate dose. Three ileal (C) and 3 duodenal (D) doses were tested (0.275, 0.55, 1.1 kcal). Values (fold amounts above saline) are means + SE. Equations for best-fit lines generated for GIP and GLP-1 are shown. All of the slopes for the best-fit lines were significantly greater than zero.
As before, cumulative GIP and GLP-1 outputs were plotted as a function of infused nutrient dose after first normalizing the data to saline levels to compare the secretory ability of the GIP- and GLP-1-producing cells to ileal-infused (Fig. 5C) or duodenal-infused carbohydrate (Fig. 5D). Since the three lowest doses were used for the ileal infusions, only the responses to the three lowest duodenal-infused doses are shown in Fig. 5D. The slope for the ileal GIP data (0.752) is lower than that for the duodenal GIP data (1.903); as stated previously, this is most likely due to the large bypass of GIP-secreting cells. On the other hand, the slopes for ileal and duodenal GLP-1 data (0.559 vs. 0.526) are almost identical, suggesting that the L cells are equally responsive to either ileal or duodenal carbohydrate; therefore, the distally located L cells are sufficiently exposed to nutrient during the duodenal infusions.
Effect of hydrolyzed protein on GIP and GLP-1 secretion.
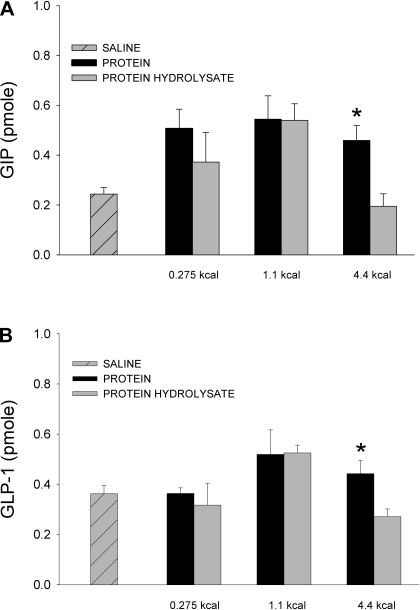
As stated previously, the five whey protein doses did not stimulate significant GIP or GLP-1 secretion. In our study, the nutrient boluses are infused directly into the small intestine; it is possible that bypassing the stomach decreases the amount of protein hydrolysis and subsequently reduces the amount of peptide and amino acids available for intestinal absorption and incretin stimulation. To test this, lymph-fistula rats were given 3-ml boluses of casein hydrolysate (0.275, 1.1, 4.4 kcal). Cumulative incretin output produced by the casein hydrolysate was compared with that following the whey protein challenges. The protein hydrolysate did not stimulate significant GIP (Fig. 6A) or GLP-1 (Fig. 6B) secretion above basal levels. Additionally, there was no difference in cumulative GIP and GLP-1 output between the whey protein and casein hydrolysate infusions at the 0.275 and 1.1-kcal doses. At the 4.4-kcal dose, however, GIP and GLP-1 output was significantly lower for the casein hydrolysate infusions compared with the whey protein infusions (GIP: whey protein 0.46 ± 0.06 pmol, casein hydrolysate 0.19 ± 0.05 pmol; GLP-1: whey protein 0.44 ± 0.06 pmol, casein hydrolysate 0.27 ± 0.03 pmol).
Fig. 6.
Cumulative lymphatic GIP (A) and GLP-1 (B) output following either a duodenal whey protein, protein hydrolysate, or saline bolus (0.275, 1.1, 4.4 kcal for each nutrient). Cumulative secretion was calculated by summing together the hourly GIP or GLP-1 output values during the 3-h collection period. Values are means + SE. *P < 0.05: protein vs. protein hydrolysate at that dose.
DISCUSSION
In this study, we used the lymph fistula rat model to investigate the effects of increasing doses of carbohydrate and protein on GIP and GLP-1 secretion. Here, we demonstrated that both lymphatic GIP and GLP-1 cumulative 3-h outputs, as well as peak hourly outputs, respond dose dependently to increasing amounts of dietary carbohydrate (with the exception of GIP secretion for the 2.2-kcal dose). On the other hand, there was no effect on GIP and GLP-1 secretion to protein doses of equal caloric value. Additionally, our data suggest that the GIP-secreting K cells are more sensitive than the GLP-1-secreting L cells to changes in intraluminal carbohydrate content.
To our knowledge, this is the first report investigating the in vivo relationships between increasing doses of carbohydrate and protein on lymphatic incretin secretion. The benefits of using the lymph fistula rodent model to measure the secretion of GIP and GLP-1 are numerous. The concentration of both incretin hormones is higher in intestinal lymph than in portal or peripheral plasma due to less degradation by DPP-IV and less dilution by the smaller circulating fluid compartment (9, 21, 22). Additionally, this model allows the continuous collection of lymph from conscious animals, eliminating any potential side effects of anesthesia. Furthermore, the hormone concentrations measured in lymph may more accurately mimic the concentrations sensed by the enteric neurons.
In agreement with our carbohydrate data, other investigators have recorded dose-dependent increases in GLP-1 secretion following a glucose stimulus from the GLUTag cell line (16, 29). More recently, primary isolated K and L cells have been shown to respond dose dependently to a glucose challenge (28, 30). Xiao and colleagues (38) also demonstrated that GLP-2, a hormone produced in the L cell and cosecreted with GLP-1, is released in a calorically dependent manner following a dextrose challenge. Furthermore, Schirra and colleagues (32) reported a dose-dependent trend in both glucose-induced GIP and GLP-1 secretion in humans. However, in both the Xiao and Schirra studies, only two nutrient doses were tested. The present study, on the other hand, includes five carbohydrate doses, which provides a more accurate picture of the relationship between increasing carbohydrate loads and incretin hormone secretion.
Despite observing a dose-dependent trend for both carbohydrate-induced GIP and GLP-1 secretion, we did not detect whey protein-induced incretin secretion for any of the five doses that was significantly different from saline or from each other. Similarly, GLP-2 has been found not to respond to a protein-only meal, despite having calorically dependent responses to both carbohydrate and fat (38). However, peptides (7) and amino acids (31) stimulate GLP-1 secretion dose dependently from STC-1 and GLUTag cells, respectively. Moreover, several studies have demonstrated successful in vivo incretin release following a protein-only meal (6, 15, 18). In these studies, the protein meals were ingested orally, whereas we provided our protein doses as bolus meals via an intraduodenal feeding tube. Despite choosing an easily digestible and absorbable protein source (whey) (3, 10), bypassing the stomach may have decreased the amount of protein hydrolysis and thus reduced the amount of peptides/amino acids available for intestinal absorption and incretin stimulation. Yet when this hypothesis was tested with intraduodenal infusions of casein hydrolysate, the secretion of GIP and GLP-1 was not greater than saline for any dose nor was it increased compared with doses of whey protein of equal caloric value, further suggesting that protein is not a potent incretin secretagogue.
Although we did not observe differences between protein-induced GIP and GLP-1 secretion, our data did suggest that GIP-secreting K cells were more sensitive than GLP-1-secreting L cells to changes in the amount of dietary carbohydrate. Plotting the cumulative 3-h outputs against the amount of infused carbohydrate generated best-fit lines with slopes of 1.634 for GIP and 0.611 for GLP-1. The steeper slope for the GIP data suggests that the GIP-secreting cells are more responsive to changes in intraluminal carbohydrate content. In our previously published studies, there was a similar trend: in response to a 4.4-kcal carbohydrate bolus, the peak stimulation for GIP was 11-fold greater than fasting levels vs. 9-fold greater than fasting levels for GLP-1 (21, 22).
Less distal exposure to nutrients is one explanation for the difference in K cell and L cell responsiveness to changes in dietary carbohydrate; however, the GIP response was still greater than the GLP-1 response to increasing doses of carbohydrate delivered directly into the distal small intestine (slope of best-fit lines: GIP 0.752, GLP-1 0.559). Additionally, cumulative GLP-1 secretion was similar regardless whether the carbohydrate boluses were infused via the duodenal or the ileal feeding tube [slope: 0.526 (duodenal) vs. 0.559 (ileal)]. Because the ileal infusions expose the distal L cells to the same nutrient concentration as the proximal K cells during the duodenal infusions, the results further support that the K cells are more sensitive than the L cells to changes in intraluminal carbohydrate content. In light of these results, the data suggest that different mechanisms may control the release of GIP and GLP-1 in response to carbohydrate. Currently, it is proposed that absorption of nutrients is required for GIP release, whereas the presence of nutrients in the intestinal lumen is sufficient to stimulate GLP-1 secretion. Compared with healthy controls, GIP secretion was reduced in patients with intestinal malabsorption (2). Moreover, inhibitors of carbohydrate digestion (12) and absorption (13) decreased GIP secretion without affecting GLP-1 levels. Wachters-Hagedoorn and colleagues (36) also found that the rate of glucose absorption was correlated with plasma concentrations of GIP but not GLP-1. Taken together, these studies and our data suggest that the differential responses of GIP and GLP-1 to increasing doses of dietary carbohydrate are most likely due to the different mechanisms regulating the release of these hormones.
Similar to the carbohydrate results, we have previously reported that both GIP and GLP-1 responded dose dependently to increasing amounts of dietary lipid. However, the GLP-1-secreting cells were more responsive than the GIP-secreting cells to changes in intraluminal lipid content (39). Comparing our present results to the previous study, it appears that both carbohydrate and lipid are equally potent GLP-1 secretagogues (slope of best-fit lines: carbohydrate 0.611, lipid 0.657), whereas carbohydrate is more effective than lipid at stimulating GIP secretion (slope of best-fit lines: carbohydrate 1.634, lipid 0.474). We had previously proposed that the enhanced sensitivity of GLP-1 to changes in dietary lipid reflects the hormone's role in the ileal brake reflex (39). The ileal brake is a distal-to-proximal feedback system that slows the intestinal transit of nutrients to aid digestion and absorption, and GLP-1 is considered to be a putative mediator of this effect (23). Although we did not measure the effect of the nutrient doses on intestinal motility, the data suggest that, compared with lipid, carbohydrate may be contributing equally to the ileal brake reflex via a GLP-1-based mechanism. As lipid or carbohydrate reaches the distal portion of the gut, GLP-1 is secreted in a dose-dependent manner to reduce intestinal transit and enhance proximal nutrient absorption. Indeed, Layer and colleagues (20) have successfully demonstrated the effect of both carbohydrate and fat on the ileal brake reflex and indicate that these responses may not be specific for particular food components but rather a nonspecific effect to the presence of unabsorbed nutrients in the distal small intestine.
Whereas carbohydrate and lipid appear to be equally effective at stimulating GLP-1 secretion, we found that carbohydrate is the more potent GIP secretagogue. The enhanced sensitivity of the GIP-producing K cells to changes in carbohydrate intake reflects the insulinotropic potential of the infused nutrient. Both glucose and fatty acids are capable of stimulating insulin release (8, 27). However, fatty acids induce moderate insulin secretion and are not considered primary insulin secretagogues (41). When challenged with a lipid-based meal, the need to produce insulin is low, and the added insulinotropic effect of GIP-signaling is not essential. In contrast, when provided with a carbohydrate-based meal, regulating glucose homeostasis is necessary; in this scenario, the enhancement of glucose-stimulated insulin secretion via GIP is advantageous. As stated in the introduction, GIP also plays an important role in adipogenesis. Whereas the need to produce insulin may be low when provided a fat-meal, the lipogenic properties of GIP, however, do promote lipid storage in adipocytes.
In conclusion, using our lymph fistula rat model, we have shown that both GIP and GLP-1 respond dose dependently to increasing amounts of dietary carbohydrate, but the incretin response following protein doses (intact whey protein or protein hydrolysate) of equal caloric value was no greater than the saline control. Additionally, we found that the GIP-secreting K cells were more sensitive than the GLP-1-secreting L cells to changes in intestinal carbohydrate content. To our knowledge, this is the first study that uses multiple nutrient doses to investigate in vivo lymphatic incretin secretion. Compared with our previously published data, in which we investigated the simulation of lymphatic incretin secretion by increasing amounts of dietary lipid (39), we hypothesize that the similar GLP-1 responses to lipid and carbohydrate demonstrate the hormone's role in the ileal brake reflex, whereas the much larger effect of carbohydrate on GIP secretion in rodents reflects the insulinotropic potential of the infused nutrient.
GRANTS
We are extremely grateful for support from the American Heart Association Predoctoral Fellowship 09PRE2400160 (S. M. Yoder) and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK082205 (T. L. Kindel), DK056863 (P. Tso), DK059360 (P. Tso), and DK076928 (P. Tso).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 131–157, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Besterman HS, Cook GC, Sarson DL, Christofides ND, Bryant MG, Gregor M, Bloom SR. Gut hormones in tropical malabsorption. Br Med J 2: 1252–1255, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 94: 14930–14935, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollman JL. A cage which limits the activity of rats. J Lab Clin Med 33: 1348, 1948. [PubMed] [Google Scholar]
- 5.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med 33: 1349–1352, 1948 [PubMed] [Google Scholar]
- 6.Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF, Ahrén B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab 295: E779–E784, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Cordier-Bussat M, Bernard C, Levenez F, Klages N, Laser-Ritz B, Philippe J, Chayvialle JA, Cuber JC. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes 47: 1038–1045, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Crespin SR, Greenough WB, Steinberg D. Stimulation of insulin secretion by long-chain free fatty acids. A direct pancreatic effect. J Clin Invest 52: 1979–1948, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. Fasting and postprandial concentrations of glucagon-like peptide 1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol 293: R2163–R2169, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Dangin M, Boirie Y, Guillet C, Beaufrère B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr 132: 3228S–3233S, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Frid AH, Nilsson M, Holst JJ, Björck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr 82: 69–75, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Fukase N, Takahashi H, Manaka H, Igarashi M, Yamatani K, Daimon M, Sugiyama K, Tominaga M, Sasaki H. Differences in glucagon-like peptide-1 and GIP responses following sucrose ingestion. Diabetes Res Clin Pract 15: 187–195, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Fushiki T, Kojima A, Imoto T, Inoue K, Sugimoto E. An extract of Gymnema sylvestre leaves and purified gymnemic acid inhibits glucose-stimulated gastric inhibitory peptide secretion in rats. J Nutr 122: 2367–2373, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Getty-Kaushik L, Song DH, Boylan MO, Corkey BE, Wolfe MM. Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity (Silver Spring) 14: 1124–1131, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, Reimann F, Holst JJ, Gribble FM. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr 89: 106–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52: 1147–1154, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Gunnarsson PT, Winzell MS, Deacon CF, Larson MO, Jelic K, Carr RD, Ahrén B. Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology 147: 3173–3180, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Karamanlis A, Chaikomin R, Doran S, Bellon M, Bartholomeusz FD, Wishart JM, Jones KL, Horowitz M, Rayner CK. Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am J Clin Nutr 86: 1364–1368, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Winter K, Nian C, Tsuneoka M, Koda Y, McIntosh CH. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem 280: 22297–22307, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Layer P, Peschel S, Schlesinger T, Goebell H. Human pancreatic secretion and intestinal motility: effects of ileal nutrient perfusion. Am J Physiol Gastrointest Liver Physiol 258: G196–G201, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am J Physiol Gastrointest Liver Physiol 293: G963–G971, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. Using the lymph fistula rat model to study the potentiation of GIP secretion by the ingestion of fat and glucose. Am J Physiol Gastrointest Liver Physiol 294: G1130–G1138, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Maljaars PW, Peters HP, Mela DJ, Masclee AA. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav 95: 271–281, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8: 738–742, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 114: 189–196, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol Endocrinol Metab 273: E981–E988, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 55: S16–S23, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52: 289–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1 secreting cells. Diabetes 51: 2757–2763, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab 8: 532–539, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 47: 1592–1601, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, Göke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 97: 92–103, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiller RC, Trotman IF, Adrian TE, Bloom SR, Misiewicz JJ, Silk DB. Further characterisation of the ‘ileal brake’ reflex in man effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut 29: 1042–1051, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 290: E550–E559, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav 96: 675–682, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Wachters-Hagedoorn RE, Priebe MG, Heimweg JA, Heiner AM, Englyst KN, Holst JJ, Stellaard F, Vonk RJ. The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J Nutr 136: 1511–1516, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Montrose-Rafizadeh C, Adams L, Raygada M, Nadiv O, Egan JM. GIP regulates glucose transporters, hexokinases, and glucose-induced insulin secretion in RIN 1046-38 cells. Mol Cell Endocrinol 116: 81–87, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Xiao Q, Boushey RP, Drucker DJ, Brubaker PL. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology 117: 99–105, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Yoder SM, Yang Q, Kindel TL, Tso P. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am J Physiol Gastrointest Liver Physiol 297: G299–G305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou H, Yamada Y, Tsukiyama K, Miyawaki K, Hosokawa M, Nagashima K, Toyoda K, Naitoh R, Mizunoya W, Fushiki T, Kadowaki T, Seino Y. Gastric inhibitory polypeptide modulates adiposity and fat oxidation under diminished insulin action. Biochem Biophys Res Commun 335: 937–942, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest 93: 870–876, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]